Abstract
There is growing evidence that nonalcoholic fatty liver disease (NAFLD) is associated with a higher risk of urolithiasis, but it has not yet been determined that this association is reproducible and consistent across different studies. We performed a systematic review and meta-analysis of these studies to examine the association between NAFLD and the risk of urolithiasis.
We searched PubMed, EMBASE, and Google scholar using terms “fatty liver” (OR “non-alcoholic fatty liver disease” OR “non-alcoholic steatohepatitis” OR “NAFLD” OR “NASH”) AND “urolithiasis” (OR “nephrolithiasis” OR “kidney stone” OR “urinary calculi” OR “renal colic” OR “urologic disease”). Observational studies in which NAFLD and urolithiasis were diagnosed by either ultrasonography or computerized tomography were included.
A total of 7 observational studies with 226,541 individuals (24.7% with NAFLD) and 19,184 urolithiasis (8.5%). NAFLD was significantly associated with an increased risk of urolithiasis (random effect odds ratio, OR 1.73, 95% confidence interval, CI 1.24–2.40, I2=94.5%). Sensitivity analyses revealed the robustness of the results. Egger test and Begg test suggested no publication bias (P > .05).
NAFLD is associated with an increased risk of urolithiasis. Therefore, patients with NAFLD should be carefully monitored for the development of urolithiasis.
Keywords: meta-analysis, nonalcoholic fatty liver disease, urolithiasis
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of disease that ranges from steatosis to non-alcoholic steatohepatitis (NASH) with varying stage of fibrosis and cirrhosis.[1–3] NAFLD is one of the most common causes of chronic liver disease worldwide, affecting up to 25% of the population globally.[4–6] Worryingly, the estimated prevalence of NASH among NAFLD patients has been reported to be 59.1% or 6.67% for those with or without specific clinical indication, respectively.[4] During the past decade, the recognition of the importance of NAFLD and its interaction with metabolic syndrome has stimulated a growing interest in the potential role of NAFLD in the development of cardiovascular disease (CVD) and chronic kidney disease (CKD).[1,7–10] Accumulating evidence shows that NAFLD is not only linked to an increased risk of liver-related morbidity or mortality, but also NAFLD affects some extra-hepatic organs as a multisystem disease, including the cardiovascular and renal systems.[11–14]
Population-based studies have demonstrated a higher risk of developing CVD and CKD among NAFLD patients, with the more advanced forms of NAFLD predicting a higher risk of future CVD and CKD events.[15–17] Similarly, the putative link between NAFLD and urolithiasis has also attracted scientific interest. Several cross-sectional and prospective studies have demonstrated that the prevalence of urolithiasis was also significantly increased among patients with NAFLD.[18–24] Recently, a large cohort study involving a total of 208,578 Korean adults who underwent a health checkup examination from January 2002 to December 2014, suggesting that NAFLD was significantly associated with an increased incidence of urolithiasis.[21] Collectively, there is currently growing evidence suggesting a close link between NAFLD and a higher risk of urolithiasis, the available data on the association between NAFLD and urolithiasis; however, is quantitatively limited. Also, it has not yet been determined that this association is reproducible and consistent across different studies, although the cross-sectional association between NAFLD and increased prevalence of urolithiasis. Moreover, the exact mechanisms linking NAFLD to urolithiasis remains unclear, although several potential mechanisms have been proposed concerning the hepatic steatosis, insulin resistance, and oxidative stress.[25–29]
In the present study, we performed a systematic review and meta-analysis of cross-sectional and prospective studies to determine the magnitude of the association between NAFLD and the risk of urolithiasis. Clarification of these issues may have critical implications for managing patients with NAFLD and provide evidence of screening for urolithiasis in NAFLD patients.
2. Methods
2.1. Literature search strategy and study selection
PubMed, EMBASE, and Google Scholar were searched for relevant articles published through May 2018. The keywords or MeSH terms used for the strategy were “fatty liver” (OR “non-alcoholic fatty liver disease” OR “non-alcoholic steatohepatitis” OR “NAFLD” OR “NASH”) AND “urolithiasis” (OR “nephrolithiasis” OR “kidney stone” OR “urinary calculi” OR “renal colic”). Also, we identified literature cited by the articles retrieved from the databases. Studies were included and excluded according to the preferred reporting items for systematic reviews and meta-analyses) flow diagram.
Studies were included and excluded following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.[30] Also, we followed the meta-analysis of observational studies in epidemiology (MOOSE) guidelines for the meta-analysis of observational studies, because of the observational design of included studies.[31] All analyses were based on previously published studies, thus no ethical approval and patient consent are required.
2.2. Study selection criteria
Two researchers independently inspected all studies identified through the search. Eligible studies met the following criteria: the design of studies was observational, prospective or retrospective studies. The studies that reported urolithiasis events among adult patients with NAFLD using subjects without NAFLD as a control. The diagnosis of NAFLD was based on ultrasonography, CT or histology in the absence of other causes of steatosis, such as alcohol consumption. Only studies published in English were included. Studies that met any of following criteria were excluded: non-original articles (including reviews, letters, and editorials); studies conducted in the adolescent population (< 18 years).
2.3. Data extraction and quality assessment
Data extraction was performed using predefined forms. Data extracted from these publications were verified by another researcher. The disagreement was resolved by consensus. The extracted data included the following items: authors, publication year, country or region of the study, sample size, the diagnosis criteria of NAFLD and urolithiasis, the number of participants in the group of NAFLD and control, the prevalence or incidence of urolithiasis in both groups. Observational studies were evaluated based on the Newcastle–Ottawa scale (NOS) as recommended by the Cochrane collaboration.[32] NOS was developed to assess the quality of nonrandomized studies with its design, content, and usability. A 'star system’ has been proposed in which a study is assessed in three domains: selection (maximum 4 stars), comparability (maximum 2 stars), and exposure/outcome (maximum 3 stars).
2.4. Statistical analysis
Statistical analyses were performed using the Stata version 12.0 software program (StataCorp LP in College Station, TX). Odds ratios (ORs) or relative risks (RR) or hazard ratios (HRs) were pooled with their 95% confidence intervals (CI), with the assumption that these are comparable measures of association because of the relatively rare prevalence of urolithiasis.[33] For dichotomous data, summary statistics are expressed as an OR with a 95% CI. The Z-test determined the significance of the pooled ORs, and a value of P < .05 was defined as statistically significant. The statistical heterogeneity among studies was assessed with I2-statistics and Cochran's Q statistic.[34] The fixed-effects model was used to estimate the summary OR if no significant heterogeneity was present (P ≥ .10). Otherwise, the random-effects model was used when significant heterogeneity existed (P < .10). Publication bias was evaluated with Egger test and Begg test.[35]
3. Results
3.1. Characteristics of included studies
Searches of the PubMed and EMBASE databases yielded 1063 citations. We identified 386 potentially relevant articles. Of these, we excluded 279 studies for the reasons reported in the PRISMA diagram (Fig. 1). Finally, 7 observational studies, including 8 comparisons, were eligible for inclusion in the meta-analysis and were assessed for quality. Among all the eligible studies, there are 6 cross-sectional studies and 1 cohort study. The only cohort study included in the meta-analysis reported the incident or prevalent urolithiasis stratified by gender in NAFLD patients compared to in those without NAFLD, without available data on the overall incidence of urolithiasis (Table 1).[18–24] The diagnosis of NAFLD and urolithiasis was determined by imaging (either ultrasonography or computed tomography).
Figure 1.
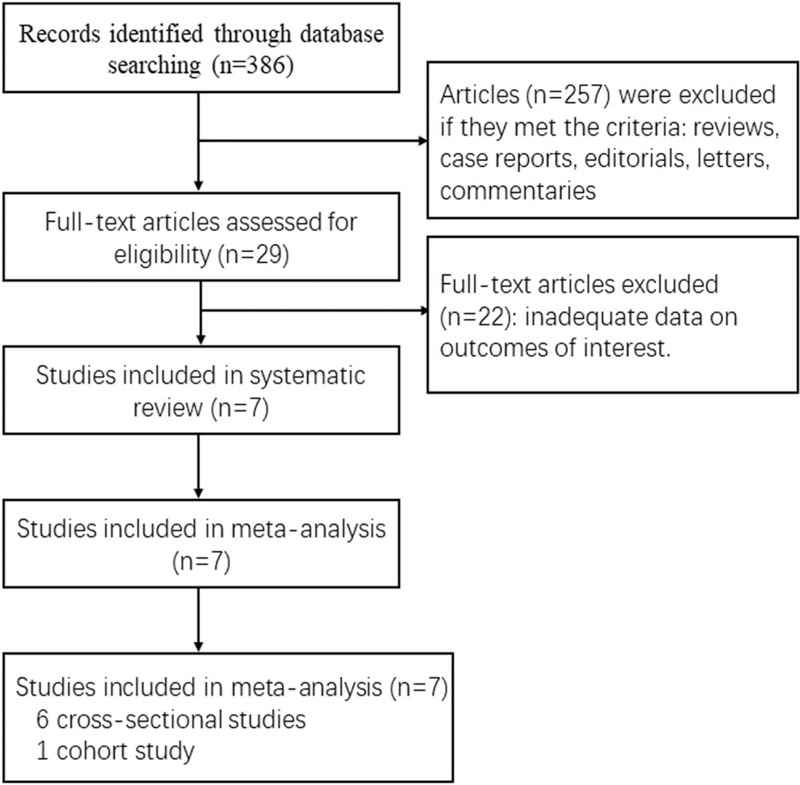
Included and excluded studies: the PRISMA flow diagram.
Table 1.
Characteristics of the studies included in the meta-analysis.
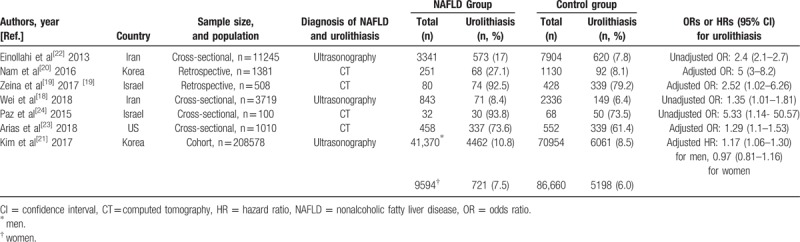
Overall, in the 7 observational studies included in the meta-analysis, there were 226,541 individuals (24.7% with NAFLD), with a urolithiasis prevalence of 8.5% (n = 19184). Studies were carried out in Iran, Korea, Israel, and the United States. All of 8 comparisons employed imaging-diagnosed urolithiasis as an outcome measure.
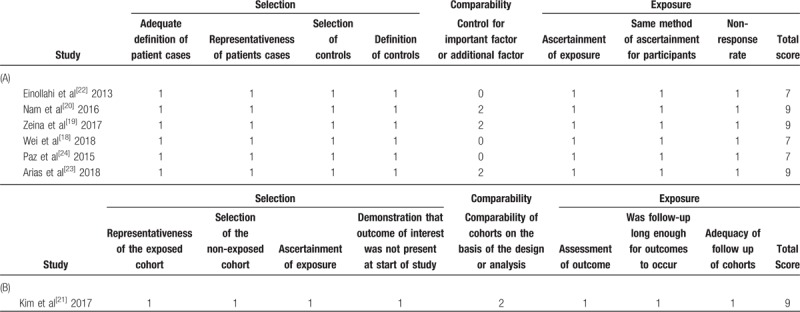
Of the 7 included studies, 4 studies receive nine stars and 3 studies seven stars at the NOS, demonstrating a low risk of bias (Table 2).[18–24] Comparability of 2 studies where the OR for urolithiasis risk was not adjusted by potential confounding factors was judged at high risk of bias in 2 studies.
Table 2.
Methodological quality of studies included in the final analysis based on the Newcastle–Ottawa Scale for assessing the quality of (a) case–control studies; (b) cohort studies.
3.2. The association between NAFLD and the risk of urolithiasis
Seven studies (8 comparisons) reported data on the association between the presence of NAFLD, defined either by ultrasonography or computed tomography and the risk of urolithiasis. NAFLD was significantly associated with an increased risk of urolithiasis (random effect OR 1.73, 95% CI 1.24–2.40, I2 = 94.5%) (Fig. 2). Both the Egger regression test and the Begg test suggested no publication bias in the meta-analysis of the link between NAFLD and urolithiasis (P > .05) (Fig. 3).
Figure 2.
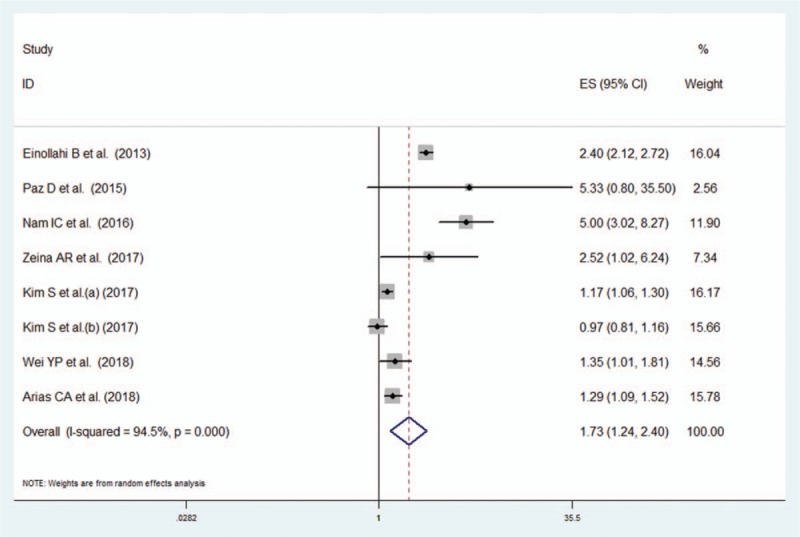
Meta-analysis on the risk of urolithiasis associated with NAFLD. Forest plot of the comparison of patients with NAFLD versus those without NAFLD.
Figure 3.
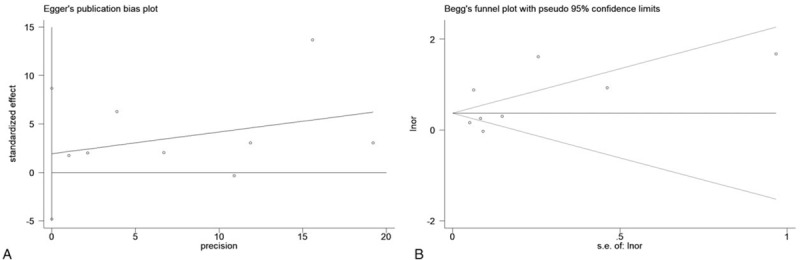
Egger test and Begg's test for examination of publication bias.
3.3. Sensitivity analyses
A sensitivity analysis was conducted by sequentially omitting each study to analyze the effect of individual research on the overall results of the meta-analysis. The omission of any single study had no significant impact on the comparison models of urolithiasis associated with NAFLD, suggesting a high level of integrity of our meta-analysis (Fig. 4).
Figure 4.

Sensitivity analysis of the association between NAFLD and urolithiasis.
4. Discussion
The present systematic review and meta-analysis investigated the association between NAFLD and the risk of urolithiasis, representing a comprehensive assessment of this association to date. The data provide evidence suggesting an increased risk of urolithiasis among patients with NAFLD. Indeed, the present study involves a total of 7 observational studies, in which 6 cross-sectional and 1 cohort studies were included. Finally, data on 26541 individuals (24.7% with NAFLD) with 19184 (8.5%) urolithiasis events were available in this meta-analysis. We found a 1.73-fold increased risk of the development of urolithiasis in patients with NAFLD than those without NAFLD.
Several studies have assessed the relationship between NAFLD and the risk of developing urolithiasis. A cross-sectional study which involved a total of 3719 Chinese men suggested that NAFLD was related to a higher prevalence of urinary calculi, independently of several traditional risk factors, such as physical activity, serum uric acid, and body mass index (BMI).[18] Similarly, a retrospective study in Israel found a 3.24-fold increased risk of CT diagnosed renal colic among NAFLD patients than individuals without NAFLD.[19] A cross-sectional study examining a total of 11245 ultrasonography reports revealed an increased prevalence of urolithiasis in NAFLD patients compared to subjects without NAFLD (OR: 2.4, 95% CI, 2.1–2.7).[22] Again, a population-based retrospective study involving 1812 patients showed that the prevalence of renal stone disease in patients with NAFLD was markedly higher than those without NAFLD in multivariate analysis (OR: 5, 95% CI, 3–8.2) (P < .05).[20] Also, a large cohort study involving 208,578 Korean adults who underwent a comprehensive health examination between January 2002 and December 2014 showed that the presence of NAFLD was significantly linked to an increased incidence of urolithiasis among in male subjects, independently of possible confounders.[21] Collectively, an increasing number of studies have shown consistent evidence that the presence of NAFLD, defined as either ultrasonography or computed tomography, was closely linked to a higher risk of urolithiasis.
The plausible biologic mechanism by which NAFLD may contribute to increasing the risk for urolithiasis remains unclear. Reactive oxygen species (ROS) and oxidative stress (OS) have been implicated in the pathogenesis of NAFLD.[28,36,37] Furthermore, increased levels of γ-glutamyl transpeptidase and renal enzymes observed in the urine of idiopathic CaOx stone patients suggest the involvement of ROS in the pathogenesis of the idiopathic stone disease.[38] A study involving adult participants of 1988 to 1994 NHANES III examined serum levels of antioxidants found that decreased antioxidant capacities, which indicated as lower levels of antioxidants, α-carotene, β-cryptoxanthin, β-carotene, predisposed to the development of kidney stones, furtherly supporting the role of ROS in nephrolithiasis.[39] Collectively, clinical and experimental data provide evidence of the involvement of ROS production and OS development in the patients with NAFLD and urolithiasis, and OS may represent shared pathogenesis for both NAFLD and urolithiasis.[28,40,41] Again, accumulating evidence suggested that kidney stones are associated with metabolic syndrome (MetS) characterized by insulin resistance.[42] A Japanese study examining the association between insulin resistance, adiponectin, and kidney stones showed that women with kidney stones had significantly higher HOMA-IR and insulin than in women without kidney stones, indicating a greater risk of kidney stones resulting from MetS components by insulin resistance or subclinical hyperinsulinemia.[43]
This meta-analysis has limitations. It is critical to underline that a causal relationship between NAFLD and urolithiasis could not be established as the nature of observational studies included in this meta-analysis. Moreover, whether the advanced form of NAFLD (NASH) is associated with an even higher risk of urolithiasis remains undetermined. Future studies investigating the severity of NAFLD and urolithiasis are needed. In addition, there was high heterogeneity between studies. The high heterogeneity was likely to result from a mix of the difference of participants with NAFLD with the various stage of fibrosis, which was not clarified in the original studies included in this meta-analysis. Although the high heterogeneity existed, a sensitivity analysis did not alter the finding of these studies, suggesting the robustness of result.
Although there are limitations, this meta-analysis has strengths. This present study identified NAFLD patients as a subset of the population at an increased risk for urolithiasis and necessitated a screening strategy for this disease among individuals with NAFLD. Again, the large number of total subjects with urolithiasis provides adequate statistical power in the detection of the association between NAFLD and urolithiasis. Finally, there is no selective reporting of studies in our study, as Egger test and Begg test showed no statistical evidence of publication bias.
5. Conclusion
The findings of this meta-analysis of observational studies indicate that the presence of NAFLD is significantly linked to an increased risk of urolithiasis. Some uncertainty, however, remains concerning whether NAFLD severity has an impact on a higher risk of urolithiasis. Furthermore, it had not yet been determined whether a scoring system based on NAFLD could be established to improve urolithiasis risk prediction. Future prospective studies and randomized, double-blind placebo-controlled trials examining the causal relationship between NAFLD and urolithiasis risk are warranted.
Author contributions
Data curation: Xu Wang.
Formal analysis: Xu Wang.
Funding acquisition: Jiangbin Wang.
Investigation: Xu Wang.
Methodology: Xu Wang.
Project administration: Jiangbin Wang.
Resources: Jiangbin Wang.
Software: Song Wang.
Supervision: Jiangbin Wang.
Validation: Song Wang.
Visualization: Song Wang.
Writing – original draft: Shaoyou Qin.
Writing – review & editing: Jiangbin Wang.
Footnotes
Abbreviations: CKD = chronic kidney disease, CT = computed tomography, CVD = cardiovascular disease, MetS = metabolic syndrome, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatosis hepatitis, OS = oxidative stress, ROS = reactive oxygen species.
This study is funded by the Science and Technology Department of Jilin Province (Grant No. 20160414036GH, 20170414031GH), the Education Department of Jilin Province (Grant No. 2015–517), the Development and Reform Commission of Jilin Province (2015y031–2) and the Wu Jieping Medical Foundation (320.6750.14028).
The authors declare no conflicts of interest.
References
- [1].Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol 2015;62(1 Suppl):S47–64. [DOI] [PubMed] [Google Scholar]
- [2].Glen J, Floros L, Day C, et al. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ 2016;354:i4428. [DOI] [PubMed] [Google Scholar]
- [3].European Association for the Study of the, L., European Association for the Study of, D., European Association for the Study of O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–402. [DOI] [PubMed] [Google Scholar]
- [4].Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–57. [DOI] [PubMed] [Google Scholar]
- [5].Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- [6].Araujo AR, Rosso N, Bedogni G, et al. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int 2018;38:47–51. [DOI] [PubMed] [Google Scholar]
- [7].Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. New Engl J Med 2010;363:1341–50. [DOI] [PubMed] [Google Scholar]
- [8].Targher G, Byrne CD. Non-alcoholic fatty liver disease: an emerging driving force in chronic kidney disease. Nat Rev Nephrol 2017;13:297–310. [DOI] [PubMed] [Google Scholar]
- [9].Agrawal V, Shah A, Rice C, et al. Impact of treating the metabolic syndrome on chronic kidney disease. Nat Rev Nephrol 2009;5:520–8. [DOI] [PubMed] [Google Scholar]
- [10].Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–44. [DOI] [PubMed] [Google Scholar]
- [11].Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313:2263–73. [DOI] [PubMed] [Google Scholar]
- [12].Musso G, Cassader M, Cohney S, et al. Fatty liver and chronic kidney disease: novel mechanistic insights and therapeutic opportunities. Diabetes Care 2016;39:1830–45. [DOI] [PubMed] [Google Scholar]
- [13].Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017;66:1138–53. [DOI] [PubMed] [Google Scholar]
- [14].Targher G, Lonardo A, Byrne CD. Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol 2018;14:99–114. [DOI] [PubMed] [Google Scholar]
- [15].Targher G, Byrne CD, Lonardo A, et al. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol 2016;65:589–600. [DOI] [PubMed] [Google Scholar]
- [16].Targher G, Bertolini L, Rodella S, et al. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin J Am Soc Nephrol 2010;5:2166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu HW, Hsu YC, Chang CH, et al. High FIB-4 index as an independent risk factor of prevalent chronic kidney disease in patients with nonalcoholic fatty liver disease. Hepatol Int 2016;10:340–6. [DOI] [PubMed] [Google Scholar]
- [18].Wei YP, Lin XG, He RQ, et al. Epidemiologic association of nonalcoholic fatty liver disease and urinary calculi: a population-based cross-sectional study in Southern China. Iran J Kidney Dis 2018;12:112–9. [PubMed] [Google Scholar]
- [19].Zeina AR, Goldenberg L, Nachtigal A, et al. Association between nephrolithiasis and fatty liver detected on non-enhanced CT for clinically suspected renal colic. Clin Imaging 2017;43:148–52. [DOI] [PubMed] [Google Scholar]
- [20].Nam IC. Association of non-alcoholic fatty liver disease with renal stone disease detected on computed tomography. Eur J Radiol Open 2016;3:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim S, Chang Y, Sung E, et al. Non-alcoholic fatty liver disease and the development of nephrolithiasis: a cohort study. PloS One 2017;12:e0184506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Einollahi B, Naghii MR, Sepandi M. Association of nonalcoholic fatty liver disease (NAFLD) with urolithiasis. Endocr Regul 2013;47:27–32. [DOI] [PubMed] [Google Scholar]
- [23].ARIAS C, Lubinus F, RAMIREZ L, ORITZ O. Renal lithiasis and Fatty Liver Disease, is there any relationship? European Congress of Radiology (ECR) 2018;Poster No.:C-3221. [Google Scholar]
- [24].Paz D, Guralnik L. Association of renal stone (urolithiasis) with nonalcoholic fatty liver (NAFL). European Congress of Radiology (ECR) 2015;Poster No.:C-2056. [Google Scholar]
- [25].Takaki A, Kawai D, Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int J Mol Sci 2013;14:20704–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tariq Z, Green CJ, Hodson L. Are oxidative stress mechanisms the common denominator in the progression from hepatic steatosis towards non-alcoholic steatohepatitis (NASH)? Liver Int 2014;34:e180–90. [DOI] [PubMed] [Google Scholar]
- [27].Sakaida I, Okita K. The role of oxidative stress in NASH and fatty liver model. Hepatol Res 2005;33:128–31. [DOI] [PubMed] [Google Scholar]
- [28].Sumida Y, Niki E, Naito Y, et al. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic Res 2013;47:869–80. [DOI] [PubMed] [Google Scholar]
- [29].Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol 2017;13:509–20. [DOI] [PubMed] [Google Scholar]
- [30].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. [DOI] [PubMed] [Google Scholar]
- [32].John Wiley & Sons, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. 2011. [Google Scholar]
- [33].Gargiullo PM, Rothenberg RB, Wilson HG. Confidence intervals, hypothesis tests, and sample sizes for the prevented fraction in cross-sectional studies. Stat Med 1995;14:51–72. [DOI] [PubMed] [Google Scholar]
- [34].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [35].Kicinski M, Springate DA, Kontopantelis E. Publication bias in meta-analyses from the Cochrane Database of Systematic Reviews. Stat Med V 34 2015;2781–93. [DOI] [PubMed] [Google Scholar]
- [36].Morita M, Ishida N, Uchiyama K, et al. Fatty liver induced by free radicals and lipid peroxidation. Free Radic Res 2012;46:758–65. [DOI] [PubMed] [Google Scholar]
- [37].Musso G, Anty R, Petta S. Antioxidant therapy and drugs interfering with lipid metabolism: could they be effective in NAFLD patients? Curr Pharm Des 2013;19:5297–313. [PubMed] [Google Scholar]
- [38].Baggio B, Gambaro G, Ossi E, et al. Increased urinary excretion of renal enzymes in idiopathic calcium oxalate nephrolithiasis. J Urol 1983;129:1161–2. [DOI] [PubMed] [Google Scholar]
- [39].Holoch PA, Tracy CR. Antioxidants and self-reported history of kidney stones: the National Health and Nutrition Examination Survey. J Endourol 2011;25:1903–8. [DOI] [PubMed] [Google Scholar]
- [40].Abhishek A, Benita S, Kumari M, et al. Molecular analysis of oxalate-induced endoplasmic reticulum stress mediated apoptosis in the pathogenesis of kidney stone disease. J Physiol Biochem 2017;73:561–73. [DOI] [PubMed] [Google Scholar]
- [41].Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 2013;189:803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Domingos F, Serra A. Metabolic syndrome: a multifaceted risk factor for kidney stones. Scand J Urol 2014;48:414–9. [DOI] [PubMed] [Google Scholar]
- [43].Ando R, Suzuki S, Nagaya T, et al. Impact of insulin resistance, insulin and adiponectin on kidney stones in the Japanese population. Int J Urol 2011;18:131–8. [DOI] [PubMed] [Google Scholar]


