Abstract
Rationale:
Vanishing bile duct syndrome (VBDS) consists of a series of diseases characterized by the loss of >50% bile duct in portal areas. Many factors are associated with VBDS including infections, neoplasms, and drugs. Antibiotic is one of the most frequently reported causes of VBDS.
Patient concerns:
A 29-year-old female was admitted because of liver injury for over 3 months. Tests for viruses that can cause hepatitis and autoantibodies were all negative. She was prescribed with antibiotics approximately a week before liver injury while there was no history of alcohol consumption.
Diagnoses:
Liver biopsy demonstrated a loss of intrahepatic bile duct in most of the portal tracts.
Interventions:
This patient was treated with ursodeoxycholic acid, polyene phosphatidylcholine, and bicyclol. Most importantly, the treatments in our hospital were proved by the ethics committee of Department of Infectious Disease, Anhui Provincial Hospital.
Outcomes:
The symptoms were improved. She is still under treatment.
Lessons:
VBDS is rare but can be severe. A liver biopsy offers an important evidence for the diagnosis of VBDS, especially for those with a history of susceptible drugs taking.
Keywords: antibiotics, liver biopsy, vanishing bile duct syndrome
1. Introduction
Vanishing bile duct syndrome (VBDS) is a group of diseases that is characterized by the missing of bile duct in portal area. Multiple causes have been convinced to be associated with VBDS, including congenital and genetic diseases, neoplasms, infection, and drugs.[1] Diagnosis of VBDS depends mostly on clinical and pathological presentations. Here we reported a case of VBDS with a history of exposure to antibiotics.
2. Case report
A 29-year-old female was admitted to our hospital because of liver injury for over 3 months.
This patient had a history of antibiotics administration for 4 to 5 days after the removal of her intrauterine device over 3 months ago. The drugs she took included cephalosporin, metronidazole, and clotrimazole. Six days later, she underwent a health examination. The report was not available until 40 days after the examination and the result showed a mild liver injury with alanine aminotransferase (ALT) of 220 U/L. She got her liver function rechecked at the local hospital the next day, which showed an increase of ALT (Table 1) and that the hepatitis B surface antibody was positive. Diammonium glycyrrhizinate enteric-coated capsules were prescribed and her liver function had improved slightly 3 weeks later. Then she took some traditional Chinese medicine for several days. Six days before admission, she went to the local hospital because of dark urine and pruritus. The results showed an elevated total bilirubin (TBil) level, so she was admitted to our hospital for further examination and treatment.
Table 1.
Laboratory data of liver function.
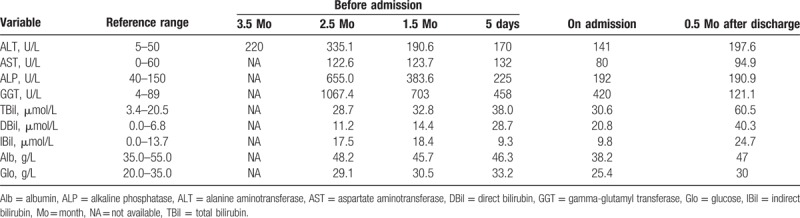
On admission, the patient was conscious, with mild jaundice, no enlargement of the thyroid, no abdominal pain, no hepatomegaly and splenomegaly, and no other abnormalities of the physical examination. Her vital signs were stable. She used to be diagnosed with intrahepatic cholestasis of pregnancy 4 years ago and had been treated with ursodeoxycholic acid. Her liver injury completely recovered after the delivery. She had no history of chronic liver diseases, no hypertension or diabetes mellitus, no other diseases, no history of alcohol addiction, no exposure to toxic substances, or infectious disease. The laboratory data of her liver function before and after admission are summarized in Table 1. Her prothrombin time was 11.4 seconds and the international normalized ratio (INR) was 0.95. Antibodies to hepatitis A, C, D, E, and G viruses; cytomegalovirus (CMV); Epstein–Barr virus (EBV); and herpes simplex virus (HSV) were all negative. Autoantibodies like antinuclear antibody (ANA), antismooth muscle antibody (ASMA), and antimitochondrial antibody (AMA) were also negative. The levels of alpha fetoprotein (AFP) and ceruloplasmin were under normal. And her thyroid function showed no abnormalities.
Since there were no abnormal findings of the abdominal ultrasound, the patient took a magnetic resonance cholangiopancreatography (MRCP) and it showed no constriction or expansion of the bile ducts both intra- and extra- the liver, except for some calculus of cystic duct. Six days after admission, she underwent a biopsy of the liver. A total of 10 portal areas could be seen on the pathological sections and there was no obvious inflammation or fibrosis. Histology showed interlobular arteries and veins without bile ducts in >50% portal areas (Fig. 1). According to her laboratory data and pathological results, the patient was finally diagnosed with VBDS. She was administrated with ursodeoxycholic acid (0.25 g 3 times a day), polyene phosphatidylcholine (456 mg 3 times a day) as well as bicyclol (25 mg 3 times a day), and then was discharged home. Her liver function was monitored regularly during the follow-up and the last report showed a significantly improved liver function: ALT 34.8 U/L, aspartate aminotransferase 79.2 U/L, alkaline phosphatase (ALP) 250.3 U/L, gamma-glutamyl transferase (GGT) 452.9 U/L, and TBil 19 μmol/L. She is still under treatment.
Figure 1.
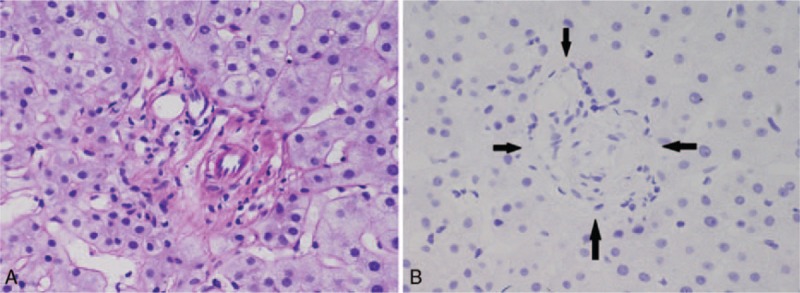
(A) Portal tract without a bile duct that should accompanied with the interlobular hepatic arteries, no obvious inflammation, or fibrosis (D-PAS staining, 60×). (B) Vanishing interlobular bile duct in portal area (CK19, 60×, arrows). CK19 = cytokeratin 19, D-PAS = periodic acid–Schiff–diastase.
3. Discussion
The loss of intrahepatic bile duct, described to be pathological changes of several diseases like primary biliary cirrhosis (PBC) and graft-versus-host disease, was firstly defined as ductopenia. It was not until 1987 when Ludwig introduced the definition of VBDS.[2] This uncommon disease is reported mostly in isolated cases. Factors associated with VBDS include congenital and genetic diseases that can affect bile duct development;[1] neoplasms, especially lymphoma;[3–5] virus infection like CMV, HCV, and EBV;[6–8] immune disorder such as PBC, primary sclerosing cholangitis (PSC), and graft-versus-host disease.[1,9] Drugs and toxins can also lead to VBDS. Up to now, several kinds of drugs have been reported to cause VBDS, including antibiotics, nonsteroidal anti-inflammatory drugs (NSAIDs), antiviral antigens, drugs for hypertension, hyperlipidemia, diabetes mellitus, and antipsychotics (Table 2).[10–15] Jaundice and itching are the most frequent symptoms for those with drug-induced VBDS. Other manifestations like fatigue and anorexia are also common.[10] Laboratory test may show elevated levels of ALP and GGT. A liver biopsy is important for the diagnosis of VBDS as it is defined as the loss of intrahepatic bile duct in >50% portal areas.[16] At least 10 portal tracts are needed for the confirmation of VBDS whereas 20 is better. In our case, the patient had no history of alcohol addiction and tests for virus hepatitis, CMV, EBV, and HSV were all negative. Her MRCP was normal and the serum levels of ANA, ASMA, and AMA were all under normal. Moreover, liver pathology showed mild inflammation while no fibrosis scar in the portal area, which lead to the exclusion of autoimmune hepatitis, PBC, and PSC. The patient had liver injury 6 days after she took the medication, and her ALP elevated obviously. According to Roussel Uclaf causality assessment method (RUCAM),[17] she got 7 scores and was diagnosed with drug-induced liver injury (DILI). She was classified into cholestatic DILI since her ALP >2ULN (upper limits of normal) and R ≤ 2 (R = (actual ALT/ALT ULN)/(actual ALP/ALP ULN)).[18] Pathological examination of her liver showed a loss of bile duct in >50% of the portal tracts and she was finally diagnosed with drug-induced VBDS.
Table 2.
Antibiotics reported to cause VBDS.
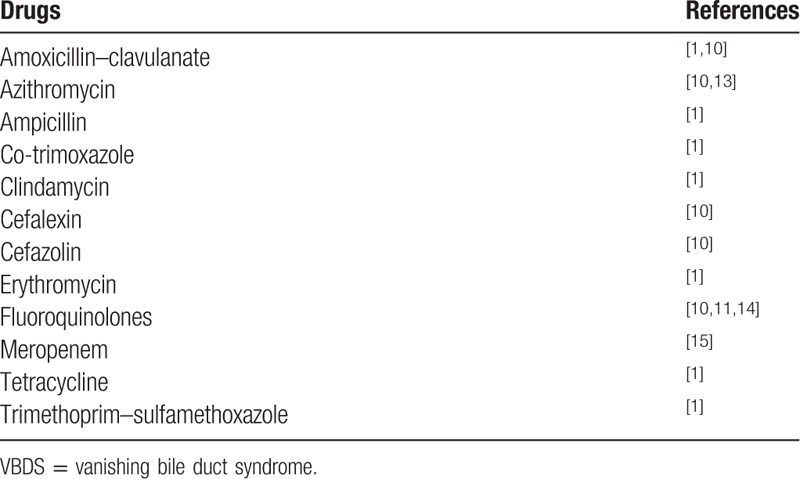
Mechanisms of VBDS are still not well understood. However, immunological injuries can play important roles in the loss of bile duct. T-cell-mediated immunological reaction may lead to biliary epithelial cells apoptosis.[19] In this case, the patient has a history of using antibiotics just 1 week before the liver injury, including cephalosporin, metronidazole, and clotrimazole. Several kinds of cephalosporin have already been reported to be associated with VBDS, as listed in Table 2. Clotrimazole has also been found to have the ability of inhibiting multidrug resistance protein 3 (MDR3), which is a kind of phospholipid export pump that was expressed on the membranes of cholangioles. Defects of MDR3 can lead to bile duct damage and are associated with DILI and VBDS.[10,20,21]
There is no effective therapy that can induce the regeneration of bile duct. Current treatments focus on improving cholestasis and suppressing immune reaction. Prognosis of VBDS depends on the degree of bile duct loss, and there is also a trend of poor outcome in younger patients and Africa–America race.[10] Even though the liver function abnormalities can continue for years, some patients may have clinical symptoms improvement. For those who had developed cirrhosis or liver failure, liver transplantation would be the last choice. Severe liver injury can also lead to death. Our patient has been under treatment for 9 months and she is still under follow-up. The liver function had no obvious improvement, but her symptoms released a lot.
In summary, we presented a case of VBDS in a patient with a history of antibiotics administration. Liver biopsy plays an important role in the diagnosis of VBDS. The mechanisms of VBDS remained unknown. Further study is needed and may help us to treat this disease more effectively.
Acknowledgment
This study was approved by the ethics committee of Department of Infectious Disease, Anhui Provincial Hospital (Geliang Xu, Xiaoling Xu, Zuojun Shen, Xiaoling Ma, Yi Li, Chunmei Yang, and Yin Chen).
Footnotes
Abbreviations: ALT = alanine aminotransferase, AMA = anti-mitochondrial antibody, ANA = antinuclear antibody, ASMA = antismooth muscle antibody, DILI = drug-induced liver injury, MDR3 = multidrug resistance protein 3, MRCP = magnetic resonance cholangiopancreatography, PBC = primary biliary cirrhosis, PSC = primary sclerosing cholangitis, VBDS = vanishing bile duct syndrome.
ZZ, LB, and XY contributed equally to this work.
Funding: This work was supported by Anhui Provincial Natural Science Foundation (No.1508085MH172), WBE Liver Fibrosis Foundation (No. CFHPC20161005), and International Cooperative Project of Anhui Province (No. 1704e1002231).
The authors have no conflicts of interest to disclose.
References
- [1].Reau NS, Jensen DM. Vanishing bile duct syndrome. Clin Liver Dis 2008;12:203–17. [DOI] [PubMed] [Google Scholar]
- [2].Ludwig J, Wiesner RH, Batts KP, et al. The acute vanishing bile-duct syndrome (acute irreversible rejection) after orthotopic liver-transplantation. Hepatology 1987;7:476–83. [DOI] [PubMed] [Google Scholar]
- [3].Bakhit M, McCarty TR, Park S, et al. Vanishing bile duct syndrome in Hodgkin's lymphoma: a case report and literature review. World J Gastroenterol 2017;23:366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bakhit M, McCarty TR, Park S, et al. Vanishing bile duct syndrome in Hodgkin's lymphoma: a single center experience and Clinical pearls. J Clin Gastroenterol 2016;50:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gill RM, Ferrell LD. Vanishing bile duct syndrome associated with peripheral T cell lymphoma, not otherwise specified, arising in a posttransplant setting. Hepatology 2010;51:1856–7. [DOI] [PubMed] [Google Scholar]
- [6].Hoffmann RM, Gunther C, Diepolder HM, et al. Hepatitis C virus infection as a possible risk factor for ductopenic rejection (vanishing bile duct syndrome) after liver transplantation. Transpl Int 1995;8:353–9. [DOI] [PubMed] [Google Scholar]
- [7].Kikuchi K, Miyakawa H, Abe K, et al. Vanishing bile duct syndrome associated with chronic EBV infection. Dig Dis Sci 2000;45:160–5. [DOI] [PubMed] [Google Scholar]
- [8].Tyagi I, Puri AS, Sakhuja P, et al. Co-occurrence of cytomegalovirus-induced vanishing bile duct syndrome with papillary stenosis in HIV infection. Hepatol Res 2013;43:311–4. [DOI] [PubMed] [Google Scholar]
- [9].Yeh KH, Hsieh HC, Tang JL, et al. Severe isolated acute hepatic graft-versus-host disease with vanishing bile duct syndrome. Bone Marrow Transplant 1994;14:319–21. [PubMed] [Google Scholar]
- [10].Bonkovsky HL, Kleiner DE, Gu J, et al. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology 2017;65:1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Orman ES, Conjeevaram HS, Vuppalanchi R, et al. Clinical and histopathologic features of fluoroquinolone-induced liver injury. Clin Gastroenterol Hepatol 2011;9:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dousset B, Conti F, Houssin D, et al. Acute vanishing bile duct syndrome after interferon therapy for recurrent HCV infection in liver-transplant recipients. N Engl J Med 1994;330:1160–1. [DOI] [PubMed] [Google Scholar]
- [13].Juricic D, Hrstic I, Radic D, et al. Vanishing bile duct syndrome associated with azithromycin in a 62-year-old man. Basic Clin Pharmacol Toxicol 2010;106:62–5. [DOI] [PubMed] [Google Scholar]
- [14].Robinson W, Habr F, Manlolo J, et al. Moxifloxacin associated vanishing bile duct syndrome. J Clin Gastroenterol 2010;44:72–3. [DOI] [PubMed] [Google Scholar]
- [15].Schumaker AL, Okulicz JF. Meropenem-induced vanishing bile duct syndrome. Pharmacotherapy 2010;30:953. [DOI] [PubMed] [Google Scholar]
- [16].Ludwig J, Wiesner RH, LaRusso NF. Idiopathic adulthood ductopenia. A cause of chronic cholestatic liver disease and biliary cirrhosis. J Hepatol 1988;7:193–9. [DOI] [PubMed] [Google Scholar]
- [17].Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 2014;109:950–66. [DOI] [PubMed] [Google Scholar]
- [18].Yu YC, Mao YM, Chen CW, et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int 2017;11:221–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Woolf GM, Vierling JM. Disappearing intrahepatic bile ducts: the syndromes and their mechanisms. Semin Liver Dis 1993;13:261–75. [DOI] [PubMed] [Google Scholar]
- [20].He K, Cai L, Shi Q, et al. Inhibition of MDR3 activity in human hepatocytes by drugs associated with liver injury. Chem Res Toxicol 2015;28:1987–90. [DOI] [PubMed] [Google Scholar]
- [21].Trauner M, Fickert P, Wagner M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin Liver Dis 2007;27:77–98. [DOI] [PubMed] [Google Scholar]


