Abstract
Background:
Human Cripto-1 (CR-1), a member of the epidermal growth factor-Cripto-1/FRL-1/Cryptic protein family (EGF-CFC), is highly expressed in a variety of human cancers. We aimed to detect serum CR-1 level in liver diseases especially in hepatocellular carcinoma (HCC) patients.
Methods:
Serum CR-1 level was Sandwich-type enzyme-linked immuno sorbent assay (ELISA) detected in 330 patients with liver diseases including HCC, cirrhosis, and chronic hepatitis and 50 volunteers without hepatitis B virus (HBV) or hepatitis C virus (HCV) infection as control.
Results:
The serum CR-1 level was significantly higher in HCC patients than volunteer controls and it was also significantly higher in HBV-related HCC than HCV-related HCC. In addition, serum CR-1 level was correlated with serum alpha-feto-protein (AFP) in HBV-related HCC patients. The serum CR-1 was also higher in cirrhosis and chronic hepatitis than volunteer controls. The serum CR-1 in HBV-related cirrhosis was higher than chronic hepatitis B, but there was no significant difference between HCV-related cirrhosis and chronic hepatitis C.
Conclusions:
Serum CR-1 was higher in HCC patients and might serve as a complementary biomarker to clinical diagnosis of HBV-related HCC. The high level of serum CR-1 in HBV-related liver disease might be partly attributed to HBV infection.
Keywords: Cripto-1, hepatitis B virus, hepatocellular carcinoma, liver cirrhosis
1. Introduction
Human Cripto-1 (CR-1) is the founding member of the epidermal growth factor-Cripto-1/FRL-1/Cryptic protein family (EGF-CFC), also known as teratocarcinoma-derived growth factor-1 (TDGF-1).[1] It is involved in the activation of several different signaling pathways during embryonic development and cellular transformation.[2] The major signaling pathways activated by CR-1 are the Nodal/ALK4/Smad-2 signaling pathway and the Glypican-1/c-Src/ mitogen-activated protein kinase (MAPK)/AKT signaling pathway.[2–4] CR-1 protein structure consists of 2 functional domains. One is a modified EGF-like domain, which is divergent from the canonical EGF-domain possessed by EGF, transforming growth factor α, and heregulins. The other is called a CFC domain, which carries a cysteine-rich region. CR-1 also functions as a cell membrane anchored protein through its COOH terminal glycosylphosphatidylineositol (GPI) anchoring site. Once cleaved by phosphatidy inositol-phospholipase C. GPI can be secreted into serum or conditioned medium by several cell lines.
CR-1 expression has been associated with the pluripotential capacity and self-renewal of human and mouse embryonic stem cells and is a stem cell marker.[5,6] Cancer stem cells (CSCs), also known as tumor-initiating cells, share several characteristics with normal tissue stem cells. CSCs have been identified in human tumors, and they possess long-term self-renewal potential, quiescent properties, and resistance to chemotherapy and radiotherapy.[7] A high level of CR-1 expression was detected in different human primary carcinomas, possibly promoting angiogenesis, cell proliferation, invasion, and migration. CR-1 is overexpressed in bladder, colon, breast, lung, and gastric cancer,[8–13] while a low or absence of CR-1 expression was noted in normal tissues, suggesting a high level of CR-1 expression in the tissue may indicate malignant transformation. Hepatocellular carcinoma (HCC) is one of the most common human cancers, ranking as a third leading cause for cancer-related death worldwide.[14] However, information on the serum CR-1 level in HCC patients remains scarce. In this study, we aimed to investigate the serum CR-1 level in patients with liver diseases including HCC.
2. Materials and methods
2.1. Patients
A total of 330 inpatients were recruited from 2012 to 2017 from the First Hospital of Jilin University (Changchun, China). Among them, there were 115 HCC patients who did not receive treatment such as resection or liver transplant. Among the remaining patients, 77 were diagnosed with hepatitis B virus (HBV)-related liver cirrhosis, 62 with chronic hepatitis B, 32 with hepatitis C virus (HCV)-related liver cirrhosis, 34 with chronic hepatitis C, and 10 with metastatic hepatic carcinoma. Among the 115 HCC patients, 74 patients were HBV infected, 34 HCV infected, and 7 without HBV or HCV infection. Serum samples from above patients as well as 50 volunteers (no HBV or HCV infection) from Changchun epidemiological investigation in 2016 were collected as normal controls were stored at −80°C until analysis. Written informed consent was obtained from all participants. This study was approved by the Ethics Committee of the First Hospital of Jilin University.
2.2. Serum samples
The serum level of CR-1 was detected by commercialized human CR-1 DuoSet enzyme-linked immuno sorbent assay (ELISA) kit (R&D System Inc.). The 96-well microtiter plates were coated with mouse anti-human CR-1 100 μL/well and incubated overnight at 4°C. Wash the plate with wash buffer for 3 times after each step. The plates were then blocked with 300 μL/well of 1% bull Serum Albumin (BSA) for 1 hour at room temperature and incubated with 100 μL serum samples for 2 hours at room temperature. Then, 100 μL of biotinylated mouse anti-human CR-1 to each well was added and incubated for 2 hours at room temperature, followed by addition of 100 μL of streptavidin horse radish peroxidase (Streptavidin-HRP) to each well for 20 minutes at room temperature. Finally, 100 μL of Substrate Solution were added to each well. The reaction was stopped with 50 μL of stop solution and OD values were read at 450 nm. A standard curve was generated and the concentrations of CR-1 were calculated. CR-1 standards ranged from 62.5 to 4000 pg/mL were included in each plate.
2.3. Statistical analysis
Statistical software (SPSS, version 18) was used for the analysis. The non-parametric Mann–Whitney U test was used to assess the statistical significances of the difference between the various groups. Receiver operating characteristics (ROC) curves were constructed to assess sensitivity, specificity, and respective areas under the curves (AUCs) with 95% CI. Correlation between different variables and CR-1 was assessed by Spearman correlation coefficient. All statistical tests were 2-sided, and P < .05 was considered statistically significant.
3. Results
3.1. Human CR-1 serum level was higher in HCC patients
Serum CR-1 level of 115 HCC serum samples was detected, as shown in Figure 1. Data are expressed as median and range. The serum level of CR-1 was significantly higher in HBV-related HCC patients [218.5 (62.5–4000) pg/mL, n = 74, P < .001] than that in volunteer controls [62.5 (62.5–74) pg/mL]. Serum CR-1 level in HCV-related HCC patients [107.5 (62.5–1085) pg/mL, n = 34, P < .001] and HCC patients without HBV or HCV infection [97.3 (62.5–261.6) pg/mL, n = 7, P = .023] was also higher than that in volunteer controls. However, there was no significant difference in serum CR-1 level between metastatic hepatic carcinoma [71.95 (62.5–2035.2) pg/mL, n = 10, P = .149] and volunteer controls. Interestingly, the serum CR-1 level of HBV-related HCC patients was significantly higher than that in HCV-related HCC patients (P = .015, Table 1).
Figure 1.
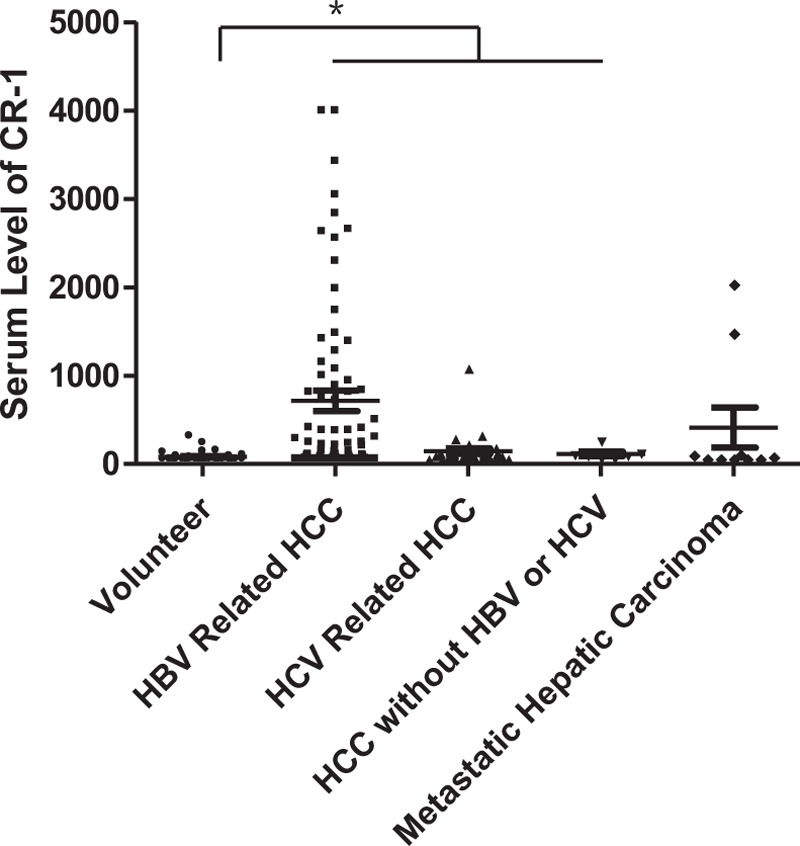
Serum CR-1 level detected by sandwich ELISA in volunteer controls (n = 50), HBV-related HCC (n = 74), HCV related HCC (n = 34), HCC without HBV or HCV infection (n = 7), and metastatic hepatic carcinoma (n = 10). The serum CR-1 levels in HBV-related HCC, HCV related HCC, and HCC without HBV or HCV infection group showed significant differences with volunteer (both P < .05). CR-1 = cripto-1, ELISA = enzyme-linked immuno sorbent assay, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, and HCV = hepatitis C virus.
Table 1.
Concentration of serum CR-1 in patients.
3.2. Serum CR-1 level in HBV infected patients
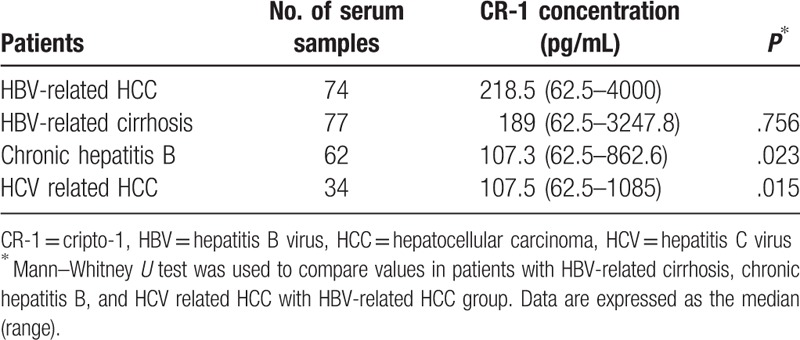
Serum CR-1 level in 77 patients with HBV-related liver cirrhosis [189 (62.5–3247.8) pg/mL] and 62 chronic hepatitis B patients [107.3 (62.5–862.6) pg/mL] (Fig. 2) were significantly higher than volunteer controls (both P < .001). Serum CR-1 level in patients with HBV-related HCC and HBV-related liver cirrhosis was significantly higher than in patients with chronic hepatitis B (P = .023 and P = .010, respectively, Table 1). But there was no significant difference in serum CR-1 level between HBV-related HCC with HBV-related liver cirrhosis (P = .756, Table 1).
Figure 2.
Serum CR-1 level detected by sandwich ELISA in HBV-related liver cirrhosis (n = 77) and chronic HBV infection (n = 62). Serum CR-1 level in HBV-related liver cirrhosis was significantly higher than chronic HBV infection (P < .05). But serum CR-1 level had no statistically difference between HBV-related liver cirrhosis with HBV-related HCC (P = .756). CR-1 = cripto-1, ELISA = enzyme-linked immuno sorbent assay, HBV = hepatitis B virus, and HCC = hepatocellular carcinoma.
3.3. Serum CR-1 level in HCV infected patients
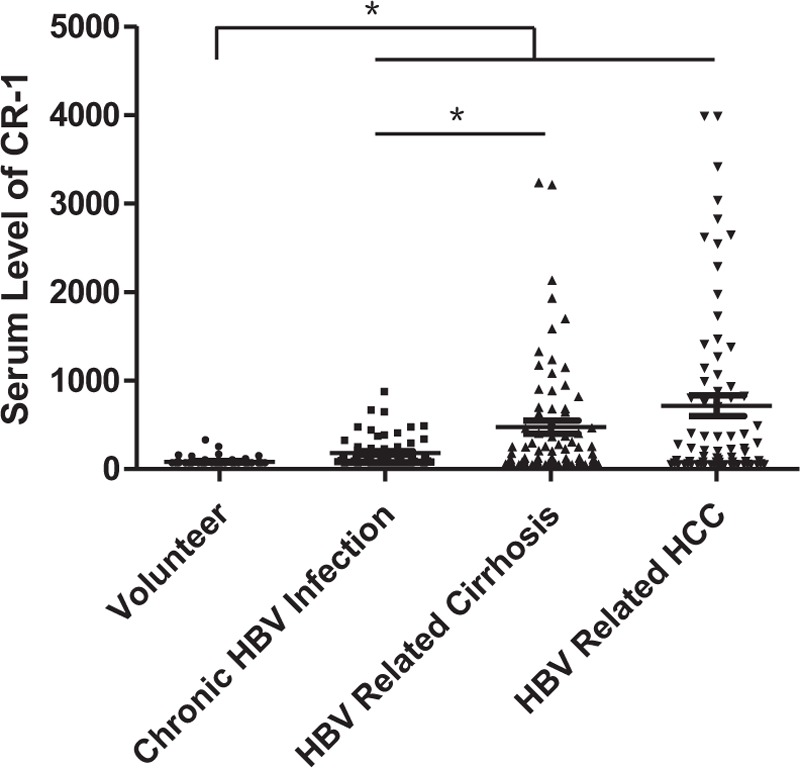
Serum CR-1 level in the 32 HCV-related liver cirrhosis patients was 91 (62.5–2345.4) pg/mL and 82.9 (62.5–674.6) pg/mL in 34 chronic hepatitis C patients (Fig. 3). They were all have significant differences compared with volunteer controls (both P < .001). However, serum CR-1 level in HCV-related cirrhosis was not significantly different from that in HCV-related hepatitis and HCV-related HCC (P = .509 and P = .801, respectively).
Figure 3.
Serum CR-1 level detected by sandwich ELISA in HCV related liver cirrhosis (n = 32) and chronic hepatitis C (n = 34). Serum CR-1 level in HCV related cirrhosis was not significantly different from that in chronic hepatitis C and HCV related HCC (P = .509 and P = .801, respectively). CR-1 = cripto-1, ELISA = enzyme-linked immuno sorbent assay, HCC = hepatocellular carcinoma, and HCV = hepatitis C virus.
3.4. Sensitivity and specificity for diagnosis of HBV-related HCC by serum CR-1
ROC curves of serum CR-1 were constructed to determine cutoff values. We used the level of serum CR-1 of HBV-related HCC and all of the volunteers, chronic hepatitis B and HBV-related liver cirrhosis patients as control to generate ROC curves. As shown in Figure 4, the sensitivity and specificity for diagnosis of HBV-related HCC were 33.80% and 91.50%, respectively, when the cutoff value was set at the level of 714.20 pg/mL. The area under the curve for serum CR-1 was 0.623. Positive rates of serum CR-1 in HBV-related HCC was 33.78%. In addition, positive rates of serum CR-1 for HBV-related liver cirrhosis and chronic hepatitis B patients were 19.48% and 1.61%, respectively.
Figure 4.
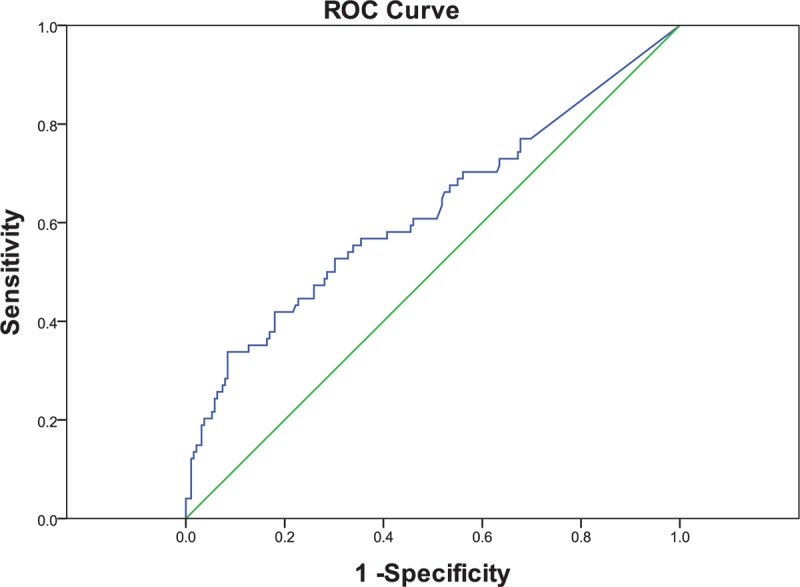
ROC curves for CR-1 in differentiating the HBV-related HCC and control group. The area under the curve was 0.623. The sensitivity and specificity at the cutoff value of 714.20 pg/mL were 33.80% and 91.50%, respectively. ROC curves = receiver operating characteristics curves, CR-1 = cripto-1, HBV = hepatitis B virus, and HCC = hepatocellular carcinoma.
When the cutoff value was adjusted to 177.27 pg/mL (mean + 2SD, volunteer controls), the positive rates were 52.70%, 51.95%, and 29.03% for HBV-related HCC, HBV-related liver cirrhosis, and chronic hepatitis B patients, respectively.
3.5. Correlation of serum CR-1 level with clinicopathological parameters
To investigate the relationship between serum CR-1 and other biochemical markers for HCC, we found that serum CR-1 level was correlated with alpha-feto-protein (AFP) (r = 0.265, n = 68, P = .029) in HBV-related HCC patients, but not in HCV-related HCC patients (r = 0.041, n = 29, P = .833). The value of AFP was not available for 6 HBV-related HCC patients and 5 HCV-related HCC patients. There was no correlation between serum CR-1 level and AFP in HBV-related liver cirrhosis patients or between serum CR-1 level and log (HBV deoxyribonucleic acid [DNA]) in HBV-related HCC patients.
4. Discussion and conclusions
In this study, we investigated serum CR-1 level in patients with liver diseases including chronic hepatitis, cirrhosis, and HCC. There were several interesting findings: serum CR-1 was significantly higher in HCC patients independent of HBV or HCV infection; serum CR-1 level positively correlated with serum AFP level in HBV-related HCC patients; high serum CR-1 might be partly attributed to HBV infection.
HCC accounts for 70% to 80% of all liver cancers. Improvement in the management of HCC has been made, but its prognosis remains poor. This is partly related to the fact that HCC diagnosis in more than two-thirds of patients was made at an advanced stage, which significantly limits therapy options.[15] An early diagnosis of HCC will enable curative surgical and ablative treatments more effective, with median survival reaching 50% to 70% at 5 years.[16,17] Therefore, identifying a simple biomarker for early diagnosis of HCC patients or predicting patients at high risk for advancing liver disease to cirrhosis was urgently needed.
There are many risk factors associated with HCC occurrence, including hepatitis B or C viral infection, alcohol consumption, aflatoxin B1 exposure, and genetic predisposition. Accordingly, we divided patients into 3 groups according to virus infection.[18–21]
CR-1 is a typical example of an oncofetal protein that promotes cell migration, angiogenesis, and stem cell maintenance during embryonic development and oncogenic transformation in vitro and in vivo.[1,22–24] CR-1 is usually expressed at low levels in normal adult tissues, a significant increase in a variety of human tumors, including colorectal, breast, gastric, pancreatic, ovarian, and lung carcinomas.[3,25] But, serum CR-1 level in HCC patients remains unclear. In this study, serum CR-1 level in HCC patients was significantly elevated compared with volunteers. Liver cirrhosis is an important cause of mortality in the world.[26] Most (70%–90%) HCC patients are developed following cirrhosis.[27] Thus, it had been reported that CR-1 expression has been detected in premalignant lesions of the colon, stomach, and breast.[28–30] The significantly high serum CR-1 in HBV-related cirrhosis patients suggests that the high serum CR-1 might indicate high risk for HCC. And it appeared to be a new biomarker candidate for screening HBV-related HCC. HBV can cause HCC in the absence of cirrhosis because of its oncogenic viruses feature. And in our study, the serum level of CR-1 in HBV-related HCC patients, as well as HBV-related cirrhosis patients, were higher than that in HCV-related HCC. We assumed that the correlation of the serum CR-1 with HBV infection might suggest that CR-1 expression could be part of the response to HBV infection.
AFP is a marker commonly used as part of HCC diagnosis, but sensitivity is low (25%–65%), particularly in the detection of early stage HCC at 20 ng/mL cutoff.[31–33] In addition, AFP concentration can also be raised in many patients with non-malignant chronic liver disease, including 15% to 58% of patients with chronic hepatitis and 11% to 47% with liver cirrhosis.[31,34] In this study, we found that serum CR-1 was significantly correlated with AFP in HBV-related HCC patients. This also suggested that CR-1 might be a supplemental biomarker that might help detecting HCC, especially in HBV-related HCC.
As noted, average serum CR-1 level in HCV-related or non-HBV/HCV-related HCC patients were differed significantly from volunteer controls, but were much lower than HBV-related HCC and HBV-related cirrhosis patients, thus, we only used HBV infected patients and volunteers to generate ROC curves. And the specificity was high, though low in the sensitivity. When replacing the ROC curve derived cutoff value with a mean + 2SD of voluntary control, the positive rate in HBV-related HCC patients and HBV-related cirrhosis was similar, so we considered this method was not suitable for our study.
Serum CR-1 in HCC patients without HBV or HCV infection was higher than that in volunteer controls. This might suggest the elevated serum CR-1 was associated with HCC, but independent of etiology. Serum CR-1 level in metastatic hepatic carcinoma was not higher than voluntary control. This might represent that serum level of CR-1 was elevated in primary liver tumor growth but not in metastasis. However, the small number of serum samples in HCC patients without HBV or HCV infection and metastatic hepatic carcinoma patients was one of the limitations to our study. So these suggestions require further validation in new studies. In our study, the serum level of CR-1 in HBV-related HCC was statistically higher than that in chronic hepatitis B, but not significantly higher than HBV-related cirrhosis. However, the median level of CR-1 in HBV-related HCC and HBV-related cirrhosis was 218.5 pg/mL and 189 pg/mL, respectively, suggesting the difference might be significant if the sample size was large enough. And another limitation to the study was the detection limits of our ELISA kit. The detection limits of our ELISA kit were set between 62.5 pg/mL and 4000 pg/mL, it was possible that the CR-1 level may have been overestimated if < 62.5 pg/mL or underestimated if >4000 pg/mL, and was considered 62.5 pg/mL or 4000 pg/mL in this study.
In summary, we found that serum CR-1 was higher in HCC patients, especially in HBV-related HCC, also in HBV-related liver cirrhosis and chronic hepatitis B, suggesting serum CR-1 level could be increased with the progression of HBV-related liver disease and high level of serum CR-1 might be a potential biomarker for screening HCC in HBV infected patients. In addition, a higher level of serum CR-1 was detected in HBV infected HCC and cirrhosis comparing HCV infection, suggesting a possibility that elevation of CR-1 expression could be part of the response to HBV infection.
Author contributions
Data curation: Yingyu Zhang, Hongqin Xu.
Formal analysis: Xiumei Chi.
Funding acquisition: Junqi Niu.
Investigation: Yingyu Zhang.
Methodology: Yingyu Zhang, Xiumei Chi, Yuxiang Fan, Ying Shi.
Project administration: Junqi Niu.
Resources: Junqi Niu.
Software: Yingyu Zhang, Hongqin Xu.
Supervision: Junqi Niu.
Validation: Ying Shi.
Writing – original draft: Yingyu Zhang.
Writing – review & editing: Yingyu Zhang, Ying Shi, Junqi Niu.
Footnotes
Abbreviations: AFP = alpha-feto-protein, ALK4 = type I activin receptor-like kinase receptor 4, AUCs = areas under the curves, BSA = bull Serum Albumin, CR-1 = cripto-1, CSCs = cancer stem cells, DNA = deoxyribonucleic acid, EGF-CFC = epidermal growth factor-Cripto-1/FRL-1/Cryptic protein family, ELISA = enzyme-linked immuno sorbent assay, GPI = glycosylphosphatidylineositol, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, MAPK = mitogen-activated protein kinase, ROC curves = receiver operating characteristics curves, Streptavidin-HRP = streptavidin horse radish peroxidase, TDGF-1 = teratocarcinoma-derived growth factor-1.
The study was supported by a grant of National Science and Technology Major Project (2014) (ZX100002002) and the science and technology development project of Jilin Province (20170520001JH).
The authors report no conflict of interest.
References
- [1].Bianco C, Castro NP, Baraty C, et al. Regulation of human Cripto-1 expression by nuclear receptors and DNA promoter methylation in human embryonal and breast cancer cells. J Cell Physiol 2013;228:1174–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bianco C, Normanno N, Salomon DS, et al. Role of the cripto (EGF-CFC) family in embryogenesis and cancer. Growth Factors 2004;22:133–9. [DOI] [PubMed] [Google Scholar]
- [3].Strizzi L, Bianco C, Normanno N, et al. Cripto-1: a multifunctional modulator during embryogenesis and oncogenesis. Oncogene 2005;24:5731–41. [DOI] [PubMed] [Google Scholar]
- [4].Yoon HJ, Hong JS, Shin WJ, et al. The role of Cripto-1 in the tumorigenesis and progression of oral squamous cell carcinoma. Oral Oncol 2011;47:1023–31. [DOI] [PubMed] [Google Scholar]
- [5].Calhoun JD, Rao RR, Warrenfeltz S, et al. Transcriptional profiling of initial differentiation events in human embryonic stem cells. Biochem Biophys Res Commun 2004;323:453–64. [DOI] [PubMed] [Google Scholar]
- [6].Noaksson K, Zoric N, Zeng X, et al. Monitoring differentiation of human embryonic stem cells using real-time PCR. Stem Cells 2005;23:1460–7. [DOI] [PubMed] [Google Scholar]
- [7].Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell 2014;14:275–91. [DOI] [PubMed] [Google Scholar]
- [8].Wei B, Jin W, Ruan J, et al. Cripto-1 expression and its prognostic value in human bladder cancer patients. Tumour Biol 2015;36:1105–13. [DOI] [PubMed] [Google Scholar]
- [9].Castro NP, Fedorova-Abrams ND, Merchant AS, et al. Cripto-1 as a novel therapeutic target for triple negative breast cancer. Oncotarget 2015;6:11910–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xu CH, Sheng ZH, Hu HD, et al. Elevated expression of Cripto-1 correlates with poor prognosis in non-small cell lung cancer. Tumour Biol 2014;35:8673–8. [DOI] [PubMed] [Google Scholar]
- [11].Zhong XY, Zhang LH, Jia SQ, et al. Positive association of up-regulated Cripto-1 and down-regulated E-cadherin with tumour progression and poor prognosis in gastric cancer. Histopathology 2008;52:560–8. [DOI] [PubMed] [Google Scholar]
- [12].Bianco C, Strizzi L, Mancino M, et al. Identification of Cripto-1 as a novel serologic marker for breast and colon cancer. Clin Cancer Res 2006;12:5158–64. [DOI] [PubMed] [Google Scholar]
- [13].Xu CH, Wang Y, Qian LH, et al. Serum Cripto-1 is a novel biomarker for non-small cell lung cancer diagnosis and prognosis. Clin Respir J 2017;6:765–71. [DOI] [PubMed] [Google Scholar]
- [14].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245–55. [DOI] [PubMed] [Google Scholar]
- [15].Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907–17. [DOI] [PubMed] [Google Scholar]
- [16].Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008;48(suppl 1):S20–37. [DOI] [PubMed] [Google Scholar]
- [17].Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The liver cancer study groupof Japan. Hepatology 2000;32:1224–9. [DOI] [PubMed] [Google Scholar]
- [18].Seeff LB, Hoofnagle JH. Epidemiology of hepatocellular carcinoma in areas of low hepatitis B and hepatitis C endemicity. Oncogene 2006;25:3771–7. [DOI] [PubMed] [Google Scholar]
- [19].Feo F, De Miglio MR, Simile MM, et al. Hepatocellular carcinoma as a complex polygenic disease. Interpretive analysis of recent developments on genetic predisposition. Biochim Biophsy Acta 2006;1765:126–47. [DOI] [PubMed] [Google Scholar]
- [20].Kwon OS, Jung YK, Kim YS, et al. Effect of alcohol on the development of hepatocellular carcinoma in patients with hepatitis B virus-related cirrhosis: a cross-sectional case-control study. Korean J Hepatol 2010;16:308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen Ban K, Singh H, Krishnan R, et al. Comparison of the expression of beta-catenin in hepatocellular carcinoma in areas with high and low levels of exposure to aflatoxin B1. J Surg Oncol 2004;86:157–63. [DOI] [PubMed] [Google Scholar]
- [22].Nagaoka T, Karasawa H, Turbyville T, et al. Cripto-1 enhances the canonical Wnt/beta-catenin signaling pathway by binding to LRP5 and LRP6 co-receptors. Cell Signal 2013;25:178–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bianco C, Rangel MC, Castro NP, et al. Role of Cripto-1 in stem cell maintenance and malignant progression. Am J Pathol 2010;177:532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].de Castro NP, Rangel MC, Nagaoka T, et al. Cripto-1: an embryonic gene that promotes tumorigenesis. Future Oncol 2010;6:1127–42. [DOI] [PubMed] [Google Scholar]
- [25].Bianco C, Strizzi L, Normanno N, et al. Cripto-1: an oncofetal gene with many faces. Curr Top Dev Biol 2005;67:85–133. [DOI] [PubMed] [Google Scholar]
- [26].Méndez-Sánchez N, Villa AR, Zamora-Valdés D, et al. Worldwide mortality from cirrhosis. Ann Hepatol 2007;6:194–5. [PubMed] [Google Scholar]
- [27].Sherman M. Hepatocellular carcinoma: epidemiology, surveillance, and diagnosis. Semin Liver Dis 2010;30:3–16. [DOI] [PubMed] [Google Scholar]
- [28].Saeki T, Stromberg K, Qi CF, et al. Differential immunohistochemical detection of amphiregulin and cripto in human normal colon and colorectal tumors. Cancer Res 1992;52:3467–73. [PubMed] [Google Scholar]
- [29].Saeki T, Salomon DS, Johnson GR, et al. Association of epidermal growth factor-related peptides and type I receptor tyrosine kinase receptors with prognosis of human colorectal carcinomas. Jpn J Clin Oncol 1995;25:240–9. [PubMed] [Google Scholar]
- [30].Panico L, D’Antonio A, Salvatore G, et al. Differential immunohistochemical detection of transforming growth factor alpha, amphiregulin and CRIPTO in human normal and malignant breast tissues. Int J Cancer 1996;65:51–6. [DOI] [PubMed] [Google Scholar]
- [31].Taketa K. Alpha-fetoprotein: reevaluation in hepatology. Hepatology 1990;12:1420–32. [DOI] [PubMed] [Google Scholar]
- [32].Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol 2006;101:524–32. [DOI] [PubMed] [Google Scholar]
- [33].El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008;134:1752–63. [DOI] [PubMed] [Google Scholar]
- [34].Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis 2001;5:145–59. [DOI] [PubMed] [Google Scholar]


