Abstract
Duodenal carcinoid tumors, a type of neuroendocrine tumors, are relatively rare and are usually found incidentally during endoscopy. Small duodenal carcinoid tumors (≤10–20 mm), embedded in the submucosa, can be resected endoscopically because of the low risk of metastasis. The aim of this study was to assess the safety and efficacy of ligation-assisted endoscopic mucosal resection (EMR) for the treatment of small duodenal carcinoid tumors. The clinical outcomes of the endoscopic procedures were also evaluated.
Between November 2008 and November 2017, a total of 15 duodenal carcinoid tumors embedded in the submucosa were resected using EMR. Two types of EMR (conventional EMR and ligation-assisted EMR) were performed according to tumor morphology (narrow-based and broad-based).
The mean tumor size was 6.6 ± 3.9 mm and the mean procedure time was 11.0 ± 11.2 minutes. Most of the lesions (80.0%) were located in the duodenal 1st portion. Broad-based tumors were more common than narrow-based tumors (66.7% vs 33.3%). All broad-based tumors were resected successfully using ligation-assisted EMR. Although en-bloc resection and complete resection rates were higher in ligation-assisted EMR than in conventional EMR ([100% vs 87.5%], and [85.7% vs 62.5%], respectively), the difference was not significant (P = .333 and P = .310, respectively). Moreover, there was no evidence of local or distant metastasis during the follow-up (26.1 ± 20.7 months).
Ligation-assisted EMR showed a higher complete resection rate than conventional EMR. Ligation-assisted EMR may be an optimal treatment option for duodenal carcinoid tumors with a broad base.
Keywords: endoscopic mucosal resection, ligation, neoplasia, neuroendocrine tumor
1. Introduction
Carcinoid tumors are relatively rare, slow-growing neuroendocrine tumors, which originate from the cells of the neuroendocrine system.[1] The gastrointestinal tract is their most common site of occurrence,[2] and they are usually found incidentally during endoscopy, most frequently in the rectum followed by the stomach and duodenum.[3] A microcarcinoid (<1 mm) is formed when an intraglandular hyperplastic proliferation of argyrophil cells develops into an extraglandular budding in the mucosal layer, which penetrates the submucosa layer through the muscularis mucosae.[4] The optimal treatment for duodenal carcinoid tumors remains controversial—whether to perform surgical resection with or without lymph node dissection or to perform an endoscopic resection, where possible.[5,6]
The metastatic rate of duodenal carcinoid tumors embedded in the submucosa was reported as 12.5%.[4] The characteristics of benign duodenal carcinoid tumors are nonangioinvasive with a low mitotic rate, ≤10 mm in size and limited to the submucosa.[4,7] Endoscopic resection may be the optimal treatment option for duodenal carcinoid tumors without malignancy features. Carcinoid tumors in the rectum or stomach can be resected easily via endoscopic submucosal dissection (ESD) maneuver.[8] However, because of the higher perforation rate associated with ESD than that with endoscopic mucosal resection (EMR),[9] EMR has been the commonly used endoscopic maneuver for duodenal carcinoid tumors. We previously reported that ligation-assisted EMR showed a higher complete resection rate for rectal or esophageal submucosa tumors than conventional EMR.[8,10]
In the present study, we used conventional EMR and ligation-assisted EMR for small duodenal carcinoid tumors embedded in the submucosa layer. In addition, we compared the treatment outcomes and clinical characteristics according to the treatment modalities.
2. Patients and methods
2.1. Patients
Between November 2008 and November 2017, we retrospectively reviewed the medical records of patients who underwent EMR for duodenal submucosal tumors at the Pusan National University Yangsan Hospital, Korea. After applying the exclusion criteria, only 15 patients with duodenal neuroendocrine tumors resected via endoscopic maneuvers were enrolled (Fig. 1). We performed abdominal computed tomography to evaluate possible lymph node metastasis. All lesions were located in the submucosa (as confirmed on endoscopic ultrasound (EUS) and pathologic review), without lymph node metastasis.
Figure 1.
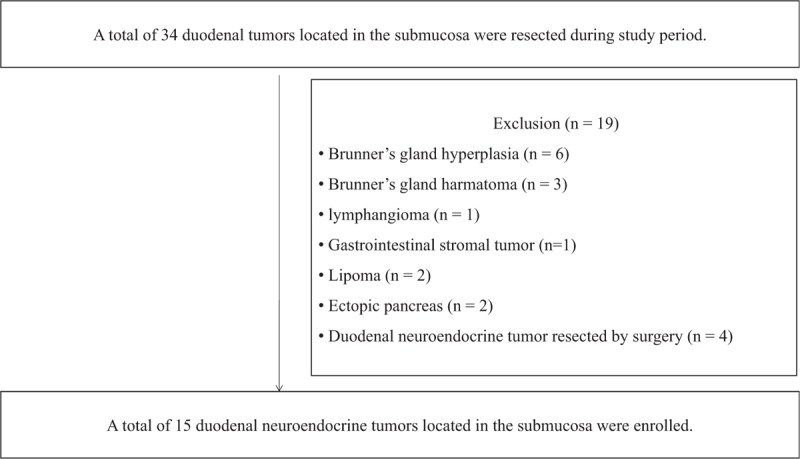
Study flow.
Written informed consent was obtained from all patients prior to the endoscopic procedure. The study was approved by the ethics committee of the institutional review board.
2.2. Procedures
All endoscopic examinations or procedures were performed by 4 endoscopists (CCW, KHW, PSB, and KSJ) who have experience performing more than a hundred therapeutic endoscopies (ESD and EMR). Diagnostic EUS was performed using a mini-probe catheter (UM-DP20-25R; Olympus, Tokyo, Japan). During the EUS, we evaluated the echogenicity of the lesions and the invasive pattern. We performed preliminary endoscopic forceps biopsy for solid duodenal submucosal tumors. For duodenal neuroendocrine tumors (≤10 mm in size) within the submucosa without evidence of malignancy (invasion of the muscularis propria layer, surface ulceration, and lymph node metastasis), we recommend preliminary endoscopic resection. If the tumor size > 10 mm which had narrow base, we tried to do EMR according to the endoscopists’ decision.
In the present study, 2 kinds of endoscopic techniques were performed: conventional EMR and ligation-assisted EMR. All procedures were performed using a single-channel endoscope (H260 or H290; Olympus Optical Co. Ltd., Tokyo, Japan) with a transparent cap attached. After injecting normal saline with a mixture of epinephrine and indigo carmine into the submucosa, endoscopic maneuvers were selected based on the endoscopists’ decision and the tumor morphology. Conventional EMR was performed after the submucosa injection, using the endoscopic electrosurgical snare without the ligation device (Fig. 2A–D). If it was difficult to resect the elevated tumor, after the submucosal saline injection, using the electrosurgical snare, we performed ligation-assisted EMR (Fig. 2E–L). For ligation-assisted EMR, we inserted an endoscope with a band ligation device attached to its tips (Stiegmann-Goff ClearVue; ConMed, Boston, MA). After the tumor had been aspirated into the ligator device, we performed elastic band ligation. Subsequently, we performed endoscopic resection beneath the elastic band using the conventional endoscopic electrosurgical snare and electrosurgical generator (Endocut Q current, effect 3, cut duration 2, cut interval 5, VIO300D electrosurgical unit, ERBE, Tuebingen, Germany).[8,10] If perforation occurred after the resection, we used endoscopic clips to close the perforation hole. After successful endoscopic resection, the patient was started on a soft diet the day after the procedure.
Figure 2.
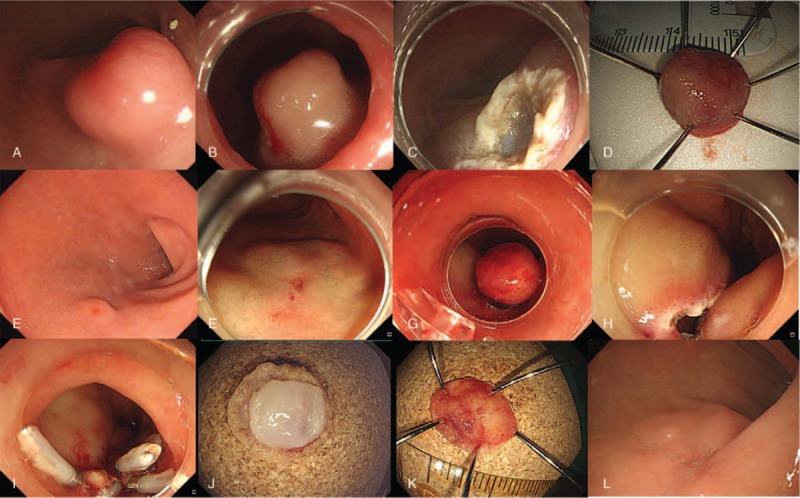
Endoscopic mucosal resection of duodenal neuroendocrine tumor. Conventional endoscopic mucosal resection (A–D) and ligation assisted endoscopic mucosal resection (E–L). (A) A neuroendocine tumor is observed in the duodenal bulb about 12 mm in size. (B) submucosal injection was done. (C) Artificial ulcer is observed after endoscopic mucosal resection. (D) Resected specimen by en-bloc maneuver. (E) A neuroendocine tumor is observed in the duodenal bulb about 5 mm in size. (F) Submucosal injection was done. (G) After ligation using elastic band. (H) A round perforated hole was observed after endoscopic resection. (I) Endoscopic closure using clips for perforated hole. (J–K) Inner and outer surface of the resected specimen. (L) Complete healing of perforated hole after 2 months.
Tumor morphology was classified as narrow-based or broad-based (Fig. 3). Narrow-based tumors were defined as elevated lesions with a clear notched base or peduncle, and broad-based tumors were defined as elevated lesions without a notch or peduncle. The procedure time (the time before submucosal injection to the time after endoscopic hemostasis [for artificial ulcers] after the endoscopic resection) was calculated from the photographs of the endoscopic procedure. Perforation could be diagnosed during the endoscopic procedure. After the resected specimens were sliced at 2-mm intervals, the histopathologic type, invasion depth, and lateral and vertical resection margins were evaluated. The specimens were fixed in formalin, embedded in paraffin, and stained with hematoxylin and eosin for histologic examination. Pathologic diagnosis was based on the hematoxylin and eosin staining and additional immunohistochemical staining using antibodies against chromogranin A, synaptophysin, and CD56. En-bloc resection was defined as the resection of the tumor in one piece. Complete resection was defined as the absence of tumor cells at the resection margins (vertical and lateral margins) and incomplete resection was defined as the presence of tumor cells at the resection margins or when the pathologists could not determine the marginal status.
Figure 3.
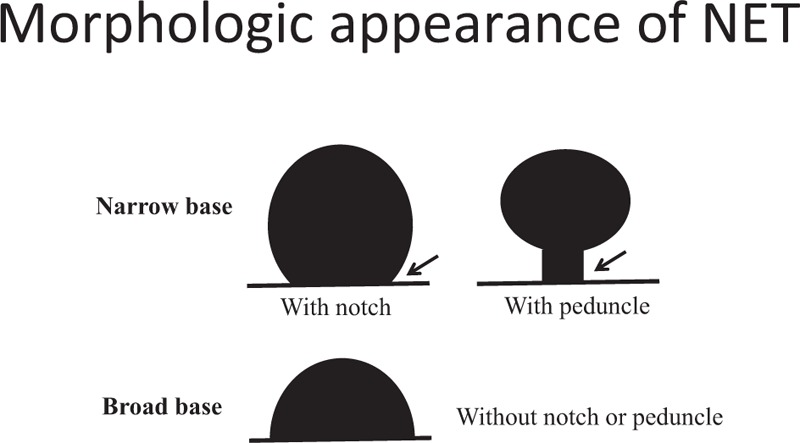
Morphologic appearance of duodenal neuroendocrine tumor.
We recommend periodic follow-up with endoscopic examinations (6–12 months after the first resection and annually, after the first follow-up examination) to evaluate local tumor recurrence and synchronous or metachronous lesions.
2.3. Statistical analysis
Our analysis was based on individual patient outcomes. Univariate analysis was performed using chi-square test or Fisher's exact test for categorical variables and Student's t test for continuous variables. A P value of <.05 was considered to be statistically significant. The statistical calculations were performed using PASW Statistics for Windows, Version 21.0 (SPSS Inc., Chicago, IL).
3. Results
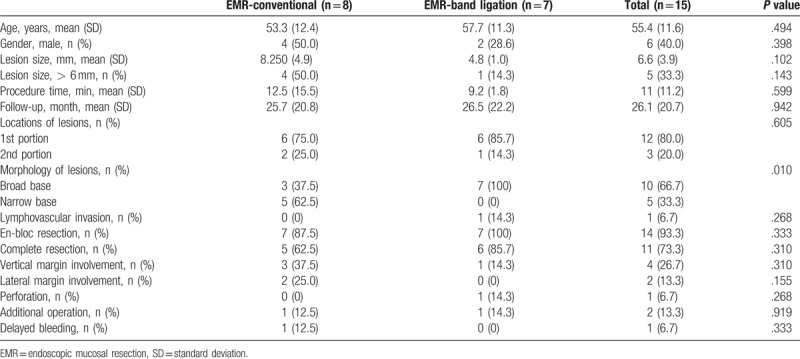
We enrolled 15 patients with duodenal carcinoid tumors embedded in the submucosa layer. The patients’ mean age was 55.4 ± 11.6 years. The men comprised 40.0% of the patients. The mean tumor size and the mean procedure time were 6.6 ± 3.9 mm and 11.0 ± 11.2 min, respectively. Most of the lesions (80.0%) were located in the duodenal 1st portion. The broad-based tumors were more common than the narrow-based tumors (66.7% and 33.3%, respectively) and ligation-assisted EMR was performed in 46.7% of cases. The overall en-bloc and complete resection rates were 93.3% and 73.3%, respectively. The mean follow-up time was 26.1 ± 20.7 months. Lymphovascular invasion was found in only 1 patient (Table 1).
Table 1.
Baseline characteristics of early duodenal neuroendocrine tumors who underwent endoscopic resection.
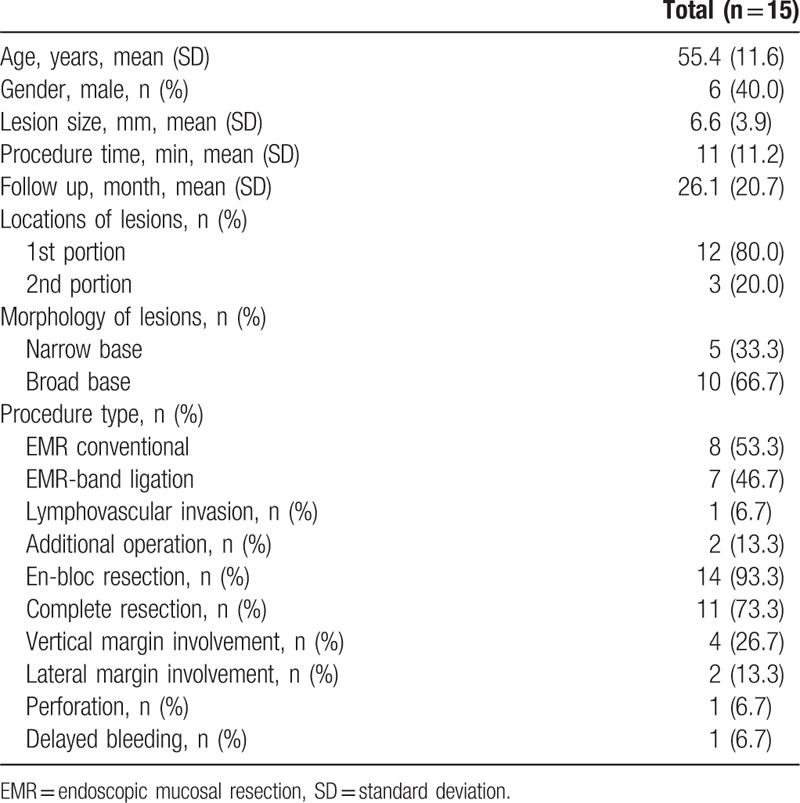
Table 2 shows the comparison between conventional and ligation-assisted EMR. The lesion morphology was the only significant difference between the treatment modalities. Although en-bloc resection and complete resection rates were higher in ligation-assisted EMR than in conventional EMR ([100% vs 87.5%] and [85.7% vs 62.5%], respectively), the difference was not significant. Although one perforation occurred after ligation-assisted EMR, we successfully closed the perforated hole endoscopically using clips (Fig. 2H–I). Two additional surgeries were performed after the endoscopic resection for the lymphovascular invasive lesion that was discovered after the ligation-assisted EMR and the lesion on the vertical margin, which was noted after conventional EMR (Tables 1 and 2). There was no evidence of lymph node metastasis after the surgeries and there was no evidence of local or distant metastasis during the follow-up.
Table 2.
Treatment outcomes after endoscopic resection of early duodenal neuroendocrine tumors according to the procedure types.
4. Discussion
The optimal treatment strategy for duodenal carcinoid tumors embedded in the submucosa is controversial. Early stage carcinoid tumors limited to the submucosa, within 10–20 mm in size, can be resected using endoscopic maneuver.[1,6,7] However, the reported rate of lymph node metastasis due to duodenal carcinoid tumors embedded in the submucosa was 8.3% to 10.5%, despite its small size (≤10 mm).[4] Another study reported that duodenal carcinoid tumors are indolent when they are small (<10–20 mm) and localized in the submucosa.[7,11] The malignancy features of duodenal carcinoid tumors embedded in the submucosa are angioinvasion, high mitotic figures, and larger lesion size.[4] In the present study, we performed EMR for duodenal carcinoid tumors without malignant features. The overall en-bloc and complete resection rates were 93.3% and 73.3%, respectively. Although 2 patients underwent additional surgeries after the EMR, there was no evidence of lymph node metastasis and no evidence of recurrence during the follow-up.
Complete resection of duodenal carcinoid tumors is difficult. The reported complete resection rate for EMR is 33% to 56%.[6] In recent years, ESD has been used for endoscopic resections in the stomach, colon, and esophagus. The benefits of ESD over EMR are higher en-bloc/complete resection rates and lower local tumor recurrence rate, and ESD has been reported to be effective in the treatment of carcinoid tumors in the stomach and rectum.[8,12,13] However, performing ESD in the duodenum is more difficult than it is in the stomach, rectum, or esophagus because the duodenal anatomy makes duodenal ESD difficult. First, maintaining adequate visual field during ESD is difficult because of the narrow and curved duodenal lumen. Second, submucosal injection is difficult because of the abundant Brunner's glands in the submucosal layer; moreover, the muscle layer is thinner than the gastric wall. These factors are associated with a possible risk of perforation during or after duodenal ESD. Third, if a delayed perforation occurs, emergency surgery may be needed because of the tissue damage caused by bile and pancreatic juice leakage.[9,14] The reported incidence rate of perforation during duodenal ESD is 16.1% to 37.5%.[9,14]
In the present study, 2 types of EMR maneuvers were used for the endoscopic treatment of duodenal carcinoid tumors, and carcinoid tumor morphology was classified as narrow-based and broad-based. After administering the submucosa injection below the tumors, snaring of the tumors was attempted first. If it was difficult to resect the tumor using the snare, we tried ligation-assisted EMR. Ligation-assisted EMR, compared with conventional EMR, achieved 100% en-bloc resection and 85.7% complete resection; however, this was statistically insignificant. One case of perforation occurred during ligation-assisted EMR, and the hole was closed successfully using an endoscopic maneuver. During the ligation-assisted EMR, the endoscopic snare was placed beneath the ligation band and then we resected the tumor. Although the snare may be placed above the ligation band to decrease perforation rate, complete resection may be difficult using this arrangement. EMR-associated perforation is different from ESD-associated perforation. Because ESD-associated perforation may occur as a linear hole and gradually enlarge during the procedure, endoscopic en-bloc resection may become difficult thereafter. However, the perforation may occur as a round hole during ligation-assisted EMR after the resection of the entire tumor including the muscularis propria. Because endoscopic tumor resection is completed, the perforation hole can be closed more easily using endoscopic clips.[9]
This study had some limitations. First, because it is a retrospective study in a single academic referral center, selection or information biases might have existed. Because the type of EMR was selected according to the endoscopists’ decision during the procedure, the procedure types were not randomized. During study period, we excluded 4 duodenal neuroendocrine tumors between 10 and 15 mm in size which resected by surgical maneuver and had no evidence of lymph node metastasis. Third, because of the small sample size of our study, multivariate analysis was impossible and the sample size was inadequate to generalize the study results. Fourth, the experience of the endoscopists could not be analyzed because of the small sample size. Further, multicenter prospective studies comparing other therapeutic modalities may provide more accurate information.
In summary, both conventional and ligation-assisted EMR are simple and safe resection modalities for the resection of small duodenal carcinoid tumors embedded in the submucosa layer. Ligation-assisted EMR showed a higher complete pathologic resection rate than conventional EMR. Retrospective analysis of present study for duodenal neuroendocrine tumor ≤ 15 mm in size regardless of treatment modalities showed no evidence of lymph node metastasis. Therefore, we suggest endoscopic resection for duodenal neuroendocrine tumor ≤ 15 mm in size which feasible for endoscopic resection. An ligation-assisted EMR may be the appropriate treatment option for broad-based duodenal carcinoid tumors.
Author contributions
Investigation: Hyung Wook Kim, Su Bum Park.
Resources: Su Jin Kim.
Supervision: Dae Hwan Kang.
Writing – original draft: Cheol Woong Choi.
Writing – review & editing: Cheol Woong Choi.
Footnotes
Abbreviations: EMR = endoscopic mucosal resection, ESD = endoscopic submucosal dissection, EUS = endoscopic ultrasound, SD = standard deviation.
SBP and CWC share the 1st authorship.
Disclosures: The authors, CWC, DHK, HWK, SBP, and SJK have no conflicts of interest or financial ties to disclose.
References
- [1].Maroun J, Kocha W, Kvols L, et al. Guidelines for the diagnosis and management of carcinoid tumours. Part 1: the gastrointestinal tract A statement from a Canadian National Carcinoid Expert Group. Curr Oncol 2006;13:67–76. [PMC free article] [PubMed] [Google Scholar]
- [2].Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer 1997;79:813–29. [DOI] [PubMed] [Google Scholar]
- [3].Caldarola VT, Jackman RJ, Moertel CG, et al. Carcinoid tumors of the rectum. Am J Surg 1964;107:844–9. [DOI] [PubMed] [Google Scholar]
- [4].Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer 2005;103:1587–95. [DOI] [PubMed] [Google Scholar]
- [5].Tsujimoto H, Ichikura T, Nagao S, et al. Minimally invasive surgery for resection of duodenal carcinoid tumors: endoscopic full-thickness resection under laparoscopic observation. Surg Endosc 2010;24:471–5. [DOI] [PubMed] [Google Scholar]
- [6].Kim GH, Kim JI, Jeon SW, et al. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol 2014;29:318–24. [DOI] [PubMed] [Google Scholar]
- [7].Burke AP, Sobin LH, Federspiel BH, et al. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med 1990;114:700–4. [PubMed] [Google Scholar]
- [8].Choi CW, Kang DH, Kim HW, et al. Comparison of endoscopic resection therapies for rectal carcinoid tumor: endoscopic submucosal dissection versus endoscopic mucosal resection using band ligation. J Clin Gastroenterol 2013;47:432–6. [DOI] [PubMed] [Google Scholar]
- [9].Shibagaki K, Ishimura N, Kinoshita Y. Endoscopic submucosal dissection for duodenal tumors. Ann Transl Med 2017;5:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Choi CW, Kang DH, Kim HW, et al. Endoscopic resection for small esophageal submucosa tumor: band ligation versus conventional endoscopic mucosal resection. Medicine (Baltimore) 2017;96:e7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shroff SR, Kushnir VM, Wani SB, et al. Efficacy of endoscopic mucosal resection for management of small duodenal neuroendocrine tumors. Surg Laparosc Endosc Percutan Tech 2015;25:e134–9. [DOI] [PubMed] [Google Scholar]
- [12].Lee DS, Jeon SW, Park SY, et al. The feasibility of endoscopic submucosal dissection for rectal carcinoid tumors: comparison with endoscopic mucosal resection. Endoscopy 2010;42:647–51. [DOI] [PubMed] [Google Scholar]
- [13].Sato Y, Takeuchi M, Hashimoto S, et al. Usefulness of endoscopic submucosal dissection for type I gastric carcinoid tumors compared with endoscopic mucosal resection. Hepatogastroenterology 2013;60:1524–9. [DOI] [PubMed] [Google Scholar]
- [14].Kim TW, Kim GH, Park DY, et al. Endoscopic resection for duodenal subepithelial tumors: a single-center experience. Surg Endosc 2017;31:1936–46. [DOI] [PubMed] [Google Scholar]


