Abstract
Objective:
This meta-analysis assessed the long-term efficacy of deep brain stimulation (DBS) of the subthalamic nucleus (STN) and globus pallidus interna (GPi) for Parkinson disease (PD).
Methods:
PubMed, Cochrane Library, and Clinical Trials databases were searched. Outcomes were unified Parkinson disease rating scale section (UPDRS) III off-medication score, Parkinson's disease questionnaire: 39 activities of daily living (PDQ-39 ADL) score, and levodopa-equivalent dosage after DBS.
Results:
During the off-medication state, pooled weighted mean difference (WMD) of UPDRS III score was .69 (95% confidence interval [CI] = −1.77 to 3.16, P = .58). In subgroup analysis, WMD of UPDRS III off-medication scores from baseline to 2 years and 3 years post-DBS were −.61 (95% CI = −2.97 to 1.75, P = .61) and 2.59 (95% CI = −2.30 to 7.47, P = .30). Pooled WMD of changes in tremor, rigidity, and gait scores were 1.12 (95% CI = −0.05 to 2.28, P = .06), 1.22 (95% CI = −0.51 to 2.94, P = .17) and .37 (95% CI = −0.13 to 0.87, P = .15), respectively. After DBS, pooled WMD of PDQ-39 ADL and LED were −3.36 (95% CI = −6.36 to −0.36, P = .03) and 194.89 (95% CI = 113.16 to 276.63, P < .001).
Conclusions:
STN-DBS and GPi-DBS improve motor function and activities of daily living for PD. Differences in the long-term efficacy for PD on motor symptoms were not observed.
Keywords: deep brain stimulation, globus pallidus internus, meta-analysis, Parkinson disease, subthalamic nucleus
1. Introduction
Parkinson disease (PD) is a progressive debilitating neurodegenerative disease. As the second most common neurodegenerative disorder, PD was found to affect approximately 7,000,000 people globally and 1,000,000 people in the United States in 2012.[1] It is characterized by resting tremor, bradykinesia, rigidity, gait disturbances, and postural instability. Currently, the anti-Parkinson medication levodopa and dopamine agonists are the first-line treatment method for PD. These medications could improve the early symptoms of PD,[2] but they become ineffective and even produce adverse effects, such as dyskinesias and psychotic symptoms.[3] Deep brain stimulation (DBS) surgery is a well-accepted treatment for medication-refractory PD.[4,5] The possible mechanism of DBS was that the chronic and high-frequency stimulation of brain areas might have comparable effect to the surgical ablation of these areas.[6] The globus pallidus interna (GPi) and the subthalamic nucleus (STN) are both accepted targets for DBS.[7] With optimized stimulation settings, DBS typically improves the motor symptoms of tremor, limb rigidity, bradykinesia, and akinesia.[8] In addition, DBS surgery could also improve overall quality of life of PD patients.[9]
Recently, many systematic reviews and meta-analyses assessing the efficacy of GPi-DBS and STN-DBS on patients suffering from PD have been published. Liu et al [10] reported that differences in therapeutic efficacy on motor symptoms for PD were not observed between the GPi-DBS and STN-DBS, STN-DBS allowed greater reduction in medication for patients, whereas GPi-DBS provided greater relief from psychiatric symptoms. Xu et al pointed out that during the off-medication state, the STN-DBS might be superior to GPi-DBS in improving the motor function and activities of daily living for PD patients, but during the on-medication state, the opposite result is observed. Meanwhile, the STN-DBS is superior at reducing the levodopa-equivalent dosage (LED), whereas the GPi-DBS shows a significantly greater reduction in the Beck depression inventory (BDI) score after DBS.[11] Xie et al reported that the unified Parkinson disease rating scale section (UPDRS) scores measuring Parkinsonian symptoms revealed no significant difference between GPi-DBS and STN-DBS. STN-DBS was more effective for reduction in medication than GPi-DBS. Alternatively, GPi-DBS was more effective for improving the Parkinson disease questionnaire: 39 activities of daily living (PDQ-39 ADL) score than STN-DBS.[12] However, most of these studies focused on the short- term (≤1 year) efficacy of DBS for the PD patients. As we know, there is no meta-analysis and systemic review used to evaluate the long-term efficacy of DBS for the treatment of PD patients so far. Thus, our meta-analysis focused on the long-term efficacy of STN-DBS vs GPi-DBS for PD to fill a critical gap in the literature and guide some clinical treatment decisions.
2. Methods
This meta-analysis was performed following the PRISMA checklist. Ethical approval and patient consent were not required because our study was retrieved from previous published studies.
2.1. Literature search
PubMed, Cochrane Library, and Clinical Trials databases were searched up to 2017. The following key words are used: “subthalamic nucleus DBS,” “globus pallidus DBS,” “deep brain stimulation,” and “Parkinson disease.” Previous reviews were also searched to identify additional trials. The search was limited to controlled clinical trials, and there was no language restriction.
2.2. Trial selection
Trials were included if they met the following criteria: controlled clinical trials comparing STN-DBS versus GPi-DBS in treating idiopathic PD; patients were >18 years old; the outcomes assessed by UPDRS III off-medication score, PDQ-39 ADL score, or LED score; the outcomes assessed not <2 years post-surgery; studies and patients were excluded for the following reasons: they studied only a single DBS target (STN or GPi); studies assessed the short-term (<2 year) efficacy; patients had active alcohol or drug abuse, dementia. The 2 authors (Y.M. and H.H.) identified and agreed upon the studies meeting these criteria. The study quality was assessed with the “assessing risk of bias” tables provided in version 5.0.2 of the Cochrane Handbook for Systematic Reviews of Interventions.
2.3. Data extraction
The information extracted included the study design, patient selection criteria, age, the number of patients, trial duration, and clinical outcomes. Clinical outcomes included UPDRS III score, PDQ-39 ADL score, LED score. UPDRS is widely used to assess the motor performance and functional status of PD patients. The higher scores indicate more severe PD. It has 4 parts, and the UPDRS III was used to assess the motor function.[13] The LED indicates the level of anti-Parkinson medication used both before and after DBS. The therapy was considered successful if the dose of medication after the treatment was significantly reduced. In addition, we also selected PDQ-39 ADL score to evaluate the change in the quality of life both before and after DBS. Data were abstracted by 1 investigator (J.F.) and checked by a second investigator (S.Z.). Any discrepant data were reviewed again by the investigators.
Most studies provided the means and SD of pre- and post-operative results. If these values were not explicitly reported, we determined them by extracting median and the interquartile range (IQR) and then we used the equation in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0, chapter 7.7.3.5). If the study only included subgroup data, we merged the data in accordance with the formula.[14]
2.4. Statistical analysis
In our meta-analysis, all outcomes were continuous data, so we pooled data using the weighted mean difference (WMD) of changes from baseline (change scores) to compare STN-DBS and GPi-DBS. The results were expressed as the 95% CI, z scores, and P values. χ2 tests and the I2 statistic derived from the χ2 values were used to test heterogeneity among the contrasts. I2 values of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively.[15] If significant heterogeneity existed in the case, we used a random-effect model for analysis. Otherwise, a fixed-effect model was used. A sensitivity analysis was performed to rule out the possibility that any single study strongly influenced the pooled effect. The meta-analysis was conducted using RevMan 5.2 software (Cochrane Collaboration).
3. Results
3.1. Description of eligible trials
After searching PubMed, Cochrane Library, and Clinical Trials databases, and previous reviews, 5 articles met the inclusion criteria (Table 1).[7,16–19] The search flow diagram is shown in Figure 1. A total of 5 studies with 890 subjects (437 patients in the STN-DBS group and 453 patients in the GPi-DBS group) were included in our analysis (Table 1).
Table 1.
Overview of the included studies.
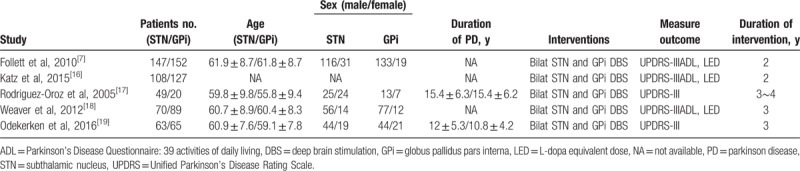
Figure 1.
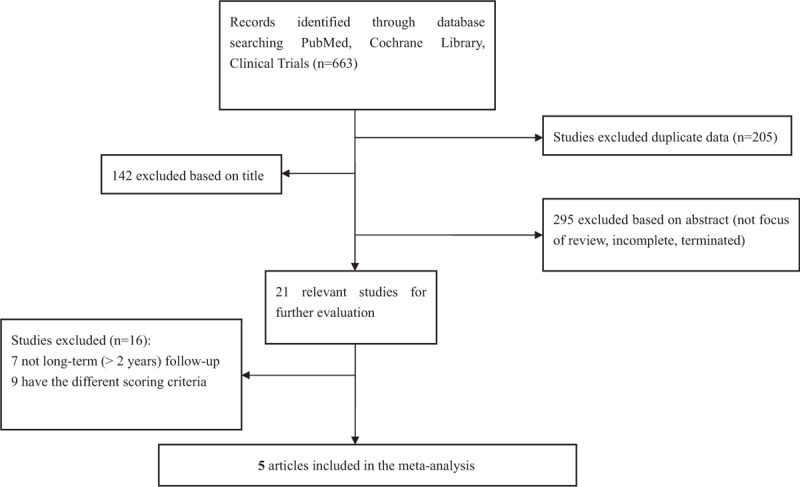
Search flow for the trial identification and selection process.
3.2. The risk of bias
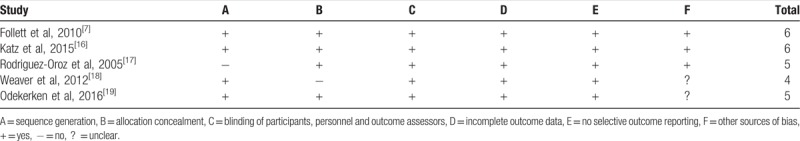
The included studies were evaluated using the “assessing risk of bias” (Table 2), which includes Sequence generation, allocation, blinding, incomplete outcome data, selective reporting, and other sources of bias. Table 2 showed that all of the included studies had a low risk of bias.
Table 2.
Risk-of-bias assessment of included studies.
3.3. Meta-analysis of the weighted mean difference
During the off-medication phase, the pooled WMD of 5 studies for UPDRS III score with STN-DBS compared to GPi-DBS was .69 (95% CI = −1.77 to 3.16, P = .58) (Fig. 2). The results showed no significant difference between the 2 procedures. Subgroup analysis was performed according to the follow-up time. The WMD of UPDRS III off-medication scores from baseline to 2 years and 3 years post-DBS were −0.61 (95% CI = −2.97 to 1.75, P = .61) and 2.59 (95% CI = −2.30 to 7.47, P = .30), respectively (Fig. 3). The results showed no significant difference between the 2 procedures. In addition, low heterogeneity was observed (I2 = 41%) (Fig. 2). However, in the subgroup analysis, I2 for the 2 years’ follow-up[7,16] showed no heterogeneity (I2 = 0%) (Fig. 3). Otherwise, I2 for the 3 or 4 year's follow-up[17–19] showed moderate heterogeneity (I2 = 62%) (Fig. 3). The sensitivity analysis indicated that the WMD of UPDRS III off-medication scores from baseline to 3 years post-DBS was significantly influenced when we excluded the study from Weaver et al (data not shown).[18]
Figure 2.
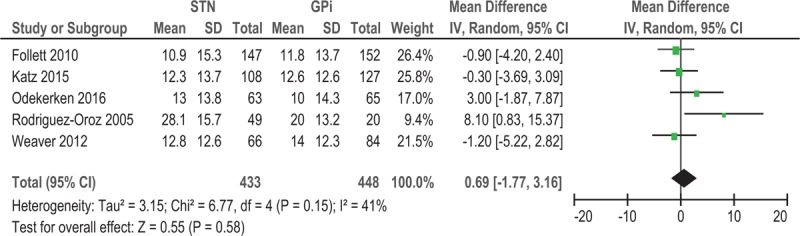
Forest plot: WMD in UPDRS III score of five studies; off-medication change and 95% CI. CI = confidence interval, GPi = globus pallidus pars interna, STN = subthalamic nucleus, UPDRS = Unified Parkinson's Disease Rating Scale, WMD = weighted mean difference.
Figure 3.
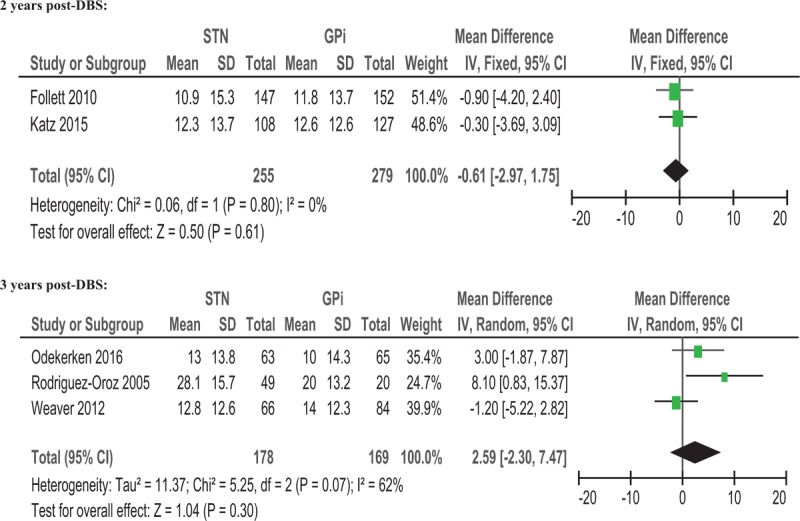
Forest plot: WMD in UPDRS III scores from baseline to 2 years and 3 years post-DBS; off-medication change and 95% CI. Two years post-DBS. CI = confidence interval, DBS = deep brain stimulation, GPi = globus pallidus pars interna, STN = subthalamic nucleus, UPDRS = Unified Parkinson's Disease Rating Scale, WMD = weighted mean difference.
3.4. Meta-analysis of motor subtypes
We also assessed the effect of DBS target (STN vs. GPi) on motor subtypes (tremor, rigidity, and gait). The results showed that responsiveness to both STN and GPi DBS was similar among different PD motor subtypes (tremor, rigidity, and gait) with change scores of 1.12 (95% CI = −0.05 to 2.28, P = .06), 1.22 (95% CI = −0.51 to 2.94, P = .17), .37 (95% CI = −0.13 to .87, P = .15), respectively (Fig. 4). There was low heterogeneity for the tremor score (I2 = 41%), and moderate heterogeneity for the rigidity score (I2 = 63%) (Fig. 4). No significant heterogeneity was observed for the gait score (I2 = 0%) (Fig. 4).
Figure 4.
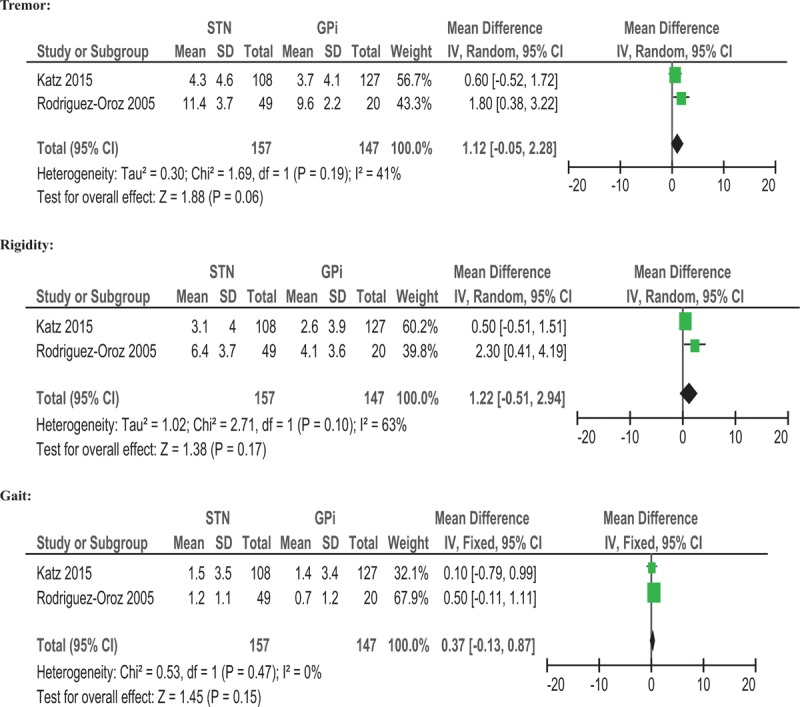
Forest plot: WMD in UPDRS III score on motor subtypes; off-medication change and 95% CI. CI = confidence interval, GPi = globus pallidus pars interna, STN = subthalamic nucleus, UPDRS = Unified Parkinson's Disease Rating Scale, WMD = weighted mean difference.
3.5. Meta-analysis of PDQ-39 ADL and LED
Use of GPi-DBS was associated with a greater improvement of PDQ-39 ADL compared with STN-DBS (−3.36, 95% CI −6.36 to −0.36, P = .03) (Fig. 5). No significant heterogeneity was observed between treatment groups (I2 = 0%). Greater improvement in LED score was observed for STN-DBS compared with GPi-DBS, with a pooled WMD of 194.89 (95% CI = 113.16 to 276.63, P < 0.001) (Fig. 6). No significant heterogeneity was observed (I2 = 0%).
Figure 5.
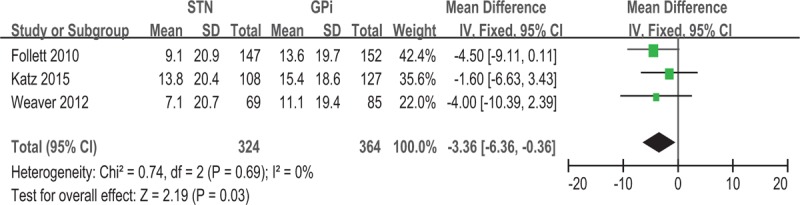
Forest plot: WMD in PDQ-39 ADL change and 95% CI. CI = confidence interval, GPi = globus pallidus pars interna, PDQ-39 ADL = Parkinson disease questionnaire: 39 activities of daily living, STN = subthalamic nucleus, WMD = weighted mean difference.
Figure 6.
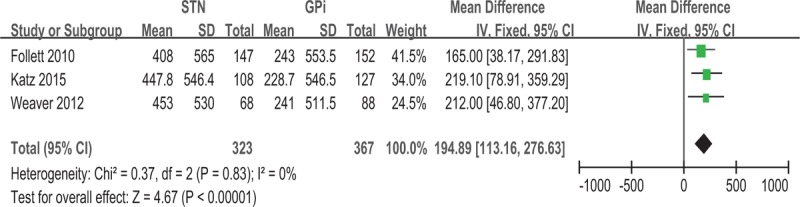
Forest plot: WMD in LED change and 95% CI. CI = confidence interval, GPi = globus pallidus pars interna, LED = levodopa-equivalent dosage, STN = subthalamic nucleus, WMD = weighted mean difference.
4. Discussion
Our results showed that differences in the long-term efficacy on motor symptoms for PD were not observed between STN-DBS and GPi-DBS. But when we excluded the study from Weaver et al,[18] the analysis indicated that the WMD of UPDRS III off-medication scores from baseline to 3 years post-DBS of STN-DBS group was significantly higher than GPi-DBS group, which indicated that off-drug phase motor symptoms improve more after STN-DBS than after GPi-DBS. In addition, excluding the study by Weaver et al[18] reduced heterogeneity to very low level (I2 = 23%) and the result was more stable. A potential explanation was that the study included more male patients, and sex-related efficacy differences might exist in the different DBS targets. Although some studies[20,21] have reported that DBS therapy results in equal degree of motor improvement for both sexes, these studies have relatively small sample size and only focus on STN-DBS. There were no relevant studies comparing sex-related efficacy differences between STN-DBS and GPi-DBS so far.
Our subanalysis examined the impacts on individual motor subtypes (tremor, rigidity, and gait) between STN-DBS and GPi-DBS and no differences were observed. Therefore, based on current data, responsiveness to both STN and GPi DBS was similar among different PD motor subtypes. Because our analysis only extracted data from 2 articles, the results should be interpreted cautiously. Besides, low heterogeneity for the tremor score (I2 = 41%) and moderate heterogeneity for the rigidity score (I2 = 63%) were observed.
We observed differences in the ADL and LED results between STN-DBS and GPi- DBS. Compared to the GPi-DBS, STN-DBS was more effective for reduction in medication. However, GPi-DBS was more effective for improving the PDQ-39 ADL scores than STN-DBS. Our results were consistent with the recent study by Xie et al.[12] Of note, a study reported by Xu et al[11] indicated that during the off-medication state, the STN-DBS might be superior to GPi-DBS in improving the motor function and activities of daily living for PD patients. But this study evaluated the short-term efficacy of STN-DBS and GPi-DBS.
In general, DBS for the treatment of PD is a relatively safe surgical procedure. Adverse events were observed less frequently and no statistically significant differences between the STN-DBS and GPi-DBS.[10,19] However, the study reported by Odekerken et al[19] showed that more reoperations after GPi-DBS, and the significant difference was observed. The electrode position of the initial surgery was considered optimal, and the need for reoperations from GPi-DBS to STN-DBS owing to waning effect. Volkmann et al[22] also described 4 patients with good initial response to GPi-DBS but waning effect that required conversion to STN-DBS. This potential adverse event warrants additional research.
There are 4 limitations of this meta-analysis. First,the small number of included studies and the relatively small sample size, which may have influenced the reliability of the results. Second, significant heterogeneity was observed among the UPDRSIII scores and motor subtype (Tremor, Rigidity) scores during the off-medication phase. Third, since primary outcomes of included studies are improvement in motor symptoms in off-drug phase. In addition, several studies[16,19] did not indicate UPDRS-III scores of patients with both stimulation and medication. Therefore, we chose to only look at UPDRS-III (off) scores. Fourth, the conversion of nonnormally distributed statistics (median and IQR) to normally distributed statistics (mean and SD) may be a source of bias in our meta-analysis. In addition, the method of merging the subgroup data may also contribute to the bias, too.
In conclusion, our results showed no significant differences between STN-DBS and GPi-DBS in the long-term efficacy of UPDRS III scores including motor subtypes. STN-DBS was more effective for reduction in medication than GPi-DBS. However, GPi-DBS was more effective for improving the PDQ-39 ADL score than STN-DBS. Owing to the small number of trials in our analysis, our results should be interpreted cautiously, and warrant additional studies to verify these findings.
Author contributions
Conceptualization: Lilei Peng, Jie Fu, Ligang Chen.
Data curation: Lilei Peng, Jie Fu, Shan Zeng, Haiping He.
Formal analysis: Lilei Peng, Jie Fu, Ligang Chen.
Methodology: Jie Fu.
Supervision: Yang Ming, Ligang Chen.
Writing – original draft: Lilei Peng.
Writing – review & editing: Lilei Peng, Jie Fu, Ligang Chen.
Footnotes
Abbreviations: BDI = Beck depression inventory, DBS = deep brain stimulation, GPi = globus pallidus interna, IQR = interquartile range, LED = levodopa-equivalent dosage, PD = Parkinson's disease, PDQ-39 ADL = Parkinson's disease questionnaire: 39 activities of daily living, STN = subthalamic nucleus, UPDRS = unified Parkinson's disease rating scale section, WMD = weighted mean difference.
Funding: This study was supported by the Science and Technology Department of Sichuan Province [grant numbers 2013SZZ002, LZ-LY-9]; the Health and Family Planning Commission of Sichuan Province [grant number 16PJ557]; and the Southwest Medical University [grant number 2013ZRQN068].
The authors report no conflicts of interest.
References
- [1].Yao SC, Hart AD, Terzella MJ. An evidence-based osteopathic approach to Parkinson disease. Osteopath Fam Physician 2013;5:96–101. [Google Scholar]
- [2].Bonuccelli U. Comparing dopamine agonists in Parkinson's disease. Curr Opin Neurol 2003;16(suppl 1):S13–9. [DOI] [PubMed] [Google Scholar]
- [3].Cieslak M, Komoszynski M, Wojtczak A. Adenosine A(2A) receptors in Parkinson's disease treatment. Purinergic Signal 2008;4:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pahwa R, Factor SA, Lyons KE, et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983–95. [DOI] [PubMed] [Google Scholar]
- [5].The Deep-Brain Stimulation for Parkinson's Disease Study Group. Deep-brain stimulation of the subthalamic nucleus or the pars interna of the globus pallidus in Parkinson's disease. N Engl J Med 2001;345:956–63. [DOI] [PubMed] [Google Scholar]
- [6].Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991;337:403–6. [DOI] [PubMed] [Google Scholar]
- [7].Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson's disease. N Engl J Med 2010;362:2077–91. [DOI] [PubMed] [Google Scholar]
- [8].St George RJ, Nutt JG, Burchiel KJ, et al. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology 2010;75:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zahodne LB, Okun MS, Foote KD, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidusas compared to the subthalamic nucleus. J Neurol 2009;256:1321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu Y, Li W, Tan C, et al. Meta-analysis comparing deep brain stimulation of the globus pallidus and subthalamic nucleus to treat advanced Parkinson disease. J Neurosurg 2014;121:709–18. [DOI] [PubMed] [Google Scholar]
- [11].Xu F, Ma W, Huang Y, et al. Deep brain stimulation of pallidal versus subthalamic for patients with Parkinson's disease: a meta-analysis of controlled clinical trials. Neuropsychiatr Dis Treat 2016;12:1435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Xie CL, Shao B, Chen J, et al. Effects of neurostimulation for advanced Parkinson's disease patients on motor symptoms: A multiple-treatments meta-analysis of randomized controlled trials. Sci Rep 2016;6:25285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 2003;18:738–50. [DOI] [PubMed] [Google Scholar]
- [14].Liu Ming. Systematic review, design and implementation of Meta- analysis[M]. 2011;Beijing: People's Medical Publishing House, 90. [Google Scholar]
- [15].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Katz M, Luciano MS, Carlson K, et al. Differential effects of deep brain stimulation target on motor subtypes in Parkinson's disease. Ann Neurol 2015;77:710–9. [DOI] [PubMed] [Google Scholar]
- [17].Rodriguez-Oroz MC, Obeso JA, Lang AE, et al. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain 2005;128(pt 10):2240–9. [DOI] [PubMed] [Google Scholar]
- [18].Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology 2012;79:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Odekerken VJ, Boel JA, Schmand BA, et al. GPi vs STN deep brain stimulation for Parkinson disease: three-year follow-up. Neurology 2016;86:755–61. [DOI] [PubMed] [Google Scholar]
- [20].Chiou SM. Sex-related prognostic predictors for Parkinson disease undergoing subthalamic stimulation. World Neurosurg 2015;84:906–12. [DOI] [PubMed] [Google Scholar]
- [21].Hariz GM, Limousin P, Zrinzo L, et al. Gender differences in quality of life following subthalamic stimulation for Parkinson's disease. Acta Neurol Scand 2013;128:281–5. [DOI] [PubMed] [Google Scholar]
- [22].Volkmann J, Allert N, Voges J, et al. Long-term results of bilateral pallidal stimulation in Parkinson's disease. Ann Neurol 2004;55:871–5. [DOI] [PubMed] [Google Scholar]


