Abstract
Lung cancer is one of the most common cancers and is associated with a poor survival rate in the Chinese Han population. Analysis of genetic variants could lead to improvements in prognosis following lung cancer treatment. Resistin (RETN) is an important mediator of metabolic diseases and tumor progression. In this study, we explored the effects of RETN single nucleotide polymorphisms (SNPs) on the susceptibility and clinicopathological characteristics of patients with lung cancer. Four RETN SNPs (rs7408174, rs1862513, rs3745367, and rs3219175) were analyzed using TaqMan SNP genotyping in 371 patients with lung cancer and 451 cancer-free controls. The results showed that the RETN SNP rs3219175 with AG or at least 1 A allele was associated with a higher risk of lung cancer than wild-type (GG) carriers. Moreover, the RETN SNP rs3219175 with AG or AG + AA alleles was associated with a higher risk of distant metastasis than that in patients carrying GG alleles. We also used genotype-tissue expression datasets to compare the correlation of the RETN SNP rs3219175 in lung tissue and whole blood. In conclusion, our study demonstrated, for the first time, that RETN polymorphisms were correlated with lung cancer progression in the Chinese Han population.
Keywords: lung cancer, polymorphism, resistin
1. Introduction
Lung cancer is one of the most common types of cancer worldwide and is associated with a poor 5-year relative survival rate.[1] The specific mechanisms underlying lung cancer development and progression are still unclear. Although cigarette smoking and alcohol consumption are known to directly induce lung cancer, various other environmental risks, such as exposure to second-hand smoke, air pollution, and genetic susceptibility, are also involved in the development of lung cancer.[2] Indeed, multiple genetic and epigenetic changes have been shown to be associated with lung cancer development.[3] Increased understanding of genetic mechanisms, including heterogeneity and DNA genotyping, is required to improve our ability to predict prognosis of lung cancer.[4] Therefore, exploration of genetic aberrations is necessary for lung cancer prognosis and risk prediction.
Resistin (RETN) is a 12.5-kDa cysteine-rich protein that is constitutively secreted by adipose tissue.[5] RETN has been reported to have function in inflammation and immune responses and to act as a pro-inflammatory mediator.[6] The RETN gene, encoding RETN, is localized on chromosome 9, and several single nucleotide polymorphisms (SNPs) have been identified in the RETN promoter and 3′-untranslated regions.[7] Several reports have connected genetic variants in RETN with the risk of various diseases, such as diabetes and colorectal cancer.[8,9] A functional RETN gene polymorphism at -420 (rs186513) has been shown to increase susceptibility to type 2 diabetes.[10] Another previous study evaluated a functional RETN gene polymorphism, rs3219175, but showed that this SNP was not associated with endometrial cancer.[11] Despite these studies, the correlation between RETN gene polymorphisms and lung cancer prognosis remains unclear.
Accordingly, in this report, we performed a case–control study to evaluate 4 SNPs in the RETN gene in patients with lung cancer.
2. Materials and methods
2.1. Patients and blood samples
We collected 371 blood specimens from the patients who had been diagnosed with lung cancer, including 279 adenocarcinoma lung cancer, 56 squamous cell carcinoma, and 36 other types of lung cancer patients, at Dongyang People's Hospital as the case group from 2014 to 2016. For the control group, 451 health participants without any history of cancer were enrolled. All patients and participants provided written informed consent, and this study was approved by the Ethics Committee of Dongyang People's Hospital. The pathological stages of lung cancer in all patients were determined according to their medical records and the Revised International System for Staging Lung Cancer. A standardized questionnaire and electronic medical record system were used to acquire data on age, sex, smoking history, and alcohol consumption.
2.2. Selection of RETN polymorphisms
Three RETN SNPs were selected from a 2-kb region upstream of RETN (rs7408174, rs1862513, and rs3219175), and 1 (rs3745367) was selected from the intron of RETN; all SNPs had minor allele frequencies of greater than 5%. Most RETN SNPs were known to be associated with type 2 diabetes mellitus or breast cancer.[12,13]
2.3. Genomic DNA extraction
Genomic DNA was extracted from peripheral blood leukocytes using a QIAamp DNA blood kit (Qiagen, CA) according to the manufacturer's instructions. Extracted DNA was stored at −20°C and prepared for genotyping by polymerase chain reaction (PCR).
2.4. Genotyping by real-time PCR
Four RETN SNP probes were purchased from Thermo Fisher Scientific Inc. (USA), and assessment of allelic discrimination for RETN SNPs was conducted using a Roche LightCycler 480 Instrument II (Roche, Mannheim, Germany). Data were further analyzed with LightCycler 480 Software, Version 1.5 (Roche). PCR was carried out in a total volume of 10 μL, containing 20 to 70 ng genomic DNA, 1 U Taqman Genotyping Master Mix (Thermo Fisher, Applied Biosystems, Foster City, CA), and 0.25 μL probes. The sequence of 4 RETN SNP probes was described as follows: rs3745367, CTCCGACTGTCCCCACCTTATCCAC[A/G]GCTCCAAACCCAA; rs7408174, TTTTACCACAAAAAGGCCCGTTGTA[C/T]TGGAAACAAAGAA; rs1862513, CCTGACCAGTCTCTGGACATGAAGA[C/G]GGAGGCCCTGTTG; rs3219175, CTCCAGCCCTTACTGTCTGCTCAGG[A/G]GCTTCCTCTTGGC. The protocol included an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.
2.5. Statistical analysis
Differences between the 2 groups were considered significant if P values were less than .05. Hardy–Weinberg equilibrium (HWE) was assessed using Chi-square goodness-of-fit tests for biallelic markers. Mann–Whitney U-tests and Fisher exact tests were used to compare differences in demographic characteristics between healthy controls and patients with lung cancer. The odds ratios (ORs) and 95% confidence intervals (CIs) for associations between genotype frequencies and the risk of lung cancer or clinicopathological characteristics were estimated by multiple logistic regression models, after controlling for other covariates. All data were analyzed using Statistical Analytic System software (v. 9.1, 2005; SAS Institute, Cary, NC).
3. Results
In this study, we evaluated differences in the general demographic characteristics of 371 patients with lung cancer and 451 cancer-free controls. All patients and participants were Chinese Han individuals (Table 1). There were no differences in age, cigarette smoking, alcohol consumption (all P < .001), and sex (P = .537). According to the American Joint Committee on Cancer (AJCC) TNM staging system, our results showed that 261 patients with lung cancer had clinical stage I + II (70.4%), and 110 patients had clinical stage III + IV (29.6%). The patients of lung cancers were divided into various subgroups, including nonsmall-cell lung cancer (NSCLC), small-cell lung cancer (SCLC), adenocarcinoma, squamous-cell carcinoma, and other carcinoma.
Table 1.
The distributions of demographical characteristics and clinical parameters in 451 controls and 371 patients with lung cancer.
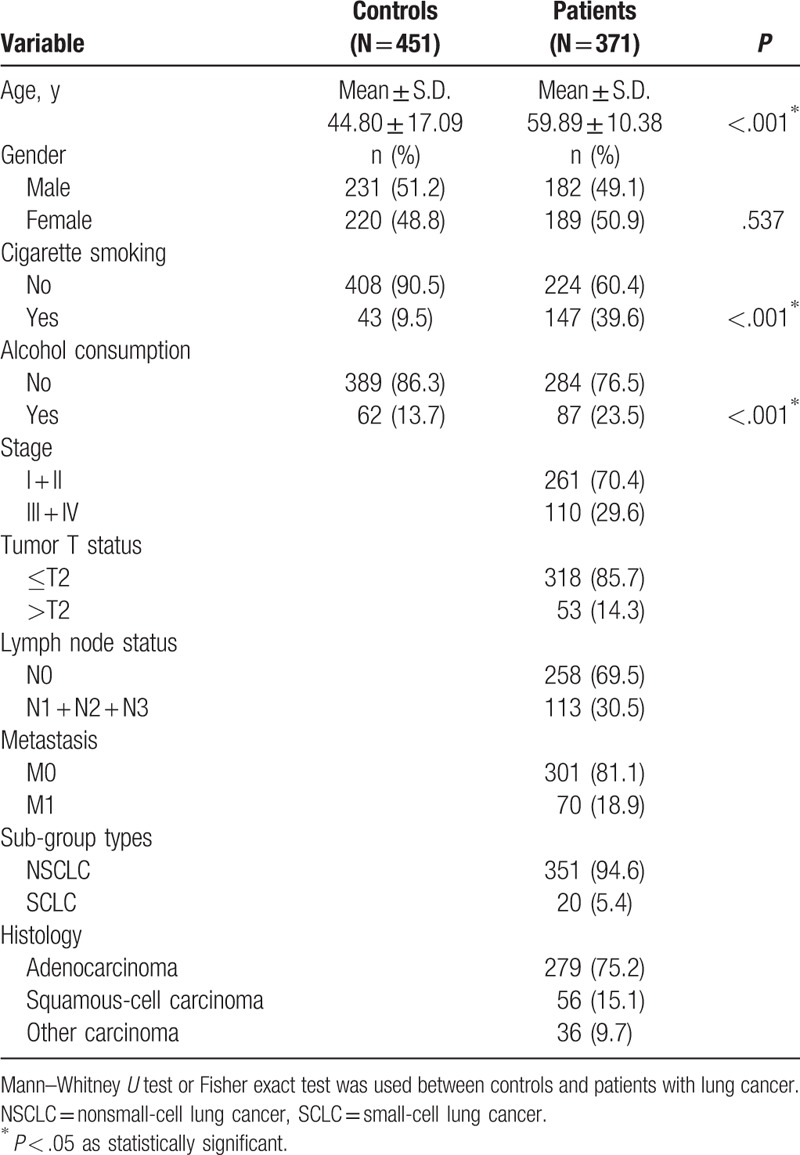
To evaluate whether the 4 RETN SNPs (rs7408174, rs1862513, rs3219175, and rs3745367) were associated with the risk of lung cancer, we genotyped controls and patients. Genotyping distributions and associations between lung cancer and RETN gene polymorphisms are illustrated in Table 2. The alleles with the highest distribution frequency at RETN rs3745367, rs7408174, rs1862513, and rs3219175 in both patients with lung cancer and control individuals were heterozygous A/G, homozygous T/T, heterozygous C/G, and homozygous G/G, respectively. Individuals carrying AG and AG + AA at rs3219175 had a 1.372-fold (95% CI: 0.533–0.961, P < .05) and 1.333-fold (95% CI: 1.010–1.761, P < .05) higher risk of lung cancer, respectively, than individuals carrying the wild-type GG polymorphic allele. Individuals with polymorphisms at rs3745367, rs7408174, and rs1862513 showed no significant associations with lung cancer risk relative to the risk in individuals with the wild-type genotype.
Table 2.
Distribution frequency of RETN genotypes in 451 controls and 371 patients with lung cancer.
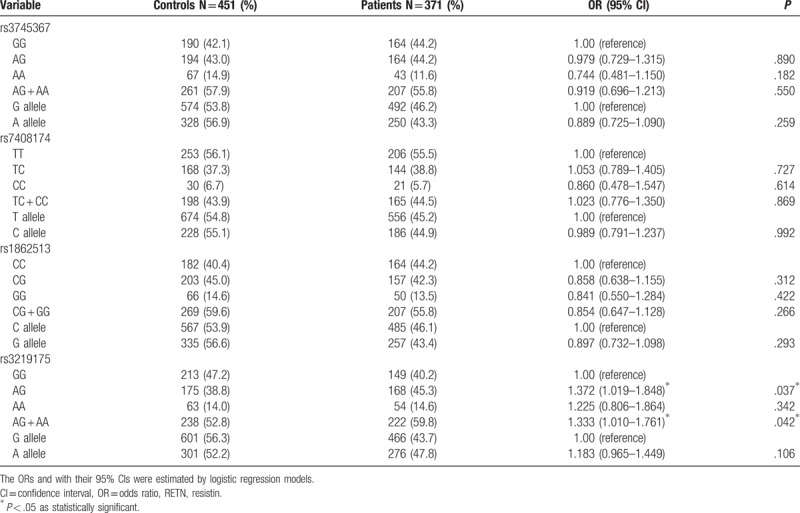
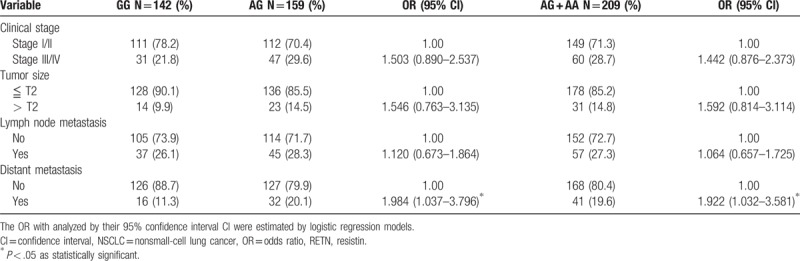
Next, RETN genotypes in patients with lung cancer were evaluated to clarify the role of RETN polymorphisms in clinical TNM stage, primary tumor size, lymph node metastasis, and distant metastasis. For 371 patients with lung cancer, a significant correlation between rs3219175 variants (GG vs AG; GG vs AG+AA) and tumor distant metastasis (OR: 1.885, 95% CI: 1.041–3.414, P < .05; OR: 1.875, 95% CI: 1.064–3.304, P < .05, respectively) was observed (Table 3). However, no significant differences were observed between RETN rs3219175 genotypes and clinicopathlogical status (data were not shown). The distribution frequency of clinical status in the NSCLC and SCLC is summarized in Table 4. A significant correlation between subgroup lung cancers (NSCLC vs SCLC) and clinical stage (OR: 54.286, 95% CI: 7.165–411.275, P < .001), tumor size (OR: 4.533, 95% CI: 1.757–11.695, P = .001), lymph node metastasis (OR: 51.947, 95% CI: 6.859–393.441, P < .001), and distant metastasis (OR: 9.579, 95% CI: 3.662–25.058, P < .001), respectively, were observed (Table 4). For 351 patients of NSCLC, a significant correlation between rs3219175 variants (GG vs AG; GG vs AG+AA) and tumor distant metastasis (OR: 1.984, 95% CI: 1.037–3.796, P < .05; OR: 1.922, 95% CI: 1.032–3.581, P < .05, respectively) was observed (Table 5). However, there was no significant difference between the lung adenocarcinoma and squamous cell carcinoma with polymorphisms of the resistin genotypes (data were not shown).
Table 3.
Odds ratio (OR) and 95% confidence interval (CI) of clinical status and RETN rs3219175 genotypic frequencies in 371 lung cancer patients.
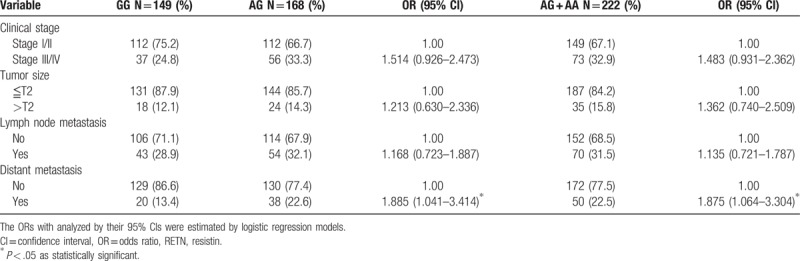
Table 4.
Distribution frequency of clinical status in 351 nonsmall-cell lung cancer (NSCLC) and 20 small-cell lung cancer (SCLC).
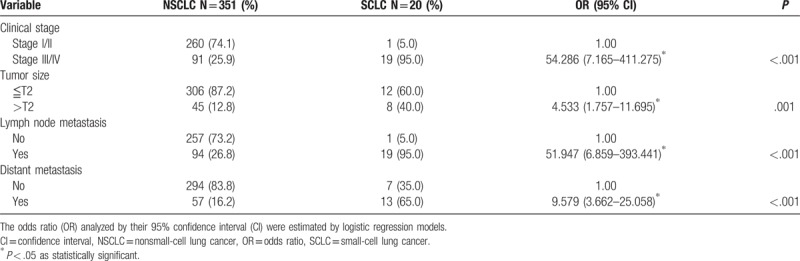
Table 5.
Association of RETN rs3219175 genotypic frequencies with laboratory status in NSCLC patients.
For the indicated correlations, we continued to employ Genotype-Tissue Expression (GTEx) datasets to further evaluate the association between rs3219175 and RETN expression. A significant alteration in RETN expression was observed in lung tissues and whole blood of patients with the polymorphic allele of RETN rs3219175 in the GTEx database. We observed that the heterozygous genotypes at rs3219175 were positively associated with RETN expression, compared with the wild-type homozygous genotypes (P < .05; Fig. 1A, B) (GTEx dataset # ENSG00000104918.4). These data suggested that the expression and function of RETN in response to genetic polymorphisms may affect lung cancer progression.
Figure 1.
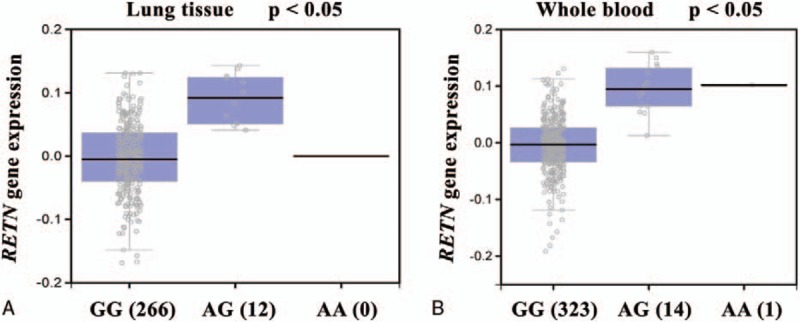
Functional analysis of the RETN SNP rs3219175 in lung cancer progression according to the Genotype-Tissue Expression (GTEx) dataset. (A) Correlation of rs3219175 genotypes with RETN mRNA expression in lung cancer tissue and (B) whole blood.
4. Discussion
Lung cancer is one of the most malignant cancers reported to date and is associated with severe morbidities and high mortality rates worldwide. Neither traditional chemotherapy nor molecular targeted therapy is efficacious in the clinical treatment of lung cancer.[14] It is imperative that increasing genetic studies and signaling mechanisms might help to clarify a proper strategy for lung cancer treatment. In this present study, we found that RETN gene polymorphisms were associated with a higher susceptibility to lung cancer. Indeed, our results showed that the ratios of cigarette smokers/nonsmokers in controls (90.5:9.5) and patients with lung cancer (60.4:39.6) were relatively normal, similar to the ratios of alcohol consumption/no alcohol consumption in controls and patients. Our findings showed that in the Chinese Han population, smoking and alcohol consumption were related to the risk of developing lung cancer.
RETN is an adipokine that is associated with obesity, inflammation, and various cancers. A recent study has reported that resistin level was found to be higher in the lung cancer (NSCLC) patients and associated with cancer cachexia.[15] Accumulating evidence has shown that the RETN gene often shows genetic or epigenetic alterations. Moreover, genetic polymorphisms in RETN have been identified in colorectal, colon, and breast cancers.[13,16,17] A recent SNP study demonstrated that RETN SNPs in the promoter region of the gene were negatively associated with DNA methylation in patients with type 2 diabetes.[18] In the present study, we showed, for the first time, that 1 of 4 RETN gene polymorphisms was associated with a high incidence of lung cancer. Indeed, our results indicated that AG or AG + AA at rs3219175 was significantly correlated with an increased lung cancer risk after 4 RETN polymorphisms genotyping in case–control subjects. In contrast, RETN polymorphisms at rs7408174, rs1862513, and rs3745367 were not significantly related to the risk of lung cancer compared with that in controls. Importantly, a previous study showed that this RETN SNP (rs3219175) positively affected the response to interferon-based anti-hepatitis C virus therapy.[19] Thus, our results may provide insights into the development of targeted therapy for lung cancer in patients with this specific SNP.
In epigenetic DNA methylation, variant polymorphisms in the promoter region could regulate gene expression and impact the risk of lung cancer.[20] The rs3219175 polymorphism is located in the promoter region and may regulate RETN gene expression. However, the reconstructed linkage disequilibrium plot of the 4 RETN SNPs showed that rs3219175 had low linkage disequilibrium with rs1862513 (data was not shown). In addition, our results demonstrated that patients with lung cancer carrying at least 1 A allele at rs3219175 were at a higher risk of tumor metastasis. A previous study revealed that the presence of the polymorphic allele of RETN rs3219175 was associated with dramatic effects on plasma resistin in patients with type 2 diabetes.[10] Another previous report had showed that rs3219175 SNPs were significant associated with log-resistin levels in Malaysian Malays.[21] In this study, we did not perform a detailed functional analysis between rs3219175 SNPs and resistin levels in Chinese Han populations, though it required to be further studied in the future. The issue of how these SNPs affect resistin gene expression in lung cancer cells is required to be further clarified. Thus, further studies are also needed to assess the correlations between RETN polymorphisms and lung cancer progression.
Although our present results showed that smoking and alcohol consumption were the risk factors for lung cancer (Table 1, P < .001), we failed to find a significant association after controlling for smoking and alcohol consumption in the Chinese Han population (data were not shown) because of poor records of smoking and alcohol consumption in these patients. In addition, some patient survival data were unavailable because patients had just recently enrolled in the study. Further studies are needed using larger populations of patients to confirm the role of RETN polymorphisms in lung cancer progression. Furthermore, the functional role of RETN in metastasis in patients with lung cancer should also be evaluated.
Taken together, our results demonstrated the association between 1 RETN gene variant and risk of lung cancer and found that RETN rs3219175 was significantly associated with tumor distant metastasis in the Chinese Han population. This study is the first to report a correlation between RETN polymorphisms and lung cancer risk. Thus, RETN could be developed as a genetic prognostic marker for lung cancer therapy.
Footnotes
Abbreviations: CI = confidence interval, GTEx = genotype-tissue expression, HWE = Hardy–Weinberg equilibrium, NSCLC = non-small-cell lung cancer, OR = odds ratio, PCR = polymerase chain reaction, RETN = resistin, SCLC = small-cell lung cancer, SNP = single nucleotide polymorphisms.
Both W-WH and C-HT have contributed equally to this work.
Funding/support: This work was supported by grants from the Dongyang People's Hospital (2015-ZD004).
The authors have no conflicts of interest to declare.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Malhotra J, Malvezzi M, Negri E, et al. Risk factors for lung cancer worldwide. Eur Respir J 2016;48:889–902. [DOI] [PubMed] [Google Scholar]
- [3].Zhu J, Zeng Y, Xu C, et al. Expression profile analysis of microRNAs and downregulated miR-486-5p and miR-30a-5p in non-small cell lung cancer. Oncol Rep 2015;34:1779–86. [DOI] [PubMed] [Google Scholar]
- [4].Fang X, Yin Z, Li X, et al. Multiple functional SNPs in differentially expressed genes modify risk and survival of non-small cell lung cancer in Chinese female non-smokers. Oncotarget 2017;8:18924–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature 2001;409:307–12. [DOI] [PubMed] [Google Scholar]
- [6].Reilly MP, Lehrke M, Wolfe ML, et al. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation 2005;111:932–9. [DOI] [PubMed] [Google Scholar]
- [7].Steppan CM, Brown EJ, Wright CM, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci U S A 2001; 98:502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Onuma H, Tabara Y, Kawamura R, et al. Dual effects of a retn single nucleotide polymorphism (SNP) at -420 on plasma resistin: genotype and DNA methylation. J Clin Endocrinol Metab 2017;102:884–92. [DOI] [PubMed] [Google Scholar]
- [9].Mahmoudi T, Majidzadeh AK, Karimi K, et al. Gly972Arg variant of insulin receptor substrate 1 gene and colorectal cancer risk in overweight/obese subjects. Int J Biol Markers 2016;31:e68–72. [DOI] [PubMed] [Google Scholar]
- [10].Onuma H, Tabara Y, Kawamura R, et al. A at single nucleotide polymorphism-358 is required for G at -420 to confer the highest plasma resistin in the general Japanese population. PLoS One 2010;5:e9718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hlavna M, Kohut L, Lipkova J, et al. Relationship of resistin levels with endometrial cancer risk. Neoplasma 2011;58:124–8. [DOI] [PubMed] [Google Scholar]
- [12].Chung CM, Lin TH, Chen JW, et al. Common quantitative trait locus downstream of RETN gene identified by genome-wide association study is associated with risk of type 2 diabetes mellitus in Han Chinese: a Mendelian randomization effect. Diabetes Metab Res Rev 2014;30:232–40. [DOI] [PubMed] [Google Scholar]
- [13].Vallega KA, Liu N, Myers JS, et al. Elevated resistin gene expression in African American estrogen and progesterone receptor negative breast cancer. PLoS One 2016;11:e0157741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gatzemeier U, Groth G, Butts C, et al. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 2004;15:19–27. [DOI] [PubMed] [Google Scholar]
- [15].Demiray G, Degirmencioglu S, Ugurlu E, et al. Effects of serum leptin and resistin levels on cancer cachexia in patients with advanced-stage non-small cell lung cancer. Clin Med Insights Oncol 2017;11:1179554917690144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mahmoudi T, Karimi K, Arkani M, et al. Resistin -420C>G promoter variant and colorectal cancer risk. Int J Biol Markers 2014;29:e233–8. [DOI] [PubMed] [Google Scholar]
- [17].Alharithy RN. Polymorphisms in RETN gene and susceptibility to colon cancer in Saudi patients. Ann Saudi Med 2014;34:334–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakatochi M, Ichihara S, Yamamoto K, et al. Epigenome-wide association study suggests that SNPs in the promoter region of RETN influence plasma resistin level via effects on DNA methylation at neighbouring sites. Diabetologia 2015;58:2781–90. [DOI] [PubMed] [Google Scholar]
- [19].Chang ML, Liang KH, Ku CL, et al. Resistin reinforces interferon lambda-3 to eliminate hepatitis C virus with fine-tuning from RETN single-nucleotide polymorphisms. Sci Rep 2016;6:30799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qin H, Zhu J, Zeng Y, et al. Aberrant promoter methylation of hOGG1 may be associated with increased risk of non-small cell lung cancer. Oncotarget 2017;8:8330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Apalasamy YD, Rampal S, Salim A, et al. Polymorphisms of the resistin gene and their association with obesity and resistin levels in Malaysian Malays. Biochem Genet 2015;53:120–31. [DOI] [PubMed] [Google Scholar]


