Supplemental Digital Content is available in the text
Keywords: adjuvant therapy, breast cancer, HER2-targeted therapy, neoadjuvant therapy, trastuzumab
Abstract
The aim of this study was to understand current trends in trastuzumab use in China as a neoadjuvant/adjuvant therapy for human epidermal growth factor receptor-2 positive (HER2+) breast cancer and identify factors influencing trastuzumab use.
This was a retrospective, multicenter, cross-sectional study of patients diagnosed with HER2+ breast cancer (stage I–III), between July 2013 and June 2014, at 155 hospitals in 29 provinces/cities in China. Demographic and clinical data, including tumor characteristics and details of adjuvant/neoadjuvant therapies used, were collected. Data analysis included univariate analysis, multivariate logistic regression, and subgroup analyses.
Of 4994 HER2+ patients (mean age 51.1 ± 9.9 years) included, only 29.8% received trastuzumab, with 30.5% in adjuvant therapy and 18.3% in neoadjuvant therapy. The highest rates of adjuvant trastuzumab were in Beijing (59.3%), Jiangsu (57.1%), and Ningxia (50.0%), while those of neoadjuvant trastuzumab were in Guangdong (24.8%), Beijing (14.1%), and Zhejiang (10.7%). Multivariate regression results revealed that factors associated with trastuzumab use were medical insurance cover for trastuzumab, residing locally to the hospital, more lymph node involvement, and more advanced tumor stage. Subgroup analysis revealed that patients receiving neoadjuvant therapy were likely to be younger, premenopausal and non-local, and had lymph node metastases, more advanced tumor, and progesterone receptor positive tumor.
Trastuzumab use in patients with HER2+ breast cancer is relatively low in China, especially for neoadjuvant therapy. Insurance coverage seems to be the most correlated factor that influences the use of trastuzumab in Chinese patients with HER2+ breast cancer.
1. Introduction
According to a recent study using data from the National Control Cancer Registry, breast cancer was the most frequently diagnosed cancer in Chinese women during 2012.[1] Breast cancer is a heterogeneous group of diseases with several different molecular subtypes, including tumors that show expression of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2).[2] Although surgery is the mainstay of treatment, the use of adjuvant (after surgery) or neoadjuvant (before surgery) therapies, including specifically targeted therapies, reduces the risk of disease recurrence and improves survival.[3] Adjuvant and neoadjuvant therapies can improve progression-free survival and can include chemotherapy, hormonal therapy, radiotherapy, and HER2-directed therapies. There have been tremendous improvements in the efficacy and tolerability of adjuvant/neoadjuvant therapies over the last half-century,[4] and these treatments now play a vital role in the management of breast cancer.
Approximately 20% of all breast cancers are HER2-positive (HER2+), which was historically indicative of a poor prognosis.[5,6] The development of trastuzumab and other therapies that target HER2, including lapatinib, pertuzumab, and trastuzumab-emtansine (T-DM1), has improved progression-free survival and overall treatment response in women with HER2+ breast cancer.[7,8] Importantly, several meta-analyses have demonstrated that adjuvant or neoadjuvant trastuzumab can enhance the complete response rate and prolong disease-free and overall survival in patients with HER2+ breast cancer.[9–13]
However, the use of trastuzumab has been restrained in certain regions of the world, particularly in developing countries, due to the high cost or limited availability of the drug. According to a recent publication by Strasser-Weippl et al,[3] resource-limited countries or populations have reduced access to trastuzumab (and other adjuvant/neoadjuvant therapies) due to economic or logistical considerations. Indeed, several studies have highlighted national, regional, age-related, and racial disparities in the use of trastuzumab in the management of HER2+ breast cancer.[14–17] However, although information is available regarding trastuzumab use in the USA, Europe, and certain other countries, no large-scale population-based surveys have been conducted in China to explore the utilization of HER2-targeted therapies in patients with HER2+ breast cancer. Therefore, the current study aims to investigate the current use of adjuvant/neoadjuvant trastuzumab therapy in patients with stage I–III HER2+ breast cancer and examine the potential factors influencing the selection of therapy.
2. Materials and methods
2.1. Study design
The Nvwa Study was a retrospective, multicenter, cross-sectional study covering 155 hospitals in 29 provinces/cities in China between July 2013 and June 2014. The registration number for this study is CBCSG024. The study was based on data extracted from patients’ hospital records. A list of the number of participating hospitals by province/city is presented in Supplementary Table 1.
The study was approved by an independent ethics committee and complied with the Declaration of Helsinki and the Good Clinical Practice (GCP) principles of the International Council on Harmonization (ICH) guidelines. The study was conducted according to the China Food and Drug Administration (CFDA) guidelines.
2.2. Patients
The study included patients diagnosed with or treated for HER2+ breast cancer. Patients had to meet the following criteria for enrollment in the study: age ≥18 years; had undergone surgery for breast cancer and received adjuvant/neoadjuvant therapy; had been diagnosed as HER2+ on the basis of a pathological report after the first operation and had histological and cytological documentary evidence; and had been staged lower than T4N3M0 (i.e., had breast cancer of stage I–III). Patients were excluded if they met any of the following criteria: their medical record contained data for <75% of the parameters analyzed in this study (see section below describing data collection); had pre-invasive carcinoma; had undetected, negative, or unclear HER2 status; or had declared that their information could not be used in the study. All patients included in the final analysis met the HER2+ diagnostic standard according to the Guidelines and Specifications for the Diagnosis and Treatment of Breast Cancer in China (2013).[18]
2.3. Data collection
Data were extracted from the medical records, and additional information was collected through telephone conversations and other means, as necessary. The following data were collected for each patient.
2.3.1. Demographic data
Patient identifier (the initials of their name); date of birth; height; weight; method of payment for treatment (health insurance, public expenditure, self-paid, or unclear); patient address (local or nonlocal); household income; and education level.
2.3.2. Clinical data
Menstruation status (premenopausal or postmenopausal); Karnofsky Performance Status (KPS) score; Eastern Cooperative Oncology Group (ECOG) score; and Breast Imaging Reporting and Data System score.
2.3.3. Surgical data
Surgical method, including sentinel lymph node biopsy and axillary lymph node dissection (for the latter, the number of lymph nodes removed and number positive for cancer were recorded); size of primary tumor and pathological staging; receptor status (whether positive or negative for the ER, PR, and HER2); and the results of immunohistochemistry and fluorescence in situ hybridization for HER2 receptor expression.
2.3.3.1. Neoadjuvant/adjuvant therapy
Whether neoadjuvant or/and adjuvant therapy was received, and which regimen was used; whether trastuzumab was administered and how the patient paid for trastuzumab.
2.4. Subgroup analyses
2.4.1. Factors influencing use of neoadjuvant therapy
HER2-positive breast cancer, found in approximately 20% of patients, is associated with shorter disease-free survival and overall survival.[19] However, there is strong evidence that the use of trastuzumab as a neoadjuvant therapy can improve the pathological complete response rate and prolong disease-free survival.[20–22] A previous study identified several potential barriers to the use of neoadjuvant therapy, including patient, system, clinician, and clinical trial related factors.[23] In the current study, we explored other potential factors that influenced the use of neoadjuvant therapy.
2.5. Statistical analysis
A descriptive statistical analysis was applied. Continuous data were analyzed by the Kolmogorov–Smirnov method to test for a normal distribution and are expressed as the mean ± standard deviation (SD) or median (quartile, maximum value, and minimum value), as appropriate. Categorical variables are expressed as n (%). Univariate analysis (comparisons of individual factors between trastuzumab and non-trastuzumab groups) was used to identify variables that were entered into a multivariate analysis. Multivariate stepwise logistic regression analysis with backward elimination was used to identify the factors influencing the use of targeted therapy, and the results are expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). For the univariate and multivariate analyses, all patients who had received trastuzumab (whether as neoadjuvant therapy, adjuvant therapy, or both) were considered as 1 group. All statistical tests were 2-sided, and P < .05 was considered to indicate statistical significance. SPSS 22.0 (IBM Corp., Armonk, NY) was used for the analyses.
3. Results
3.1. Patients
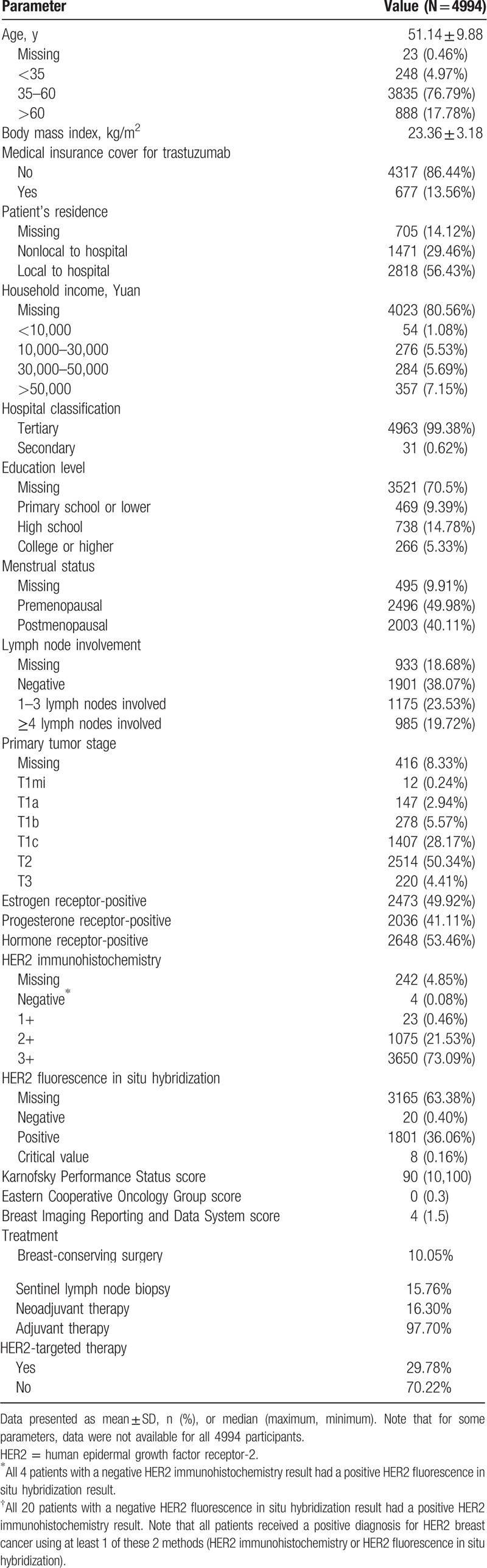
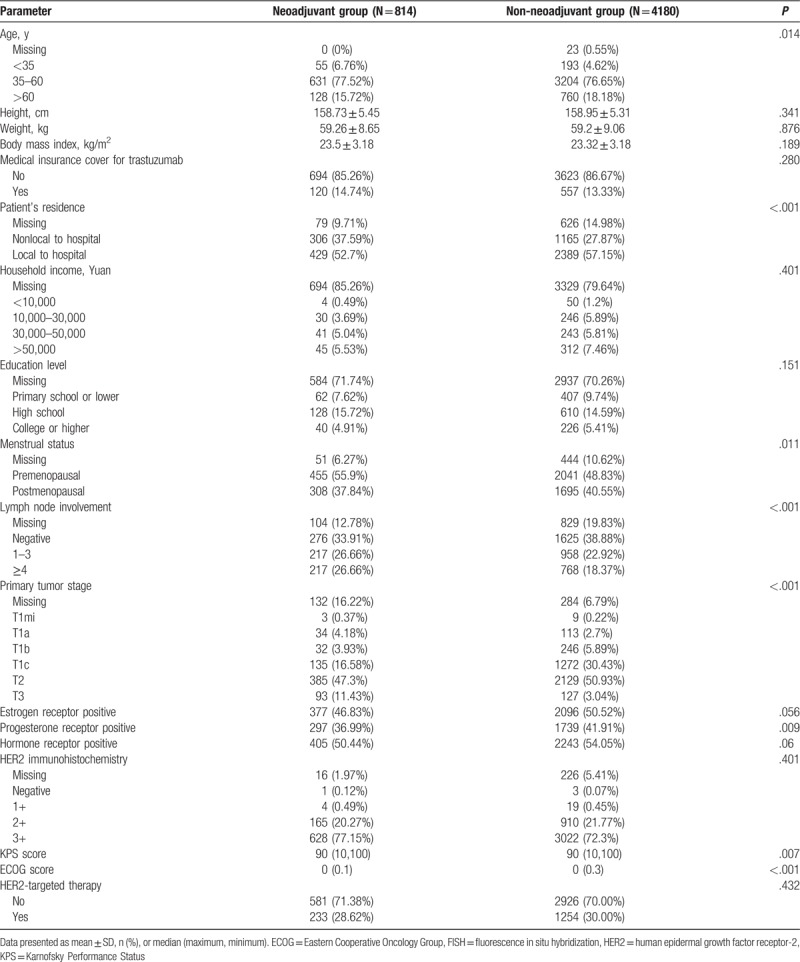
A total of 4994 adults (mean age 51.1 ± 9.9 years) diagnosed with HER2+ breast cancer were enrolled in the study, of whom 4879 received adjuvant therapy, 814 received neoadjuvant therapy, and 724 patients received both neoadjuvant and adjuvant therapy. Among all patients included, only 29.8% (1488 patients) were administered trastuzumab therapy; 30.5% of patients receiving adjuvant therapy were given adjuvant trastuzumab (1488/4879 patients), and 18.3% of those receiving neoadjuvant therapy were given neoadjuvant trastuzumab (149/814 patients). All 149 patients who received neoadjuvant trastuzumab were also given adjuvant trastuzumab. The majority of patients (86.4%) did not have medical insurance cover for trastuzumab and over half (55.5%) were premenopausal. Detailed baseline demographics for the patients included in the analysis are presented in Table 1.
Table 1.
Baseline demographic and clinical characteristics of the patients included in the analysis.
3.2. Epidemiological analysis
3.2.1. Geographical differences in the use of trastuzumab therapy
Regional differences in the use of trastuzumab therapy as an adjuvant or neoadjuvant treatment are depicted in Fig. 1. Geographical variation between provinces in the use of trastuzumab therapy was greater for neoadjuvant therapy (Fig. 1A) than for adjuvant therapy (Fig. 1B). Provinces/cities with the 3 highest rates of neoadjuvant trastuzumab therapy were Guangdong (24.8%), Beijing (14.1%), and Zhejiang (10.7%), while 10 of the 29 provinces did not use neoadjuvant trastuzumab therapy in any patients (Supplementary Table 2). Provinces/cities with the 3 highest rates of adjuvant trastuzumab therapy were Beijing (59.3%), Jiangsu (57.1%), and Ningxia (50.0%), while those with the lowest rates were Anhui (5.0%), Guizhou (11.1%), and Gansu (12.2%).
Figure 1.
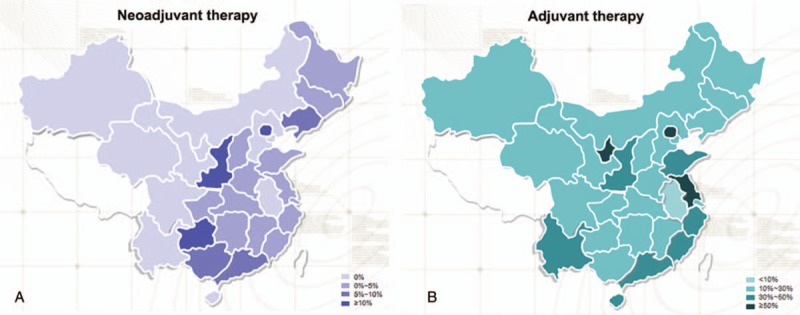
Use of HER2-targeted adjuvant and neoadjuvant therapy in patients with HER2+ breast cancer. (A) Proportion of patients with HER2+ breast cancer receiving neoadjuvant therapy. The percentage value refers to the proportion of all patients (in each province) with HER2+ breast cancer that received neoadjuvant therapy. (B) Proportion of patients with HER2+ breast cancer receiving adjuvant therapy. The percentage value refers to the proportion of all patients (in each province) with HER2+ breast cancer who received adjuvant therapy.
3.2.2. Univariate analysis of factors associated with trastuzumab use
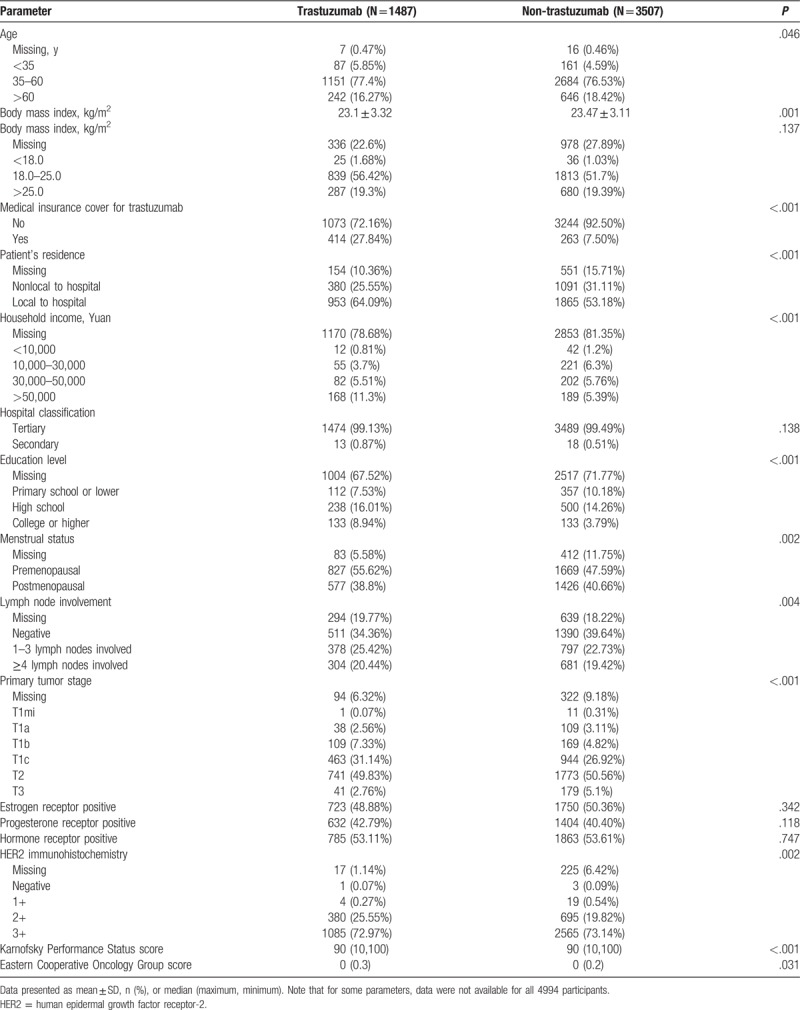
Univariate analysis revealed that patients receiving trastuzumab therapy and those not receiving trastuzumab therapy differed significantly with regard to the following factors: age, height, BMI, medical insurance cover for trastuzumab, locality of the patient's residence to the treating hospital, household income, education level, menstrual status, lymph node involvement and primary tumor stage, HER2 immunohistochemistry, KPS score, and ECOG score (all P < .05; Table 2). There were no significant differences between the 2 groups in weight, ER status, PR status, or hormone receptor status (Table 2).
Table 2.
Univariate analysis of the factors associated with the use of trastuzumab therapy.
3.3. Multivariate logistic regression analysis
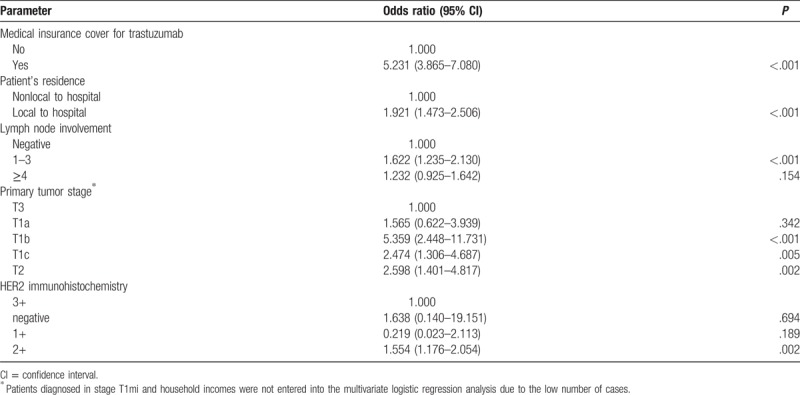
Multivariate logistic regression analysis was performed to identify the factors associated with the use of trastuzumab therapy. The factors identified by the univariate analysis as differing significantly between patients treated with trastuzumab and those not treated with trastuzumab were entered into the multivariate analysis. As height and BMI, KPS, and ECOG scores are similar, only BMI and ECOG were included in multivariate analysis. As differences in policies and standard practices between hospitals of different classifications could also potentially influence our results, hospital classification (Primary, Secondary, and Tertiary as classified in China) was also included in the multivariate analysis. The factors identified by multivariate analysis as significantly associated with trastuzumab therapy are summarized in Table 3. We found that medical insurance cover for trastuzumab was strongly correlated with trastuzumab use (OR: 4.795; 95% CI: 3.416–6.631), while those living locally to the hospital were more likely to be given trastuzumab therapy than those living nonlocally (OR: 2.01; 95% CI: 1.488–2.714). Greater lymph node involvement was also associated with higher use of trastuzumab therapy (OR of 1.426 and 1.783 for ≥4 and 1–3 compared with no lymph node involvement, respectively). Compared with advanced tumor stage (T3), patients diagnosed at early tumor stages (T1a, T1b, T1c, and T2) were also more likely to receive trastuzumab therapy (OR: 1.186, 6.094, 2.376, 2.593, respectively). All other factors, including hospital classification, showed no significant association with trastuzumab therapy. No multicollinearity was observed between the included covariates.
Table 3.
Multivariate logistic regression analysis of the factors associated with the use of trastuzumab therapy.
3.4. Subgroup analyses
3.4.1. Factors influencing use of trastuzumab as a neoadjuvant therapy
Table 4 summarizes the demographic and clinical characteristics of patients in our study who received neoadjuvant therapy with those who did not receive neoadjuvant therapy. Compared with those who did not receive neoadjuvant therapy, patients who were given neoadjuvant therapy were more likely to be younger, live less locally to the hospital, be premenopausal, have lymph node metastases, have more advanced tumor stage, and have PR-positive tumor (all P < .05; Table 4).
Table 4.
Comparison of demographic and clinical characteristics of patients who received neoadjuvant therapy with non-neoadjuvant therapy.
4. Discussion
The current study was conducted in 155 hospitals of different types and sizes that covered almost all provinces/cities of China, expect Tibet. To the best of our knowledge, this is the first large-scale, multicenter study in China exploring the utilization of trastuzumab therapy in patients with HER2+ breast cancer. It is envisaged that better knowledge of the factors influencing the use of trastuzumab therapy in China will help to improve the management of patients with HER2+ breast cancer.
Breast cancer was the most common cancer diagnosed in Chinese women in 2012,[2] and approximately 20% of those with the disease are diagnosed as HER2+. Although trastuzumab therapy is considered standard treatment for early HER2+ breast cancer,[3] inter-country and intra-country regional variations in the use of trastuzumab therapy have been reported.[14–16] Our findings demonstrate clear regional differences in the use of trastuzumab in China. Before June 2014, there were limited geographical regions in China that provided trastuzumab to patients with HER2+ breast cancer: Jiangsu province, Guangzhou City, and Qiangdao City. The data provided in the present study indicate that cities and provinces with patients holding medical insurance provide targeted therapy at higher rates. Hospitals treating patients with medical insurance offered trastuzumab treatment to 61.2% of patients (26 hospitals), whereas those in the same geographical areas that did not offer medical insurance-based treatment only provided targeted therapy to 24.9% of patients (129 hospitals). On the basis of the geographical data obtained by this study, patients who resided in more prosperous provinces/cities (with higher incomes) or in more populous urban settings (Beijing, Guangdong, and Zhejiang provinces) were more likely to be treated with neoadjuvant therapy. This is also true of the cities with the highest rates of adjuvant therapy: Beijing, Jiangsu, and Ningxia. All of these areas have populations with greater incomes and higher education levels, suggesting that patients with higher incomes were more likely to receive more comprehensive treatment.
The rate of trastuzumab use in developed countries has increased progressively during the past decade.[24,25] An important finding of the present study was that although more than 97% of patients with HER2+ breast cancer were given adjuvant therapy, only around 30% received trastuzumab therapy. The main factors revealed by the current study as predicting the use of trastuzumab therapy in China were medical insurance cover for trastuzumab, residing locally to the hospital, more lymph node involvement, and primary tumor stage.
Using certain assumptions (including the rate of HER2+ breast cancer), 1 recent study has estimated that Western Europe and the USA procure enough trastuzumab to treat virtually all patients with HER2+ breast cancer, indicating widespread use of this adjuvant therapy in developed countries.[15] By comparison, many Eastern European countries appeared not to buy sufficient quantities of trastuzumab to treat all patients who might require it,[15] suggesting that less economically developed regions of the world may show lower use of HER2-targeted therapies. A recent survey of physicians in Mexico, Turkey, Russia, Brazil, and the USA suggested that there were several barriers to the use of trastuzumab in patients with HER2+ breast cancer, such as a lack of insurance coverage, a lack of drug availability at the hospital/clinic, and a prohibitive cost to the patient.[14] Regional differences within Sweden in the utilization of trastuzumab have also been reported, with the underlying reasons suggested to include differing interpretations in clinical practice, budget-related issues, and variations in coordination, experience, and training.[16] The present study found that more than two-thirds of patients with HER2+ breast cancer in China did not receive HER2-targeted therapy, in stark contrast to the situation in Western Europe and the USA, and more in keeping with observations made in countries that are less well economically developed. Further studies are merited to compare the use of adjuvant and neoadjuvant HER2-targeted therapy between China, Europe, and the USA.
Economic factors might be one of the most important considerations in the decision to use trastuzumab therapy in patients with HER2+ breast cancer in China. This is illustrated by the observation that patients with medical insurance cover for trastuzumab were much more likely to be given trastuzumab therapy than those without; indeed, the presence or absence of medical insurance cover for trastuzumab was by far the strongest predictor of trastuzumab therapy use. This is consistent with previous studies suggesting that treatment costs are an important factor determining the administration of trastuzumab and related agents.[14–16] Particularly concerning observations were that only 14% of patients in the present study held medical insurance cover for trastuzumab and that only a quarter of those without medical insurance were administered trastuzumab therapy. However, due to the retrospective nature of this study, we were unable to collect sufficient information regarding the patients’ household income: as many of the patients’ records did not include data for household income, we did not include this factor in the multivariate analysis in order to avoid potential bias.
Interestingly, women who had to travel for their treatment were less likely to receive trastuzumab therapy. The underlying reasons for this observation remain unknown, although it is possible that those patients living nonlocally to the hospital may have resided in less prosperous areas and had lower levels of income, limiting their ability to afford the costs of adjuvant treatment. Other factors affecting the use of trastuzumab therapy included lymph node involvement and later tumor stage at diagnosis. The latter findings may indicate that a patient is more willing to accept, or the physician more willing to recommend, trastuzumab therapy when the disease is more advanced.
As a cross-sectional study, the current research has some limitations. The variables selected for the analysis were mostly based on the patients’ hospital records, which may have introduced some bias in the analyses of the relationships between the various factors and trastuzumab use. In addition, data were missing for many of the variables analyzed, particularly household income. Incomplete datasets, which may be encountered in retrospective observational studies such as this one, are another potential source of bias whose direction and effect might not be well estimated. Well-designed questionnaires will help to reduce bias in future studies.
In conclusion, the current study has analyzed the factors affecting the use of trastuzumab as an adjuvant/neoadjuvant therapy for HER2+ breast cancer in China. The results show that the main factors influencing the use of trastuzumab therapy in patients with HER2+ breast cancer were medical insurance cover for trastuzumab, household income, locality of the patient's residence to the hospital, and specific disease-related factors (lymph node metastasis status and primary tumor stage). It is hoped that highlighting these variations in trastuzumab therapy will be of help to future efforts to standardize the use of trastuzumab in the management of breast cancer in China.
Author contributions
Conceptualization: Junjie Li, Zhimin Shao.
Data curation: Junjie Li, Zhimin Shao, Binghe Xu, Zefei Jiang, Shude Cui, Jin Zhang, Ning Liao, Jun Jiang, Yongsheng Wang, Quchang Ouyang, Ziwei Ying.
Formal analysis: Junjie Li, Zhimin Shao, Binghe Xu, Zefei Jiang, Shude Cui, Jin Zhang, Ning Liao, Jun Jiang, Yongsheng Wang, Quchang Ouyang, Ziwei Ying.
Investigation: Junjie Li.
Writing – original draft: Junjie Li.
Writing – review & editing: Zhimin Shao, Binghe Xu, Zefei Jiang, Shude Cui, Jin Zhang, Ning Liao, Jun Jiang, Yongsheng Wang, Quchang Ouyang, Ziwei Ying.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, CFDA = China Food and Drug Administration, ECOG = Eastern Cooperative Oncology Group, ER = estrogen receptor, GCP = Good Clinical Practice, HER2 = human epidermal growth factor receptor-2, HER2+ = HER2-positive, ICH = International Council on Harmonization, KPS = Karnofsky Performance Status, ORs = odds ratios, PR = progesterone receptor, SD = standard deviation.
The authors declare that they have no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Chen W, Zheng R, Zuo T, et al. National cancer incidence and mortality in China, 2012. Chin J Cancer Res 2016;28:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen K, Li S, Li Q, et al. Breast-conserving surgery rates in breast cancer patients with different molecular subtypes: an observational study based on Surveillance, Epidemiology, and End Results (SEER) database. Medicine (Baltimore) 2016;95:e2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Strasser-Weippl K, Horick N, Smith IE, et al. Long-term hazard of recurrence in HER2+ breast cancer patients untreated with anti-HER2 therapy. Breast Cancer Res 2015;17:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rampurwala MM, Rocque GB, Burkard ME. Update on adjuvant chemotherapy for early breast cancer. Breast Cancer (Auckl) 2014;8:125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dendukuri N, Khetani K, McIsaac M, et al. Testing for HER2-positive breast cancer: a systematic review and cost-effectiveness analysis. CMAJ 2007;176:1429–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res 2015;5:2929–43. [PMC free article] [PubMed] [Google Scholar]
- [7].Schramm A, De Gregorio N, Widschwendter P, et al. Targeted therapies in HER2-positive breast cancer: a systematic review. Breast Care (Basel) 2015;10:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Santa-Maria CA, Nye L, Mutonga MB, et al. Management of metastatic HER2-positive breast cancer: where are we and where do we go from here? Oncology (Williston Park) 2016;30:148–55. [PubMed] [Google Scholar]
- [9].O'Sullivan CC, Bradbury I, Campbell C, et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors </= 2 cm: a meta-analysis of the randomized trastuzumab trials. J Clin Oncol 2015;33:2600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cardoso AT, Nanji L, Costa J, et al. [Analysis of the Cochrane Review: vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014, 6:CD007469]. Cochrane Database Syst Rev 2014;27:411–3. [PubMed] [Google Scholar]
- [11].Brollo J, Curigliano G, Disalvatore D, et al. Adjuvant trastuzumab in elderly with HER-2 positive breast cancer: a systematic review of randomized controlled trials. Cancer Treat Rev 2013;39:44–50. [DOI] [PubMed] [Google Scholar]
- [12].Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012;CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Valachis A, Mauri D, Polyzos NP, et al. Trastuzumab combined to neoadjuvant chemotherapy in patients with HER2-positive breast cancer: a systematic review and meta-analysis. Breast 2011;20:485–90. [DOI] [PubMed] [Google Scholar]
- [14].Lammers P, Criscitiello C, Curigliano G, et al. Barriers to the use of trastuzumab for HER2+ breast cancer and the potential impact of biosimilars: a physician survey in the United States and emerging markets. Pharmaceuticals (Basel) 2014;7:943–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moraes FA, Senterre C, Zardavas D, et al. Patterns and discrepancies of trastuzumab use in the European Union and the USA. Ann Oncol 2014;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wilking U, Jonsson B, Wilking N, et al. Trastuzumab use in breast cancer patients in the six Health Care Regions in Sweden. Acta Oncol 2010;49:844–50. [DOI] [PubMed] [Google Scholar]
- [17].Reeder-Hayes K, Peacock Hinton S, Meng K, et al. Disparities in use of human epidermal growth hormone receptor 2-targeted therapy for early-stage breast cancer. J Clin Oncol 2016;34:2003–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Commission NHaFP. Guidelines and specifications for the diagnosis and treatment of breast cancer in China. Chin J Front Med Sci 2013;5:641–8. [Google Scholar]
- [19].Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009;14:320–68. [DOI] [PubMed] [Google Scholar]
- [20].Hamy-Petit AS, Belin L, Bonsang-Kitzis H, et al. Pathological complete response and prognosis after neoadjuvant chemotherapy for HER2-positive breast cancers before and after trastuzumab era: results from a real-life cohort. Br J Cancer 2016;114:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Palmieri C, Macpherson IR, Yan K, et al. Neoadjuvant chemotherapy and trastuzumab versus neoadjuvant chemotherapy followed by post-operative trastuzumab for patients with HER2-positive breast cancer. Oncotarget 2016;7:13209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pernas Simon S. Neoadjuvant therapy of early stage human epidermal growth factor receptor 2 positive breast cancer: latest evidence and clinical implications. Ther Adv Med Oncol 2014;6:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zdenkowski N, Butow P, Mann GB, et al. A survey of Australian and New Zealand clinical practice with neoadjuvant systemic therapy for breast cancer. Intern Med J 2016;46:677–83. [DOI] [PubMed] [Google Scholar]
- [24].de Munck L, Schaapveld M, Siesling S, et al. Implementation of trastuzumab in conjunction with adjuvant chemotherapy in the treatment of non-metastatic breast cancer in the Netherlands. Breast Cancer Res Treat 2011;129:229–33. [DOI] [PubMed] [Google Scholar]
- [25].Kurebayashi J, Miyoshi Y, Ishikawa T, et al. Clinicopathological characteristics of breast cancer and trends in the management of breast cancer patients in Japan: based on the Breast Cancer Registry of the Japanese Breast Cancer Society between 2004 and 2011. Breast Cancer 2015;22:235–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


