Abstract
Background:
There has always been a controversy on the hepatectomy for huge hepatocellular carcinoma (HCC). Therefore, we aim to explore the hepatectomy efficacy of huge HCC and its risk factors.
Methods:
A systematic research was performed using PubMed, MedLine, Web of Knowledge, and Cochrane Library from their establishment to August 2017. The major endpoints were overall survival (OS) rate and recurrence-free survival (RFS) rate, and the secondary ones were the morbidity of complications and mortality of hepatectomy.
Results:
About 13 studies with a total of 7609 patients were included in this meta-analysis. The hepatectomy efficacy of huge HCC was inferior to non-huge HCC both in OS (hazard ratio [HR] = 2.18, 95% confidence interval [CI] = 1.90–2.50, P < .00001; I2 = 66%, P = .003) and RFS (HR = 1.97, 95% CI = 1.76–2.19, P < .00001; I2 = 74%, P = .0001). However, the risk difference[RD] of the 1-year, 3-year and 5-year OS tended to be acceptable (RD = −0.05, 95% CI = −0.11–0.00, P = .05; RD = −0.13, 95% CI = −0.21–−0.05, P = .002; RD = −0.10, 95% CI = −0.19–−0.01, P = .03; respectively). Moreover, there were also no significant differences between huge HCC and non-huge HCC in the morbidity of complication and mortality of hepatectomy (RD = 0.07, 95% CI = −0.09–0.23, P = .38; RD = −0.01, 95% CI = −0.00–−0.03, P = .06; respectively). Related risk factors were measured to explore the differences, and the results showed that the level of alpha fetal protein (AFP) and the margin-positive rate were higher (standard mean difference [SMD] = 0.57, 95% CI = 0.26–0.88, P = .0003; odd radio[OR] = 32.52, 95% CI = 1.02–6.22, P = .04; respectively), the characteristic of huge HCC tended to be worse such as lower clinical or pathological stage, incomplete capsule and incorporate satellite metastases (OR = 2.91, 95% CI = 1.68–5.04, P = .001; OR = 3.99, 95% CI = 3.40–4.67, P < .00001; OR = 2.52, 95% CI = 1.66–3.83, P < .0001; respectively), and the rate of micorvascular invasion (MVI) including portal vein tumor thrombus (PVTT) were higher (OR = 3.36, 95% CI = 1.61–7.02, P = .001; OR = 2.75, 95% CI = 2.29–3.31, P < .00001; respectively) in the huge HCC.
Conclusion:
The hepatectomy efficacy of huge HCC was inferior to non-huge HCC, but its survival benefits and feasibility were confirmed in this meta-analysis. In addition, higher level of AFP, positive margin, lower clinical or pathological stage, incomplete capsule, incorporate satellite metastasis and MVI were significantly correlated with poor OS.
Keywords: hepatectomy, huge hepatocellular carcinoma, meta-analysis
1. Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancer in the world.[1] With the development of comprehensive therapy including locoregional and systemic treatment, HCC has not more than a lethal cancer, especially in eastern Asia.[2] Up to now, surgical resection has been considered as the most efficient therapy.[3] While, as for huge HCC (i.e., >10 cm in diameter), it is hard to say.
Firstly, huge HCC is the absolute contraindication of the liver transplantation.[4] Secondly, radiofrequency ablation (RFA)[5] and trans-arterial chemoembolization (TACE)[6] has been proved to be of little efficacy. Moreover, Sorafenib, as the only therapeutic targeted drug approved by the FDA, could not achieve tumor regression.[7] Hence, surgical resection is the only option for huge HCC.
In this meta-analysis, not only the efficacy of hepatectomy of huge HCC was evaluated, but also the safety and feasibility were assessed. And in addition, the risk factors associated with the results were also analyzed.
2. Material and methods
2.1. Literature search
A comprehensive search was conducted to clarify all the published researches of hepatectomy on huge HCC versus non-huge HCC. Both English electronic databases such as PubMed, MedLine, the Cochrane Library, Web of Knowledge, and Chinese databases including Wan Fang, CNKI, and SinoMed were used to seek the literature, from September 1990 to September 2017. Keywords including “hepatocellular carcinoma ” and “hepatectomy” combined with free text words such as “huge” or “giant” or “larger more than 10 cm” or “≥10 cm in diameter” appeared in the electronic search.
2.2. Selection criteria
Huge HCC was defined as the diameter of the HCC tumor exceeding 10 cm.
Inclusion criteria: ① cohort studies and randomized controlled trials were both considered; ② hepatectomy for huge HCC and non-huge HCC; ③ survival was analyzed in the study; ④ sufficient data such as the baseline of characteristic were depicted.
Exclusion criteria: ① in vitro or animal studies; ② case reports, letters, reviews and conference reports; ③ studies based on overlapping cohorts derived from the same center; ④ sample size was not more than 10.
In case of results reported from the same center more than once, the latest was extracted.
2.3. Data extraction
All data were extracted and assessed by 2 independent investigators with predefined forms such as baseline characteristics and outcomes from each study. In the case of disagreement, a third investigator intervened for a conclusion. Hazard ratios (HRs) of overall survival (OS) and recurrence-free survival (RFS) were calculated according to Tierney’ algorithm.[8]
2.4. Intervention definition
Partial resection was divided into anatomical and nonanatomical resection according to whether complianced with anatomical structure, minor and major hepatectomy according to the size of the liver resection, and open and laparoscopic hepatectomy according to whether performed by laparoscope.
Minor hepatectomy was defined as the liver resections <3.
Major hepatectomy was defined as the liver resections no fewer than 3.
2.5. Quality assessment
Cohort studies were assessed by Newcastle–Ottawa scale (NOS),[9] and studies scored as ≥ 6 were considered high quality.[10]
2.6. Statistical analysis
The systematic review and meta-analysis were registered at http://www.researchregistry.com and performed using RevMan Version 5.3. HR was applied as a summary statistic for time-to-event outcomes like OS and RFS, odd ratio (OR) was for the dichotomous outcomes and standard mean difference (SMD) was for the continuous outcomes, and all the results followed with 95% confidence intervals (CI). The χ2 test and I2 statistics were used to assess the heterogeneity; P < .05 or I2>50% were considered as significant heterogeneity. When the hypothesis of homogeneity was not rejected, the fixed-effects model was used to estimate the case with homogeneity, and the random-effects model was used for the cases with significant heterogeneity.[10]
3. Results
3.1. Search
Initially, 324 reports were identified initially by 2 independent reviewers including both English and Chinese. A total of 18 articles were excluded after duplicate removal by NoteExpress 3.1. After browsing the titles and abstracts, 283 records were excluded. Among the remaining 13 articles,[11–23] one record was excluded for lack of enough cases.[13] So, there were 12 reports included in this meta-analysis.[11,12,14–23] In total, 7049 patients were enrolled in this meta-analysis, with 1770 cases in the huge HCC group and 5279 cases in the with non-huge HCC group (Fig. 1)
Figure 1.
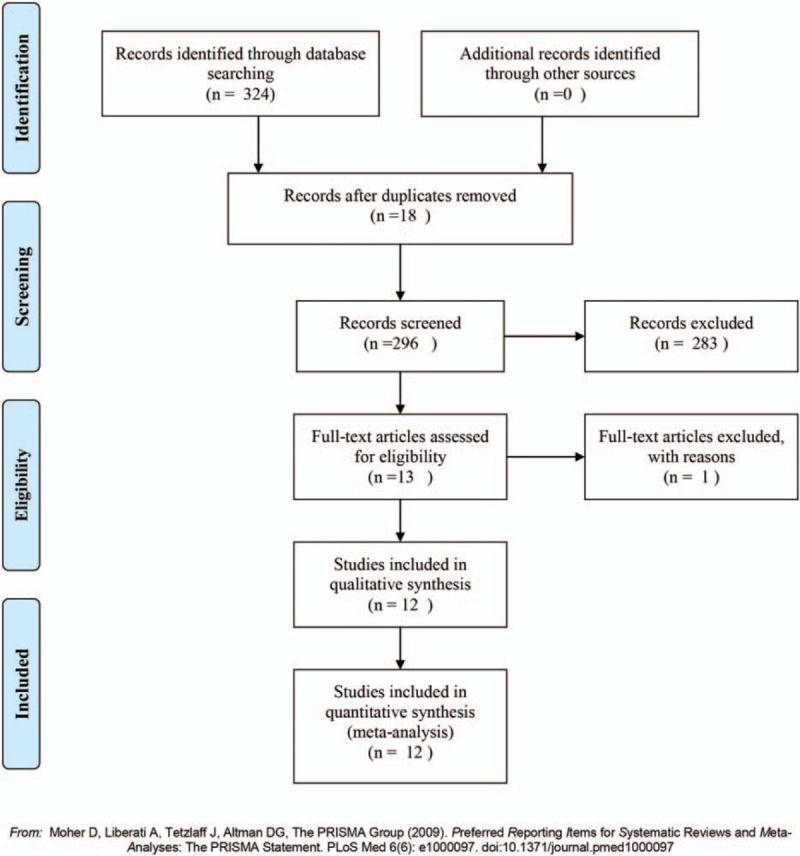
Flowchart of study selection process for meta-analysis.
3.2. Trial characteristics
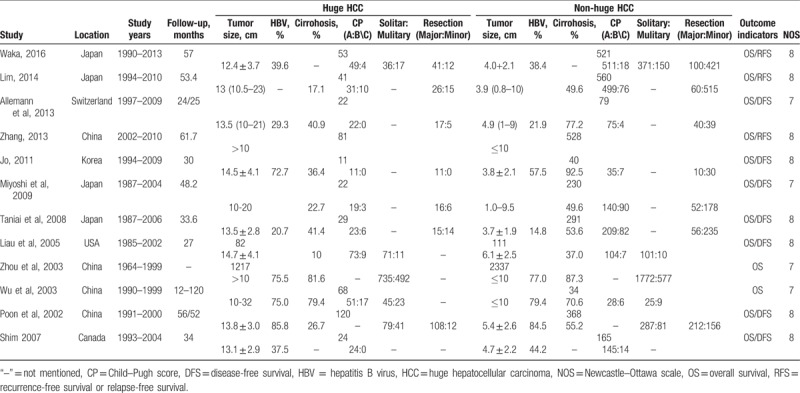
The characteristics and quality of the included trials are shown in Table 1. All the 12 studies included huge HCC group and non-huge HCC group. Follow-up was not mentioned in 1 study, and the remaining had complete follow-up (Table 1). Except for one study, risk factors such as gender, age, preoperative AFP level, tumor characteristic and vascular invasion and so on were involved in all the 12 studies. All the studies included in this meta-analysis were nonrandomized studies and were assessed by NOS. The scores ranged from 7 to 8, indicating that all the studies were high quality (Table 1).
Table 1.
Characteristics of trials included.
3.3. Long-term outcome of hepatectomy for huge HCC and non-huge HCC
Long-term outcome of hepatectomy including OS and RFS rates were reported in the nine studies, and there were significantly differences in the rate of OS (HR = 2.18, 95% CI = 1.90–2.50, P < .00001; I2 = 66%, P = .003. Fig. 2A) and RFS (HR = 1.97, 95% CI = 1.76–2.19, P < .00001; I2 = 74%, P = .0001. Fig. 2B) between huge HCC group and non-huge HCC group. And, there were also significantly decreases in the 1-year, 3-year, and 5-year OS (75% vs 90%, P = .002; 52% vs 74%, P = .002; 40% vs 60%, P = .03; respectively) and RFS (46% vs 73%, P < .0001; 30% vs 47%, P < .00001; 20% vs 35%, P = .0002; respectively. Tables 2 and 3). However, the RDs were considerably small (ranged from −0.20 to −0.05, Table 3), which meant that the long-term outcome of hepatectomy for huge HCC was acceptable.
Figure 2.
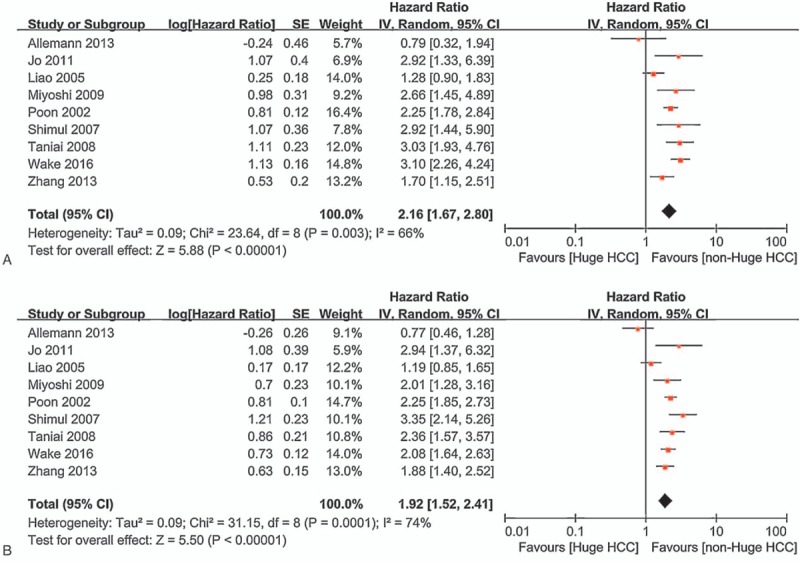
Long-term outcome of hepatectomy for huge HCC and non-huge HCC. HCC = huge hepatocellular carcinoma.
Table 2.
The comparison of 1-year, 3-year, 5-year OS or RFS between huge HCC and non-huge HCC.

Table 3.
Risk factors of the hepatectomy efficacy of huge hepatocellular carcinoma.
3.4. Short-term outcome of hepatectomy for huge HCC and non-huge HCC
Short-term outcome of hepatectomy was assessed by treatment-related complications and hepatectmoy mortality. And, there were no significant differences in the rates of treatment-related complications (RD = 0.07, 95% CI = −0.09–0.23, P = .38; I2 = 66%, P = .05, Fig. 3A) and hepatectmoy mortality (RD = 0.01, 95% CI = −0.00–0.03, P = .06; I2 = 30%, P = .22, Fig. 3B) between huge HCC group and non-huge HCC group.
Figure 3.
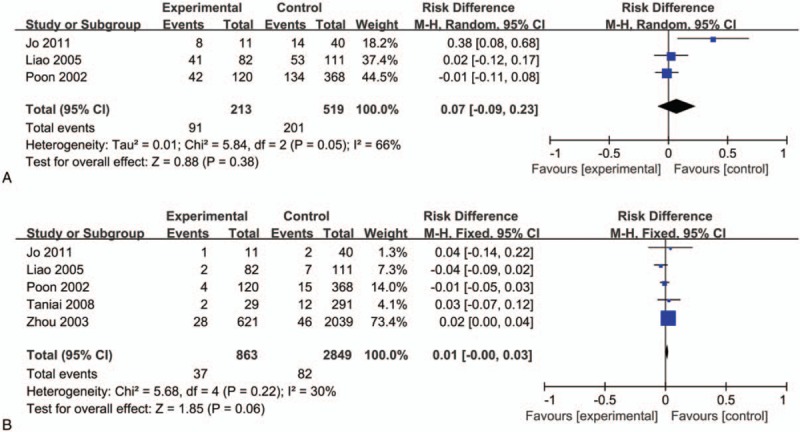
Short-term outcome of hepatectomy for huge HCC and non-huge HCC. HCC = huge hepatocellular carcinoma.
3.5. Risk factors of hepatectomy for huge HCC and non-huge HCC
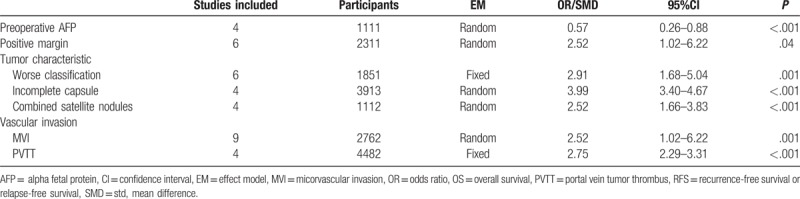
Risk factors such as gender, age, tumor characteristic and so on were analyzed. Results showed that as for the hepatectomy, there were significant differences in the preoperative AFP levels (SMD = 0.57, 95% CI = 0.26–0.88, P = .0003), the rate of positive margin (OR = 32.52, 95% CI = 1.02–6.22, P = .04), the rate of worse classification (OR = 2.91, 95% CI = 1.68–5.04, P = .0001), the rate of incomplete capsule (OR = 3.99, 95% CI = 3.40–4.67, P < .00001), the rate of combined satellite nodules (OR = 2.52, 95% CI = 1.66–3.83, P < .0001), the rate of MVI (OR = 2.52, 95% CI = 1.66–3.83, P = .001), and the rate of PVTT (OR = 2.75, 95% CI = 2.29–3.31, P < .00001) between huge HCC group and non-huge HCC group (Table 3).
3.6. Sensitivity analysis
Significant heterogeneities were observed among the included studies for OS and RFS rate. The studies conducted by Liau et al[19] and Allemann et al[14] showed results were significantly different from others, which likely contributed to the heterogeneities. After excluding these two studies, the pooled HRs for OS rate and RFS rate using the fixed-effect model were 2.47 (95% CI 2.13–2.86, P < .00001; I2 = 18%, P = .29) and 2.21 (95% CI 1.96–2.48, P < .00001; I2 = 0%, P = .47), respectively.
4. Discussion
Hepatectomy is still the most effective therapy for the huge HCC,[12] although the clinical value has always been questioned. In this meta-analysis, the feasibility and efficacy of hepatectomy for huge HCC has been demonstrated by evidence-based medicine.
It is prerequisite for the feasibility of hepatectomy. Tumor size was used to be the restriction of hepatectomy for HCC.[24–26] Hwang et al[27] found that it was resectable for huge HCC, and the rate of anatomical hepatectomy and R0 resection were 91.9% and 89.4%, respectively. Compared with the non-huge HCC, the patients of huge HCC tended to be younger epidemiologically and rarely combined with serious cirrhosis. Hence, it is absolutely feasible of hepatectmoy for huge HCC, especially for those young patients with favorable liver function.
It is essential to evaluate the efficacy of hepatectomy for huge HCC. A propensity score analysis showed that hepatectomy for large HCC (>5 cm in diameter) including huge HCC was superior to local-regional therapy. Similarly, the efficacy of hepatectomy for large HCC including huge HCC was reported to superior to TACE in the latest meta-analysis. And, exceeding two liver sections was considered to be independent prognostic factor for huge HCC. In this meta-analysis, we found that the rates of 1-year, 3-year, and 5-year OS were 75%, 52%, and 40%, which were acceptable, although they were significantly lower than those of non-huge HCC.
Safety is the key of hepatectomy for huge HCC. Severe complications did not increase after hepatectomy, compared with local-regional therapy such as TACE.[28,29] On the other hand, the median mortality was reported to be 3.5% in a systematic review,[30] which was much lower in the latest report.[31] In this meta-analysis, there were no significant differences on the post-operative complications and mortality between huge HCC group and non-huge HCC group, which indicated that it was safe to perform hepatectomy for huge HCC.
Based on these above, huge HCC was not considered to be a contraindication for hepatectomy. However, the criteria should not be expanded unlimitedly. Both tumor characteristic such as classification, capsule, and operative variables including intraoperative estimated blood loss,[32] anatomical or non-anatomical hepatectomy[33–35] were reported to be risk factors of hepatectomy for huge HCC. Other factors including AFP dynamic changes,[36] MVI,[37] and cirrhosis[38] were also considered to affect the efficacy of hepatectomy seriously. All of the risk factors were enrolled in this meta-analysis, and results showed that in the huge HCC group, the level of preoperative AFP was higher, the rate of positive margin increased, the tumor characteristic tended to be worse including lower classification, incomplete capsule and combined satellite nodules, and the incidence of MVI and PVTT increased, too, compared with non-huge HCC. And therefore, it should be prudent for us to conduct hepatectomy for huge HCC patients accompanied with such factors.
There were several limitations in this study. First, there were no RCTs included in this meta-analysis, which made the conclusion sound weaken because cohort data had selection bias. Second, survival data such as OS and DFS/RFS were extracted from survival curves, which might be brought with several tiny errors. Third, the definition of MVI varied due to the lack of a golden standard.[39–41] Fourth, measurement data and enumeration data were mixed up to record the same variable such AFP level.[11,12,15,16] Fifth, the baseline characteristics varied from each other, caution should be taken when interpreting these results. Finally, it was hard to avoid publication bias, because the journals tend to publish positive results.
5. Conclusion
In summary, we concluded that hepatectomy for huge HCC was feasible and effective in this meta-analysis. And, the survival will be improved in the future with the advent of precision hepatectomy, administration of newly targeted drugs and combination with systematic therapy. However, hepatectomy is not suitable for all the huge HCC patients, criteria should be worked out in future, and related risk factors should be taken into consideration.
Footnotes
Abbreviations: DFS = disease-free survival, HCC = huge hepatocellular carcinoma, MVI = micorvascular invasion, OS = overall survival, RFS = recurrence-free survival.
This study was supported by the Science and Technology Infrastructure Construction Program of Fujian Province (Grant No. 2014Y2005)
The authors have no conflicts of interest to disclose.
References
- [1].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- [2].Bertuccio P, Turati F, Carioli G, et al. Global trends and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67:302–9. [DOI] [PubMed] [Google Scholar]
- [3].Kim H, Ahn SW, Hong SK, et al. Survival benefit of liver resection for Barcelona Clinic Liver Cancer stage B hepatocellular carcinoma. Br J Surg 2017;104:1045–52. [DOI] [PubMed] [Google Scholar]
- [4].Parikh ND, Yopp A, Singal AG. Controversies in criteria for liver transplantation in hepatocellular carcinoma. Curr Opin Gastroenterol 2016;32:182–8. [DOI] [PubMed] [Google Scholar]
- [5].Kim YS, Lim HK, Rhim H, et al. Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 2014;28:897–908. [DOI] [PubMed] [Google Scholar]
- [6].Huang YH, Wu JC, Chen SC, et al. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther 2006;23:129–35. [DOI] [PubMed] [Google Scholar]
- [7].Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378–90. [DOI] [PubMed] [Google Scholar]
- [8].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schunemann HJ, Tugwell P, Reeves BC, et al. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Res Synth Methods 2013;4:49–62. [DOI] [PubMed] [Google Scholar]
- [10].Liu W, Zhou JG, Sun Y, et al. Hepatic resection improved the long-term survival of patients with BCLC stage B hepatocellular carcinoma in Asia: a systematic review and meta-analysis. J Gastrointest Surg 2015;19:1271–80. [DOI] [PubMed] [Google Scholar]
- [11].Wakayama K, Kamiyama T, Yokoo H, et al. Huge hepatocellular carcinoma greater than 10 cm in diameter worsens prognosis by causing distant recurrence after curative resection. J Surg Oncol 2017;115:324–9. [DOI] [PubMed] [Google Scholar]
- [12].Lim C, Mise Y, Sakamoto Y, et al. Above 5 cm, size does not matter anymore in patients with hepatocellular carcinoma. World J Surg 2014;38:2910–8. [DOI] [PubMed] [Google Scholar]
- [13].Goh BK, Teo JY, Chan CY, et al. Importance of tumor size as a prognostic factor after partial liver resection for solitary hepatocellular carcinoma: Implications on the current AJCC staging system. J Surg Oncol 2016;113:89–93. [DOI] [PubMed] [Google Scholar]
- [14].Allemann P, Demartines N, Bouzourene H, et al. Long-term outcome after liver resection for hepatocellular carcinoma larger than 10 cm. World J Surg 2013;37:452. [DOI] [PubMed] [Google Scholar]
- [15].Zhang H, Yuan SX, Dai SY, et al. Tumor size does not independently affect long-term survival after curative resection of solitary hepatocellular carcinoma without macroscopic vascular invasion. World J Surg 2014;38:947–57. [DOI] [PubMed] [Google Scholar]
- [16].Jo S. Outcome of hepatectomy for huge hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg 2011;15:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Miyoshi A, Takahashi T, Otsuka T, et al. Efficacy of major hepatectomy for large hepatocellular carcinoma. Hepatogastroenterology 2009;56:768–72. [PubMed] [Google Scholar]
- [18].Taniai N, Yoshida H, Tajiri T. Adaptation of hepatectomy for huge hepatocellular carcinoma. J Hepatobiliary Pancreat Surg 2008;15:410–6. [DOI] [PubMed] [Google Scholar]
- [19].Liau KH, Ruo L, Shia J, et al. Outcome of partial hepatectomy for large (>10 cm) hepatocellular carcinoma. Cancer 2005;104:1948–55. [DOI] [PubMed] [Google Scholar]
- [20].Zhou XD, Tang ZY, Ma ZC, et al. Surgery for large primary liver cancer more than 10 cm in diameter. J Cancer Res Clin Oncol 2003;129:543–8. [DOI] [PubMed] [Google Scholar]
- [21].Wu H, Liang L. Surgical treatment of large hepatocellular carcinoma. Chin J Hepatobiliary Surg 2003;9:13–5. [Google Scholar]
- [22].Poon RT, Fan ST, Wong J. Selection criteria for hepatic resection in patients with large hepatocellular carcinoma larger than 10 cm in diameter. J Am Coll Surg 2002;194:592–602. [DOI] [PubMed] [Google Scholar]
- [23].Shah SA, Wei AC, Cleary SP, et al. Prognosis and results after resection of very large (> or=10 cm) hepatocellular carcinoma. J Gastrointest Surg 2007;11:589–95. [DOI] [PubMed] [Google Scholar]
- [24].Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol 2002;20:1527–36. [DOI] [PubMed] [Google Scholar]
- [25].Vauthey JN, Klimstra D, Blumgart LH. A simplified staging system for hepatocellular carcinomas. Gastroenterology 1995;108:617–8. [DOI] [PubMed] [Google Scholar]
- [26].Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hwang S, Lee YJ, Kim KH, et al. Long-term outcome after resection of huge hepatocellular carcinoma >=10 cm: single-institution experience with 471 patients. World J Surg 2015;39:2519–28. [DOI] [PubMed] [Google Scholar]
- [28].Stevens CL, Awad A, Abbas SM, et al. Systematic review and meta-analysis of hepatic resection versus transarterial chemoembolization for solitary large hepatocellular carcinoma. HPB (Oxford) 2017;19:653–8. [DOI] [PubMed] [Google Scholar]
- [29].Zhu SL, Zhong JH, Ke Y, et al. Efficacy of hepatic resection vs transarterial chemoembolization for solitary huge hepatocellular carcinoma. World J Gastroenterol 2015;21:9630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhou YM, Li B, Xu DH, et al. Safety and efficacy of partial hepatectomy for huge (≥10 cm) hepatocellular carcinoma: A systematic review. Med Sci Monit 2011;17:76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hwang S, Lee YJ, Kim KH, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg 2015;19:1281–90. [DOI] [PubMed] [Google Scholar]
- [32].Lee EC, Kim SH, Park H, et al. Survival analysis after liver resection for hepatocellular carcinoma: a consecutive cohort of 1002 patients. J Gastroenterol Hepatol 2017;32:1055–63. [DOI] [PubMed] [Google Scholar]
- [33].Li SQ, Huang T, Shen SL, et al. Anatomical versus non-anatomical liver resection for hepatocellular carcinoma exceeding Milan criteria. Br J Surg 2017;104:118–27. [DOI] [PubMed] [Google Scholar]
- [34].Zhao H, Chen C, Gu S, et al. Anatomical versus non-anatomical resection for solitary hepatocellular carcinoma without macroscopic vascular invasion: a propensity score matching analysis. J Gastroenterol Hepatol 2017;32:870–8. [DOI] [PubMed] [Google Scholar]
- [35].Kim JM, Kwon CH, Joh JW, et al. Nonanatomical resection is comparable with anatomical resection in solitary hepatocellular carcinoma <5 cm in the right posterior section. Medicine (Baltimore) 2016;95:e5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Shen JY, Li C, Wen TF, et al. Alpha fetoprotein changes predict hepatocellular carcinoma survival beyond the Milan criteria after hepatectomy. J Surg Res 2017;209:102–11. [DOI] [PubMed] [Google Scholar]
- [37].Zhao H, Chen C, Fu X, et al. Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget 2017;8:5474–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhou YM, Sui CJ, Zhang XF, et al. Influence of cirrhosis on long-term prognosis after surgery in patients with combined hepatocellular-cholangiocarcinoma. BMC Gastroenterol 2017;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Sumie S, Kuromatsu R, Okuda K, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375–82. [DOI] [PubMed] [Google Scholar]
- [40].Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Feng LH, Dong H, Lau WY, et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J Cancer Res Clin Oncol 2017;143:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]


