Abstract
Transmembrane 4 L6 family member 1 (TM4SF1) belongs to the 4-transmembrane-domain family and functions as an oncogene in multiple human cancers. In this work, we aim to determine TM4SF1 expression and its prognostic impact on patients with invasive breast cancer.
Overall, we enrolled 209 invasive breast cancer patients and immunohistochemically examined the expression of TM4SF1 in tumor specimens. The relationship between TM4SF1 expression and clinicopathological parameter and patient survival of breast cancer patients was analyzed.
Among the 209 cases, 137 (65.6%) exhibited high expression of TM4SF1. High TM4SF1 expression was significantly associated with advanced histological grade and negative estrogen receptor and progesterone receptor status. Triple-negative breast cancer (TNBC) tumors were more likely to express high levels of TM4SF1 than non-TNBC cases. Patients with high tumoral expression of TM4SF1 had a significantly shorter disease-free survival (DFS; P = .00) and overall survival (OS; P = .01) than those with low expression of TM4SF1. When survival analysis was restricted to the 167 patients (79.9%) receiving adjuvant chemotherapy, TM4SF1 expression was also correlated with poorer DFS and OS (P = .00). In multiple Cox regression analysis TM4SF1 expression remained an independent prognostic indicator for OS and DFS.
TM4SF1 is upregulated and serves as an independent poor prognostic indicator in invasive breast cancer.
Keywords: aggressive parameters, breast cancer, overexpression, prognosis, TM4SF1
1. Introduction
Breast cancer is the most common malignancy among females and associated with high rates of mortality.[1] It is a heterogeneous disease and has at least 4 molecular subtypes including luminal A and B, human epidermal growth factor receptor 2+ (HER2+), and basal-like.[2] Each subtype has distinct biological characteristics and varying response to treatment, providing a rationale for personalized therapy.[3] Estrogen receptor (ER)/progesterone receptor (PR)-positive luminal tumors can be treated by hormonal therapy, whereas HER2-positive tumors can be controlled with anti-HER2 therapies. The majority of basal-like tumors lack hormone receptors and HER2 and are also called triple-negative breast cancer (TNBC). TNBC tumors often show rapid growth and early metastasis.[4] Systemic chemotherapy is usually used to treat TNBC.[4] Identification of effective prognostic markers for breast cancer, especially TNBC tumors, is of significance in making a treatment decision and improving therapeutic outcomes.
Transmembrane 4 L6 family member 1 (TM4SF1) belongs to the 4-transmembrane-domain family and acts as an oncogene in many cancers.[5–7] It has been reported that TM4SF1 is upregulated in pancreatic cancer tissues and its knockdown in pancreatic cancer cells leads to reduced migration and invasion capacity.[7] Overexpression of TM4SF1 was reported to promote cell motility and reduces apoptosis in the TNBC cell line MDA-MB-231.[6] In contrast, silencing of TM4SF1 was found to result in decreased invasiveness of ER-positive T47D and BT474 cells,[8] confirming the implication of TM4SF1 in the pathogenesis of breast cancer. Dysregulation of TM4SF1 has exhibited prognostic values in pancreatic ductal adenocarcinoma[9] and glioma.[10] However, the expression and prognostic relevance of TM4SF1 in breast cancer is still unclear.
In this study, we immunohistochemically analyzed the expression of TM4SF1 in 209 invasive breast cancer patients and examined the associations between TM4SF1 expression and clinicopathologic parameters and patient survival.
2. Materials and methods
2.1. Patients
The current study involved a total of 209 invasive breast cancer patients who underwent surgical resection at the First Affiliated Hospital of China Medical University (Shenyang, China) between January 2007 and March 2011. All cases were confirmed by pathological diagnosis. None of them received neoadjuvant chemotherapy or preoperative radiation therapy. Patients with any other malignant diseases were excluded. Clinicopathologic characteristics were extracted from patient records, including age at diagnosis, T and N stage, histological grade, expression status of ER, PR, and HER2, and adjuvant therapy status. The median follow-up period was 62 months (range, 6–115 ms). Three patients (1.4%) were lost to follow-up. As a normal control, we collected 40 breast tissues adjacent to tumor specimens. This study was approved by the ethics committee of China Medical University. The reference number is AF-SOP-07–1.0–01. All subjects were informed in advance, and signed explicit informed consent. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2.2. Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded tumor sections were subjected to routine IHC staining. In brief, sections were deparaffinized, rehydrated, and incubated with 3% H2O2 to block endogenous peroxidase activity. After antigen retrieval, sections were incubated with rabbit anti-TM4SF1 polyclonal antibody (ab113504, Abcam, Cambridge, UK; 1:1000) at 4°C overnight. Sections were then incubated with biotinylated goat anti-rabbit IgG and peroxidase-conjugated streptavidin. The sections were developed with 3,3′-diaminobenzidine and counterstained with hematoxylin. Negative controls were included by omitting the primary antibody.
Evaluation of IHC was performed by 2 expert clinicians (HD and QL) who were blinded to the patient's clinical data. Twelve independent microscopic fields for each tissue sample were evaluated. Immunostaining intensity was scored on a scale of 0 to 3: 0 = no staining, 1 = weak staining, 2 = moderate staining, and 3 = strong staining. Percentage of positively stained cells was classified into the following 4 categories: 0%–10%, 11%–50%, 51%–75%, and 76%–100%. IHC scores ranging from 0 to 12 were calculated by multiplying the 2 parameters.[11] The IHC score of ≥4 was defined as high TM4SF1 expression and the IHC score of < 4 as low TM4SF1 expression.
2.3. Statistical analysis
Statistical analyses were performed with the SPSS software program for windows (version 17.0; SPSS, Inc., Chicago, IL). The relationship between TM4SF1 expression and clinicopathological parameters was analyzed using the χ2 test and Fisher exact test. Disease-free survival (DFS) and overall survival (OS) curves were calculated by the Kaplan-Meier method, and differences in survival curves were determined by the Log-rank test. DFS was defined as the time from the date of surgery to the date of recurrence. OS was defined as the time from the date of diagnosis to the date of death from any cause. If a patient did not have an event, the surviving time was censored at the date of last follow-up. Hazard ratios (HR) and their 95% confidence intervals (95% CI) were calculated using Cox proportional hazards models to determine the associations between survival and possible risk factors. Multivariate Cox regression analysis was performed on the significant variables in univariate survival analysis to identify independent prognostic factors of survival. A P < .05 was considered to be statistically significant.
3. Results
3.1. Characteristics of patients and tumors
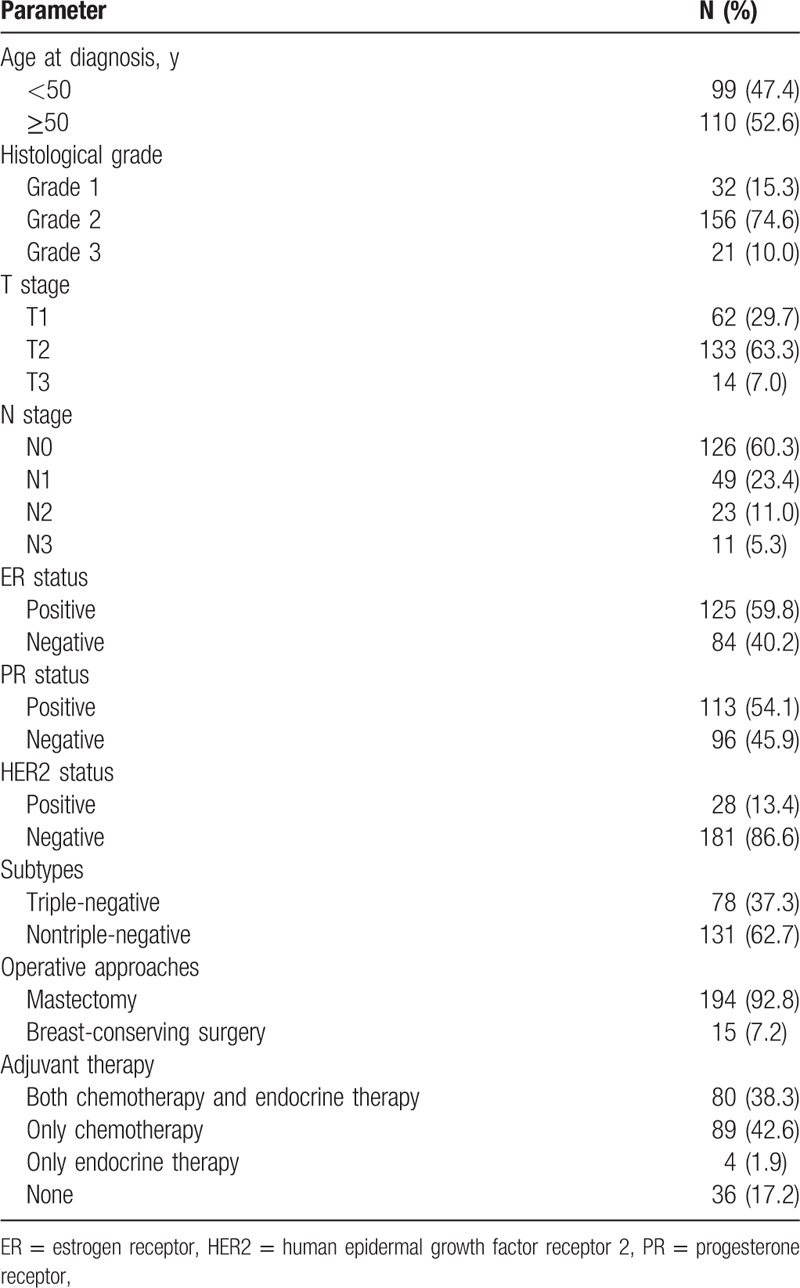
Table 1 summarizes characteristics of patients and tumors. This cohort consisted of 209 breast cancer patients with a median age of 50 years (range, 31–78 ys). Histological grade 1 tumors were detected in 32 patients (15.3%), grade 2 tumors in 156 patients (74.6%), and grade 3 tumors in 21 patients (10.0%). Pathological T1, T2, and T3 disease was identified in 62 patients (29.7%), 133 patients (63.3), and 14 (7.0%), respectively. The majority of patients (60.3%) had a pathological N0 disease. ER, PR, and HER2 were positive in 125 patients (59.8%), 113 (54.1%), and 28 (13.4%), respectively. Seventy-eight patients (37.3%) were classified as TNBC. With regard to operative approaches, 194 patients (92.8%) underwent mastectomy, and 15 (7.2%) underwent breast-conserving surgery. Concerning adjuvant therapy, 79 patients (37.8%) received both chemotherapy and endocrine therapy, 88 patients (42.1%) only received chemotherapy, 5 patients (2.4%) received endocrine therapy, and the remaining 37 patients (17.7%) did not receive adjuvant therapy.
Table 1.
Characteristics of patients and tumors.
3.2. Associations between TM4SF1 expression and clinicopathologic parameters
Immunohistochemical staining demonstrated that TM4SF1 was abundantly expressed in the cell membrane and cytoplasm in breast cancer specimens (Fig. 1). Among the 209 breast cancer cases, 137 (65.6%) showed high expression of TM4SF1. In contrast, high TM4SF1 was detected in only 12.5% of adjacent normal breast tissues.
Figure 1.
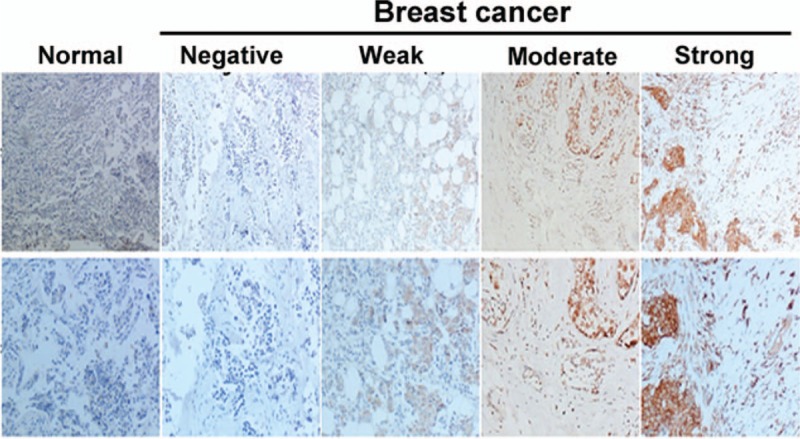
Immunohistochemical analysis of TM4SF1 in breast cancer tissues and normal breast tissues. TM4SF1 showed membranous and cytoplasmic staining. Representative images of sections with different immunostaining intensity. Upper panels: 100×; lower panels: 200×.
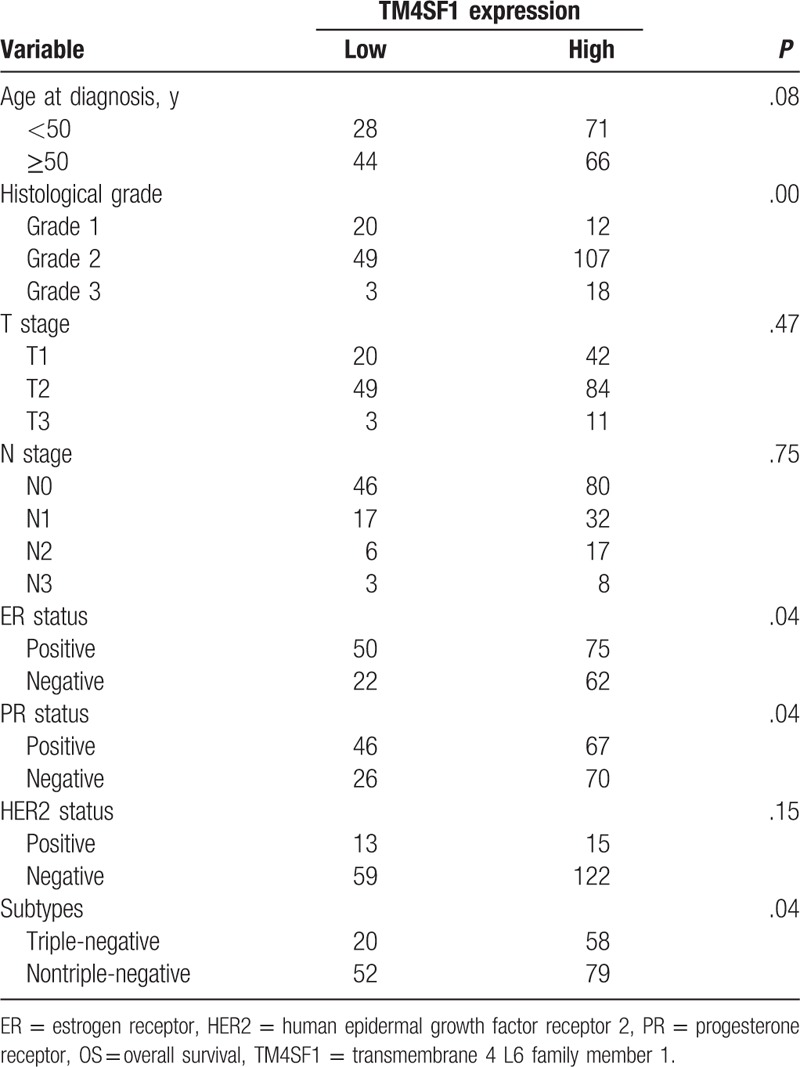
Table 2 shows associations between TM4SF1 expression and clinicopathologic parameters. High TM4SF1 expression was more common in tumors with advanced histological grade (P = .00). High TM4SF1 expression was also significantly associated with negative ER (P = .04) and PR (P = .04) status. Compared to non-TNBC tumors, TNBC counterparts were more likely to express high levels of TM4SF1 (P = .04). However, there was no significant correlation between TM4SF1 expression and T stage, N stage, or HER2 status (P > .05).
Table 2.
Associations of TM4SF1 expression with clinicopathologic parameters in 209 breast cancer patients.
3.3. Associations between TM4SF1 expression and survival
Kaplan-Meier survival analysis revealed that breast cancer patients with high tumoral expression of TM4SF1 had a significantly shorter DFS (P = .00) and OS (P = .01) than those with low expression of TM4SF1 (Fig. 2A). When non-TNBC patients were excluded from this survival analysis, the DFS (P = .09) and OS (P = .07) remained lower in the high-TM4SF1 subgroup than in the low-TM4SF1 subgroup, but the difference was not significant (Fig. 2B). In addition, we evaluated the associations of TM4SF1 expression with survival in the 167 patients (79.9%) receiving adjuvant chemotherapy. As shown in Figure 2C, high levels of TM4SF1 were significantly associated with poorer DFS (P = .00) and OS (P = .00).
Figure 2.
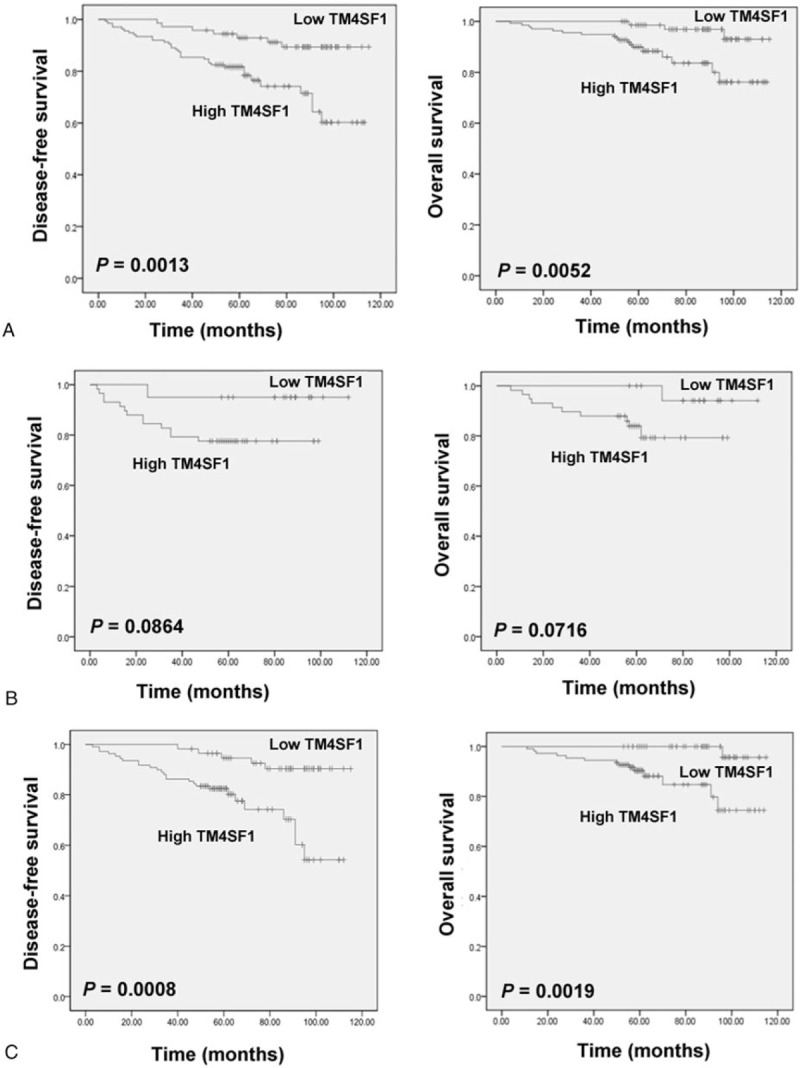
Kaplan-Meier analysis of disease-free survival and overall survival in different cohorts of patients according to TM4SF1 expression status. A, Survival analysis in all the 209 breast cancer patients. B, Survival analysis in a subset of TNBC patients (n = 78). C, Survival analysis in a subset of patients receiving adjuvant chemotherapy (n = 167). Differences were determined using the Log-rank test.
In univariable Cox regression analyses, TM4SF1 expression and N stage were significantly associated with OS and DFS (Table 3). Multiple Cox regression analysis (Table 4) revealed that TM4SF1 expression remained an independent prognostic indicator for OS [hazard ratio (HR) = 4.17; 95% confidence interval (CI): 1.21–14.40; P = .02] and DFS (HR = 2.76; 95% CI: 1.20–6.36; P = .02).
Table 3.
Univariable Cox regression analyses.
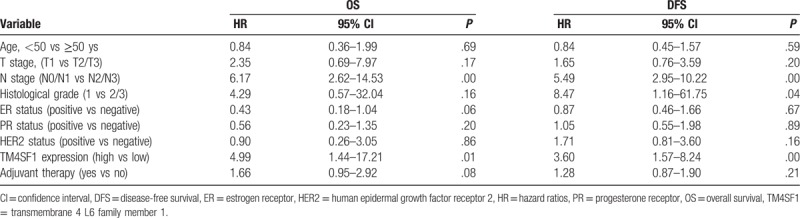
Table 4.
Multiple Cox regression analyses.

4. Discussion
TM4SF1 has been shown to be deregulated in many types of cancers.[12] For example, it was reported that tumor tissue from glioma patients had significantly greater levels of TM4SF1 than adjacent normal brain tissues.[10] Another study demonstrated that TM4SF1 is upregulated in prostate cancer relative to benign prostatic hyperplasia specimens.[13] In agreement with these studies, we found that TM4SF1 was also overexpressed in invasive breast cancer, compared with normal breast tissues. Moreover, our data indicated that high TM4SF1 expression was significantly associated with advanced histological grade and negative ER and PR status of tumors. TNBC tissues showed significantly higher levels of TM4SF1 than non-TNBC tumors, suggesting that TM4SF1 may be a novel biomarker to identify TNBC tumors. Schiedeck et al[14] analyzed the mRNA expression of TM4SF1 in serum samples from 187 patients with colorectal cancer and found that 79% of the cases showed abundant TM4SF1 mRNA expression. This study suggests that TM4SF1 upregulation may be a result of enhanced transcription. However, the mechanism for the upregulation of TM4SF1 needs to be further clarified.
Previous studies have revealed that TM4SF1 plays a critical role in tumor development and progression.[5,15,16] Overexpression of TM4SF1 was found to promote endothelial cell migration and tumor angiogenesis.[15] In lung cancer cells, TM4SF1 displays the ability to facilitate cell migration and invasion.[16] The association between TM4SF1 and CD13 governs the invasive ability of lung cancer cells.[17] Recruitment of TM4SF1 to tetraspanin-enriched microdomains is important in modulating tumor cell migration.[18] Of note, ectopic expression of TM4SF1 enhances breast cancer cell migration and reduces cell survival.[6] These in vitro findings highlight an oncogenic activity of TM4SF1, which provides a biological explanation for the upregulation of TM4SF1 in breast cancer.
Survival analysis revealed that breast cancer patients with high tumoral expression of TM4SF1 had an inferior survival than those with low expression of TM4SF1. In multivariate regression analyses, high TM4SF1 expression showed an independent prognostic impact on the OS and DFS of patients with invasive breast cancer. Our results are consistent with those of previous studies on pancreatic ductal adenocarcinoma[9] and glioma.[10] When survival analysis was restricted to patients with TNBC tumors, the high TM4SF1 group remained a trend for shorter DFS and OS compared with the low TM4SF1 group. Adjuvant chemotherapy is commonly employed to manage TNBC tumors, yielding survival benefits.[4] We also determined the prognostic value of TM4SF1 expression in breast cancer patients receiving adjuvant chemotherapy. The results demonstrated that high TM4SF1 expression had a significant prognostic impact in this subset of patients. Taken together, tumoral expression of TM4SF1 shows a prognostic value in invasive breast cancer patients, in particular those receiving adjuvant chemotherapy after surgery.
However, this work is a retrospective study conducted at a single institution, likely resulting in patient selection bias. In addition, the adjuvant therapy regimens were not standardized, which may have an undeniable impact on patient survival.
Despite these limitations, our results indicate that TM4SF1 is overexpressed in invasive breast cancer, in particular TNBC, and that high expression of TM4SF1 is an independent adverse prognostic factor in breast cancer.
Footnotes
Abbreviations: ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2, PR = progesterone receptor, TM4SF1 = transmembrane 4 L6 family member 1, TNBC = triple-negative breast cancer.
Author Contribution: PX contributed in the study conceptualization and writing-original draft. HD contributed in the funding acquisition. QL contributed in the software; TZ contributed in the formal analysis; and FY, YX, BC, and XZ contributed in the data curation. YW and JL contributed in the supervision; and FJ contributed in the writing-review and editing. This work was financially supported by a research grant from China Medical University (No. LK201629).
The authors declare no conflicts of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 2009;27:1168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Polyak K. Heterogeneity in breast cancer. J Clin Invest 2011;121:3786–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Collignon J, Lousberg L, Schroeder H, et al. Triple-negative breast cancer: treatment challenges and solutions. Breast Cancer (Dove Med Press) 2016;8:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang YK, Fan XG, Qiu F. TM4SF1 promotes proliferation, invasion, and metastasis in human liver cancer cells. Int J Mol Sci 2016;17:ii: E661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sun Y, Xu Y, Xu J, et al. Role of TM4SF1 in regulating breast cancer cell migration and apoptosis through PI3K/AKT/mTOR pathway. Int J Clin Exp Pathol 2015;8:9081–8. [PMC free article] [PubMed] [Google Scholar]
- [7].Cao J, Yang JC, Ramachandran V, et al. TM4SF1 regulates pancreatic cancer migration and invasion in vitro and in vivo. Cell Physiol Biochem 2016;39:740–50. [DOI] [PubMed] [Google Scholar]
- [8].McFall T, Patki M, Rosati R, et al. Role of the short isoform of the progesterone receptor in breast cancer cell invasiveness at estrogen and progesterone levels in the pre- and post-menopausal ranges. Oncotarget 2015;6:33146–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zheng B, Ohuchida K, Cui L, et al. TM4SF1 as a prognostic marker of pancreatic ductal adenocarcinoma is involved in migration and invasion of cancer cells. Int J Oncol 2015;47:490–8. [DOI] [PubMed] [Google Scholar]
- [10].Wang P, Bao W, Zhang G, et al. Transmembrane-4-L-six-family-1, a potential predictor for poor prognosis, overexpressed in human glioma. Neuroreport 2015;26:455–61. [DOI] [PubMed] [Google Scholar]
- [11].Zhu A, Yuan P, Du F, et al. SPARC overexpression in primary tumors correlates with disease recurrence and overall survival in patients with triple negative breast cancer. Oncotarget 2016;7:76628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marken JS, Schieven GL, Hellström I, et al. Cloning and expression of the tumor-associated antigen L6. Proc Natl Acad Sci U S A 1992;89:3503–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Allioli N, Vincent S, Vlaeminck-Guillem V, et al. TM4SF1, a novel primary androgen receptor target gene over-expressed in human prostate cancer and involved in cell migration. Prostate 2011;71:1239–50. [DOI] [PubMed] [Google Scholar]
- [14].Schiedeck TH, Wellm C, Roblick UJ, et al. Diagnosis and monitoring of colorectal cancer by L6 blood serum polymerase chain reaction is superior to carcinoembryonic antigen-enzyme-linked immunosorbent assay. Dis Colon Rectum 2003;46:818–25. [DOI] [PubMed] [Google Scholar]
- [15].Shih SC, Zukauskas A, Li D, et al. The L6 protein TM4SF1 is critical for endothelial cell function and tumor angiogenesis. Cancer Res 2009;69:3272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kao YR, Shih JY, Wen WC, et al. Tumor-associated antigen L6 and the invasion of human lung cancer cells. Clin Cancer Res 2003;9:2807–16. [PubMed] [Google Scholar]
- [17].Chang YW, Chen SC, Cheng EC, et al. CD13 (aminopeptidase N) can associate with tumor-associated antigen L6 and enhance the motility of human lung cancer cells. Int J Cancer 2005;116:243–52. [DOI] [PubMed] [Google Scholar]
- [18].Lekishvili T, Fromm E, Mujoomdar M, et al. The tumour-associated antigen L6 (L6-Ag) is recruited to the tetraspanin-enriched microdomains: implication for tumour cell motility. J Cell Sci 2008;121:685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]


