Abstract
This study investigated the effect of boiling on the inhibitory action of spices on digestive enzymes. Unboiled extracts of Trigonella foenum‐graecum (seed) (25.42%), Myristica fragrans (seed) (22.70%), and Cuminum cyminum (seed) (19.17%) showed significantly (p < 0.05) a higher lipase inhibitory activity than their respective boiled extracts (20.23%, 15.74%, and 12.57%). Unboiled extracts of Cinnamomum zeylanicum (stem bark) (−16.98%) and Foeniculum officinale (seed) (−16.05%) showed an activation of lipase enzyme, and boiling significantly (p < 0.05) changed the activity into lipase inhibition as 8.47% and 9.54%, respectively. Unboiled extracts of Coriandrm sativum (seed), C. cyminum, and Elettaria cardamomum (seed) showed an activation of amylase enzyme, and boiling these extracts significantly reduced the enzyme activation. The other unboiled extracts showed a higher amylase inhibition than the boiled extracts, whereas the boiled extracts of C.longa (rhizome) and Syzygium aromaticum (flower) exhibited significantly (p < 0.05) lower values. Unboiled extracts of C. zeylanicum, M. fragrans, and S. aromaticum showed an insignificantly higher glucosidase inhibitory activity than the boiled extracts. Inhibition of digestive enzymes by nutritional intervention is one avenue to be considered in treating diet‐induced obesity and in the management of postprandial hyperglycemia. Spices, used as food additives, could be a potential source of digestive enzyme inhibitors. The current study revealed that unboiled extracts of T. foenum‐graecum (seed), C. cyminum (seed), and M. fragrans (seed) are more effective than the boiled extracts as an antiobesity therapy. Moreover, it endorses the use of infusion of T. foenum‐graecum seeds as an antiobesity therapy.
Keywords: α‐amylase inhibition, boiling, glucosidase inhibition, lipase inhibition, Spices
1. INTRODUCTION
Herbs and spices have been used for thousands of years by many ancient cultures for curing ailments and for the promotion of good health (Kochhar, 2008). Dietary spices, used as flavoring and coloring agents and as preservatives, are obtained from the dried aroma parts of plants—generally seeds, berries, roots, pods, and leaves. Spices such as cinnamon, pepper, cloves, nutmeg, cumin, saffron, garlic, and ginger have been used in Asia and the Middle East for many centuries. Sri Lanka is well known for a variety of spices such as cinnamon, pepper, cloves, cardamoms, nutmeg, mace, and vanilla that grow in abundance all over the island in different types of soil and climatic conditions. These spices are an important part of the agricultural exports of Sri Lanka.
Obesity has become an epidemic increasing at an alarming rate (Katulanda, Jayawardena, Sheriff, Constantine, & Matthews, 2010; WHO; 2014) and is a major risk factor contributing to chronic diseases such as type 2 diabetes, cardiovascular diseases, and certain cancers (Jacobson, Miller, & Schaefer, 2007; Kopelman, 2000). Pancreatic lipase, which hydrolyzes triglycerides into glycerol and fatty acids, is the key enzyme for dietary fat digestion in the small intestine (Mukherjee & Sengupta, 2013). An inhibitor of pancreatic lipase can reduce fat absorption and can be used as a therapeutic agent for treating diet‐induced obesity in humans. Orlistat is the only clinically approved pharmacological agent used as a pancreatic lipase inhibitor (Weibel, Hadvary, Houchuli, Kupfer, & Lengsfeld, 1987).
Inhibition of carbohydrate hydrolyzing enzymes such as α‐glucosidase and pancreatic α‐amylase is one of the therapeutic approaches for delaying carbohydrate digestion, resulting in reduced postprandial hyperglycemia which is critical in the management of diabetes mellitus (Sudhir & Mohan, 2002). α‐Amylase catalyzes the hydrolysis of α‐1,4‐glucosidic linkages in starch and related polysaccharides. α‐Glucosidase secreted from intestinal epithelium is responsible for the degradation of oligosaccharides, trisaccharides, and disaccharides into monosaccharides. Inhibition of amylase and glucosidase enzymes would delay carbohydrate digestion and glucose absorption and thereby reduce postprandial hyperglycemia. Drugs that target carbohydrate hydrolyzing enzymes include acarbose, miglitol, voglibose, nojirimycin, and 1‐deoxynojirimycin, which reduce postprandial hyperglycemia by delaying glucose absorption (Bischoff, 1994).
The synthetic inhibitors of digestive enzymes lead to several adverse events in the gastrointestinal tract (Chiasson et al., 2002; Kaila & Raman, 2008). In this context, spices are an attractive source for the identification of newer digestive enzyme inhibitors that lack some of the adverse reactions of these synthetic enzyme inhibitors. Spices are usually used as food additives and in many recipes worldwide “cooking, baking, and roasting were applied to the spices.” Digestive enzyme inhibitors belonging to a variety of secondary metabolite groups such as polyphenols (flavonoids, phenolic acids, and proanthocyanidins) (Karamać & Amarowicz, 1996; Kim, Kwon, & Son, 2000; Shimura et al., 1994; You, Chen, Wang, Luo, & Jiang, 2011) and saponins (Zhao & Kim, 2004) have been identified, and these metabolites have been isolated in spices as well (Villupanoor, Chempakam, & Zachariah, 2008).
Therefore, the heat stability of inhibitors is vital for the inhibitory action to persist after cooking (Adefegha, Oboh, Oyeleye, & Osumo, 2016). The heat stability of the lipase, amylase, and glucosidase inhibitors of spices after processing (cooking at 100°C) has not been clearly investigated. Therefore, the investigation of the effects of boiling of spices on the lipase, amylase, and glucosidase inhibitory activities is important.
Hence, this study was designed to determine the lipase inhibitory activity, amylase inhibitory activity, and glucosidase inhibitory activity of unboiled and boiled crude methanol extracts of ten popular spices in Sri Lanka. Moreover, the heat stability of antioxidants of these spices was determined. The real‐time lipase inhibition assay used in this study was also optimized using the lipase assay developed by Choi et al. in 2003.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
Porcine pancreatic lipase (E.C.3.1.1.3, Type II) (L‐0382), porcine pancreatic α‐amylase (EC 3.2.1.1, Type VI) (A3176), α‐glucosidase from Saccharomyces cerevisiae (EC 3.2.1.20, Type I) (9001‐41‐7), 2,3‐dimercapto‐1‐propanol tributyrate substrate (DMPTB, 97%) (282413), 5,5‐dithiobis (2‐nitrobenzoic acid) (DTNB, Ellman's reagent) (D218200), 3,5‐dinitrosalicylic acid (128848), p‐nitrophenyl α‐D‐glucopyranoside (pNPG) (N‐1377), orlistat (O4139), acarbose (A8980), and 2,2‐diphenyl‐l‐picrylhydrazyl (DPPH) (D9132) were purchased from Sigma‐Aldrich, USA. Phosphate‐buffered saline (PBS) (003002), Tris‐HCl buffer (15504020), butylated hydroxyl anisole (BHA) (817023), and all the other chemicals used in this study were of analytical grade.
2.2. Plant collection and extraction
Brassica juncea, Cinnamomum zeylanicum, Coriandrm sativum, Cuminum cyminum, Curcuma longa, Elettaria cardamomum, Foeniculum officinale, Myristica fragrans, Syzygium aromaticum, and Trigonella foenum‐graecum, were purchased from a traditional drug store in Kandy, Sri Lanka. Voucher specimens were deposited at the Department of Biochemistry, Faculty of Medicine, University of Peradeniya, Sri Lanka. All the plant materials (Table 1) were washed with water and air‐dried in the shade. Dried and powdered plant material (100 g) was successively extracted using an ultrasonicator (VWR ultrasound cleaner, model‐USC 1700D—USA) with methanol (MeOH, 100 ml × 3). The extracts were evaporated to dryness using a rotary evaporator (Büchi Rotvapor® R II—Switzerland) under reduced pressure at 40°C and kept in a vacuum oven at room temperature.
Table 1.
Percentage yield (%) of crude methanol extracts of spices
| Scientific name | Local name | Plant part used | Yield (%W/W)a |
|---|---|---|---|
| Brassica juncea | Aba | Seed | 3.5 |
| Cinnamomum zeylanicum | Kurundu | Stem bark | 3.0 |
| Coriandrum sativum | Kottamalli | Seed | 2.7 |
| Cuminum cyminum | Suduru | Seed | 5.7 |
| Curcuma longa | Kaha | Rhizome | 8.0 |
| Elettaria cardamomum | Ensal | Seed | 2.1 |
| Foeniculum officinalis | Ma'duru | Seed | 4.8 |
| Myristica fragrans | Sadikka | Seed | 6.8 |
| Syzygium Aromaticum | Karabu | Flower | 4.7 |
| Trigonella foenum‐graecum | Ulu‐hal | Seed | 8.4 |
Yield (%) of MeOH extracts of dry weight of plant material. Percentage extract yield (w/w) was calculated as (dry extract weight/dry starting material weight × 100).
2.3. Lipase inhibition assay
Lipase inhibition assay used during the current study was adapted from the lipase assay reported by Choi, Hwang, and Kim (2003). In this assay, free thiol groups that are generated by the lipase hydrolysis of DMPTB reduce DTNB to create a yellow color, which is spectrophotometrically quantified. The substrate mixture contained 0.2 mM DMPTB in 50 mM Tris‐HCl, pH 7.2, 0.001% EDTA, 0.06% Triton X‐100, and 0.8 mM DTNB. The porcine pancreatic lipase was prepared as a stock solution at 50 mg/ml in 50 mM Tris‐HCl, pH 7.2, and bovine serum albumin (BSA; 1 mg/ml), and stored at −40°C.
Optimization of lipase inhibition assay was conducted by evaluating the linearity of the method. Different reaction mixtures were prepared with different substrate concentrations (0.004 mM, 0.008 mM, and 0.016 mM), incubated with lipase (8 U) at 37°C, and the absorbances were recorded for 30 min at 412 nm.
For the screening of the extracts, porcine pancreatic lipase (8 U/ml) and plant extract (1 mg/ml; Tris‐HCl buffer or 4% v/v DMSO) were preincubated at 37°C. After preincubation, DMPTB (0.008 mM) standard substrate mixture was added to all tubes and incubated for 15 min. The absorbances were recorded after 15 min at 412 nm. Orlistat was used as the control/standard inhibitor.
The percentage lipase inhibition was calculated by the following formula;
2.4. Amylase inhibition assay
The amylase inhibition assay was performed using the preincubation chromogenic method adapted from Geethalakshmi, Sarada, Marimuthu, and Ramasamy (2010). The plant extract (40 μl, 20 mg/ml in dimethylsulfoxide, DMSO), 160 μl of distilled water, was preincubated with the addition of 200 μl of the enzyme solution for 5 min at 37°C before reacting with the starch solution (400 μl) for 15 min. The mixture (200 μL) was removed and added into a separate tube containing 100 μL 3,5‐dinitrosalicylic acid (DNS) color reagent solution and placed in a 85°C water bath. After 15 min, this mixture was diluted with 900 μl distilled water and removed from the water bath. α‐Amylase activity was determined by measuring the absorbance of the mixture at 540 nm. Acarbose was used as the control/standard inhibitor.
Percentage maltose generated was calculated from the equation obtained from the maltose standard calibration curve (0%–0.2%, w/v maltose)
% reaction = (mean maltose in test/mean maltose in control) × 100.
% Amylase inhibition activity = (100−% reaction)
2.5. Glucosidase inhibition assay
The inhibition of α‐glucosidase activity was determined using the modified published method (Elya et al., 2012). 10 mM pNPG solution (400 μl), 400 μl PBS buffer, and 100 μl plant extract (10 mg/ml in dimethylsulfoxide, DMSO) were preincubated for 3 min at 37°C. After preincubation, 100 μl glucosidase enzyme (0.15 U/ml) was added to all and incubated for 15 min. The reaction was terminated by the addition of 2000 μl Na2CO3 (200 mM). The absorbance of the mixture at 405 nm was measured. Acarbose was used as the control/standard inhibitor
The percentage lipase inhibition was calculated by the following formula;
2.6. Determination of the heat stability of the inhibitory effect
The extracts were dissolved in the corresponding buffer, and the solutions were boiled for 20 min in a boiling water bath at 100°C. Then, the extract solutions were cooled, and volumes were adjusted to original volumes with buffer and assayed for lipase or amylase or glucosidase inhibitory activities.
2.7. 2,2‐Diphenyl‐1‐picrylhydrazyl (DPPH) radical scavenging assay
DPPH solution (1.8 ml) of 0.004% was mixed with 200 μl extract solution (10 mg/ml in methanol) to get a final concentration of 1 mg/ml. These solution mixtures were kept in the dark for 30 min, and absorbance was measured at 517 nm. The absorbance was recorded and % scavenging activity was calculated (Tepe, Eminagaoglu, Akpulat, & Aydin, 2005). BHA (1 mg/ml) was used as the control/standard for the assay.
The extracts were boiled in a water bath at 100°C for 20 min. Then, the extract solutions were cooled, and volumes were adjusted to original volumes with buffer and assayed for the antioxidant activity by conducting the DPPH radical scavenging assay.
2.8. Statistical analysis
All experiments were performed in three different sets, with each set in triplicates. Results were expressed as the mean ± SD of n experiments. The data were analyzed by repeated measurements of one‐way analysis of variance (ANOVA) using Minitab version 17. Differences between means were considered significant if p < 0.05 as denoted in applicable tables, figures, and the text.
3. RESULTS
3.1. Yield of plant extracts
The yields of crude methanol extracts obtained from 100 g of each plant material from the spices studied are tabulated in Table 1. The highest yield (8.4%) was obtained from the crude methanol extract of T. foenum‐graecum.
3.2. Optimization of lipase inhibition assay
The lipase concentration employed in the assay was restricted to a maximum of 8U/ml due to the lack of solubility of lyophilized powder of lipase at higher concentrations. As shown in Figure 1, the initial reaction rate increased with the increasing concentration of the substrate. The reaction mixture that contained 0.008 mM DMPTB showed a linear absorbance change up to 22 min, with absorbance reaching a plateau above 1.326 absorbance unit after further incubation (Figure 1), whereas the reaction mixture that contained 0.004 mM DMPTB showed a linear absorbance change only up to 10 min, reaching a plateau above 0.793 absorbance unit. But the reaction mixture that contained 0.016 mM DMPTB showed no linear absorbance change (Figure 1).
Figure 1.
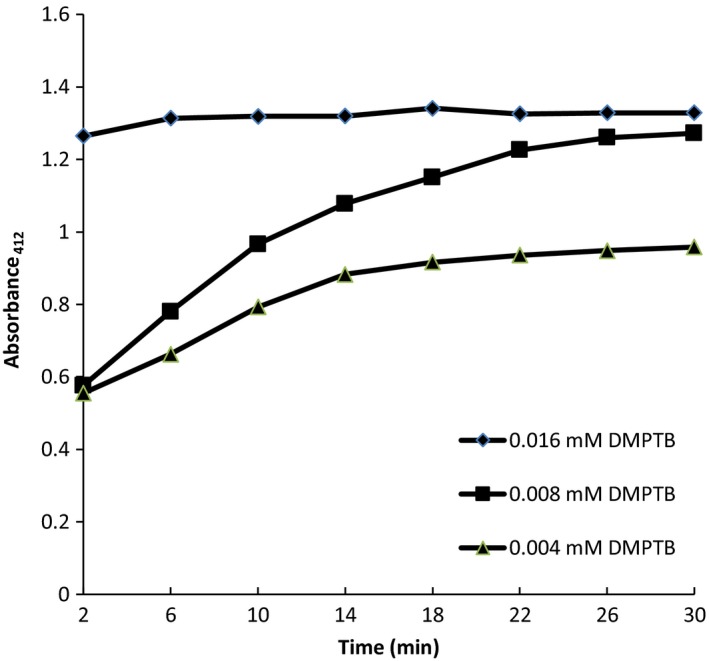
Linearity evaluation. Reaction mixtures were prepared with different amounts (0.004, 0.008, and 0.016 mM) of 2,3‐dimercapto‐1‐propanol tributyrate (DMPTB) and incubated with porcine pancreatic lipase at 37°C. The absorbances were recorded for 30 min at 412 nm
3.3. Inhibition of lipase activity
Among the unboiled extracts tested, the crude methanol extract of Trigonella foenum‐graecum was the most effective inhibitor of pancreatic lipase activity, while M. fragrans, C. cyminum, E. cardamomum, and C. sativum also showed an inhibitory activity (Table 2). The extract from S. aromaticum did not inhibit pancreatic lipase, whereas C. longa, F. officinalis, and C. zeylanicum crude methanol extracts showed an activation of the lipase enzyme (Table 2).
Table 2.
Percentage lipase inhibitory activity of unboiled and boiled crude methanol extracts of spices
| Plant species | % Inhibition activity unboiled | % Inhibition activity boiled |
|---|---|---|
| Brassica juncea | 8.80 ± 1.56 | 6.96 ± 1.76 |
| Cinnamomum zeylanicum | −16.98 ± 1.34 | 8.47 ± 1.43a |
| Coriandrum sativum | 6.19 ± 1.15 | 4.03 ± 1.21 |
| Cuminum cyminum | 19.17 ± 1.54 | 12.57 ± 1.87a |
| Curcuma longa | −8.4 ± 1.54 | −6.79 ± 1.34 |
| Elettaria cardamomum | 11.76 ± 1.55 | 8.74 ± 1.54 |
| Foeniculum officinale | −16.05 ± 1.63 | 9.54 ± 1.31a |
| Myristica fragrans | 22.7 ± 1.45 | 15.74 ± 1.44a |
| Syzygium aromaticum | 0.12 ± 1.13 | 0.15 ± 1.12 |
| Trigonella foenum‐graecum | 25.42 ± 1.32 | 20.23 ± 1.78a |
| Orlistat | 98.8 ± 0.91 | NA |
2,3‐Dimercapto‐1‐propanol tributyrate (DMPTB) was used as the substrate, and the final concentration of the crude extracts was at 1 mg/ml. The amount of thiol released was measured after the incubation for 6 mins at 37°C with 412 nm. Orlistat is taken as standard inhibitor. Results were presented as mean ± standard deviation, and mean was taken as the average of three readings of three different experiments. “‐” indicates a promotion of pancreatic lipase activity.
NA, not applicable.
The inhibitory activity in boiled extract is significantly (p < 0.05) different to the corresponding unboiled extract.
All the boiled extracts exhibited a pancreatic lipase inhibitory activity (Table 2) except crude methanol extract of C. longa. The pancreatic lipase inhibitory activities of the boiled extracts of C. cyminum, M. fragans, and T. foenum‐graecum were significantly (p < 0.05) lower than their unboiled extracts. The unboiled methanol extracts of C. zeylanicum and F. officinale showed an activation of pancreatic lipase enzyme but boiling these extracts significantly (p < 0.05) changed the activity into an inhibition of the lipase enzyme (Table 2).
3.4. Inhibition of amylase activity
All ten unboiled crude methanol extracts were subjected to the amylase inhibition assay, using the preincubation method and only six crude extracts exhibited inhibition activity (Table 3). In those extracts, the pancreatic amylase inhibitory activity was in the following order, from the highest to the lowest: S. aromaticum > C. longa > C. zeylanicum > F. officinale > B. juncea > T. foenum‐graecum (Table 3). Other four unboiled extracts showed an activation of the pancreatic amylase enzyme (Table 3).
Table 3.
Percentage amylase inhibitory activity of unboiled and boiled crude methanol extracts of spices
| Plant species | % Inhibition activity unboiled | % Inhibition activity boiled |
|---|---|---|
| Brassica juncea | 20.1 ± 1.50 | 16.6 ± 1.67 |
| Cinnamomum zeylanicum | 32.39 ± 1.91 | 28.59 ± 1.78 |
| Coriandrum sativum | −33.33 ± 1.43 | −23.98 ± 1.27a |
| Cuminum cyminum | −7.14 ± 1.12 | 1.16 ± 1.14a |
| Curcuma longa | 52.2 ± 1.65 | 47.78 ± 1.98a |
| Elettaria cardamomum | −33.33 ± 1.67 | −26.89 ± 1.67a |
| Foeniculum officinale | 28.79 ± 1.22 | 26.73 ± 1.75 |
| Myristica fragrans | −5.81 ± 1.45 | −5.74 ± 1.44 |
| Syzygium aromaticum | 58.10 ± 1.24 | 52.82 ± 1.45a |
| Trigonella foenum‐graecum | 8.69 ± 1.35 | 7.78 ± 1.19 |
| Acarbose | 87.67 ± 1.76 | NA |
Preincubation chromogenic method from Geethalakshmi et al. (2010) was adapted, and the final concentrations of the crude extracts were 1 mg/ml. The amylase inhibition was analyzed by amount of maltose production from starch at 517 nm after incubation at 37°C. Acarbose was used as the standard inhibitor. Results were presented as mean ± standard deviation, and mean was taken as the average of three readings of three different experiments. “‐” indicates a promotion of pancreatic amylase activity.
NA, not applicable.
The inhibitory activity in boiled extract is significantly (p < 0.05) different to the corresponding unboiled extract.
All the unboiled crude extracts which showed an amylase inhibitory activity exhibited a lower inhibitory activity in the boiled form (Table 3). Among them, the boiled extracts of S. aromaticum and C. longa showed a significantly (p < 0.05) lower inhibitory activity (Table 3). Further, the unboiled extract of C.cyminum, which exhibited an activation of amylase enzyme, inhibited the enzyme in the boiled form (Table 3). The other three boiled extracts were showing activation of the pancreatic amylase enzyme (Table 3), whereas C. sativum and E. cardamomum extracts showed a significant (p < 0.05) reduction in the enzyme activation in the boiled form.
3.5. Inhibition of glucosidase activity
Among the seven unboiled crude methanol extracts with glucosidase inhibitory activity, C. zeylanicum, M. fragans, and S. aromaticum had the strongest effect on inhibiting α‐glucosidase enzyme from Saccharomyces cerevisiae (Table 4). Unboiled crude extract of C. zeylanicum showed the highest α‐glucosidase inhibition of 96.78 ± 0.45% at 1 mg/ml. Unboiled extracts of C. sativum, C. longa, and T. foenum‐graecum showed a mild inhibition of the glucosidase enzyme (Table 4). Unboiled extracts of B. juncea, C.cyminum, and F.officinale showed neither activation nor inhibition of the α‐glucosidase enzyme (Table 4).
Table 4.
Percentage glucosidase inhibitory activity of unboiled and boiled crude methanol extracts of spices
| Plant species | % Inhibition activity unboiled | % Inhibition activity boiled |
|---|---|---|
| Brassica juncea | 0.0 | 0.0 |
| Cinnamomum zeylanicum | 96.78 ± 12.45 | 95.64 ± 1.47 |
| Coriandrum sativum | 2.58 ± 1.76 | 0.0 |
| Cuminum cyminum | 0.0 | 0.0 |
| Curcuma longa | 3.72 ± 1.62 | 1.78 ± 0.98 |
| Elettaria cardamomum | 22.87 ± 1.98 | 21.90 ± 1.01 |
| Foeniculum officinale | 0.0 | 0.0 |
| Myristica fragrans | 90.64 ± 1.23 | 89.65 ± 1.31 |
| Syzygium aromaticum | 88.91 ± 1.12 | 86.89 ± 1.98 |
| Trigonella foenum‐graecum | 3.2 ± 1.76 | 2.54 ± 1.21 |
| Acarbose | 99.52 ± 0.54 | NA |
p‐nitrophenyl‐α‐D‐glucopyranoside was used as the substrate, and the final concentrations of the crude extracts were at 1 mg/ml. The amount of p‐nitro phenol released was measured after the incubation for 15 mins at 37°C with 405 nm. Acarbose was used as the standard inhibitor. Results were presented as mean ± standard deviation, and mean was taken as the average of three readings of three different experiments.
NA, not applicable.
Boiled extracts of six spices exhibited a glucosidase inhibitory activity, and those activities were not significantly (p < 0.05) lower than the unboiled extracts (Table 4). Meanwhile, the boiled extracts of B. juncea, C. sativum, C. cyminum, and F. officinale showed neither activation nor inhibition of the α‐glucosidase enzyme.
3.6. Antioxidant activity
All the ten unboiled and boiled extracts of spices had pronounced radical scavenging antioxidant activity (Table 5). The antioxidant activity of the unboiled extracts of C sativum, C. cyminum, and E. cardamomum was 94.80%, 92.92%, and 91.61%, respectively, at 1 mg/ml and were statistically similar (p < 0.05) to that of BHA (94.97%).
Table 5.
Percentage antioxidant activity of unboiled and boiled crude methanol extracts of spices
| Plant species | % Scavenging activity unboiled | % Scavenging activity boiled |
|---|---|---|
| Brassica juncea | 59.01 ± 1.10 | 61.23 ± 1.54 |
| Cinnamomum zeylanicum | 83.41 ± 1.30 | 76.97 ± 1.89a |
| Coriandrum sativum | 94.80 ± 1.15 | 96.56 ± 1.12 |
| Cuminum cyminum | 92.92 ± 1.13 | 93.43 ± 1.34 |
| Curcuma longa | 76.55 ± 1.13 | 71.45 ± 1.23a |
| Elettaria cardamomum | 91.61 ± 1.32 | 93.87 ± 1.15 |
| Foeniculum officinale | 90.11 ± 1.22 | 91.23 ± 1.51 |
| Myristica fragrans | 83.41 ± 1.21 | 89.76 ± 1.56a |
| Syzygium aromaticum | 89.25 ± 1.24 | 90.53 ± 1.23 |
| Trigonella foenum‐graecum | 90.76 ± 1.22 | 84.67 ± 1.13a |
| BHA | 94.97 ± 1.01 | NA |
2,2‐Diphenyl‐1‐picrylhydrazyl (DPPH) radical scavenging assay, by measuring the absorbance at 517 nm after incubation for 30 min in the dark. The final concentrations of the crude extracts were at 1 mg/ml. BHA was used as the standard. Results were presented as mean ± standard deviation, and mean was taken as the average of three readings of three different experiments.
NA, not applicable.
The inhibitory activity in boiled extract is significantly (p < 0.05) different to the corresponding unboiled extract.
The boiled extracts of B. juncea, C. sativum, C. cyminum, E.cardamomum, F. officinale, M. fragans, and S. aromaticum had a higher antioxidant activity than the unboiled extracts. However, only the M. fragans showed a significantly (p < 0.05) higher antioxidant activity. Alternatively, the boiled extracts of C. zeylanicum, C. longa, and T. foenum‐graecum had significantly (p < 0.05) lower antioxidant activity than their unboiled extracts.
4. Discussion
Cooking represents an indispensable prerequisite in obtaining quality, palatability, and digestibility of food (Adefegha & Oboh, 2011). It is well known that thermal processing such as boiling, frying, and microwave cooking might be able to modulate the secondary metabolites present in plant materials (Kaur & Kapoor, 2001; Rohn, Buchner, Driemel, Rauser, & Kroh, 2007) and also may modulate the biological activity of plant secondary metabolites (Estbeyoglu, Ulbrich, Rehberg, Rogn, & Rambach, 2015). Studies conducted to investigate the thermal stability of secondary metabolites of spices are very scarce. In such studies, thermal processing was reported to cause reduction in flavonoid and polyphenol contents of spices based on the magnitude and extent of heat and duration of heating (Adefegha et al., 2016; Settharaksa, Jongjareonrak, Hmadhlu, Chansuwan, & Siripongvutikorn, 2012).
Despite the large number of research studies conducted to investigate inhibitory potential of spice crude extracts on lipase, amylase, and glucosidase enzymes, the current study is the first reported study revealing the heat stability of the lipase, amylase, and glucosidase inhibitors in crude spice extracts. The results of the current study revealed that unboiled spice extracts had a higher lipase and amylase inhibitory activities than boiled extracts. Major compounds present in spices such as apegenins in T. foenum‐graecum (seed) (Fernando, 2016), eugenol in M. fragrans and S. aromaticum (Mnafgui et al., 2013), and cucuminoids in C. longa (Lekshmi, Arimboor, Raghu, & Menon, 2012) have inhibited the digestive enzymes. Several researchers have identified changes in the concentration of these secondary metabolites due to thermal processing such as boiling (Baker, Chogan, & Opara, 2013; Tomaino et al., 2005), and this might be the reason for the change in the inhibitory activities between the boiled and the unboiled extracts observed in this study. Further, flavonoids in spices exist in free and conjugated forms and may breakdown by enzyme, acid, or heat treatment (Cartea, Francisco, Soengas, & Velasco, 2011), and change in the inhibitory activities of boiled and unboiled extracts may be due to the variation of the inhibitory activities in these two forms. However, recent literature data did not show a consistent trend for the effects of thermal processing on secondary metabolite contents in spices (Baker et al., 2013; Khatun, Eguchi, Yamaguchi, Takamura, & Matoba, 2006). This suggests that the effect of thermal processing on secondary metabolites varies in different spices and deserves further research. Therefore, the reason for the modulation in the inhibition of lipase, amylase, and glucosidase activities as a result of boiling of the spices could not be categorically stated.
Among crude extracts, T. foenum‐graecum (seed), C. cyminum (seed), and M. fragrans (seed) extracts showed lipase inhibitory activity in unboiled and boiled forms. Further, the unboiled form of these extracts showed a significantly higher inhibitory activity than the boiled form, and this suggested the extraction procedures which do not involve heating to be more effective than the extraction procedures which involve heating in extracting lipase inhibitors from these spices. Unboiled extracts of T. foenum‐graecum (seed), C. cyminum (seed), and M. fragrans (seed) had more potential in inhibiting the major lipid digesting enzyme pancreatic lipase. Therefore, these extracts may have more potential as dietary therapy in controlling obesity and dyslipidemias.
In Asian countries, a drink prepared from T. foenum‐graecum (seed) is consumed as an infusion or as a decoction. Therefore, considering results from the current study, the use of T. foenum‐graecum (seed) extract in the form of infusion could be recommended to be more beneficial than the decoction in controlling obesity and dyslipidemias. In the past, seeds of M. fragrans were added to puddings as a flavoring agent but, at present, this practice has been discontinued in Sri Lanka. This study shows the hypolipidemic potential of M. fragrans seeds in the boiled form and endorses the need of resuscitation of the old culinary practice.
Research has revealed that stronger inhibition of α‐glucosidase activity and mild inhibition of α‐amylase activity of drugs/extracts could address the major drawbacks of currently used synthetic α‐glucosidase and α‐amylase inhibitors (Bischoff, 1994). The current study shows the potential of unboiled and the boiled forms of M. fragrans seed to address this issue, and therefore, M. fragrans seed could be beneficial in the management of postprandial hyperglycemia in diabetic patients and healthy individuals.
Antioxidants are molecules that slow down or prevent oxidation of biomolecules. Antioxidant activity was increased in E.cardamomum, M. fragans, and S. aromaticum boiled extracts in the present study and in support of this finding, Chan et al. (2015), Baker et al. (2013), and Tomaino et al. (2005) have reported increased antioxidant activity in E.cardamomum, M. fragans, and S. aromaticum even after boiling, suggesting that the active components are relatively stable during thermal treatment. Large numbers of studies that have been conducted to evaluate the effect of temperature on the antioxidant activity of spices could not be compared with the present study because those studies used different solvent systems and/or different extraction procedures (Khatun et al., 2006).
5. CONCLUSIONS
Boiling the spices modulated the inhibitory potentials for lipase, amylase, and glucosidase enzymes that are already present in the spices. Conducting in vivo studies using unboiled and boiled extracts to prove the hypothesis developed by the in vitro studies is beneficial. Overall, the properties investigated in this study will be of value in the use of spices as antiobesity and antidiabetic agents.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Ethics approval and consent to participate is not applicable to this manuscript, since it does not report on or involve the use of any animal or human data or tissues.
CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGEMENTS
Technical assistance by Mrs. I. U. Wijethungarachchi, Mr. A. M. P. S. T. M. Bandara, and Ms. T. R. T. Weerasinghe of the Department of Biochemistry, Faculty of Medicine, University of Peradeniya, Sri Lanka, and grammatical guidance in manuscript writing by Mrs. Shyamali Mapa Senanayake of English Teaching Unit, Faculty of Medicine, University of Peradeniya, Sri Lanka, are highly appreciated.
Fernando IT, Perera KI, Athauda SBP, Sivakanesan R, Kumar NS, Jayasinghe L. Heat stability of the in vitro inhibitory effect of spices on lipase, amylase, and glucosidase enzymes. Food Sci Nutr. 2019;7:425–432. 10.1002/fsn3.797
Funding information
The work was financially assisted by University of Peradeniya, Sri Lanka, and University Grants Commission, Sri Lanka
REFERENCES
- Adefegha, S. A. , & Oboh, G. (2011). Cooking enhances the antioxidant properties of some tropical green leafy vegetables. African Journal of Biotechnology, 10, 632–639. [Google Scholar]
- Adefegha, S. A. , Oboh, G. , Oyeleye, S. I. , & Osumo, K. (2016). Alteration of starch hydrolyzing enzyme inhibitory properties, antioxidant activities, and phenolic profile of clove buds (Syzygium aromaticum L.) by cooking duration. Food Science & Nutrition, 4, 250–260. 10.1002/fsn3.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, I. , Chogan, M. , & Opara, E. I. (2013). Impact of cooking and digestion, in vitro, on the antioxidant capacity and anti‐Inflammatory activity of cinnamon, clove and nutmeg. Plant Food for Human Nutrition, 68, 364–369. 10.1007/s11130-013-0379-4 [DOI] [PubMed] [Google Scholar]
- Bischoff, H. (1994). Pharmacology of glucosidase inhibitor. European Journal of Clinical Investigation, 24, 3–10. [PubMed] [Google Scholar]
- Cartea, M. E. , Francisco, M. , Soengas, P. , & Velasco, P. (2011). Phenolic compounds in Brassica vegetables. Molecules, 16, 251–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, E. W. C. , Chan, H. J. , Lim, J. E. , Yik, S. H. , Tan, S. F. , Goh, P. C. , … Yee, S. Y. (2015). Effects of different cooking methods on the bioactivities of some spices. Emirates Journal of Food and Agriculture, 27(8), 610–616. 10.9755/ejfa. [DOI] [Google Scholar]
- Chiasson, J. L. , Josse, R. G. , Gomis, R. , Hanefeld, M. , Karasik, A. , & Laakso, M. (2002). Acarbose for prevention of type 2 diabetes mellitus: The STOP‐NIDDM randomised trial. Lancet, 359, 2072–2077. 10.1016/S0140-6736(02)08905-5 [DOI] [PubMed] [Google Scholar]
- Choi, S.‐J. , Hwang, J. M. , & Kim, S. I. (2003). A colorimetric microplate assay method for high throughput analysis of lipase activity. Journal of Biochemistry and Molecular Biology, 36(4), 417–420. [DOI] [PubMed] [Google Scholar]
- Elya, B. , Basah, K. , Mun'im, A. , Yuliastuti, W. , Bangun, A. , & Septiana, E. K. (2012). Screening of α‐glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. Journal of Biomedicine and Biotechnology, 2012, 281078–281082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estbeyoglu, T. , Ulbrich, K. , Rehberg, C. , Rogn, S. , & Rambach, G. (2015). Thermal stability, antioxidant, and anti‐inflammatory activity of curcumin and its degradation product 4‐vinyl guaiacol. Food and Function, 6, 887–893. 10.1039/C4FO00790E [DOI] [PubMed] [Google Scholar]
- Fernando, W.I.T. , 2016. Inhibitors of digestive enzymes from some medicinal plants and the isolation and identification of lipase inhibitors from Trigonella foenum‐graecum(Ph. D. thesis). University of Peradeniya, Peradeniya, Sri Lanka.
- Geethalakshmi, R. , Sarada, D. V. L. , Marimuthu, P. , & Ramasamy, K. (2010). α‐amylase inhibitory activity of Trianthema decandra L. International Journal of Biotechnology and Biochemistry, 6, 369–376. [Google Scholar]
- Jacobson, T. A. , Miller, M. , & Schaefer, E. J. (2007). Hypertriglyceridemia and cardiovascular risk reduction. Clinical Therapeutics, 29, 763–777. 10.1016/j.clinthera.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Kaila, B. , & Raman, M. (2008). Obesity: A review of pathogenesis and management strategies. Canadian Journal of Gastroenterology, 22(1), 61–68. 10.1155/2008/609039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamać, M. , & Amarowicz, R. (1996). Inhibition of pancreatic lipase by phenolic acids – examination in vitro. Zeitschrift für Naturforschung, 51, 903–905. 10.1515/znc-1996-11-1222 [DOI] [PubMed] [Google Scholar]
- Katulanda, P. , Jayawardena, M. A. , Sheriff, M. H. , Constantine, G. R. , & Matthews, D. R. (2010). Prevalence of overweight and obesity in Sri Lankan adults. Obesity Review, 11, 751–756. 10.1111/j.1467-789X.2010.00746.x [DOI] [PubMed] [Google Scholar]
- Kaur, C. , & Kapoor, H. C. (2001). Antioxidants in fruits and vegetables – the millennium's health. International Journal of Food Science and Technology, 36, 703–725. 10.1046/j.1365-2621.2001.00513.x [DOI] [Google Scholar]
- Khatun, M. , Eguchi, S. , Yamaguchi, T. , Takamura, H. , & Matoba, T. (2006). Effect of thermal treatment on radical‐scavenging activity of some spices. Food Science and Technology Research, 12, 178–185. 10.3136/fstr.12.178 [DOI] [Google Scholar]
- Kim, J.‐S. , Kwon, C.‐S. , & Son, K. H. (2000). Inhibition of alpha glucosidase and amylase by Luteolin, a flavanoid. Bioscience Biotechnol Biochem, 64, 2458–2461. 10.1271/bbb.64.2458 [DOI] [PubMed] [Google Scholar]
- Kochhar, K. P. (2008). Dietary spices in health and diseases (II). Indian Journal of Physiology and Pharmacology, 52(4), 327–354. [PubMed] [Google Scholar]
- Kopelman, P. G. (2000). Obesity as a medical problem. Nature, 404, 635–643. 10.1038/35007508 [DOI] [PubMed] [Google Scholar]
- Lekshmi, P. C. , Arimboor, R. , Raghu, K. G. , & Menon, A. N. (2012). Turmerin, the antioxidant protein from turmeric (Curcuma longa) exhibits antihyperglycaemic effects. Natural Product Research: Formerly Natural Product Letters, 26(17), 1654–1658. 10.1080/14786419.2011.589386 [DOI] [PubMed] [Google Scholar]
- Mnafgui, K. , Kaanich, F. , Derbali, A. , Hamden, K. , Derbali, F. , Slama, S. , … Elfeki, A. (2013). Inhibition of key enzymes related to diabetes and hypertension by Eugenol in vitro and in alloxan‐induced diabetic rats. Archives of Physiology and Biochemistry, 119(5), 225–233. [DOI] [PubMed] [Google Scholar]
- Mukherjee, A. , & Sengupta, S. (2013). Indian medicinal plants known to contain intestinal glucosidase inhibitors also inhibit pancreatic lipase activity‐An ideal situation for obesity control by herbal drugs. Indian Journal of Biotechnology, 12(1), 32–39. [Google Scholar]
- Rohn, S. , Buchner, B. , Driemel, G. , Rauser, M. , & Kroh, L. (2007). Thermal degradation of onion quercetin glucosides under roasting conditions. Journal of Agricultural and Food Chemistry, 55, 1568–1573. 10.1021/jf063221i [DOI] [PubMed] [Google Scholar]
- Settharaksa, S. , Jongjareonrak, A. , Hmadhlu, P. , Chansuwan, W. , & Siripongvutikorn, S. (2012). Flavonoid, phenolic contents and antioxidant properties of Thai hot curry paste extract and its ingredients as affected of pH, solvent types and high temperature. International Food Research Journal, 19, 1581–1587. [Google Scholar]
- Shimura, S. , Itoh, Y. , Yamashita, A. , Kitano, A. , Hatano, T. , Yoshida, T. , & Okuda, T. (1994). Inhibitory effects of flavonoids on lipase. Nippon Shokuhin Kogyo Gakkaishi, 41, 847–850. 10.3136/nskkk1962.41.847 [DOI] [Google Scholar]
- Sudhir, R. , & Mohan, V. (2002). Postprandial hyperglycemia in patients with Type 2 diabetes mellitus. Treat Endocrinology, 1(2), 105–116. 10.2165/00024677-200201020-00004 [DOI] [PubMed] [Google Scholar]
- Tepe, B. , Eminagaoglu, O. , Akpulat, H. A. , & Aydin, E. (2005). Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia verticillata (L.) subsp. verticillata and S. verticillata (L.) subsp. amasiaca (Freyn and Bornm.) Bornm. Food Chemistry, 100, 985–989. [Google Scholar]
- Tomaino, A. , Cimino, F. , Zimbalatti, V. , Venuti, V. , Sulfaro, V. , De Pasquale, A. , & Saija, A. (2005). Influence of heating on antioxidant activity and the chemical composition of some spice essential oils. Food Chemistry, 89, 549–554. 10.1016/j.foodchem.2004.03.011 [DOI] [Google Scholar]
- Villupanoor, A. , Chempakam, B. , & Zachariah, T. J. (2008). Chemistry of Spices. King’s Lynn, UK: C A B Intl. [Google Scholar]
- Weibel, E. K. , Hadvary, P. , Houchuli, E. , Kupfer, E. , & Lengsfeld, H. (1987). Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. 1. Producing organism, fermentation, isolation and biological activity. Journal of Antibiotics, 40, 1082–1085. [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2014). Obesity and overweight. Fact sheet No 311, 2014. http://www.who.int/mediacentre/factsheets/fs311/en/. Accessed 11 May 2015.
- You, Q. , Chen, F. , Wang, X. , Luo, P. G. , & Jiang, Y. (2011). Inhibitory effects of muscadine anthocyanins on α‐glucosidase and pancreatic lipase activities. Journal of Agricultural Food Chemistry, 59, 9506–9511. 10.1021/jf201452v [DOI] [PubMed] [Google Scholar]
- Zhao, H. , & Kim, Y. (2004). Determination of the kinetic properties of platycodin D for the inhibition of pancreatic lipase using a 1,2‐diglyceride‐based colorimetric assay. Archives of Pharmacology Research, 27, 1048–1052. 10.1007/BF02975430 [DOI] [PubMed] [Google Scholar]


