Abstract
Rational:
The therapeutic effect of deep brain stimulation (DBS) for refractory obsessive–compulsive disorder (OCD) has been studied, but complications after this treatment have rarely been noted.
Patient Concerns:
A 28-year-old man with treatment-resistant OCD received bilateral ventral capsule/ventral striatum stimulation for 12 months.
Diagnosis:
Compulsive skin-picking behavior and infection were noted following 12-month DBS treatment.
Intervention:
We removed the implanted right-side pulse generator.
Outcomes:
The local inflammation and skin-picking behavior gradually improved. The stimulator device was re-implanted 4 months later.
Lessons:
We suggest that patients with the OC spectrum should be evaluated for skin-picking behaviors during presurgical and postsurgical follow-up to reduce the infection and device removal rates.
Keywords: deep brain stimulation, obsessive–compulsive disorder, skin-picking behavior
1. Introduction
The therapeutic effect of deep brain stimulation (DBS) for treatment-resistant obsessive–compulsive disorder (OCD) has been studied, and the results support that this strategy is therapeutically promising.[1] However, postsurgical infection has been noted.[2–6] In this report, we describe a patient with refractory OCD who developed compulsive skin-picking behavior and infection after 1 year of DBS.
2. Case report
2.1. History
A 28-year-old ethnically Chinese man had an 8-year history of refractory OCD. His main obsessive thought was “the fear of contamination,” and his compulsive behavior included hand washing and using ethanol to sterilize furniture. He was referred to the Neurosurgery Department because of severe symptoms and no response to at least 3 serotonin reuptake inhibitors, cognitive behavioral therapies, and electroconvulsive therapy. He fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnostic criteria for major depressive disorder. He had no family history of bipolar disorder.
2.2. Operation
The study was approved by the Institutional Review Board (IRB) of Buddhist Tzu-Chi General Hospital (IRB094–33). After he had discussed DBS treatment with his family and signed the informed consent form, we implanted Medtronic Model 3387 leads (Minneapolis, MN) bilaterally in an area spanning the ventral anterior limb of the internal capsule and the adjacent ventral striatum referred to as the ventral capsule/ventral striatum (VC/VS). Each lead was 1.27 mm in diameter with four 1.5-mm long electrode contacts, and adjacent contacts were separated by a distance of 1.5 mm. The contacts were at the tip of the lead and were numbered from 0 (deepest) to 3 (most superficial). The left lead was implanted 7.0 mm lateral to the midline, 14.7 mm anterior to the midcommissural point (MCP), and 4.0 mm inferior to the anterior commissure–posterior commissure (AC–PC) plane. The right lead was implanted 7.0 mm lateral to the midline, 14.7 mm anterior to MCP, and 4.0 mm inferior to the AC–PC plane. The AC–PC line was 24.7.
2.3. Methods
We performed psychiatric evaluations preoperatively, postoperatively, and at follow-up visits every 3 months. We used the Yale–Brown obsessive–compulsive scale (Y-BOCS) to evaluate the severity of OCD. Secondary outcome assessments included the Hamilton Depression Rating Scale (HAM-D) and the Global Assessment of Function (GAF) scale.
2.4. Postoperative course
The patient's medications, including propranolol (30 mg/d), triazolam (0.5 mg/d), bupropion (300 mg/d), venlafaxine (150 mg/d), and quetiapine (300 mg/d), were kept constant. The preoperative Y-BOCS, HAM-D, and GAF scores were 36, 30, and 51, respectively. Two weeks after a brief stimulation test, we selected the following settings considered to be effective because they improved his symptoms: bilateral contact 2, 2 to 4 V, pulse width 210 μs, and stimulation frequency 130 Hz.
At the follow-up 1 year after implantation, his Y-BOCS, HAM-D, and GAF scores were 25, 30, and 61, respectively. However, signs of minor inflammation (an erythematous plaque of approximately 1.5 cm2) were noted over the right pectoral area where a Kinetra pulse generator (Medtronic, Dublin, Ireland) had been implanted. He had no fever, and laboratory findings revealed a normal complete blood count. An antibiotic ointment was prescribed to treat the infection. However, 1 month later, the inflammation had progressed. He was anxious that the implanted device seemed not to be fixed in his body, and that it was movable and easily infected. His family noted that he exhibited impulsive behaviors such as picking at the skin in the area where the pulse generator was implanted. At the 15-month follow-up, his Y-BOCS, HAM-D, and GAF scores were 25, 28, and 65, respectively.
To avoid further infection, the right-side pulse generator was removed; thereafter, the local inflammation resolved. His habit of picking the skin in the area of pulse generator implantation gradually disappeared, and the stimulator device was re-implanted 4 months later. At the 24-month follow-up, infection had not re-occurred, and his Y-BOCS, HAM-D, and GAF scores were 32, 33, and, 61, respectively (Fig. 1).
Figure 1.
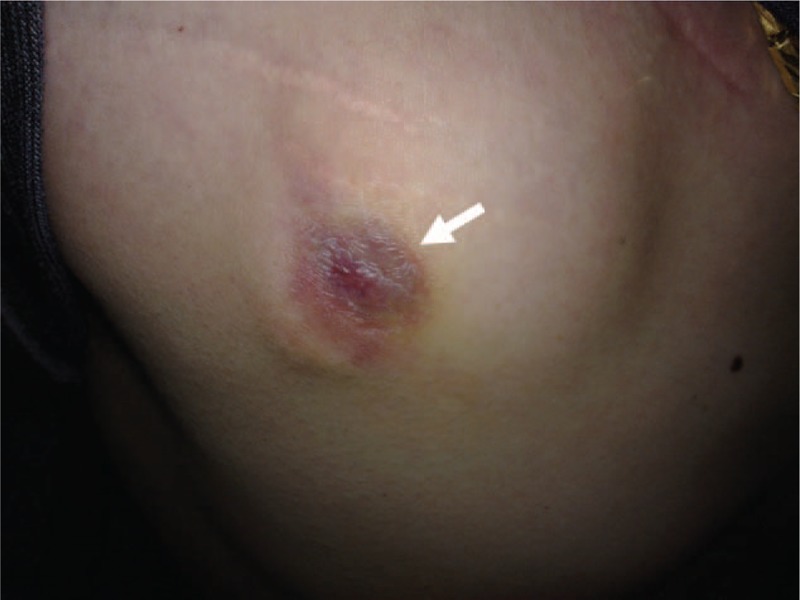
Image of the wound (white arrow).
3. Discussion
Previous studies have reported the occurrence of infection after DBS surgery in patents with OCD.[2–6] (Table 1) Dell’Osso et al[2] reported scar-picking behaviors in a patient with treatment-resistant OCD after DBS surgery, which resulted in infection and DBS device removal. Skin picking is categorized as an impulse-control disorder (ICD) not classified elsewhere or otherwise specified; skin picking does not meet the criteria for any specific ICDs or other mental disorders.[7] It is characterized by the repetitive and compulsive picking of the skin, leading to tissue damage.[8] It is also conceptualized as belonging to OC spectrum disorders.[9] Skin-picking disorder may occur with other disorders including major depressive disorder (12.5%–48%), anxiety disorders (8%–23%), and substance use disorders (14%–36%). Physicians must evaluate skin-picking disorder by using broader physical and psychiatric examinations. Cognitive behavioral interventions and pharmacology are the main treatments.[8]
Table 1.
Infection after DBS surgery for treatment-resistant OCD.
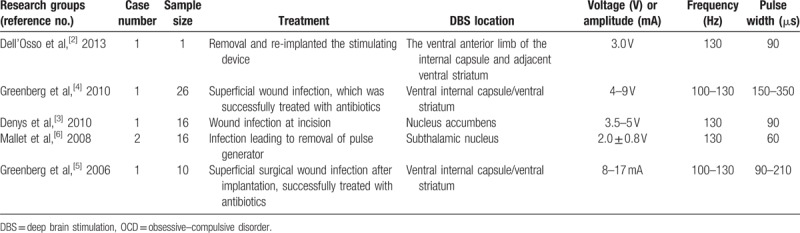
Compared with small trials of DBS in patients with OCD, a large-scale review of DBS in patients with Parkinson disease reported that infection rates for DBS surgery vary widely, from less than 1% to more than 15%. Skin-picking behaviors typically present within 3 months of surgery and most often occur at the site of the implanted pulse generator.[10] Postoperative hardware-related infection requiring further surgery occurs in 4.5% of cases.[11] Furthermore, the risk of hardware-related infection in DBS surgery is greater at impulse generator replacement than at the primary procedure.[12] A higher rate of inflammatory complications after DBS surgery has also been reported in patients with Tourette syndrome,[13] which also belongs to OC spectrum disorders.[9] Therefore, awareness of the predictive factors for postsurgical infection in a specific group of patients will become paramount as the implementation of DBS treatment becomes more extensive.
4. Conclusions
In summary, we described a patient with refractory OCD who developed infection and skin picking after 1 year of DBS. Patients with the OC spectrum may have higher rates of postsurgical infection and device removal due to skin picking. Because DBS is now being extended to treatment of more neuropsychiatric disorders such as OCD, refractory depression, and Tourette syndrome, further research is necessary to evaluate skin-picking behavior during presurgical and postsurgical follow-up to reduce infection and device removal rates.
Footnotes
Abbreviations: DBS = deep brain stimulation, OCD = obsessive–compulsive disorder, VC/VS = ventral capsule/ventral striatum.
Author contributions: C-HC conceptualized and designed the study and drafted the initial manuscript; H-CT, S-YC, and S-TT provided expert opinions and reviewed the final submitted manuscript; H-CT was in charge of this study, including conducting the data analysis, and critically reviewed the manuscript, and approved the final submitted manuscript.
Funding: This study was supported by a grant from Buddhist Tzu-Chi General Hospital.
Conflicts of interest: The authors report no conflict of interest concerning the materials or methods used in this study, nor concerning the findings reported in this paper.
References
- [1].Krack P, Hariz MI, Baunez C, et al. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci 2010;33:474–84. [DOI] [PubMed] [Google Scholar]
- [2].Dell’Osso B, Porta M, Servello D, et al. Deep brain stimulation device removal after scar picking behaviors in a patient with treatment-resistant obsessive compulsive disorder. Brain Stimul 2013;6:96–8. [DOI] [PubMed] [Google Scholar]
- [3].Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry 2010;67:1061–8. [DOI] [PubMed] [Google Scholar]
- [4].Greenberg BD, Gabriels LA, Malone DA, Jr, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 2010;15:64–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 2006;31:2384–93. [DOI] [PubMed] [Google Scholar]
- [6].Mallet L, Polosan M, Jaafari N, et al. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 2008;359:2121–34. [DOI] [PubMed] [Google Scholar]
- [7].Kaplan BJ, Kaplan VA. Kaplan and Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry, 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:783–784. [Google Scholar]
- [8].Grant JE, Odlaug BL, Chamberlain SR, et al. Skin picking disorder. Am J Psychiatry 2012;169:1143–9. [DOI] [PubMed] [Google Scholar]
- [9].Simeon D, Favazza AR. Self-injurious Behaviors, Phenomenology and Assessment. Washington, DC: American Psychiatric Press; 2001. [Google Scholar]
- [10].Benabid AL, Chabardes S, Mitrofanis J, et al. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol 2009;8:67–81. [DOI] [PubMed] [Google Scholar]
- [11].Sillay KA, Larson PS, Starr PA. Deep brain stimulator hardware-related infections: incidence and management in a large series. Neurosurgery 2008;62:360–6. [discussion 366–367]. [DOI] [PubMed] [Google Scholar]
- [12].Pepper J, Zrinzo L, Mirza B, et al. The risk of hardware infection in deep brain stimulation surgery is greater at impulse generator replacement than at the primary procedure. Stereotact Funct Neurosurg 2013;91:56–65. [DOI] [PubMed] [Google Scholar]
- [13].Servello D, Sassi M, Gaeta M, et al. Tourette syndrome (TS) bears a higher rate of inflammatory complications at the implanted hardware in deep brain stimulation (DBS). Acta Neurochir (Wien) 2011;153:629–32. [DOI] [PubMed] [Google Scholar]


