Abstract
Background:
Weaning failure is common in mechanically ventilated patients. Whether ultrasound can predict weaning outcome remains controversial. This meta-analysis was performed to assess the accuracy of diaphragmatic ultrasonography for predicting reintubation within 48 hours of extubation.
Methods:
Literature search was performed in PubMed, Embase, and Cochrane Library to identify all the relevant papers, published in English up to July 16, 2017. Eligible studies were included if data were in adequate details to rebuild 2 × 2 contingency tables. Methodological quality of the included studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) in Review Manager 5.3. The sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR), and summary receiver operating characteristic (SROC) curve were pooled using the fixed or random effects model, meanwhile, the heterogeneity was evaluated using Cochran Q test and I2 statistics in Meta-DiSc 1.4. Publication bias was assessed using Deeks funnel plot in Stata 12.0.
Results:
Thirteen studies with 742 subjects were included in this meta-analysis. The pooled sensitivities for diaphragm excursion (DE) and diaphragm thickness fraction (DTF) were 0.786 and 0.893, and the pooled specificities were 0.711 and 0.796, respectively. The area under curve (AUC) for DE and DTF were 0.8590 and 0.8381. The DORs for DE and DTF were 10.623 and 32.521. No publication bias was observed among these studies.
Conclusions:
Diaphragmatic ultrasonography is a promising tool for predicting reintubation within 48 hours of extubation. However, due to heterogeneities among the included studies, large-scale studies are warranted to confirm our findings.
Keywords: diagnosis, diagnostic accuracy, diaphragmatic displacement, diaphragmatic thickening, mechanical ventilator weaning, ultrasonography, ventilator weaning
1. Introduction
After the recovery of underlying conditions, most patients can be weaned from the mechanical ventilation successfully, given that they have sufficient gas exchange, good neurological and muscular status, and hemodynamic stability. However, weaning failure, defined as the requirement of invasive or noninvasive mechanical ventilation within 48 hours after extubation,[1] is extremely common. About 20% of mechanically ventilated patients confront weaning failure and require reintubation.[2] Weaning failure is associated with prolonged mechanical ventilation and ICU stay, as well as increased hospital mortality[2,3] ranging between 40% and 50%.[4] Delay in weaning from ventilator increases the inherent risks of mechanical ventilation, such as barotrauma, ventilator-associated pneumonia, and ventilator-induced diaphragmatic atrophy.[5] Thus, patients should be weaned from mechanical ventilation once they are able to cope with the respiratory load to avoid diaphragmatic dysfunction and infection, as well as to decrease the length of ICU and hospital stay.[6,7]
Current guidelines for weaning[8,9] recommend spontaneous breathing trial (SBT) as a tool to predict weaning outcome. However, 13% to 26% of patients who are extubated following a successful SBT need to be reintubated within 48 hours.[10,11] Many other weaning parameters have been used to predict weaning failure, including rapid shallow breathing index (RSBI), minute ventilation (VE), and maximum inspiratory pressure (PImax), but none has shown great prognostic accuracy.[2,12]
The diaphragm is the principal respiratory muscle. With an excursion of 1 to 2 cm, the diaphragm provides nearly 75% of the resting pulmonary ventilation, while during the forced breathing, its amplitude is up to 7 to 11 cm.[13] However, the diaphragm is vulnerable to damage from hypotension, hypoxia, and sepsis, all of which are very common in critically ill patients.[14,15] While in surgical patients, diaphragm dysfunction is often caused by acute insults such as trauma or surgical procedures. In addition, mechanical ventilation itself can decrease the force of the diaphragm and induce diaphragmatic dysfunction, named as ventilator-induced diaphragmatic dysfunction.[16,17] Many studies have shown that diaphragm dysfunction might lead to weaning failure and long-term mechanical ventilation.[18–21] Diaphragm dysfunction is responsible for a number of pulmonary complications, including atelectasis and pneumonia, which are risk factors for extubation failure. Hence, an early diagnosis of diaphragm dysfunction (before extubation) is mandatory to avoid weaning failure.
In recent years, diaphragmatic ultrasonography has emerged as a safe, no radiation, bed-side tool for assessment of diaphragm function and prediction of weaning outcome.[19] It provides both morphological and functional information in real time. Furthermore, it allows repeated measurements over time. Several ultrasound techniques, such as B-mode and M-mode, have been used to assess diaphragm sonographic predictors: diaphragm excursion (DE), which measures the distance that the diaphragm is able to move during the respiratory cycle, and diaphragm thickness fraction (DTF), which is the ratio between the difference in thickness from inspiration and expiration divided by the thickness on expiration [(Tinsp − Texp)/Texp].[22,23] DTF and DE during respiration reflect diaphragm function and are similar to “thickening fractions and motion amplitude” of the heart. Diaphragm thickness measured at end inspiration correlates with maximal inspiratory pressure[24] and the change in diaphragm thickness during respiration is strongly related to lung volume.[25]
However, the results are still controversial. For instance, some studies reported that DE and DTF had high sensitivity and specificity in predicting weaning outcome,[26,27] while others had opposite results.[28,29] To gain a more reliable conclusion, we performed this meta-analysis to summarize the overall performance of DE and DTF on predicting reintubation within 48 hours of extubation.
2. Methods
We carried out this systematic review following a predefined protocol, which was published in the International Prospective Register for Systematic Reviews with the registration number CRD42017072593. As it is a meta-analysis of the previous works of literature, approval of the ethics committee was not required.
2.1. Literature search
A comprehensive and systematic literature search was conducted by 2 independent researchers (CL and XL) in PubMed, Embase, and Cochrane Library up to July 16, 2017, using the following terms: “Ventilator Weaning,” “Respirator Weaning,” “Mechanical Ventilator Weaning,” “Ultrasonography,” “Ultrasound Imaging,” “Diaphragm,” “Diaphragmatic displacement,” “Diaphragmatic thickening,” “Sensitivity and Specificity,” “Diagnosis,” and “Diagnostic accuracy.” In addition, the reference lists of the included studies were hand-screened to find potentially related papers.
2.2. Study selection
Studies that met the following criteria were included: participants aged ≥18 years; focused on ability or accuracy of diaphragmatic ultrasonography on predicting weaning outcome; published in English; provided sufficient data to calculate true-positive, false-positive, false-negative and true-negative numbers; defined weaning failure as the requirement of mechanical ventilation within 48 hours after extubation; protocols[30] and conference proceedings published as abstracts[31,32] were excluded.
2.3. Data extraction and quality assessment
Two researchers (CL and XL) independently carried out a primary screening of studies based on the titles and abstracts, then they conducted an examination of full texts in accordance with the eligibility criteria. Data were abstracted from included studies, including first author, year of publication, study period, location, setting, study design, target patient, number of patients, age, gender, patient position, the delay between diaphragmatic ultrasonography and weaning, cutoff value, right or left hemidiaphragm, machine and probe, probe position, number and skills of operator. Study quality was assessed by QUADAS-2,[33] which consisted of 4 domains: patient selection, index test, reference standard, flow and timing. A third author (HC) was invited to resolve any discrepancies between the 2 researchers.
2.4. Statistical analysis
Review Manager 5.3 (Cochrane Collaboration, Oxford, England) was used for quality assessment. Meta-DiSc 1.4 (Romany Cajal Hospital, Madrid, Spain) and Stata 12.0 (Stata Corporation, College Station, TX) were used for analysis. Sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) were pooled independently. The threshold effect was tested using Spearman correlation analysis. Heterogeneity was judged by Cochran Q test and I2 statistics. If significant heterogeneity existed (P < .05 or I2 > 50%), the random effect model was used to calculate the pooled effect size; otherwise, the fixed effect model was applied.[34] Source of heterogeneities was detected by meta-regression analysis. Sensitivity analysis was also performed by removing the trials one by one to find the source of heterogeneities and evaluate the reliability of the results. Probable publication bias was estimated by Deeks funnel plot.
3. Results
3.1. Literature search
The process of literature search and selection is shown in Figure 1. Forty-two papers were obtained according to the search strategy, then 5 duplicates were removed. After screening for the titles and abstracts, 23 papers were excluded due to the irrelevant contents. Among the remaining articles, 8 were excluded because of reviews (n = 5), abstracts (n = 2), and protocol (n = 1). Finally, 6 studies[1,23,27,29,35,36] and 7 hand-screened studies,[22,26,28,37–40] which met the selected criteria, were included in our meta-analysis.
Figure 1.
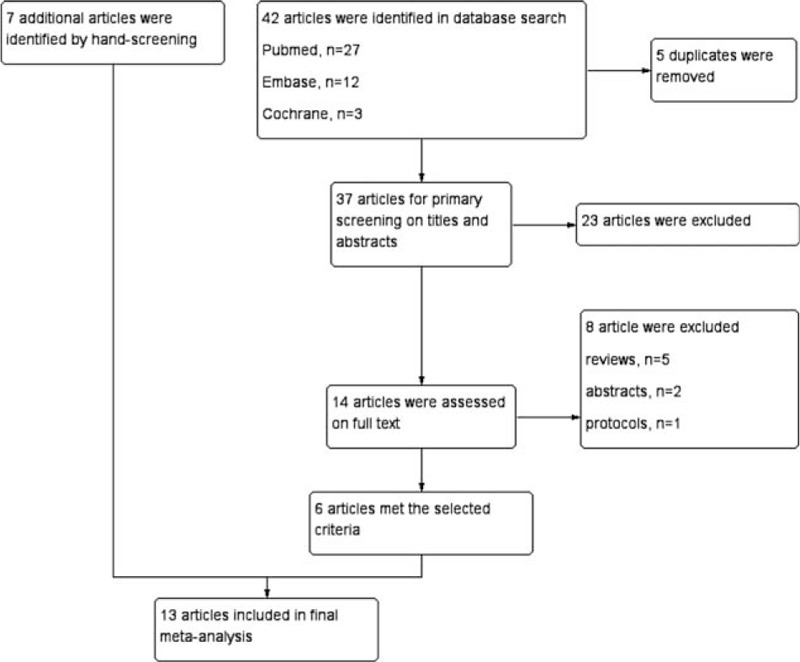
The flowchart of literature search and selection.
3.2. Characteristics and qualities of included studies
Thirteen prospective observational trials, with 742 adult participants, were included in the final meta-analysis and systematic review. These studies, published between 2011 and 2017, were from different countries, such as Egypt, France, Greece, Italy, Korea, and the USA. Nine studies reported data for DE (n = 469), while 8 studies for DTF (n = 485). Most of the studies were conducted in ICU, while 3 studies[26,28,38] took place in the respiratory ICU, 1 study[22] was conducted in a high dependency unit. Usually ultrasounds were performed on patients during the SBT,[1,22,23,26,28,35–38] 3 studies[29,39,40] had ultrasounds carried out before SBT, and only 1[27] had it done after SBT. Most studies reported DE or DTF during the resting breath, while only 2 studies[22,29] reported maximal DE or DTF during the forced breath. Ultrasounds and measurements were conducted in the supine position in 4 studies,[23,26,37,40] but another 9 studies[1,22,27–29,35,36,38,39] have performed the procedure in the semi-recumbent position. The ultrasound beam was directed to the diaphragmatic dome in 6 studies,[23,26,28,29,36,40] While 2 studies[22,35] observed the zone of apposition of the muscle. The characteristics of eligible studies are presented in Table 1. The methodological qualities of included studies were evaluated according to QUADAS-2. The results of quality assessment are shown in Figure 2.
Table 1.
Characteristics of included studies.
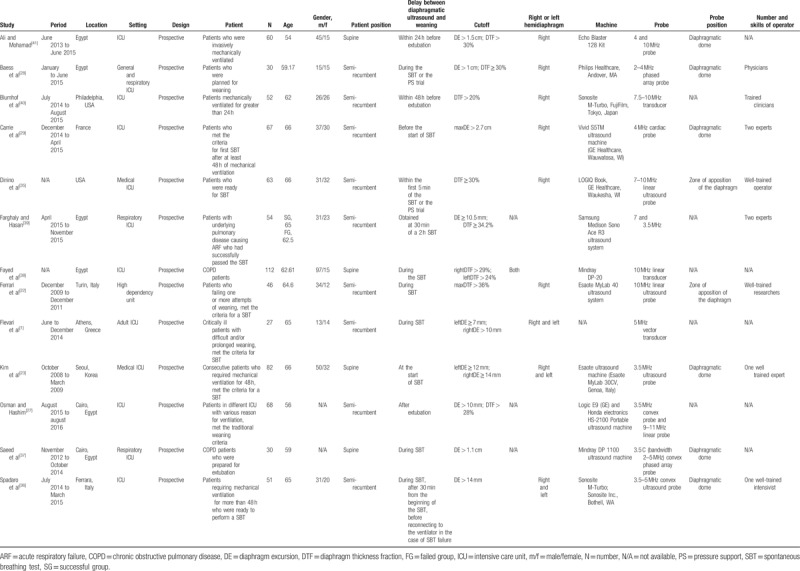
Figure 2.

The methodological quality of included studies.
3.3. Predicting value of DE and DTF on weaning outcome
The threshold effect was estimated using the Spearman correlation coefficient. No threshold effect was detected in DE (Spearman correlation coefficient: −0.234, P = .544) and DTF (Spearman correlation coefficient: 0.144, P = .734), therefore, a further combined analysis could be performed. The sensitivities of DE were homogeneous (P = .075, I2 = 44.0%), thus, the fixed effect model was used and the pooled specificity of DE was 0.786 [95% confidence interval (CI): 0.734–0.832]. Due to significant heterogeneity in sensitivities of DTF (P = .009, I2 = 62.6%), the random effect model was used and the pooled sensitivity of DTF was 0.893 (95% CI: 0.854–0.924). Similarly, because of significant heterogeneity in specificities of DE (P = .000, I2 = 76.3%), the random effect model was used and the pooled specificity of DE was 0.711 (95% CI: 0.637–0.777); however, the specificities of DTF were homogeneous (P = .075, I2 = 45.6%), thus, the fixed effect model was used and the pooled specificity of DTF was 0.796 (95% CI: 0.723–0.857). Significant heterogeneities were detected in NLRs of DE (P = .015, I2 = 57.9%) and DTF (P = .029, I2 = 55.2%), so the random effect model was performed, the pooled NLRs of DE and DTF were 0.287 (95% CI: 0.190–0.435) and 0.157 (95% CI: 0.094–0.259). Owing to significant heterogeneity in PLRs of DE (P = .000, I2 = 84.6%), the random effect model was used, and the pooled PLR of DE was 2.854 (95% CI: 1.474–5.527); conversely, PLRs of DTF were not heterogeneous (P = .393, I2 = 4.8%), the fixed effect model was applied, and the pooled PLR of DTF was 4.257 (95% CI: 3.113–5.822). Moreover, owing to heterogeneity (P = .003, I2 = 65.2%) in DORs of DE, the random effect model was used, and the pooled DOR of DE was 10.623 (95% CI: 4.169–27.068). On the contrary, the DORs of DTF exhibited consistency (P = .127, I2 = 37.9%), therefore, the fixed effect model was used and the combined DOR of DTF was 32.521 (95% CI: 18.618–56.807). The diagnostic performances of DE and DTF on weaning outcome are presented in Table 2 and Figure 3. The summary receiver operating characteristic (SROC) curve was applied to evaluate the comprehensive diagnostic performance. Figure 4 shows the SROC curves, with area under curve (AUC) of 0.8590 for DE and 0.8381 for DTF, indicating the high level of overall accuracy. Fagan nomogram, presented in Figure 5, was also used to analyze the diagnostic value of DE and DTF on weaning outcome.
Table 2.
The diagnostic performance of DE and DTF on weaning outcome.
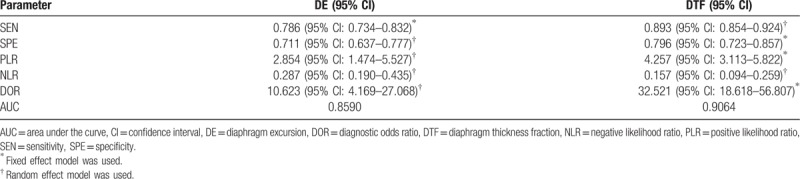
Figure 3.
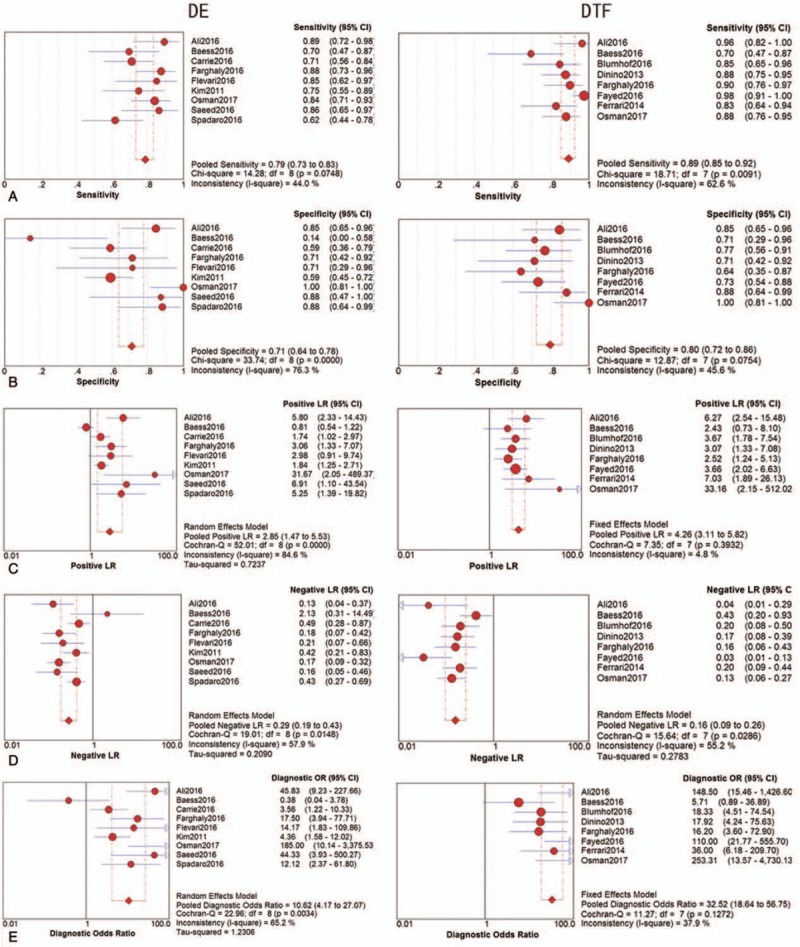
The diagnostic performance of DE and DTF on weaning outcome: (A) sensitivity, (B) specificity, (C) PLR, (D) NLR, (E) DOR. CI = confidence interval, DE = diaphragm excursion, DOR = diagnostic odds ratio, DTF = diaphragm thickness fraction, NLR = negative likelihood ratio, PLR = positive likelihood ratio.
Figure 4.
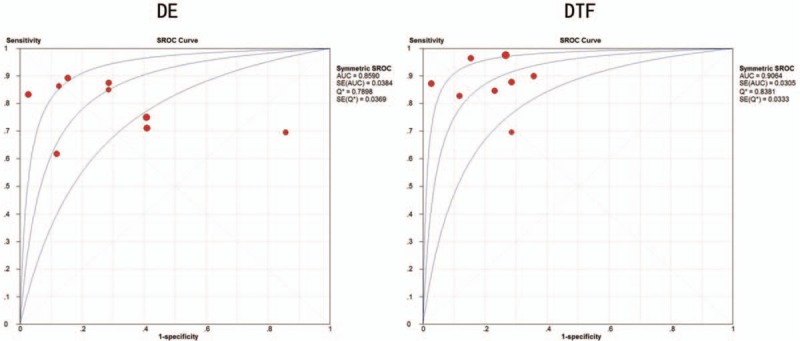
Summary receiver operating characteristic curves of DE and DTF on weaning outcome. DE = diaphragm excursion, DTF = diaphragm thickness fraction, SROC = summary receiver operating characteristic.
Figure 5.
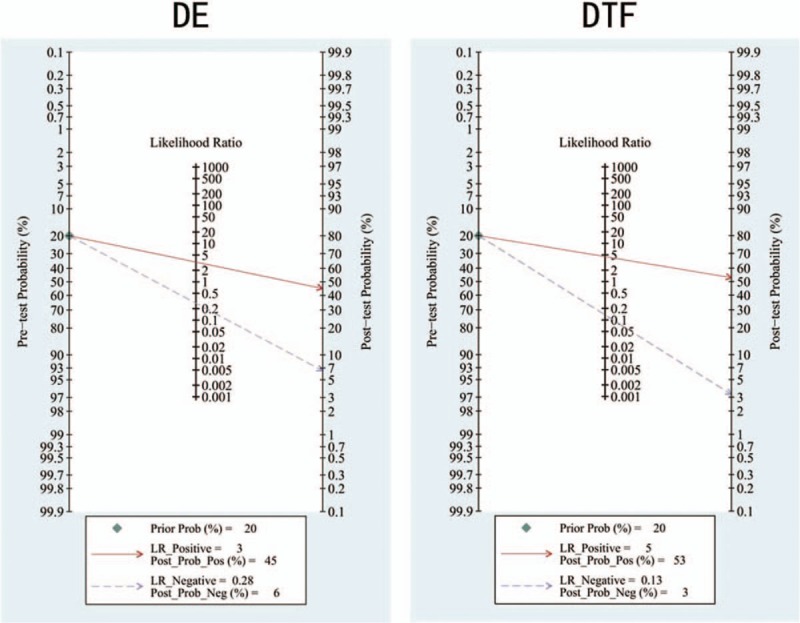
Fagan nomograms of DE and DTF on weaning outcome. DE = diaphragm excursion, DTF = diaphragm thickness fraction, LR = likelihood ratio.
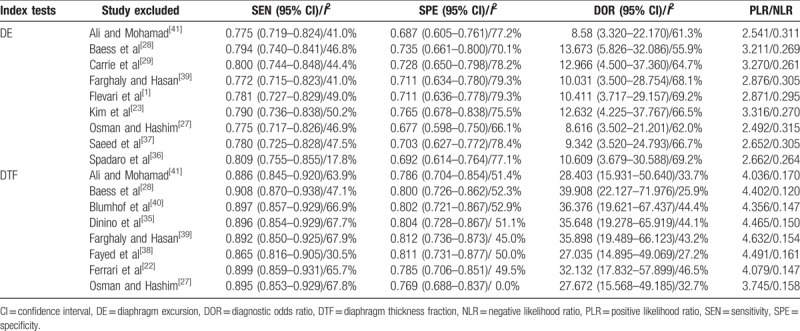
In our meta-analysis, significant heterogeneities were observed. Meta-regression analysis was carried out to find the source of heterogeneities, however, due to the small number of included studies, no significant result was found in target patient (the difficult weaning patient or not), sample size (N ≥ 50 or not), index test (maxDE/maxDTF during the forced breath or DE/DTF during the resting breath) and timing of index test (before/during SBT or after extubation). The results of meta-regression analysis are presented in Table 3. Sensitivity analysis, exhibited in Table 4, was also performed to explore the source of heterogeneities. After removing the trials one by one, the results remained consistent; however, the heterogeneities between studies were not significantly decreased, thus, additional large-scale trials are needed to confirm the findings of this study.
Table 3.
Possible sources of heterogeneity (meta-regression analysis).
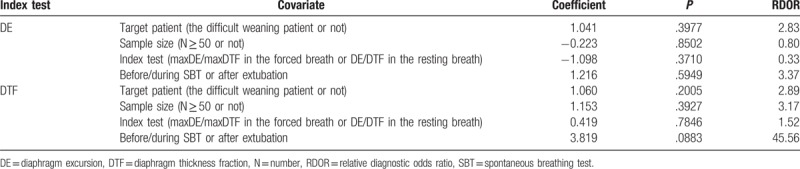
Table 4.
The diagnostic performance of DE and DTF after omitting each trial (sensitivity analysis).
3.4. Publication bias
Publication bias was evaluated by Deeks funnel plot. Shown in Figure 6, the slope coefficient was −5.345298 and −32.12883, the associated P-value was P = .782 and P = .054, respectively, indicating no evidence of publication bias.
Figure 6.

Deeks funnel plots for publication bias.
4. Discussion
Diaphragmatic ultrasonography has gained more and more attention from clinicians all over the world. As it is a noninvasive, painless and convenient bedside method, it might provide more accurate and reliable information regarding the prediction of weaning outcome.[41,42] However, the findings of related studies are inconsistent and lack statistical power, and the clinical significances of DE and DTF still remain controversial. Lerolle et al[18] demonstrated that DE correlated well with trans-diaphragmatic pressure and suggested that DE could reflect diaphragmatic dysfunction. On the contrary, Umbrello et al[43] believed that DTF rather than DE was a reliable index of respiratory effort and diaphragmatic contractile function.
In our meta-analysis, the pooled sensitivities for DE and DTF were 0.786 and 0.893, and the pooled specificities were 0.711 and 0.796, respectively. The SROC curve and the AUC were used to assess the overall diagnostic performance. The AUC for DE and DTF were 0.8590 and 0.8381, respectively. Our data indicate a satisfactory diagnostic accuracy in predicting extubation outcome. The DOR was also analyzed to evaluate the diagnostic accuracy. The DOR is the ratio between PLR and NLR, the larger the DOR is, the greater the diagnostic accuracy is. In this meta-analysis, the DORs for DE and DTF were 10.623 and 32.521, illustrating a high diagnostic accuracy.
Our data suggest a lower sensitivity and specificity for DE as compared to DTF in predicting weaning outcome. DTF reflects the active contraction of the diaphragm during the mechanical ventilation,[44] whereas DE is primarily related to the inspired volume,[45] regardless of whether it depends on the muscle effort or the ventilatory support. Therefore, DTF should be measured to estimate the diaphragm function in patients undergoing mechanical ventilation, while DE is meaningful in the absence of the ventilatory support. Moreover, diaphragm dysfunction is the main cause of weaning failure, but not the only one, it is essential to contextualize the information derived from diaphragmatic ultrasound with clinical and laboratory data, as well as information obtained from other imaging technologies such as X-ray, CT scans, and echocardiography.
RSBI and VE, widely used in clinical practices, are the weaning parameters that measure the volume generated by all the respiratory muscles without especially assessing the contribution of the diaphragm.[23] Under the circumstance of diaphragm dysfunction, the diaphragm motion is inhibited and the accessory muscles take on a greater role in the production of tidal volume (VT).[46,47] Therefore, diaphragm dysfunction can be disguised by the compensatory action of other respiratory muscles during SBT.[30] However, the accessory muscles are weaker and more fatigable than the diaphragm, so the compensatory effect cannot be sustained for a long time.[48,49] Thus, weaning failure may occur despite an initially acceptable RSBI and VT.[35] Spadaro et al[36] reported many patients failed the weaning attempt, though RSBI was much lower than the threshold predicting weaning failure described in the paper by Yang and Tobin.[50] Poor endurance is an important cause of failed weaning. DE and DTF reflect the ability of the diaphragm to generate inspiratory volume, hence, the true diaphragmatic contribution to VT.[43] There are other traditional ways to evaluate diaphragm dysfunction, such as fluoroscopy, electrical or magnetic phrenic nerve stimulation, and trans-diaphragmatic pressure measurement. However, all of these methods possess serious shortcomings, for instance, ionizing radiation, high cost, difficult execution, invasiveness, and inconvenience.[22] What is more, transportation of critical patients to the radiology department is difficult and dangerous.
Similar systemic reviews and meta-analyses have been published recently[8,51]; however, the inclusion criteria, reference standard and major clinical endpoints of these meta-analyses are different from our study. Our study focuses on diaphragmatic ultrasound for predicting weaning failure within 48 hours after extubation in adults, which makes it much more specific and reliable. In addition, the latest trials are included in our paper, so the results are more powerful.
In this meta-analysis, significant heterogeneities were observed. However, sensitivity analysis showed the result of this meta-analysis was robust and reliable. Although meta-regression analysis was performed, we could not explore the source of potential heterogeneities due to the limited number of included studies. It is generally known that the measuring result could deviate as the position of probe changes. Moreover, DE may differ relying on the posture, demonstrating higher values when patients are supine than seated.[52] In addition, DTF may vary as STB progresses owing to diaphragmatic fatigue, that is to say, an early test would show lower accuracy to predict weaning failure. In our meta-analysis, with a coefficient of 3.819 and RDOR of 45.56, the timing of measurement was very nearly significant. Therefore, the exact position of probe, patient posture and the timing of ultrasound should be considered as factors that need to be solved in future studies. Moreover, we might hold that the accuracy of diaphragmatic ultrasound on predicting weaning outcome depends on the number and experience of the operators. Despite being an observer-reliant technique, the available evidence shows that both DTF and DE are replicable measures.[53,54]
In addition, we were also concerned about the effect of publication bias because positive results were more likely to be published. However, Deeks funnel plot indicates no publication bias in our meta-analysis.
There are some limitations in our meta-analysis. First, only articles published in English were included. Second, the influence of gender was not considered, since the excursion and thickness fraction were larger in men than in women. Third, the number of included studies was small and may not have fully assessed the diagnostic accuracy and could not find the source of heterogeneities. Thus, further large-scale studies are needed to investigate the diagnostic accuracy of DE and DTF.
5. Conclusion
Despite the limitations mentioned above, the results of our meta-analysis indicated that diaphragmatic ultrasonography may be a reliable, noninvasive and convenient way to predict reintubation within 48 hours of extubation. However, due to significant heterogeneities among the included studies, clinicians should be aware of the utilities and limitations of this tool. Additional high-quality, large-scale studies are required.
Acknowledgment
We would like to thank Ms. Pratiksha Paudel and Ms. Kalpana for the great help with writing the manuscript in English.
Author contributions
Conceived and designed the experiments: CL. Performed the experiments: CL, XL, HC. Analyzed the data: CL. Contributed reagents/materials/analysis tools: CL, XL, HC, HH, GW, ZW. Wrote the manuscript: CL.
Conceptualization: Caifeng Li, Guolin Wang, Zhiqiang Wang.
Data curation: Caifeng Li.
Formal analysis: Caifeng Li, Xin Li.
Investigation: Caifeng Li, Xin Li, Hongqiu Han, Hailong Cui.
Methodology: Caifeng Li, Hailong Cui, Guolin Wang.
Project administration: Caifeng Li.
Resources: Caifeng Li, Hongqiu Han.
Software: Caifeng Li, Xin Li.
Supervision: Guolin Wang, Zhiqiang Wang.
Validation: Caifeng Li.
Visualization: Caifeng Li.
Writing – original draft: Caifeng Li.
Writing – review & editing: Caifeng Li, Guolin Wang.
Footnotes
Abbreviations: AUC = area under curve, CI = confidence interval, DE = diaphragm excursion, DOR = diagnostic odds ratio, DTF = diaphragm thickness fraction, NLR = negative likelihood ratio, PImax = maximum inspiratory pressure, PLR = positive likelihood ratio, QUADAS = Quality Assessment of Diagnostic Accuracy Studies, RSBI = rapid shallow breathing index, SBT = spontaneous breathing test, SROC = summary receiver operating characteristic, VE = minute ventilation, VT = tidal volume.
Funding: This work was not supported by any funding.
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Availability of data and material: The datasets used during the present study are available from the corresponding author on a reasonable request.
The authors have no conflicts of interest to disclose.
References
- [1].Flevari A, Lignos M, Konstantonis D, et al. Diaphragmatic ultrasonography as an adjunct predictor tool of weaning success in patients with difficult and prolonged weaning. Minerva Anestesiol 2016;82:1149–57. [PubMed] [Google Scholar]
- [2].Epstein SK. Extubation. Respir Care 2002;47:483–92. [PubMed] [Google Scholar]
- [3].Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345–55. [DOI] [PubMed] [Google Scholar]
- [4].Funk G, Anders S, Breyer M, et al. Incidence and outcome of weaning from mechanical ventilation according to new categories. Eur Respir J 2010;35:88–94. [DOI] [PubMed] [Google Scholar]
- [5].Hudson MB, Smuder AJ, Nelson WB, et al. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med 2012;40:1254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Conti G, Montini L, Pennisi MA, et al. A prospective, blinded evaluation of indexes proposed to predict weaning from mechanical ventilation. Intensive Care Med 2004;30:830–6. [DOI] [PubMed] [Google Scholar]
- [7].Sellares J, Ferrer M, Cano E, et al. Predictors of prolonged weaning and survival during ventilator weaning in a respiratory ICU. Intensive Care Med 2011;37:775–84. [DOI] [PubMed] [Google Scholar]
- [8].Ouellette DR, Patel S, Girard TD, et al. Liberation From Mechanical Ventilation in Critically Ill Adults: An Official American College of Chest Physicians/American Thoracic Society Clinical Practice Guideline: Inspiratory Pressure Augmentation During Spontaneous Breathing Trials, Protocols Minimizing Sedation, and Noninvasive Ventilation Immediately After Extubation. Chest 2017;151:166–80. [DOI] [PubMed] [Google Scholar]
- [9].Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J 2007;29:1033–56. [DOI] [PubMed] [Google Scholar]
- [10].Ouanes-Besbes L, Dachraoui F, Ouanes I, et al. NT-proBNP levels at spontaneous breathing trial help in the prediction of post-extubation respiratory distress. Intensive Care Med 2012;38:788–95. [DOI] [PubMed] [Google Scholar]
- [11].Frutos-Vivar F, Ferguson ND, Esteban A, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest 2006;130:1664–71. [DOI] [PubMed] [Google Scholar]
- [12].MacIntyre NR, Cook DJ, Ely EW, Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001;120(6 suppl):s375–95. [DOI] [PubMed] [Google Scholar]
- [13].Yamaguti WP, Paulin E, Shibao S, et al. Ultrasound evaluation of diaphragmatic mobility in different postures in healthy subjects. J Bras Pneumol 2007;33:407–13. [DOI] [PubMed] [Google Scholar]
- [14].Khan J, Harrison TB, Rich MM. Mechanisms of neuromuscular dysfunction in critical illness. Crit Care Clin 2008;24:165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Herridge MS, Batt J, Hopkins RO. The pathophysiology of long-term neuromuscular and cognitive outcomes following critical illness. Crit Care Clin 2008;24:179–99. [DOI] [PubMed] [Google Scholar]
- [16].Schild K, Neusch C, Schonhofer B. [Ventilator-induced diaphragmatic dysfunction (VIDD)]. Pneumologie 2008;62:33–9. [DOI] [PubMed] [Google Scholar]
- [17].Vassilakopoulos T. Ventilator-induced diaphragm dysfunction: the clinical relevance of animal models. Intensive Care Med 2008;34:7–16. [DOI] [PubMed] [Google Scholar]
- [18].Lerolle N, Guérot E, Dimassi S, et al. Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 2009;135:401–7. [DOI] [PubMed] [Google Scholar]
- [19].Matamis D, Soilemezi E, Tsagourias M, et al. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med 2013;39:801–10. [DOI] [PubMed] [Google Scholar]
- [20].Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care 2010;16:19–25. [DOI] [PubMed] [Google Scholar]
- [21].Vassilakopoulos T, Zakynthinos S, Roussos C. The tension–time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med 1998;158:378–85. [DOI] [PubMed] [Google Scholar]
- [22].Ferrari G, De FG, Elia F, et al. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 2014;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim WY, Suh HJ, Hong SB, et al. Diaphragm dysfunction assessed by ultrasonography: influence on weaning from mechanical ventilation. Crit Care Med 2011;39:2627–30. [DOI] [PubMed] [Google Scholar]
- [24].Mccool FD, Conomos P, Benditt JO, et al. Maximal inspiratory pressures and dimensions of the diaphragm. Am J Respir Crit Care Med 1997;155:1329–34. [DOI] [PubMed] [Google Scholar]
- [25].Cohn D, Benditt JO, Eveloff S, et al. Diaphragm thickening during inspiration. J Appl Physiol 1997;83:291–6. [DOI] [PubMed] [Google Scholar]
- [26].Saeed A, El Assal G, Ali T, et al. Role of ultrasound in assessment of diaphragmatic function in chronic obstructive pulmonary disease patients during weaning from mechanical ventilation. Egypt J Bronchol 2016;10:167–72. [Google Scholar]
- [27].Osman AM, Hashim RM. Diaphragmatic and lung ultrasound application as new predictive indices for the weaning process in ICU patients. Egypt J Radiol Nucl Med 2017;48:61–6. [Google Scholar]
- [28].Baess A, Abdallah T, Emara D, et al. Diaphragmatic ultrasound as a predictor of successful extubation from mechanical ventilation: thickness, displacement, or both? Egypt J Bronchol 2016;10:162–6. [Google Scholar]
- [29].Carrie C, Gisbert-Mora C, Bonnardel E, et al. Ultrasonographic diaphragmatic excursion is inaccurate and not better than the MRC score for predicting weaning-failure in mechanically ventilated patients. Anaesth Crit Care Pain Med 2016;36:9–14. [DOI] [PubMed] [Google Scholar]
- [30].Zhou P, Zhang Z, Hong Y, et al. The predictive value of serial changes in diaphragm function during the spontaneous breathing trial for weaning outcome: a study protocol. BMJ Open 2017;7:e015043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Palkar A, Darabaner R, Singh K, et al. Serial lung and diaphragm ultrasonography to predict successful discontinuation of mechanical ventilation. Chest 2016;150:465A. [DOI] [PubMed] [Google Scholar]
- [32].Liu LX, Su D, Hu ZJ. [The value of the excursion of diaphragm tested by ultrosonography to predict weaning from mechanical ventilation in ICU patients]. Zhonghua Nei Ke Za Zhi 2017;56:495–9. [DOI] [PubMed] [Google Scholar]
- [33].Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. [DOI] [PubMed] [Google Scholar]
- [34].Zamora J, Abraira V, Muriel A, et al. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol 2006;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].DiNino E, Gartman EJ, Sethi JM, et al. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014;69:423–7. [DOI] [PubMed] [Google Scholar]
- [36].Spadaro S, Grasso S, Mauri T, et al. Can diaphragmatic ultrasonography performed during the T-tube trial predict weaning failure? The role of diaphragmatic rapid shallow breathing index. Crit Care 2016;20:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fayed AM, Abd ElHady MA, Shaaban M, et al. Use of ultrasound to assess diaphragmatic thickness as a weaning parameter in invasively ventilated chronic obstructive pulmonary disease patients. J Am Sci 2016;12:96–105. [Google Scholar]
- [38].Farghaly S, Hasan AA. Diaphragm ultrasound as a new method to predict extubation outcome in mechanically ventilated patients. Aust Crit Care 2016;30:37–43. [DOI] [PubMed] [Google Scholar]
- [39].Blumhof S, Wheeler D, Thomas K, et al. Change in diaphragmatic thickness during the respiratory cycle predicts extubation success at various levels of pressure support ventilation. Lung 2016;194:519–25. [DOI] [PubMed] [Google Scholar]
- [40].Ali ER, Mohamad AM. Diaphragm ultrasound as a new functional and morphological index of outcome, prognosis and discontinuation from mechanical ventilation in critically ill patients and evaluating the possible protective indices against VIDD. Egypt J Chest Dis Tuberculosis 2016;66:339–51. [Google Scholar]
- [41].Goligher EC, Laghi F, Detsky ME, et al. Erratum to: Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med 2015;41:734. [DOI] [PubMed] [Google Scholar]
- [42].Testa A, Soldati G, Giannuzzi R, et al. Ultrasound M-mode assessment of diaphragmatic kinetics by anterior transverse scanning in healthy subjects. Ultrasound Med Biol 2011;37:44–52. [DOI] [PubMed] [Google Scholar]
- [43].Umbrello M, Formenti P, Longhi D, et al. Diaphragm ultrasound as indicator of respiratory effort in critically ill patients undergoing assisted mechanical ventilation: a pilot clinical study. Crit Care 2015;19:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Goligher EC, Fan E, Herridge MS, et al. Evolution of diaphragm thickness during mechanical ventilation. impact of inspiratory effort. Am J Respir Crit Care Med 2015;192:1080–8. [DOI] [PubMed] [Google Scholar]
- [45].Houston JG, Angus RM, Cowan MD, et al. Ultrasound assessment of normal hemidiaphragmatic movement: relation to inspiratory volume. Thorax 1994;49:500–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yan S, Lichros I, Zakynthinos S, et al. Effect of diaphragmatic fatigue on control of respiratory muscles and ventilation during CO2 rebreathing. J Appl Physiol 1993;75:1364–70. [DOI] [PubMed] [Google Scholar]
- [47].Yan S, Sliwinski P, Gauthier AP, et al. J Appl Physiol 1993;75:1371–7. [DOI] [PubMed] [Google Scholar]
- [48].Hershenson MB, Kikuchi Y, Loring SH. Relative strengths of the chest wall muscles. J Appl Physiol 1988;65:852–62. [DOI] [PubMed] [Google Scholar]
- [49].Hershenson MB, Kikuchi Y, Tzelepis GE, et al. Preferential fatigue of the rib cage muscles during inspiratory resistive loaded ventilation. J Appl Physiol 1989;66:750–4. [DOI] [PubMed] [Google Scholar]
- [50].Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med 1991;324:1445–50. [DOI] [PubMed] [Google Scholar]
- [51].Llamas-Álvarez AM, Tenza-Lozano EM, Latour-Pérez J. Diaphragm and lung ultrasound to predict weaning outcome: systematic review and meta-analysis. Chest 2017;152:1140–50. [DOI] [PubMed] [Google Scholar]
- [52].Haji K, Royse A, Green C, et al. Interpreting diaphragmatic movement with bedside imaging, review article. J Crit Care 2016;34:56–65. [DOI] [PubMed] [Google Scholar]
- [53].Bobbia X, Clément A, Claret PG, et al. Diaphragmatic excursion measurement in emergency patients with acute dyspnea: toward a new diagnostic tool? Am J Emerg Med 2016;34:1653–7. [DOI] [PubMed] [Google Scholar]
- [54].Francis CA, Hoffer JA, Reynolds S. Ultrasonographic evaluation of diaphragm thickness during mechanical ventilation in intensive care patients. Am J Crit Care 2016;25:e1–8. [DOI] [PubMed] [Google Scholar]


