Abstract
The purpose of our study was to assess the safety and efficacy of recombinant adenovirus-p53 (rAd-p53) combined with surgery and chemoradiotherapy (CRT) for patients with hypopharyngeal squamous cell carcinomas (HPSCC). This study retrospectively and consecutively collected clinical data of 102 patients with primary HPSCC who were admitted to the Department of Otolaryngology of West China Hospital, Sichuan University in China between March 2010 and December 2015. A retrospective clinical study of 102 patients with HPSCC was carried out from March 2010 to December 2015. All patients were male and were divided into 3 groups based on the treatments they received, including Single Surgery, Surgery + CRT, and Surgery + CRT + rAd-p53. In the Surgery + CRT + rAd-p53 group, rAd-p53 was intratumorally injected on the 1st day preoperatively; peritumorally injected on the 7th day intraoperatively, and on the 21st, 28th, and 35th days postoperatively. Their clinical data were retrospectively collected and analyzed. In our study, for all 102 patients with HPSCC, 16 patients received Single Surgery, 44 patients received Surgery + CRT therapy, and 42 patients received Surgery + CRT + rAd-p53 therapy. In the Surgery + CRT + rAd-p53 group, all patients could tolerate rAd-p53 treatment and no serious side effect was observed. In addition, rAd-p53 application did not increase the side reactions caused by surgery and CRT. Compared with the 3-year overall survival rates of Single Surgery group and Surgery + CRT group, the 3-year overall survival rates of Surgery + CRT + rAd-p53 group was significantly enhanced (P < .05). Similar results were also observed for the 3-year disease-free survival rates. Our results indicate that rAd-p53 therapy may improve the therapeutic effect of patients with HPSCC, and is a safe and effective treatment method for patients with HPSCC. However, further prospective studies with larger sample sizes are needed to validate our findings.
Keywords: gene therapy, hypopharyngeal squamous cell carcinomas, recombinant adenovirus-p53
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is the 6th common malignancy worldwide.[1] As one of HNSCC, hypopharyngeal squamous cell carcinoma (HPSCC) only constitutes approximately 3% to 5% of HNSCC, but it is usually diagnosed in the advanced stage with poor diagnosis.[2] In the past decades, different treatments, including surgery, chemotherapy, radiotherapy, and immunotherapy, have been developed and utilized for HPSCC,[3–5] but no treatment could ever achieve a satisfactory cure effect and the survival rate has not been improved significantly.[6,7]
Gene therapy has been attracting increasing attentions and it has been investigated for cancer treatment as well. As the guardian of genome, p53 gene takes part in regulation of cell growth division and induction of cell death through apoptosis. The mutations and deletions of p53 gene have been reported in many malignant carcinomas[8–10] and also detected frequently in head and neck carcinoma.[4,11] Thus, with the development of gene therapy technology, transducting wild-type p53 gene into HPSCC cells could be expected to restore the tumor suppressor functions, which might be an attractive therapeutic method for HPSCC.
In vivo and in vitro studies have shown that adenovirus-mediated p53 gene therapy could be effective against a variety of malignancies.[12–14] Recombinant adenovirus-p53 (rAd-p53), which is an E1 substituted replication-incompleted recombinant adenovirus encoding the human p53 gene, has also been investigated for treating multiple tumors, including pancreatic cancer,[15] glioblastoma,[16] sarcoma,[17] and oral cancer.[18] However, there is no report on the role of adenovirus-p53 in the treatment of advanced HPSCC. In this study, safety and efficacy of rAd-p53 combined surgery and chemoradiotherapy (CRT) in treating advanced HPSCC was investigated.
2. Methods
2.1. Patients
This study retrospectively and consecutively analyzed the clinical data from 102 patients with primary HPSCC who were admitted to the Department of Otolaryngology of West China Hospital, Sichuan University in China between March 2010 and December 2015. The histopathologic diagnosis was carried out by the hospital's pathology department. The TNM classification was in accordance with the International Union Against Cancer (2010). Some patients received conventional surgery combined with CRT and these patients were classified into the Surgery + CRT group. Some patients had signed the agreement to reject the postoperative CRT, as a result, they received surgery only and were classified into the Single Surgery group. In addition, some patients had signed the agreement to utilize the rAd-p53, they received not only surgery and postoperative CRT, but also the rAd-p53 treatment and these patients were classified into the Surgery + CRT + rAd-p53 group. The present retrospective review of medical records was approved by the Institutional Review Board of the West China Hospital (Cheng Du, Sichuan Province, China).
2.2. P53 gene therapy
rAd-p53 (Shenzhen Sibiono Genetech Co. Ltd, Shenzhen) was stored at −20°C in concentrations of 1 × 1012 virus particles (vp)/mL and diluted by 0.9% physiologic saline solution to the concentration of 4 × 109 vp/mL before using. The whole course of treatment included 35 days. Multipoint intratumoral injections were done on the 1st day with the help of the electronic fibrolaryngoscope. Multipoint peritumoral injections were done on the 7th day during surgery after tumor resection. And the multipoint peritumoral injections were carried out on the 21st, 28th, and 35th days with the guidance of the electronic fibrolaryngoscope.
2.3. Chemoradiotherapy
For patients in the Surgery + CRT and Surgery + CRT + rAd-p53 groups, they received concurrent CRT of cisplatin (cumulative dose of 200 mg/m2) and radiotherapy (60–70 Gy) after surgery treatment or surgery + rAd-p53 treatment.
2.4. Adverse events evaluation
Patients were observed for adverse events. Toxic and adverse events were evaluated according to the NCI common toxicity standard.
2.5. Follow-up
All patients received periodic follow-up review including electronic laryngoscopy and computed tomography examination. In this study, the survival time was measured as the period from the date of surgery to the date of death or the last follow-up, and disease-free survival time as the period from the date of surgery to the date of disease progression or death.
2.6. Statistical analysis
Statistical analyses were conducted using SPSS statistics software (version 16.0, SPSS Inc, Chicago, IL). The statistical differences were assessed by the analysis of variance method. And a chi-squared method was utilized for categorical variables. The overall survival rate and disease-free survival rate were determined using the Kaplan–Meier method. In all statistical analyses, values were considered significant at a P value of <0.05.
3. Results
3.1. Patient characteristics
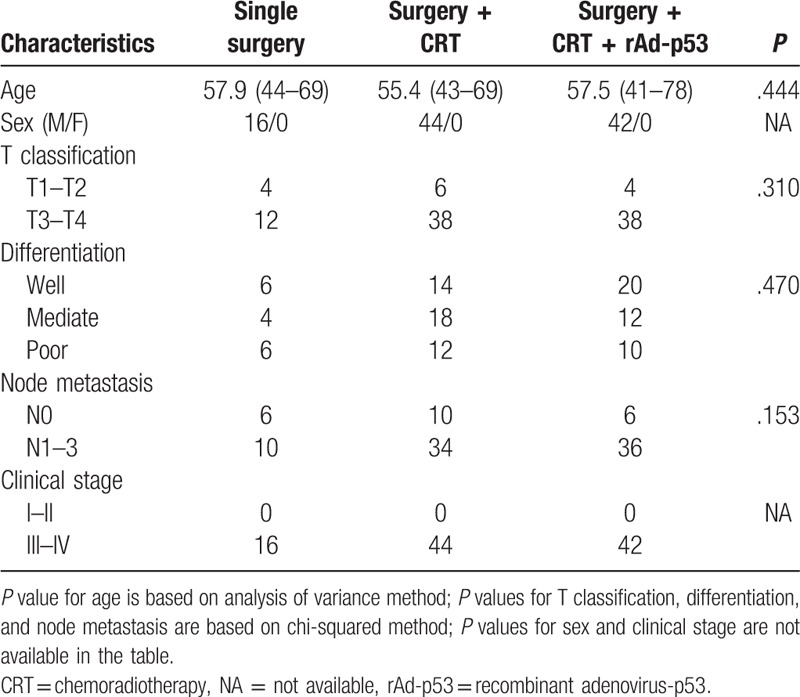
From March 2010 to December 2015, 102 patients with primary HPSCC received treatment in West China Hospital and all patients were male. Sixteen patients received Single Surgery, 44 patients received Surgery and CRT therapy, and 42 patients received Surgery, CRT, and rAd-p53 therapy. As shown in Table 1, no significant differences in age, sex, tumor differentiation, or clinical stage (including T and N stages) were observed in the 3 groups.
Table 1.
Patients of characteristics.
3.2. Adverse events
All the values in blood, urine, and liver and renal function remained within normal range before and after rAd-p53 therapy. And the heart function damage was not found for the patients who received rAd-p53 therapy. In addition, no significant difference was observed in the fields of wound infection, pharyngeal fistula, and pulmonary infection between Surgery + CRT group and Surgery + CRT + rAd-p53 group (P > .05). These results indicate that the rAd-p53 application did not increase the side reactions caused by surgery and CRT.
Among all 42 patients who received rAd-53 injections, the transient and self-limited fever events were the most common adverse events. The incidence of fever was 71.43% (30 of 42). We observed that the fever events usually happened as early as about 3 hours after rAd-p53 injections, and then disappeared spontaneously after lasting approximately 4 hours. Additionally, gastrointestinal reactions and muscle aches were observed in 2 patients after receiving rAd-p53 injections, respectively.
3.3. Treatment outcomes
All 102 patients were followed up for a period of 11 to 63 months (median 36 months). For Single Surgery, Surgery + CRT, and Surgery + CRT + rAd-p53 groups the follow-up times were 14 to 60 months (median 31 months), 11 to 63 months (median 36 months), and 14 to 60 months (median 38 months), respectively.
During 63 months of follow-up, the 3-year overall survival rates of Single Surgery, Surgery + CRT, and Surgery + CRT + rAd-p53 groups were 43.8%, 54.5%, and 78.6%, respectively. And the 3-year disease-free survival rates were 43.8%, 54.5%, and 69.0%, respectively. Compared with the 3-year overall survival rates of Single Surgery group and Surgery + CRT group, the 3-year overall survival rates of Surgery + CRT+ rAd-p53 group were significantly enhanced (P < .05). No significant difference was found in the 3-year overall survival rates between the Single Surgery group and the Surgery + CRT group (P > .05). As for the 3-year disease-free survival rates, similar results were observed. The overall survival and disease-free survival of patients in Single Surgery, Surgery + CRT, and Surgery + CRT + rAd-p53 groups were shown in Figs. 1 and 2, respectively.
Figure 1.
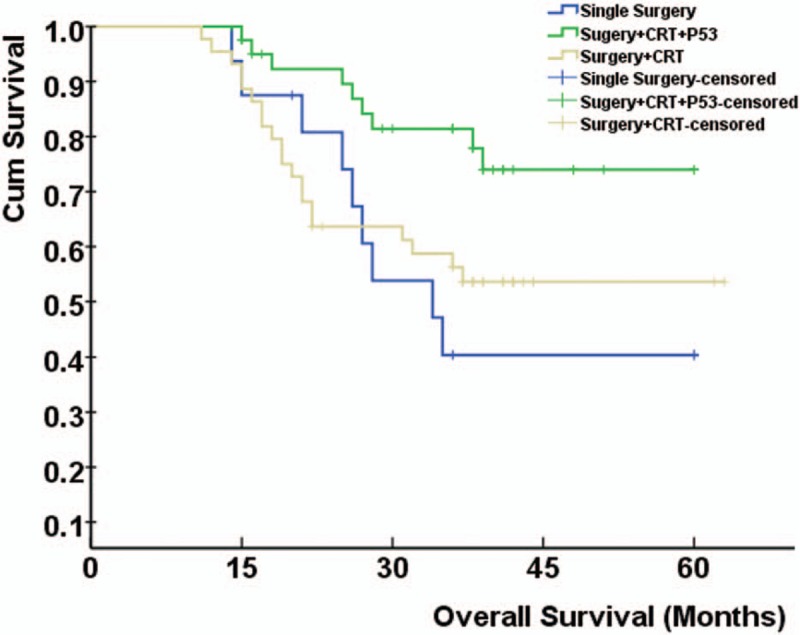
The overall survival of patients in Single Surgery, Surgery + CRT, and Surgery + CRT + rAd-p53 groups. Compared with Single Surgery, Surgery + CRT groups, the overall survival rates of Surgery + CRT + rAd-p53 groups were significantly enhanced (P < .05). CRT = chemoradiotherapy, rAd-p53 = recombinant adenovirus-p53.
Figure 2.
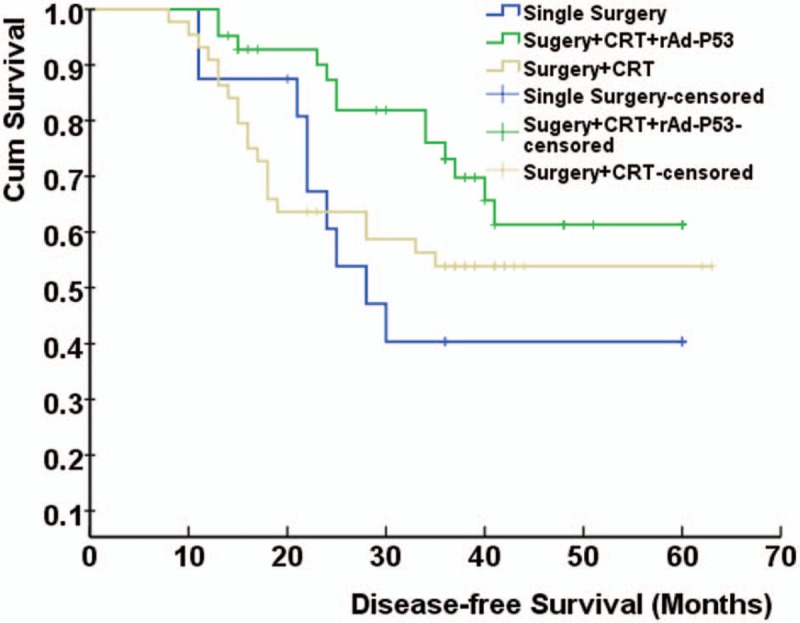
The disease-free survival of patients in Single Surgery, Surgery + CRT, and Surgery + CRT + rAd-p53 groups. Compared with Single Surgery, Surgery + CRT groups, the disease-free survival rates of Surgery + CRT + rAd-p53 groups were significantly enhanced (P < .05). CRT = chemoradiotherapy, rAd-p53 = recombinant adenovirus-p53.
4. Discussion
Squamous cell carcinoma of the hypopharynx is a highly malignant carcinoma with a very high morbidity and mortality. HPSCC has the particular trait of invasiveness through the submucosa to induce distant lesions, also known as skip lesions,[19] and thus complete tumor surgical resection with adjuvant CRT is widely carried out in advanced HPSCC[20] to improve the therapeutic effect of patients with HPSCC. In our study, we found that the 3-year overall survival rates of HPSCC patient in Surgery + CRT group (54.5%) were higher than that in Single Surgery group (43.8%); however, this gap was not statistically significant. It is noted that the number of patients in Single Surgery group is much smaller than that in Surgery + CRT group. Therefore, further clinical study with a larger sample size is needed to explore whether CRT could benefit patients with HPSCC. These results indicate that Single Surgery and/or CRT might not greatly improve the prognosis of patients with HPSCC. Obviously, additional and much more effective treatment will be needed to improve the therapeutic result of HPSCC.
Gene therapy has drawn increasing interest. It is well known that the p53 gene is an important tumor suppressor gene. Wild-type p53 gene plays an important role in inhibiting carcinogenesis by maintaining genomic integrity. If p53 gene mutation or deletion occurs, cells could not enter into stage G1 and DNA reparation via p53-regulated pathway after DNA injury. Cells with damaged genetic information could enter into proliferation and finally induce malignant tumor.[21,22] Structural alteration and abnormal expression of p53 gene was observed in HPSCC and thus contributed to the complex network of molecular events leading to tumorigenesis and development of tumor.[11] Thus, restoring normal p53 gene and its expression may be the key to p53 gene therapy. rAd-p53 is an E1 substituted replication-incompleted recombinant adenovirus encoding human p53 gene and might have the potential to achieve this goal.
Studies have shown that the molecular mechanisms of antitumor effect of rAd-p53 combined with conventional therapies might include cell cycle arrest, apoptosis/necrosis, and upregulation of innate immune defenses.[15] Therefore, rAd-p53 therapy might increase the sensitivity of HPSCC to CRT and increase HPSCC susceptibility to apoptosis. In this study, the overall survival and disease-free survival of HPSCC patients in the Surgery + CRT + rAd-p53 group were improved comparing to those of HPSCC patients in the Single Surgery group and the Surgery + CRT group, indicating that rAd-p53 therapy greatly enhanced the therapeutic effect of conventional treatments (surgery and CRT) for patients with HPSCC.
In addition, our research also showed that all patients could tolerate rAd-p53 treatment and rAd-p53 therapy did not increase the adverse reactions caused by surgery and CRT. Nonetheless, rAd-p53 treatment-related toxicities could not be ignored. The transient and self-limited fever events were the most common adverse events, and gastrointestinal reactions and muscle aches were also observed in some patients. However, these treatment-related toxicities were negligible and no patient was found to have serious toxicity or any other fatal situations.
Although the present study indicated that rAd-p53 might have a good application value in the treatment of patients with HPSCC, several limitations should be considered. First, there might be a gender bias as all patients enrolled were male. This is somehow reasonable because HPSCC incidence in female is very low[2,6,7] and with current sample size, it is possible that no female patient was encountered. Second, due to the limited number of patients in each subgroup, the stratified analyses could not be carried out according to age, sex, T classification, node metastasis, clinical stage, and pathologic differentiation. Therefore, the therapeutic role of rAd-p53 and its mechanisms in patients with HPSCC require confirmation in studies with larger groups.
In conclusion, our results indicate that rAd-p53 therapy may improve the therapeutic effect of patients with HPSCC and is a safe and effective treatment method for patients with HPSCC. However, further prospective studies with larger sample sizes are needed to validate our findings.
Author contributions
Conceptualization: Hui Yang.
Data curation: Jun Liu, Dan Lv, Haiyang Wang.
Funding acquisition: Fei Chen.
Investigation: Dan Lv, Jian Zou.
Methodology: Jun Liu, Jian Zou, Hui Yang, Fei Chen.
Project administration: Fei Chen.
Resources: Haiyang Wang, Hui Yang.
Software: Haiyang Wang, Jian Zou.
Writing – original draft: Jun Liu, Dan Lv, Hui Yang, Fei Chen.
Writing – review & editing: Jun Liu, Hui Yang, Fei Chen.
Footnotes
Abbreviations: CRT = chemoradiotherapy, HNSCC = head and neck squamous cell carcinoma, HPSCC = hypopharyngeal squamous cell carcinoma, rAd-p53 = recombinant adenovirus-p53.
Jun Liu and Dan Lv have contributed equally to this work.
This work was supported by the Science and Technology Department of Sichuan Province (No. 2012SZ0024).
The authors have no conflicts of interest to disclose.
References
- [1].Wittekindt C, Wagner S, Mayer CS, et al. Basics of tumor development and importance of human papilloma virus (HPV) for head and neck cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg 2012;11:Doc09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Song J, Chang I, Chen Z, et al. Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PLoS ONE 2010;5:e11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Belcher R, Hayes K, Fedewa S, et al. Current treatment of head and neck squamous cell cancer. J Surg Oncol 2014;110:551–74. [DOI] [PubMed] [Google Scholar]
- [4].Chan KK, Glenny AM, Weldon JC, et al. Interventions for the treatment of oral and oropharyngeal cancers: targeted therapy and immunotherapy. Cochrane Database Syst Rev 2015;CD010341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc 2016;91:386–96. [DOI] [PubMed] [Google Scholar]
- [6].Braakhuis BJ, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol 2014;50:670–5. [DOI] [PubMed] [Google Scholar]
- [7].Delagranda A, Leterme G, Chirpaz E, et al. Epidemiological features of cancers of the oral cavity, oropharynx, hypopharynx and larynx cancer in Reunion Island. Eur Ann Otorhinolaryngol Head Neck Dis 2018;135:175–81. [DOI] [PubMed] [Google Scholar]
- [8].Zulfiqar M, Bluth MH, Bhalla A. Molecular diagnostics in esophageal and gastric neoplasms: 2018 update. Clin Lab Med 2018;38:357–65. [DOI] [PubMed] [Google Scholar]
- [9].Swarts DR, Ramaekers FC, Speel EJ. Molecular and cellular biology of neuroendocrine lung tumors: evidence for separate biological entities. Biochim Biophys Acta 2012;1826:255–71. [DOI] [PubMed] [Google Scholar]
- [10].Koonrungsesomboon N, Wadagni AC, Mbanefo EC. Molecular markers and Schistosoma-associated bladder carcinoma: a systematic review and meta-analysis. Cancer Epidemiol 2015;39:487–96. [DOI] [PubMed] [Google Scholar]
- [11].Solomon B, Young RJ, Rischin D. Head and neck squamous cell carcinoma: genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin Cancer Biol 2018. [DOI] [PubMed] [Google Scholar]
- [12].Koom WS, Park SY, Kim W, et al. Combination of radiotherapy and adenovirus-mediated p53 gene therapy for MDM2-overexpressing hepatocellular carcinoma. J Radiat Res 2012;53:202–10. [DOI] [PubMed] [Google Scholar]
- [13].Shimada H, Matsubara H, Shiratori T, et al. Phase I/II adenoviral p53 gene therapy for chemoradiation resistant advanced esophageal squamous cell carcinoma. Cancer Sci 2006;97:554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pan D, Wei X, Liu M, et al. Adenovirus mediated transfer of p53, GM-CSF and B7-1 suppresses growth and enhances immunogenicity of glioma cells. Neurol Res 2010;32:502–9. [DOI] [PubMed] [Google Scholar]
- [15].Mo J, Lin M, He B, et al. Recombinant human adenovirus-p53 improves the outcome of mid-late stage pancreatic cancer via arterial infusion. Oncol Lett 2017;14:6829–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Qiao HB, Li J, Lv LJ, et al. The effects of interleukin 2 and rAd-p53 as a treatment for glioblastoma. Mol Med Rep 2018;17:4853–9. [DOI] [PubMed] [Google Scholar]
- [17].Xia Y, Du Z, Wang X, et al. Treatment of uterine sarcoma with rAd-p53 (gendicine) followed by chemotherapy: clinical study of TP53 gene therapy. Hum Gene Ther 2018;29:242–50. [DOI] [PubMed] [Google Scholar]
- [18].Li Y, Li LJ, Wang LJ, et al. Selective intra-arterial infusion of rAd-p53 with chemotherapy for advanced oral cancer: a randomized clinical trial. BMC Med 2014;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Katzenell U, Yeheskeli E, Segal S, et al. Hemilaryngeal flap for hypopharyngeal reconstruction in pyriform sinus carcinoma. Acta Otolaryngol 2007;127:4–7. [DOI] [PubMed] [Google Scholar]
- [20].Bradley PJ. Multidisciplinary clinical approach to the management of head and neck cancer. Eur Arch Otorhinolaryngol 2012;269:2451–4. [DOI] [PubMed] [Google Scholar]
- [21].Jain AK, Barton MC. p53: emerging roles in stem cells, development and beyond. Development 2018;145: [DOI] [PubMed] [Google Scholar]
- [22].Wawrzynow B, Zylicz A, Zylicz M. Chaperoning the guardian of the genome. The two-faced role of molecular chaperones in p53 tumor suppressor action. Biochim Biophys Acta 2018;1869:161–74. [DOI] [PubMed] [Google Scholar]


