Abstract
Rationale:
Coexistence of lung adenocarcinoma and polyserous effusions is quite rare. This complexity of etiology adds difficulty to the diagnosis and is likely to cause misdiagnosis and maldiagnosis.
Patient concerns:
A 43-year-old woman was admitted with symptoms of dry cough, chest suffocation, polyserous effusions, and generalized edema. Only a small number of heterocysts were detected in the ascites, and malignant cells were detected in the pleural and pericardial effusions. After cytology tests of pericardial, pleural effusions, and ascites, puncture biopsy of the left lung lesion was performed with CT guidance, and immunohistochemical tests were performed.
Diagnoses:
The diagnosis of lung adenocarcinoma was histopathologically confirmed by puncture biopsy with CT guidance of the left lower lung lesion.
Interventions:
Combined treatments(pemetrexed/cisplatin) was administered after the left lung lesion immunohistochemistry.
Outcomes:
The patient has survived more than 1 year after pemetrexed/cisplatin combination chemotherapy.
Lessons:
Coexistence of lung adenocarcinoma and polyserous effusions is quite rare. Close attention should be paid whenever a patient with coexistence of ascites, pleural effusion, and pericardial effusion. More diverse methods could be helpful to identify the diagnosis and avoid misdiagnosis. Patients with advanced lung adenocarcinoma need individualized therapy, including pemetrexed/cisplatin combination chemotherapy.
Keywords: chemotherapy, cisplatin, lung adenocarcinoma, pemetrexed, polyserous effusions
1. Introduction
Polyserous effusions refer to simultaneous occurrence of fluid collection in 2 or more serous cavities. They can be caused by multiple pathologic factors, mainly including malignant tumors and tuberculosis.[1,2] The leading underlying causes for malignant pleural effusions are lung and breast cancers; while ovarian and breast cancers are the leading underlying etiologies for malignant peritoneal effusions.[3,4] Occurrence of ascites, pleural effusion, and pericardial effusion in lung cancer patients is quite rare. The complexity of etiology for polyserous effusions adds difficulty to the diagnosis and is likely to cause misdiagnosis and maldiagnosis.[5] This article reports a clinical case of immunohistopathologically confirmed lung adenocarcinoma with coexistence of ascites, pleural effusion, and pericardial effusion.
2. Case report
A 43-year-old woman was admitted with symptoms of dry cough, suffocation, abdominal distension, and generalized edema. The serum tumor markers showed CA125 969.60 U/mL, CA153 62.30 U/mL, CEA 92.90 ng/mL, NSE 1543 μg/L, SCC 0.60 ng/mL, CA199 22.64 U/mL, AFP 5.43 ng/mL, and CA724 1.21 U/mL. Ultrasonography revealed large amounts of pericardial effusion and bilateral pleural effusion, and an echo-free area with a fluid depth of 8.9 cm in the right lower abdomen. The results of autoimmune antibody test and tuberculosis infection T cell spot test (T-SPOT) were not remarkable. Chest computer tomography (CT) plain and contrast-enhanced scans suggested a small amount of inflammation in the lingular segment of left lung, bilateral pleural effusion, compressive atelectasis of both lower lungs, and large amounts of pericardial effusion and ascites.
Abdominocentesis showed ascites CA125 >1000.0 U/mL, CA153 15.60 U/mL, CEA 30.67 ng/mL, CA199 6.39 U/mL. Ascites biochemistry showed ADA 2.0 U/L, LDH 112 U/L, glucose 7.4 mmol/L, total protein 21.0 g/L, and Cl 97 mmol/L. Ascites cytology detected only a small number of heterocysts (Fig. 1). Malignant cells were detected in both pericardial effusions and pleural effusions.
Figure 1.
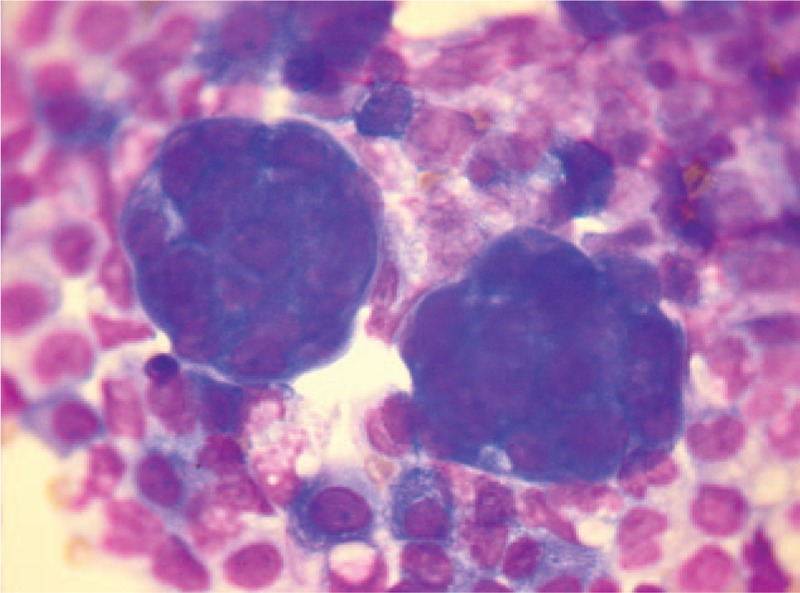
Ascites cytology (HE× 40) detected only a small number of heterocysts.
PET-CT suggested: a nodular shadow with high metabolism in the left lower lung (Fig. 2), which was suspected as lung cancer; multiple metastatic tumors in both lungs; multiple bone metastases; bilateral pleural effusion with atelectasis of both lower lungs; pericardial and pelvic effusions; thoracoabdominal subcutaneous edema; slight elevation of metabolism in bilateral cervical, axillary, retroperitoneal. and groin lymph nodes.
Figure 2.
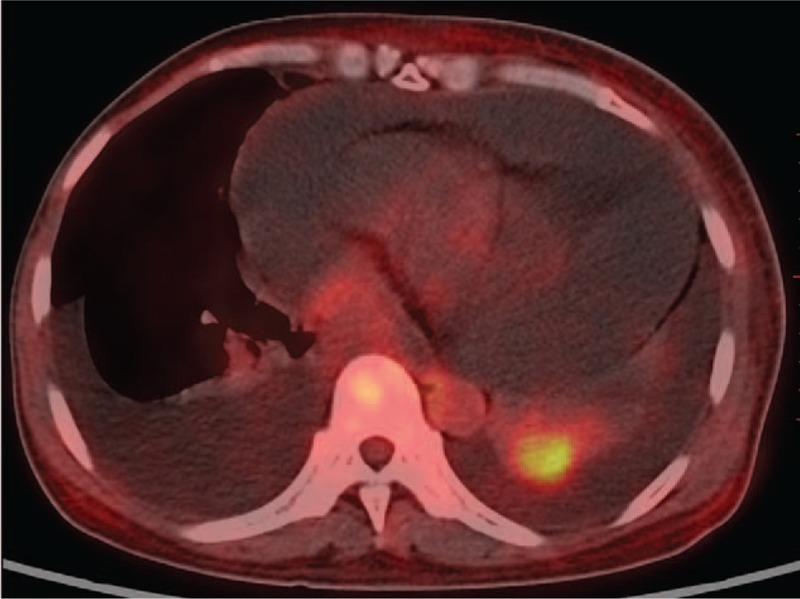
Nodular shadow with elevation of metabolism in the left lower lung.
After drainage of the pleural effusion, puncture biopsy of the left lung lesion was performed with CT guidance (Fig. 3), and immunohistopathology confirmed it as left lung adenocarcinoma (Fig. 4), excluding the diagnosis of ovarian cancer lung metastasis. Further EGFR gene testing demonstrated it as the wild type. The final diagnosis was T4N0M1a (pleura)1b (lung, pericardium, bone, abdominal cavity) in the inoperable late stage. The patient has received 5 cycles of pemetrexed/cisplatin chemotherapy, analgesia therapy, symptomatic, and supportive treatment. The patient is doing relatively well in general condition without suffocation, cough, and abnormal distension, and she can go on with her daily routine.
Figure 3.
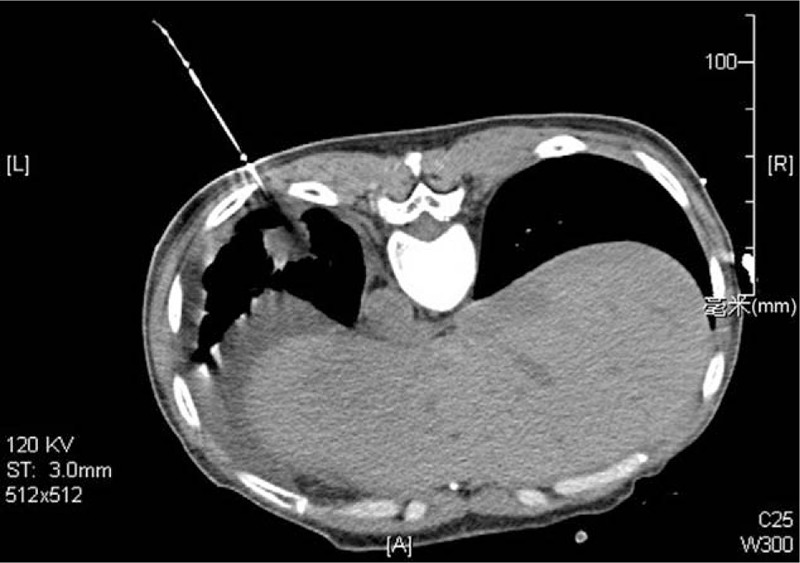
CT-guided lung puncture biopsy of the left lower lung lesion.
Figure 4.
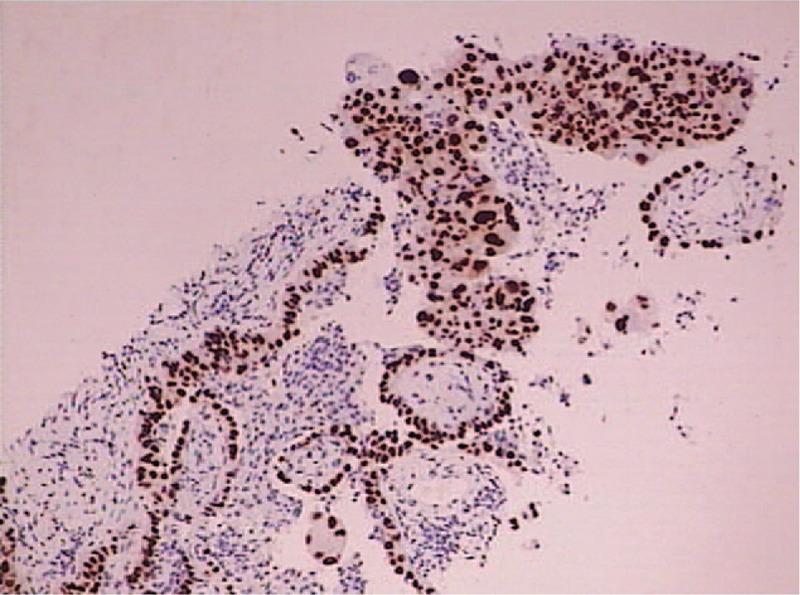
Immunohistochemical staning (TTF1 positive, DAB ×40) of left lung adenocarcinoma.
3. Discussion
Polyserous effusions can be seen in patients with malignant tumors especially lung cancer, ovarian cancer, and liver cancer, and those with connective tissue diseases, tuberculosis, cirrhosis and cardiorenal dysfunction.[6–9]
Unilateral or bilateral pleural effusion and pericardial effusion are more frequently seen malignant serous effusions in lung cancer as compared with metastatic ascites.[10,11] The reported autopsy detection rate of metastatic ascites in lung cancer is 2.7% to 16%,[12,13] while Satoh et al[14] only reported 12 (1.2%) cases of metastatic ascites in their 1041 lung cancer cases based on their 26-year statistical data. Su et al[15] reported only 30 cases of metastatic ascites in their 16-year statistics based on all general hospitals in Taiwan. Metastatic ascites in lung cancer is often accompanied with metastases in the bone, liver, brain, and adrenal glands.[11] Of the 12 cases of lung cancer with metastatic ascites reported by Satoh et al,[14] 9 cases were accompanied with thoracic implantation metastasis, and metastatic ascites occurred in only 1 case. Of all reported lung cancer cases with pleural effusion and ascites, adenocarcinoma is predominant. According to the cases reported in the literature, metastatic ascites generally occurs in the late stage of disease, later than pleural effusion and pericardial effusion.[10] Metastatic ascites as the onset symptom was reported in only four of the 30 lung cancer cases reported by Su et al.[15] In the case reported herein, lung cancer of the patient was already in the late stage, pathologically classified as adenocarcinoma with simultaneous occurrence of metastatic ascites, bilateral pleural effusion, and pericardial effusion, which is very rarely seen in clinical practice.
Occurrence of ascites, pleural effusion, and pericardial effusion in lung cancer patients indicates the late stage of disease with a relatively short median survival rate. Su et al[15] reported a median survival duration of 15 days in their lung cancer patients with ascites. Although 2 of their 30 patients benefited from gefitinib treatment, they only survived 203 and 343 days respectively. Tanriverdi et al[10] reported a lung cancer patient with simple peritoneal metastasis and ascites, whose condition continued to progress after 2-week treatment with cisplatin/gemcitabine, and died 1 week after second-line treatment with radiotherapy and taxinol. The patient in our case has survived more than a year since the confirmed diagnosis of the disease and subsequent chemotherapy.
Polyserous (pleural, pericardial, and abdominal) effusions are the onset symptom of the patient in our case. Only a small number of heterocysts were detected in the ascites, and malignant cells were detected in the pleural and pericardial effusions. The diagnosis of lung adenocarcinoma was histopathologically confirmed by puncture biopsy of the left lower lung lesion, for which pemetrexed/cisplatin was administered. Pemetrexed is a new-type chemotherapy agent with potent anti-tumor activity. As a multi-targeted folic acid antagonist, it can exert an inhibitory effect on the key enzyme involved in folic acid metabolism.[16] Treated with this chemotherapy protocol, with the addition of analgesia therapy, symptomatic and supportive treatment, the patient has survived more than 1 year, although the overall prognosis of the disease remains poor. It is reported that a combination of traditional Chinese medications and single-drug chemotherapy could improve the quality of life of patients with late-stage lung adenocarcinoma and prolong their median survival duration.[17] A combination of traditional Chinese medications and EGFR-TKIs is reported to potentiate the therapeutic efficacy and reduce the toxicity of chemotherapy,[18] and therefore the use of traditional Chinese medications on the basis of the pemetrexed/cisplatin chemotherapy protocol can be considered in this patient.
4. Conclusions
Coexistence of lung adenocarcinoma and polyserous effusions is quite rare. Close attention should be paid whenever a patient with coexistence of ascites, pleural effusion, and pericardial effusion. More diverse methods could be helpful to identify the diagnosis and avoid misdiagnosis. Patients with advanced lung adenocarcinoma need individualized therapy, which includes Pemetrexed/cisplatin combination chemotherapy.
5. Consent
We did not need ethical approval to report this case. Written informed consent was obtained from the patient in the case reported.
Acknowledgments
The authors here express many thanks to the patient for generously authorizing them to share her rare case.
Footnotes
Abbreviations: ADA = adenosine deaminase, AFP = alpha fetoprotein, CA125 = carbohydrate antigen 125, CA153 = carbohydrate antigen 153, CA199 = carbohydrate antigen 199, CA724 = carbohydrate antigen 724, CEA = carcinoma embryonic antigen, Cl = chloride, CT = computer tomography, EGFR = epidermal growth factor receptor, LDH = lactate dehydrogenase, NSE = neuron-specific enolase, PET-CT = Positron Emission Computed Tomography, SCC = squamous cell carcinoma antigen, TKI = tyrosine kinase inhibitor, TNM = TNM staging system, T-SPOT = tuberculosis infection T cell spot test.
PH and RPY contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Das DK. Age and sex distribution in malignant and tuberculous serous effusions: a study of 127 patients and review of the literature. Geriatr Gerontol Int 2015;15:1143–50. [DOI] [PubMed] [Google Scholar]
- [2].Lim MH, Garrettc J, Mowlem L, et al. Diagnosing malignant pleural effusions:how do we compare? N Z Med J 2013;126:42–8. [PubMed] [Google Scholar]
- [3].Sears D, Hajdu SI. The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol 1987;31:85–97. [PubMed] [Google Scholar]
- [4].Monte SA, Ehya H, Lang WR. Positive effusion cytology as the initial presentation of malignancy. Acta Cytol 1987;31:448–52. [PubMed] [Google Scholar]
- [5].El Hag M, Schmidt L, Roh M, et al. Utility of TTF-1 and Napsin-A in the work-up of malignant effusions. Diagn Cytopathol 2016;44:299–304. [DOI] [PubMed] [Google Scholar]
- [6].Jurcuţ C, Filişan C, Popovici C, et al. Young woman with polyserositis, ovarian cystic mass and increased level of CA-125. Case report of peritoneal and pleural tuberculosis. Rom J Intern Med 2009;47:297–9. [PubMed] [Google Scholar]
- [7].Giarnieri E, Alderisio M, Mancini R, et al. Tissue inhibitor of metalloproteinase 2 (TIMP-2) expression in adenocarcinoma pleural effusions. Oncol Rep 2008;19:483–7. [PubMed] [Google Scholar]
- [8].Feng M, Zhu J, Liang L, et al. Diagnostic value of tumor markers for lung adenocarcinoma-associated malignant pleural effusion: a validation study and meta-analysis. Int J Clin Oncol 2017;22:283–90. [DOI] [PubMed] [Google Scholar]
- [9].Hanley KZ, Facik MS, Bourne PA, et al. Utility of anti-L523S antibody in the diagnosis of benign and malignant serous effusions. Cancer 2008;114:49–56. [DOI] [PubMed] [Google Scholar]
- [10].Tanriverdi O, Barutca S, Meydan N. Relapse with isolated peritoneal metastasis in lung adenocarcinoma: case report and review of the literature. Contemp Oncol 2012;16:586–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Quraishi MA, Costanzi JJ, Hokanson J. The natural history of lung cancer with pericardial metastases. Cancer 1983;51:740–2. [DOI] [PubMed] [Google Scholar]
- [12].McNeill PM, Wagman LD, Neifeld JP. Small bowel metastases from primary carcinoma of the Lung. Cancer 1987;59:1486–9. [DOI] [PubMed] [Google Scholar]
- [13].Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer 1950;3:74–85. [DOI] [PubMed] [Google Scholar]
- [14].Satoh H, Ishikawa H, Yamashita YT, et al. Peritoneal carcinomatosis in lung cancer patients. Oncol Rep 2001;8:1305–7. [DOI] [PubMed] [Google Scholar]
- [15].Su HT, Tsai CM, Perng RP. Peritoneal carcinomatosis in lung cancer. Respirology 2008;13:465–7. [DOI] [PubMed] [Google Scholar]
- [16].Tomasini P, Barlesi F, Mascaux C, et al. Pemetrexed for advanced stage nonsquamous non-small cell lung cancer: latest evidence about its extended use and outcomes. Ther Adv Med Oncol 2016;8:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhou ZY, Xu L, Li HG, et al. Chemotherapy in conjunction with traditional Chinese medicine for survival of elderly patients with advanced non-small-cell lung cancer: protocol for a randomized double-blind controlled trial. J Integr Med 2014;12:175–81. [DOI] [PubMed] [Google Scholar]
- [18].Liu ZL, Zhu WR, Zhou WC, et al. Traditional Chinese medicinal herbs combined with epidermal growth factor receptor tyrosine kinase inhibitor for advanced non-small cell lung cancer: a systematic review and meta-analysis. J Integr Med 2014;12:346–58. [DOI] [PubMed] [Google Scholar]


