Abstract
To study the therapeutic effect of neuromuscular electrical stimulation and electromyographic biofeedback (EMG-biofeedback) therapy in improving swallowing function of Alzheimer's disease patients with dysphagia.
A series of 103 Alzheimer's disease patients with dysphagia were divided into 2 groups, among which the control group (n = 50) received swallowing function training and the treatment group (n = 53) received neuromuscular electrical stimulation plus EMG-biofeedback therapy. The mini-mental state scale score was performed in all patients along the treatment period. Twelve weeks after the treatment, the swallowing function was assessed by the water swallow test. The nutritional status was evaluated by Mini Nutritional Assessment (MNA) as well as the levels of hemoglobin and serum albumin. The frequency and course of aspiration pneumonia were also recorded.
No significant difference on mini-mental state scale score was noted between 2 groups. More improvement of swallowing function, better nutritional status, and less frequency and shorter course of aspiration pneumonia were presented in treatment group when compared with the control group.
Neuromuscular electrical stimulation and EMG-biofeedback treatment can improve swallowing function in patients with Alzheimer's disease and significantly reduce the incidence of adverse outcomes. Thus, they should be promoted in clinical practice.
Keywords: Alzheimer's disease, dysphagia, electromyographic biofeedback, neuromuscular electrical stimulation, rehabilitation
1. Introduction
Swallowing dysfunction is a common clinical presentation in patients with Alzheimer's disease (AD) in the medium and long term. It was estimated that 80% of these patients would suffer from oropharyngeal dysphagia if left untreated. In advanced stages, the incidence of dysphagia can increase up to 93%.[1] Dysphagia is frequently associated with malnutrition, dehydration, and aspiration pneumonia,[2,3] causing severe consequences of the quality of life.[4,5] Therefore, it is of great importance to seek for effective treatments for dysphagia.
Neuromuscular electrical stimulation (NMES) was proposed as a therapeutic adjunct in the treatment of dysphagia.[6–9] By stimulating the nerve as well as the motor end-plate, NMES can be used for remodeling the functional muscle contraction patterns[10] and thus improve the swallowing function. On the other hand, electromyographic biofeedback (EMG-biofeedback) has been advocated as an adjunct to swallowing therapy with prior reports of rapid progress in patients treated with this approach, even in chronic patients.[11–13] Also, it was suggested to be effective in patients with pharyngeal dysphagia after stroked.[12,13] However, the effect of NMES and EMG-biofeedback in treating AD patients with dysphagia has not been well elucidated.
In this study, combination therapy with the NMES and EMG-biofeedback was adopted to explore effective treatment for swallowing disorder in AD patients with dysphagia.
2. Materials and methods
This study was approved by Ethics Review Board of Fujian Provincial Geriatric Hospital. Forms of written consent were obtained from all to patients involved in this study.
2.1. Participants
A series of 103 AD patients with dysphagia, who were treated in neurological department of Fujian Provincial Geriatric Hospital from March 2013 to November 2016, were included in this study. After reviewing the medical records, patients were retrospectively divided into 2 groups according to the treatment they underwent. The patients treated with swallowing function training, which is a basic treatment, were enrolled in control group. In addition to swallowing function training, combination treatment with NMES and EMG-biofeedback was conducted in treatment group. Patient in control group refused further treatment with NMES and EMG-biofeedback due to the personal concerns instead of medical considerations. The control group consisted of 50 patients (30 men) with a mean age of 76.2 ± 2.3 while the treatment group included 53 patients (26 men) with a mean age of 72.5 ± 2.5.
For the diagnosis of dysphagia, videofluoroscopic swallowing assessment with the scoring criteria described by the previous literature was adopted.[14] The standard method of videofluoroscopic swallowing assessment was as described by Verin et al.[15] Since this study was conducted in AD patients, those who complained of dysphagia but did not show any abnormal findings in videofluoroscopic swallowing assessment were not included in this study.
For the diagnosis of AD, there were a set of inclusion and exclusion criteria adopted in our department. Patients were diagnosed with AD according to the Diagnostic and Statistical Manual of Mental Disorders, 4th ed (DSM-IV)[16] and National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS ADRDA)[17] criteria. Patients with the following conditions were excluded: pseudo dementia (such as depressive dementia), mental retardation, low cognitive function due to extreme poverty and limited education or drug-induced intelligence damage. The dementia syndrome caused by liver failure, pernicious anemia, reduced thyroid function or hyperthyroidism, neuro syphilis, prion diseases, or AIDS were ruled out by related serological tests.
2.2. Clinical assessments and functional assessment of dysphagia
Physical examinations including general, neurological, and dental examinations as well as routine blood tests were performed on admission. The levels of hemoglobin (Hb) and serum albumin (ALB) were viewed as adjunct indicator of nutritional status. Mini Nutritional Assessment (MNA) was performed by 2 trained nutritionists to evaluate the nutritional status of all the patients involved. The MNA consisted of 6 questions on food intake, weight lose, mobility, psychological stress or acute disease, neuropsychological problems, and body mass index or calf circumference. These 6 items yield 0 to 2 or 0 to 3 points, with 0 indicating poor function and 2 or 3 indicating normal function.[18] Scores from 12 to 14 points implicate normal nutritional status, patients with scores from 8 to 11 points are at risk of malnutrition, and those with 0 to 7 points are considered as malnourished.
Mini-mental state examination (MMSE) was used to determine the normal cognitive function in healthy controls by the following decisive criteria: the points achieved by the subjects were greater than the highest mark of the corresponding literacy level (illiteracy >17 points, primary school >20 points, more than high school >24 points).
Water swallow test (WST) were also conducted in all patients to evaluate the swallowing function before and after treatment.[15,19] Patients were asked to drink 30 mL tepid water and the scores were given accordingly as shown in Table 1. The pretreatment score was indicated as N0 and posttreatment score as N1 (12 weeks later). The difference between N0 and N1 was defined as N (N = N0–N1). The effect of treatment was interpreted as deteriorated, ineffective, effective, and excellent when N was <0, 0, 1, and >1, respectively.[7]
Table 1.
Descriptions of scoring system in water swallow test.

2.3. Rehabilitation therapy
The rehabilitation therapy was performed in each patient according to a routine rehabilitation training described in a previous reported, including tongue exercises, pharynx and larynx exercises.[19] Each exercise in rehabilitation therapy was repeatedly practiced for 15 circles at each time for a total of 45 circles per day.
2.4. NMES and EMG-biofeedback
Aside from the swallowing function training, NMES and EMG-biofeedback were also conducted in the treatment group.
Patients were comfortably seated in a reclining chair with skin electrode attached. Electrical stimulation was delivered using a dual-channel electrotherapy system of VitalStim (Chattanooga Group, Hixson, TN). The thyroid notch was identified by palpation and the first electrode was placed midline 1 mm above it, the second electrode immediately superior to the first electrode, the third electrode 1 mm below the thyroid notch, and the fourth electrode immediately inferior to the third electrode. NMES was applied at a frequency of 80 Hz with a wave width of 700 μs and wave amplitude 0 to 25 mA. The stimulus intensity was gradually increased in 0.5 mA increments until patients reported feeling a “grabbing” sensation, which was demonstrated in patients in the pretreatment to be identified. The amplitude of the electrical current was regulated according to the patients’ verbal feedback and when a grabbing sensation was reported, the amplitude was left at that level for the remainder of the 60-minutes therapy session.[20,21]
The swallowing activity was visualized with the application of EMG (MyeTrac III, Thought Technology, Canada) of the submental muscle and the data were recorded on a computer. Electrodes were placed on the submental region, which was between the mandible and the hyoid, to record the muscle activity of submental muscle (musculi stylohyoideus, musculi mylohyoideus, musculi digastricus). In the treatment of dysphagia with EMG-biofeedback, patients were instructed to make Mendelsohn maneuver, where they prolonged the laryngeal excursion to the maximum of 2 to 3 seconds.[22] The EMG signal was on a computer monitor and patients had an immediate feedback of their swallowing action. NMES and EMG-biofeedback were conducted once a day with 1 hour at a time.
All the patients in treatment group and control group were exercised and evaluated by the same therapist. Data were collected on an outpatient basis about every 4 weeks (W0, W4, W8, W12), including the evaluation of swallowing function, the levels of Hb and ALB, the results of MNA and MMSE. Meanwhile, the reported incidences of aspiration pneumonia in the 2 groups during follow-up were also recorded.
2.5. Statistical methods
SPSS13.0 software package (Chicago, IL) was used for statistical analysis. Statistical data were expressed as mean ± standard deviation and P < .05 was considered statistically significant. Comparisons of the results of MMSE, MNA, and WST, the level of Hb and ALB were made with the use of t test while demographic data were analyzed by the Pearson χ2 test. The comparison of the results of WST was made with the use of Mann–Whitney U test.
3. Results
No statistically significant difference on sex, age, the results of MMSE, MNA, and WST, the level of Hb and ALB between the 2 groups was observed on admission (Table 2).
Table 2.
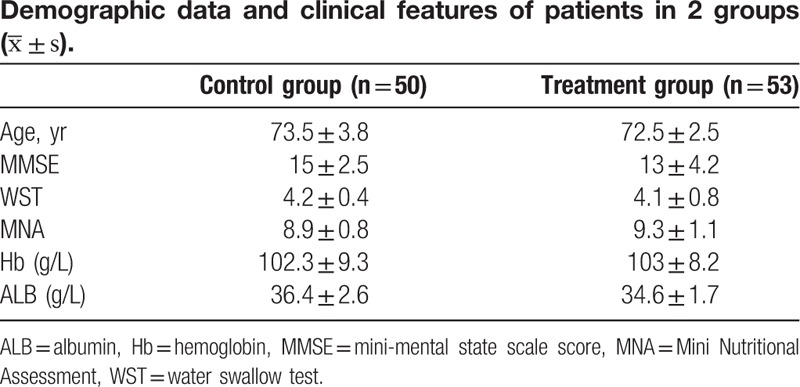
Along the whole treatment periods, no significant difference on MMSE was presented between 2 groups (P < .05). After 12 weeks of treatment, improvement of swallowing function assessed by WST was noted in both groups while more improvement was noted in treatment group than that in control group (P < .05) (Tables 3 and 4). The nutritional status, evaluated by MNA and the levels of Hb and ALB, was better achieved in treatment when compared with control group after 12 weeks of treatment (P < .05) (Table 4). As for the complication of aspiration pneumonia, less frequency and shorter course was presented in treatment group when compared with control group (Fig. 1).
Table 3.
The results of water swallow test in 2 groups.

Table 4.
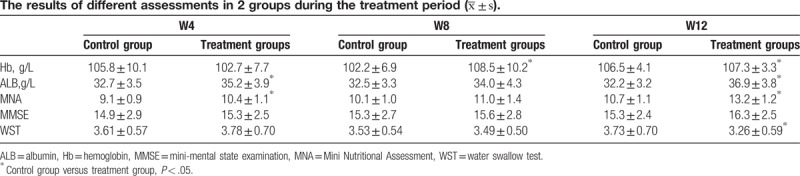
Figure 1.
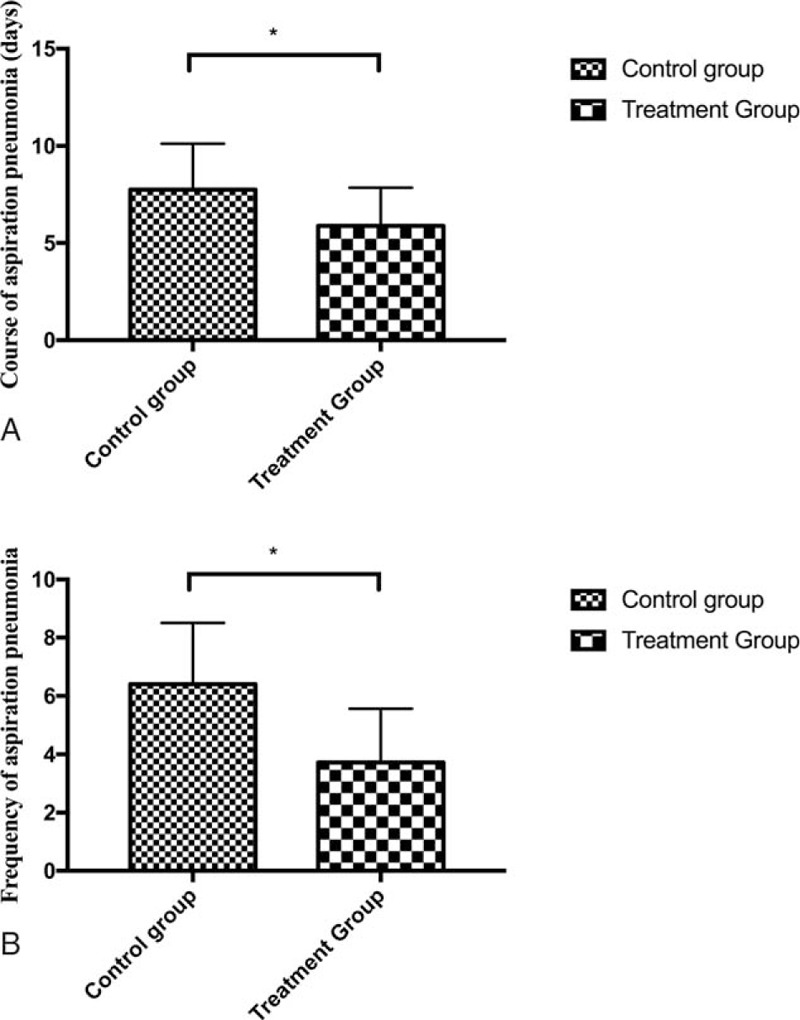
Graphs of course (A) and frequency (B) of aspiration pneumonia in both groups. A, shorter course of aspiration pneumonia was noted in treatment group when compared with control group, B, less frequency of aspiration pneumonia was noted in treatment group when compared with control group. ∗, indicates significant difference was noted between 2 groups.
4. Discussion
The reflex center of swallowing is located in the brain stem, while the start of swallowing action depends on the cortex motor area and the integrity of limbic cortex.[23] Quite a few studies have shown that swallowing function is closely related to the insula,[24–29] which controls swallowing movements with the synergistic action of precentral gyrus, postcentral gyrus, and cingulate gyrus. AD mainly involves the forebrain, including insula. AD patients in the medium and long term were associated with different degrees of swallowing disorder, usually complicated by aspiration pneumonia. It has a negative impact on the patient's recovery, extending the length of hospital stay, increasing the economic burden on patients, even the mortality.[30]
NMES stimulates muscle contraction mainly through low frequency pulse current. Contraction and expansion of pharyngeal muscle can move the food into the esophagus, and thus help to rebuild the control function of the brainstem reflex center over swallowing reflex, improve blood circulation and the pharyngeal muscle flexibility and coordination, and prevent pharyngeal muscle atrophy. Meanwhile, the appropriate pharyngeal stimulation help increase the pressure in the mouth, and improve significantly or even restore the swallowing function. NMES helps to strengthen the digestive function of oropharyngeal cavity and esophagus and prevent food regurgitation and aspiration, thereby reducing respiratory complications.[31]
EMG-biofeedback establishes a feedback path outside the body and makes each of the correct processes learnt gradually through repeatedly studying external signal conditioning, improving the regulation of the swallowing function of the cerebral cortex motor area and cortex edge. In addition to this, low-frequency pulse current induces coordinated local muscle contraction and expansion and increases the pressure within the oral cavity and pharyngeal, moving the food into the esophagus. By stimulating feedback loop, EMG-biofeedback treatment helps restore normal reflex, promoting the central conduction pathway formation.[32]
Related researches have proved that drug therapy can improve dysphagia in AD patients with swallowing disorder,[33,34] but few researches on rehabilitation treatment were reported. According to the theory of neural plasticity and individual difference, the treatment group patients in this study were, on the basis of swallowing training, treated with NMES and EMG-biofeedback therapy. The purpose is to promote hemisphere function remodeling and to establish the role of loss of motor function in feeding training. In the present study, we found that swallowing function in patients was improved gradually with the rehabilitation training. During rehabilitation training, nutritional status was improved in both groups while the treatment group was improved more obviously. Since the frequency and course of aspiration pneumonia were reduced, the nutritional status was improved, which was indicated by the results of MNA and the increased levels of the Hb in the 2 groups after treatment. It is suggested that combination therapy with NMES and EMG-biofeedback can reduce the frequency of pneumonia and shorten the course of the disease.
This study suggested that swallowing function of AD patients can be improved through NMES and EMG-biofeedback. However, the present study mainly focused on the short-term results, the long-term effect of NMES combined with EMG-biofeedback remains to be confirmed by further researches.
5. Conclusion
In summary, NMES combined with EMG-biofeedback can effectively improve the swallowing function of AD patients with dysphagia and thus should be promoted in clinical practice.
Footnotes
Abbreviations: AD = Alzheimer's disease, ALB = serum albumin, DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, 4th ed, EMG-biofeedback = electromyographic biofeedback, Hb = hemoglobin, MMSE = Mini-mental state examination, MNA = Mini Nutritional Assessment, NINCDS ADRDA = National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association, NMES = neuromuscular electrical stimulation, WST = water swallow test.
The authors report no conflicts of interest.
References
- [1].Boccardi V, Ruggiero C, Patriti A, et al. Diagnostic assessment and management of dysphagia in patients with Alzheimer's disease. J Alzheimers Dis 2016;50:947–55. [DOI] [PubMed] [Google Scholar]
- [2].Kirshner HS. Causes of neurogenic dysphagia. Dysphagia 1989;3:184–8. [DOI] [PubMed] [Google Scholar]
- [3].McMicken BL, Muzzy CL. Functional outcomes of standard dysphagia treatment in first time documented stroke patients. Disabil Rehabil 2009;31:806–17. [DOI] [PubMed] [Google Scholar]
- [4].Hansen T, Kjaersgaard A, Faber J. Measuring elderly dysphagic patients’ performance in eating—a review. Disabil Rehabil 2011;33:1931–40. [DOI] [PubMed] [Google Scholar]
- [5].Hansen T, Lambert HC, Faber J. Validation of the Danish version of the McGill Ingestive Skills Assessment using classical test theory and the Rasch model. Disabil Rehabil 2012;34:859–68. [DOI] [PubMed] [Google Scholar]
- [6].Lee HY, Hong JS, Lee KC, et al. Changes in hyolaryngeal movement and swallowing function after neuromuscular electrical stimulation in patients with dysphagia. Ann Rehabil Med 2015;39:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Long YB, Wu XP. A randomized controlled trail of combination therapy of neuromuscular electrical stimulation and balloon dilatation in the treatment of radiation-induced dysphagia in nasopharyngeal carcinoma patients. Disabil Rehabil 2013;35:450–4. [DOI] [PubMed] [Google Scholar]
- [8].Toyama K, Matsumoto S, Kurasawa M, et al. Novel neuromuscular electrical stimulation system for treatment of dysphagia after brain injury. Neurol Med Chir (Tokyo) 2014;54:521–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Power ML, Fraser CH, Hobson A, et al. Evaluating oral stimulation as a treatment for dysphagia after stroke. Dysphagia 2006;21:49–55. [DOI] [PubMed] [Google Scholar]
- [10].Freed ML, Freed L, Chatburn RL, et al. Electrical stimulation for swallowing disorders caused by stroke. Respir Care 2001;46:466–74. [PubMed] [Google Scholar]
- [11].Crary MA. A direct intervention program for chronic neurogenic dysphagia secondary to brainstem stroke. Dysphagia 1995;10:6–18. [DOI] [PubMed] [Google Scholar]
- [12].Huckabee ML, Cannito MP. Outcomes of swallowing rehabilitation in chronic brainstem dysphagia: a retrospective evaluation. Dysphagia 1999;14:93–109. [DOI] [PubMed] [Google Scholar]
- [13].Crary MA, Mann GD, Groher ME. Initial psychometric assessment of a functional oral intake scale for dysphagia in stroke patients. Arch Phys Med Rehabil 2005;86:1516–20. [DOI] [PubMed] [Google Scholar]
- [14].Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke 1999;30:744–8. [DOI] [PubMed] [Google Scholar]
- [15].Verin E, Maltete D, Ouahchi Y, et al. Submental sensitive transcutaneous electrical stimulation (SSTES) at home in neurogenic oropharyngeal dysphagia: a pilot study. Ann Phys Rehabil Med 2011;54:366–75. [DOI] [PubMed] [Google Scholar]
- [16].APA. Diagnostic and Statistical Manual of Mental Disorders. 4th ed ed. Washington: American Psychiatric Association; 1994. [Google Scholar]
- [17].McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- [18].Vellas B, Villars H, Abellan G, et al. Overview of the MNA—its history and challenges. J Nutr Health Aging 2006;10:456–63. discussion 463-455. [PubMed] [Google Scholar]
- [19].Tang Y, Shen Q, Wang Y, et al. A randomized prospective study of rehabilitation therapy in the treatment of radiation-induced dysphagia and trismus. Strahlenther Onkol 2011;187:39–44. [DOI] [PubMed] [Google Scholar]
- [20].Fowler LP, Gorham-Rowan M, Hapner ER. An exploratory study of voice change associated with healthy speakers after transcutaneous electrical stimulation to laryngeal muscles. J Voice 2011;25:54–61. [DOI] [PubMed] [Google Scholar]
- [21].Permsirivanich W, Tipchatyotin S, Wongchai M, et al. Comparing the effects of rehabilitation swallowing therapy vs. neuromuscular electrical stimulation therapy among stroke patients with persistent pharyngeal dysphagia: a randomized controlled study. J Med Assoc Thai 2009;92:259–65. [PubMed] [Google Scholar]
- [22].Kahrilas PJ, Logemann JA, Krugler C, et al. Volitional augmentation of upper esophageal sphincter opening during swallowing. Am J Physiol 1991;260(Pt 1):G450–6. [DOI] [PubMed] [Google Scholar]
- [23].Hamdy S, Rothwell JC, Aziz Q, et al. Organization and reorganization of human swallowing motor cortex: implications for recovery after stroke. Clin Sci (Lond) 2000;99:151–7. [PubMed] [Google Scholar]
- [24].Penfield W, Faulk ME., Jr The insula; further observations on its function. Brain 1955;78:445–70. [DOI] [PubMed] [Google Scholar]
- [25].Daniels SK, Foundas AL, Iglesia GC, et al. Lesion site in unilateral stroke patients with dysphagia. J Stroke Cerebrovasc Dis 1996;6:30–4. [DOI] [PubMed] [Google Scholar]
- [26].Daniels SK, Foundas AL. The role of the insular cortex in dysphagia. Dysphagia 1997;12:146–56. [DOI] [PubMed] [Google Scholar]
- [27].Hamdy S, Mikulis DJ, Crawley A, et al. Cortical activation during human volitional swallowing: an event-related fMRI study. Am J Physiol 1999;277(Pt):G219–25. [DOI] [PubMed] [Google Scholar]
- [28].Kern M, Birn R, Jaradeh S, et al. Swallow-related cerebral cortical activity maps are not specific to deglutition. Am J Physiol Gastrointest Liver Physiol 2001;280:G531–8. [DOI] [PubMed] [Google Scholar]
- [29].Martin RE, Goodyear BG, Gati JS, et al. Cerebral cortical representation of automatic and volitional swallowing in humans. J Neurophysiol 2001;85:938–50. [DOI] [PubMed] [Google Scholar]
- [30].Finlayson O, Kapral M, Hall R, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology 2011;77:1338–45. [DOI] [PubMed] [Google Scholar]
- [31].Bogaardt HC, Grolman W, Fokkens WJ. The use of biofeedback in the treatment of chronic dysphagia in stroke patients. Folia Phoniatr Logop 2009;61:200–5. [DOI] [PubMed] [Google Scholar]
- [32].Lake DA. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med 1992;13:320–36. [DOI] [PubMed] [Google Scholar]
- [33].Sonnenberg A. Cost effectiveness of competing strategies to prevent or treat GORD-related dysphagia. Pharmacoeconomics 2000;17:391–401. [DOI] [PubMed] [Google Scholar]
- [34].Geeganage C, Beavan J, Ellender S, et al. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev 2012;10:CD000323. [DOI] [PubMed] [Google Scholar]


