Abstract
Cancer patients can be well-connected to resources during treatment but become lost to follow-up and subsequently may receive treatment in free clinics for chronic morbidities. Few studies have examined outcomes for uninsured patients with cancer histories in free clinics, but research examining socioeconomic determinants emphasizes poor cancer outcomes for patients with lower socioeconomic statuses (SES).
Demographic data and chronic disease measures were extracted from medical charts of patients treated in 8 free clinics in 2016 in Tampa Bay. Descriptive statistics and Pearson correlation coefficients were used to demonstrate relationships between socioeconomic factors, cancer diagnoses, and comorbidities. Charlson Comorbidity Index (CCI) was used to assess mortality risk and severity of disease burden.
The histories of 4804 uninsured patients were evaluated, identifying 86 (1.7%) as having had cancer. They were predominantly female (65.1%) and significantly older than those without cancer histories. Average duration from initial diagnosis was approximately 8.53 years (standard deviation [SD] 7.55). Overall, cancer patients had higher CCI scores (3.04 [1.928 SD] versus 0.90 [1.209 SD]; P <.001); thus reflecting more weighted comorbidities than patients without cancer (P <.001). Other factors of chronic disease including obesity and substance abuse correlated with cancer history.
Among uninsured patients, those with cancer histories had greater mortality risk by CCI than those without. Chronic conditions such as diabetes, cerebrovascular disease, and chronic pulmonary disease existed in patients with cancer histories, affecting their mortality risk. Uninsured patients with a history of cancer are in greater need for chronic disease management and prevention.
Keywords: Charlson comorbidity, chronic disease, oncology, socioeconomic factors, uninsured
1. Introduction
There is limited research on the health outcomes and comorbidities of uninsured patients who have had cancer. While many free clinics treat patients with concomitant chronic conditions, few studies have documented the prevalence of malignancies and their associations with other diseases in patients without health insurance. Prior US studies with Medicaid patients have shown that uninsured patients had higher likelihoods of being diagnosed with advanced-stage solid cancers.[1,2] Low-income populations tend to have higher incidences of distant disease and are less likely to receive cancer-directed surgery,[3] and as a result, they also have a greater risk of death.[4] Socioeconomic determinants of cancer outcomes such as gender and race have long been researched and especially emphasize poor outcomes for African American and low-income patients.[5,6] Patients from low socioeconomic status disproportionately experience tobacco-related disease, a risk factor for most cancers;[7] yet, tobacco cessation programs that can improve cancer outcomes are usually not available to patients outside the private health insurance network system in the US.
Cancer patients who are often well-connected to resources during the treatment phase of their disease can become lost to follow-up in the extended and permanent stages of cancer survivorship and end up in free clinics for further management of chronic comorbidities.[8] The Charlson Comorbidity Index (CCI) consists of 19 categories of comorbidity and can be used to calculate the 10-year mortality risk for patients having 1 or more of the conditions included, each of which is individually assigned a weighted score.[9] This score has been used to study the severity of diseases and risks of tolerance for future therapies, such that if a cancer patient has a high CCI, the risks of treatment may outweigh the benefits. We measured the CCI of uninsured cancer patients to better characterize their mortality risks and elucidate any associations with socioeconomic disparities.
By using a patient's CCI as a proxy for the burden of chronic disease, we can better characterize the risk factors associated with uninsured patients with cancer history. Uninsured patients who are lost to follow-up are at risk of cancer recurrence and metastasis resulting in further economic burden.[10] Thus, this study highlights the need to study this under-privileged and vulnerable population. An analysis of socioeconomic and disease risk factors demonstrates increased awareness of the interplay of disease and cancer in the disenfranchised.
2. Methods
This retrospective study involved the extraction of data including demographics, chronic disease measures, and Charlson comorbidities from medical charts of uninsured patients who were seen in 8 free clinics from January through December 2016 in the Tampa Bay Area. These 8 clinics treated only uninsured patients and relied in part on volunteer services from community and academic providers. Medical and undergraduate students volunteered to conduct a thorough extraction of data from paper and electronic records. This study included all patients seen at each clinic over a 1-year period, according to each clinic's appointment schedule.
The prevalence of cancer was defined as the proportion of patients who had any history of the condition, whether active or resolved, as noted in the patient chart. Frequencies, means, Pearson correlation coefficients, and 95% confidence intervals were used to assess associations between patient socioeconomic variables and cancer diagnoses. CCI-defined comorbidities were assessed and extracted from patient charts, and the CCI score was used to compare mortality risks for patients with or without histories of cancer. This study was approved by the University of South Florida's Institutional Review Board and each clinic provided consent to access their patient data. All analyses were performed in SAS 9.3 (SAS Institute, Cary NC).
3. Results
In 2016, 4804 uninsured patients were treated in 8 free clinics. According to our manual chart review, 86 patients (1.7%) were found to have histories of cancer and 3318 (69.1%) were identified as having no history of cancer. The remaining 1400 patients (29.1%) were not specifically asked about their cancer histories, so their charts did not include this information. The most common malignancies reported among the population of patients with cancer histories included breast (19, 22.09%), prostate (8, 9.30%), melanoma (6, 6.97%), cervical (5, 5.81%), colon (5, 5.81%), skin squamous cell carcinoma (5, 5.81%), ovarian (4, 4.65%), and lung (4, 4.65%) cancers. The average follow-up time from cancer diagnosis was approximately 8.53 years (7.55 standard deviations [SDs]).
Among patients who had ever been diagnosed with cancer, most were women (65.1%), and the average age was 54.37 years (13.42 SDs), which was significantly greater than for patients without cancer histories (P <.001). Only 5.8% of the population of patients with cancer histories was black (4 patients), which was a significantly smaller proportion than was found in the population of patients without any history of cancer (16.9% [393]; P = .014). A significantly higher proportion of the patients with cancer histories was white than was found in the population of patients without cancer histories (57.97% [40] vs 29.77% [649]; P = <.001). Patients with cancer histories were less likely to be employed than patients without cancer histories (29.7% [19] vs 51.0% [817]; P = .0001). Patients with cancer histories more commonly came from smaller household sizes than patients without cancer histories (1.65 [1.003 SDs] vs 2.38 [1.571 SDs]; P >.001). There was no significant difference in monthly salaries between uninsured patients with cancer histories and those without ($509.70 [722.470 SDs] vs $741.55 [1283.298 SDs]; Table 1). In terms of obesity, patients with cancer histories weigh more than patients without cancer histories (188.11 pounds [56.123 SDs] versus 170.34 pounds [49.501 SDs]; P = .003).
Table 1.
Socioeconomic characteristics of uninsured patients with and without a history of cancer.
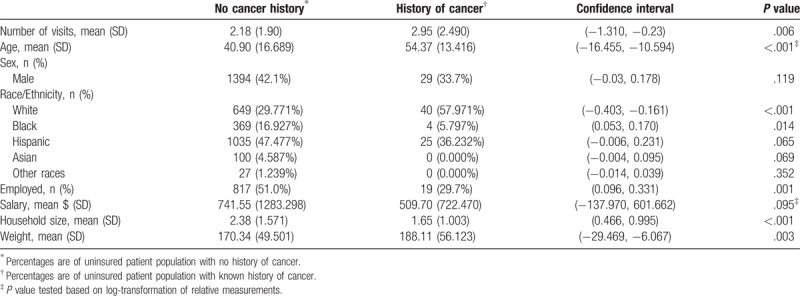
Table 2 shows the mean and standard deviation for continuous socioeconomic characteristics, frequencies, and percentages for categorical socioeconomic characteristics. It also shows both the unadjusted and multivariate odds ratio with 95% confidence interval for the odds ratio. P values are reported with statistical significance considered <.05. Due to the nature of free clinic chart reviews, missing values were noted for race, employment, salary, house size, and weight. Therefore, multiple imputation was applied to address the missing values among potential predictors.[11] We applied SAS proc MI (SAS Institute, 2007) to impute the missing data, using all the variables listed in Table 1. We imputed 5 data sets and then analyzed each imputed data set separately. SAS Proc MIANALYZE was used to summarize all the 5 findings, to reduce the uncertainty in the estimated parameters within and across the imputed data set (SAS Institute, 2007).
Table 2.
Socioeconomic characteristics of uninsured cancer patients.
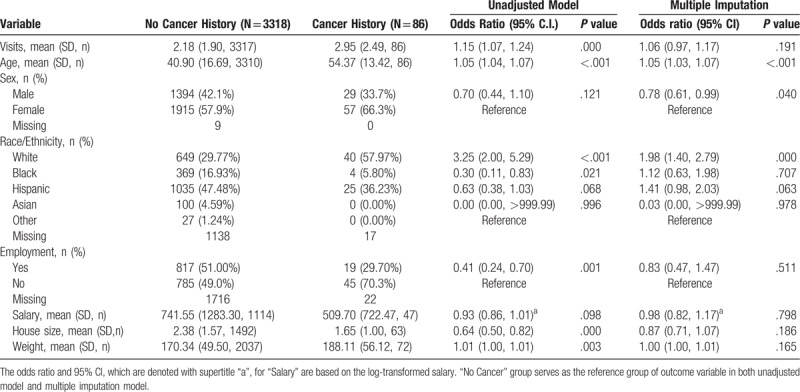
The multivariate analysis showed that age, sex, and white race were significant factors, after adjusting for other factors. According to this model, there is an about 5% increase in the odds of having cancer with every year in the age. Compared to women, men had a 22% less likelihood of having cancer in this uninsured population. Similarly, the odds of having cancer for white patients were about 98% more than other races.
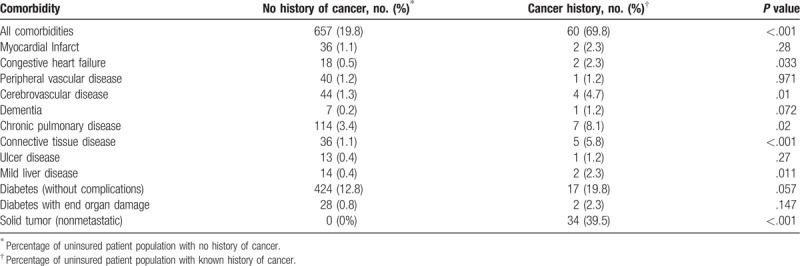
Several comorbidities per the CCI were recorded, and patients with cancer histories were associated with higher rates of comorbidities than patients without (69.8% [60] vs 19.8% [657]; P <.001; Table 3). In relative proportion, cancer patients had more cerebrovascular disease than patients without cancer (4.7% [4] vs 1.3% [44]); P = .01). They also had more chronic pulmonary disease (8.1% [7] vs 3.4% [114]; P = .02), more connective tissue disease (5.8% [5] vs 1.1% [36]; P = .001), and more uncomplicated diabetes (19.8% [17] vs 12.8% [424]; P = .057) than those without cancer histories. Overall, patients with cancer histories had a higher CCI score than patients without cancer histories (3.04 [1.928 SDs] vs 0.90 [1.209 SDs]; P <.001).
Table 3.
Frequency of comorbidities among uninsured patients with and without a history of cancer.
Tobacco and alcohol use among uninsured cancer patients is shown in Table 4. Smoking habits were categorized into 3 groups (never smokers, active smokers, and past smokers). Chi-square analysis of cancer histories revealed significant associations with smoking (P = .018). Furthermore, alcohol consumption history was categorized into 3 groups (never drinkers, active drinkers, and past drinkers). Chi-square analysis of cancer histories revealed significant associations with alcohol consumption, and alcohol consumption was significantly higher among patients with cancer histories than those without (P = .002). The same analyses were performed for drug use, with 90.48% of cancer patients denying any history of illicit drug use compared to 89.11% of patients without cancer histories. Overall, there was no significant association between cancer history and drug use (chi-square, 0.598; P = .74).
Table 4.
Incidence of substance abuse among uninsured patients with and without a history of cancer.

4. Discussion
4.1. Mortality risk for uninsured patients with cancer histories
The CCI is a standardized method of assessing health outcomes in patients with diseases such as cancer. Comorbidity indices have been used by cancer registries to demonstrate associations between different types of cancer and overall survival.[16] Although there are different standardized methods of assessing mortality risk, the CCI has proven to be more appropriate to prognostic analyses and has been used clinically in several datasets.[17] To illustrate how CCI is used to estimate mortality, a CCI of 3 points is associated with a 77% 10-year survival rate, whereas 2 points are 90%, 1 point is 96%, and zero points is 98%.[9] As evidenced by our CCI calculations, uninsured patients with cancer histories bear a greater risk of mortality than uninsured patients without cancer histories. Additionally, according to the National Cancer Institute, patients with higher burdens of disease and more comorbidities are less likely to be enrolled in clinical trials than other cancer patients, especially when they are black, thereby increasing their mortality risk.[18]
4.2. Race as a risk factor
Racial disparities in diagnosis and prognosis of cancer have been a multifactorial challenge. Although the majority of our patients were White, several historical concepts are important to consider. Cancer survival has historically been lower in black patients, specifically in breast,[12] ovarian,[13] and colon cancers.[14] According to a 2018 report by the American Cancer Society, non-Hispanic black women have a lower incidence of cancer than non-Hispanic white women, but their overall cancer mortality rate is 14% higher.[15] In our study, only 4 black patients had histories of cancer. This may have been a result of black patients not using free resources like charitable clinics when they were uninsured or those with active cancer diagnoses receiving treatment in other clinical settings in the Tampa Bay region. Another possibility is that such patients’ cancer histories were not captured in the demographics because they were burdened with many comorbid conditions, making it difficult for them to be clinically evaluated at any location. Lastly, the medical charts only reflected what was discussed or mentioned during consultations; thus, black patients either may not have disclosed their oncological histories in the free primary care setting or may utilize free and charitable clinics less than other races.
4.3. Chronic disease risk factors
The uninsured cancer population in this study may be at greater risk of secondary malignancies, especially with 19.8% (17) of patients having uncomplicated diabetes, obesity and increased at-risk behaviors such as tobacco and alcohol use. Several biological mechanisms have been proposed to explain the link between diabetes and cancer, including chronic inflammation and hyperglycemia.[19] Insulin has been highlighted as a possible oncogenic factor in malignancies,[20] and diabetes and it's higher insulin requirements are more associated with breast and colon cancers, which were among the most common cancer types identified in our study population, than elevated body-mass index (BMI) alone.[21,22] This evidence raises concern for the uninsured patients with histories of cancer in the present study, given the high proportion of them with diabetes and receiving short and/or long-acting insulin.
According to a study published in 2017 that used global cancer data, 5% to 6% of all new cancers in 2012 were attributable to the combined effects of diabetes and high BMI as independent risk factors,[23] although another study showed that obesity alone was the cause of 3% to 6% of all cancer cases globally in the same year.[24] The World Cancer Research Fund (WCRF) has concluded that there are causal associations between obesity and multiple solid and hematological malignancies, and it continues to study dietary and nutritional recommendations that can improve the outcomes of several malignancies.[23,25,26] Elevated BMI was responsible for twice as many cases of cancer than diabetes,[23] although some cancers such as breast cancer are more affected by poor metabolic health (diabetes) than by obesity alone.[27] Multiple meta-analyses have also confirmed the associations between obesity and cancer mortality.[28,29]
We were limited by a lack of height data with which to calculate BMI in our study population, but we can perhaps infer that our uninsured patients with histories of cancer were at greater risk of obesity than the patients without any history of cancer, and they were significantly heavier, on average. One study involving 18 years of follow-up of 93,000 women revealed a dose-response relationship between obesity and the medication received for several cancers.[30] In 2014, more than 55% of all cancers diagnosed in women and 24% of cancers diagnosed in men were associated with obesity, according to a report by the US Centers for Disease Control and Prevention.[31]
There is limited research about the uninsured patient population who frequent free clinics in the literature. Strengths of this study include a large sample size involving multiple free clinics in the locality, a thorough chart review of a subset population, and inclusion criteria consisting of 1 calendar year of data to account for seasonal variation. Although this study represents one of the few retrospective studies reported on patients with cancer history in free clinics, there are several limitations that need to be discussed in order to fully understand this study's results. For 1, estimated proportion of cancer patients are clinic-based and do not reflect population-based estimated proportions. Given the cross-sectional nature of this retrospective study, temporality of cancer history was difficult to determine. Missing and incomplete data was another major limitation and thus multivariate analysis with multiple imputations was used to handle this statistical drawback. Given that free clinics are not billing their patients, medical coding is not emphasized, and thus medical charts are often brief and to the point with no standardized terminology except that employed by the primary providers. Therefore, systematic and chart-by-chart review and extraction, albeit intensive, was necessary to fully capture as many clinical details about each patient.
The lack of health insurance perpetuates a cycle of reduced health-care accessibility and poor health outcomes. Data from the Third National Health and Nutrition Examination Survey demonstrated that patients with either public or no insurance had higher levels of smoking, reduced diet quality, higher BMI, and increased risks of chronic disease and cancer mortality than patients in the privately insured population.[32] Indeed, tobacco, alcohol, and drug use are often socially distributed, with the higher rates of abuse and addiction occurring among lower socioeconomic groups.[33] Although rates of tobacco use have declined in the general population, the prevalence of smoking has increased among those with substance-use disorders.[34] In addition, the patients with a history of cancer in our study were associated with greater levels of alcohol use, which is concerning given that alcohol has been established as a risk factor for several malignancies, as was recently emphasized by the American Society of Clinical Oncology.[35] Thus, if uninsured patients of lower socioeconomic statuses (SES) are at higher risk of substance abuse, their risk of cancer mortality is elevated and concerning.
5. Conclusion
There are abundant information and research already present regarding the pathogenesis of cancer and its high associations with tobacco abuse, obesity, and chronic disease, but evidence-based prevention programs are inconsistently implemented throughout the US.[36] This study confirms past research on the dynamics of socioeconomic disparities and cancer in a unique population of uninsured patients who frequent free and charitable clinics. There is limited literature on the demographics and comorbidities of uninsured patients with histories of cancer. Our findings show that patients with histories of cancer and without insurance may be in greater need than the general population for preventative and public health programs that focus on chronic disease prevention and management, weight control, and substance abuse. By describing this disparity, we hope to raise awareness about uninsured cancer patients in the free clinic setting. For current oncologists and other clinical providers, we emphasize the importance of ensuring follow-up for cancer patients due to their higher risk of mortality and disease burden, as evidenced by our study.
Acknowledgments
We thank Sonya J. Smyk of Moffitt Cancer Center for editorial support. She was not compensated beyond her regular salary.
This project was implemented by a consortium of volunteers composed of medical residents, public health, medical and pre-medical students. We thank the patients, clinic directors, and other staff for their support to this work. We also thank Shirley Smith and Kevin Casey from the Morsani College of Medicine Office of Student Diversity and Enrichment Program for incorporating our research into their pre-medical research curriculum.
Author contributions
Conceptualization: Abu-Sayeef Mirza, Aldenise Ewing.
Data curation: Noura Ayoubi.
Formal analysis: Yuanyuan Lu.
Funding acquisition: Abu-Sayeef Mirza, Michael Jaglal.
Investigation: Abu-Sayeef Mirza.
Methodology: Noura Ayoubi, Michael Jaglal.
Project administration: Abu-Sayeef Mirza, Aldenise Ewing.
Supervision: Smitha Pabbathi, Michael Jaglal, Richard Roetzheim.
Validation: Yuanyuan Lu.
Writing – original draft: Abu-Sayeef Mirza.
Writing – review & editing: Abu-Sayeef Mirza, Smitha Pabbathi, Richard Roetzheim.
Abu-Sayeef Mirza orcid: 0000-0002-1875-8966.
Footnotes
Abbreviations: BMI = body-mass index, CCI = Charlson Comorbidity Index.
This research was supported in part by the Office of Research, Innovation, and Scholarly Endeavors (RISE) at the University of South Florida, Morsani College of Medicine.
The authors declare no relevant conflicts of interest.
References
- [1].Han X, Zhu S, Tian Y, et al. Insurance status and cancer stage at diagnosis prior to the affordable care act in the United States. J Registry Manag 2016;41:143–51. [PubMed] [Google Scholar]
- [2].Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. JNCI J Nat Cancer Inst 1999;91:1409–15. [DOI] [PubMed] [Google Scholar]
- [3].Abdelsattar ZM, Hendren S, Wong SL. The impact of health insurance on cancer care in disadvantaged communities. Cancer 2017;123:1219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Roetzheim RG, Pal N, Gonzalez EC, et al. Effects of health insurance and race on colorectal cancer treatments and outcomes. Am J Public Health 2000;90:1746–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: progress and opportunities in reducing racial disparities. CA Cancer J Clin 2016;66:290–308. [DOI] [PubMed] [Google Scholar]
- [6].Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin 2004;54:78–93. [DOI] [PubMed] [Google Scholar]
- [7].Simmons VN, Pineiro B, Hooper MW, et al. Tobacco-related health disparities across the cancer care continuum. Cancer Control: J Moffitt Cancer Center 2016;23:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mullan F. Seasons of survival: reflections of a physician with cancer. New EnglJ Med 1985;313:270–3. [DOI] [PubMed] [Google Scholar]
- [9].Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- [10].Charlson M, Wells MT, Ullman R, et al. The Charlson comorbidity index can be used prospectively to identify patients who will incur high future costs. PloS One 2014;9:e112479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wiley, Little RJAR, Donald B. Statistical Analysis with Missing Data. Second Edition.2002. [Google Scholar]
- [12].Miller JW, Smith JL, Ryerson AB, et al. Disparities in breast cancer survival in the United States (2001-2009): findings from the CONCORD-2 study. Cancer 2017;123:5100–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Stewart SL, Harewood R, Matz M, et al. Disparities in ovarian cancer survival in the United States (2001-2009): findings from the CONCORD-2 study. Cancer 2017;123:5138–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].White A, Joseph D, Rim SH, et al. Colon cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer 2017;123:5014–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [16].Lichtensztajn DY, Giddings BM, Morris CR, et al. Comorbidity index in central cancer registries: the value of hospital discharge data. Clin Epidemiol 2017;9:601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang CC, Fong Y, Lin LC, et al. The age-adjusted Charlson comorbidity index is a better predictor of survival in operated lung cancer patients than the Charlson and Elixhauser comorbidity indices. Eur J Cardiothorac Surg Off J Eur Assoc Cardiothorac Surg 2018;53:235–40. [DOI] [PubMed] [Google Scholar]
- [18].Langford AT, Resnicow K, Dimond EP, et al. Racial/ethnic differences in clinical trial enrollment, refusal rates, ineligibility, and reasons for decline among patients at sites in the National Cancer Institute's Community Cancer Centers Program. Cancer 2014;120:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care 2010;33:1674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nead KT, Sharp SJ, Thompson DJ, et al. Evidence of a causal association between insulinemia and endometrial cancer: a mendelian randomization analysis. J Nat Cancer Inst 2015;107: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gunter MJ, Xie X, Xue X, et al. Breast cancer risk in metabolically healthy but overweight postmenopausal women. Cancer Res 2015;75:270–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Murphy N, Cross AJ, Abubakar M, et al. A nested case-control study of metabolically defined body size phenotypes and risk of colorectal cancer in the european prospective investigation into cancer and nutrition (EPIC). PLoS Med 2016;13:e1001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pearson-Stuttard J, Zhou B, Kontis V, et al. Worldwide burden of cancer attributable to diabetes and high body-mass index: a comparative risk assessment. Lancet Diabetes Endocrinol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol 2015;16:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Turati F, Bravi F, Di Maso M, et al. Adherence to the world cancer research fund/American institute for cancer research recommendations and colorectal cancer risk. Eur J Cancer (Oxf Engl: 1990) 2017;85:86–94. [DOI] [PubMed] [Google Scholar]
- [26].Vingeliene S, Chan DSM, Vieira AR, et al. An update of the WCRF/AICR systematic literature review and meta-analysis on dietary and anthropometric factors and esophageal cancer risk. Ann Oncol Off J Eur Soc Med Oncol 2017;28:2409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palmer JR, Castro-Webb N, Bertrand K, et al. Type II diabetes and incidence of estrogen receptor negative breast cancer in African American women. Cancer Res 2017;77:6462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev 2015;95:727–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Massetti GM, Dietz WH, Richardson LC. Excessive weight gain, obesity, and cancer: opportunities for clinical intervention. JAMA 2017;318:1975–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer—viewpoint of the IARC working group. New Engl J Med 2016;375:794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Steele CB, Thomas CC, Henley SJ, et al. Vital Signs: trends in incidence of cancers associated with overweight and obesity—United States, 2005–2014. MMWR Morb Mortal Wkly Rep 2017;66:1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bittoni MA, Wexler R, Spees CK, et al. Lack of private health insurance is associated with higher mortality from cancer and other chronic diseases, poor diet quality, and inflammatory biomarkers in the United States. Prev Med 2015;81:420–6. [DOI] [PubMed] [Google Scholar]
- [33].Menvielle G, Kulhanova I, Bryere J, et al. Tobacco-attributable burden of cancer according to socioeconomic position in France. Int J Cancer 2018;478–85. [DOI] [PubMed] [Google Scholar]
- [34].Weinberger AH, Gbedemah M, Wall MM, et al. Cigarette use is increasing among people with illicit substance use disorders in the United States, 2002–14: emerging disparities in vulnerable populations. Addiction (Abingdon, Engl) 2017;719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].LoConte NK, Brewster AM, Kaur JS, et al. Alcohol and cancer: a statement of the American society of clinical oncology. J Clin Oncol Off J Am Soc Clin Oncol 2018;36:83–93. [DOI] [PubMed] [Google Scholar]
- [36].Colditz GA, Emmons KM. Accelerating the pace of cancer prevention- right now. Cancer Prev Res (Philadelphia, Pa) 2018. [DOI] [PubMed] [Google Scholar]


