Abstract
The number of older adults requiring dialysis is increasing worldwide, whereas the use of peritoneal dialysis (PD) in this population is lower with respect to younger patients, despite the theoretical advantages of PD respect to hemodialysis. This is most likely due to the concern that older patients may not be able to correctly and safely manage PD.
We aimed to prospectively compare clinical, nutritional and metabolic outcomes and measures of quality of life between younger (<65 years old) and older (≥65 years old) patients on PD.
PD patients were enrolled and divided into 2 groups according to age (Group A < 65 years, Group B ≥ 65 years). Clinical and instrumental parameters, and quality of life were evaluated at baseline (start of PD) (T0) and at 24 months (T1). Technique survival, mortality, total number of hospitalizations, and the index of peritonitis (episodes of peritonitis/month) were also evaluated.
Fifty-one patients starting PD were enrolled. Group A included 22 patients (48.7 ± 8.3 years), and Group B consisted of 29 patients (74.1 ± 6.4 years). At baseline, the 2 groups showed no differences in cognitive status, whereas Group A showed higher total cholesterol (P = .03), LDL (P = .03), and triglycerides (P = .03) levels and lower body mass index (P = .02) and carotid intima media thickness (P < .0001) with respect to Group B. At T1 Group B showed, compared to baseline, a significant reduction in albumin (P < .0001) and phosphorus (P = .045) levels, while no significant differences on body composition, technique survival, total number of hospitalizations, index of peritonitis, and quality of life indices were observed.
Our data do not show clinically relevant barriers to use PD in older adult patients, supporting its use in this population. Nutritional and metabolic parameters should be carefully monitored in older PD patients.
Keywords: older adults, peritoneal dialysis, peritonitis, quality of life, renal replacement therapy
1. Introduction
The prevalence of chronic kidney disease (CKD) is increasing in older adults worldwide [1,2] and the most prevalent causes of loss of kidney function are diabetes, hypertension, and vascular disease.[3] As cardiovascular disease is better managed in the general population, patients live longer and the risk to develop CKD in the older adults is significantly higher.[3] Therefore, the number of older adults needing renal replacement therapy (RRT) is steadily increasing.[4] Studies have shown that dialysis can significantly impact on life expectancy and quality of life, but only in a limited number of patients the peritoneal dialysis (PD) is considered as the first therapeutic option.[5,6] This is probably due to the concern that older adult patients may not be able to safely manage this home-based treatment or due to a perception that PD is associated with greater risk of complications.[7] From the other side, hemodialysis (HD) can be negatively perceived by patients because of the long time to be spent in the HD facilities.[8,9] A large number of older patients do not positively accept to start a HD treatment, while the proportion of older adults on PD is considerably lower with respect to younger patients. Despite different studies have shown that survival and quality of life of older adults on PD do not differ from HD, the option to implement PD is still largely lacking.[7–10] Furthermore, the North Thames Dialysis Study, a prospective study conducted on patients ≥ 70 years old, has shown that survival, hospitalization, and quality of life were similar between patients on HD and those on PD.[5,11,12] Older patients are often socially isolated and often depressed due to loss of independence. Moreover, the associated vascular disease results in a high risk of failure for vascular access for the HD treatment. This results in increased reliance on venous access, with higher risk of infection.[5,8,11–13] Failure of vascular access can necessitate frequent hospital admissions for radiological and surgical procedures. Also, cardiac disease can cause hypotension and arrhythmias during HD session and older adults often feel “weakened” after a HD treatment. In addition, many patients cannot reach the dialysis center independently and require transportation provided by the hospital.[8,12,14] The problem is to determine whether older adults can safely manage PD. Several older adults can be trained to do their own PD, although this may take longer time. Family members are often willing to help with all or part of the procedure, and in some parts of Europe, the use of community nurses enables older adults to be on PD in their own homes.[15–17] Furthermore, we should consider that PD is a less expensive treatment than HD.[18]
The aim of this study was to compare clinical, instrumental parameters, quality of life, and hospitalizations between younger (< 65 years old) and older (≥65 years old) patients on PD.
2. Patients and methods
The study protocol was approved by the Local Clinical Research Ethics Committee. The study conforms to the principles outlined in the Declaration of Helsinki and we obtained a written consent by all patients before the enrollment.
2.1. Study design
We performed a prospective study on clinically stable patients starting PD at the Peritoneal Dialysis Unit, Policlinico Umberto I, Sapienza University of Rome, Rome, Italy.
We enrolled consecutive patients from January 2009 to August 2014, eligible to perform PD.[6] Patients were divided into 2 groups in accordance to age: the first group (Group A) included patients aged < 65 years and the second group patients aged ≥ 65 years (Group B). Clinical, laboratory, instrumental parameters and quality of life were evaluated at baseline (start of PD) (T0) and at 24 months (T1). Kt/V urea and creatinine clearance (L/week/1.73 m2) were calculated. These values were measured by collecting the dialytic effluent over a 24-hour period and by a plasma sample. Residual renal function (RRF) (glomerular filtration rate; mL/minute) was calculated as mean of renal clearances of urea and creatinine, collecting all urine output over the same 24-hour period and dialysate creatinine clearance was obtained from 24-hour collections of dialysate. We also used normalized protein catabolic rate (nPCR) (grams per kilogram per day) calculated from dialysis kinetic modeling.[19–21] We evaluated the technique survival, the mortality, the “index of peritonitis,” expressed as months free from peritonitis (episodes of peritonitis/months), and the number of hospitalizations at T0 and T1 in both groups. Peritoneal dialysis-associated peritonitis was defined as a symptom or sign (abdominal pain, fever, and turbid dialysate) combined with an effluent cell count of more than 100/μL leukocytes, with at least 50% polymorphonuclear neutrophilic cells.[13] The Kidney Disease Quality of Life Short Form (KDQOL-SF) was also administered in both groups.
2.2. Inclusion criteria
Patients aged >18 years starting PD.
2.3. Exclusion criteria
Patients with cancer, liver disease, human immunodeficiency virus, and those who had been on HD (before starting PD) for more than 30 days and patients with previous renal transplantation were excluded. Patients who refused to give consent and patients with missing clinical data were also excluded.
2.4. Anthropometric assessments
Patients were evaluated before the first replacement with empty peritoneum in the morning. During routine visits, height and weight were measured with the patient wearing indoor clothing before intraperitoneal dialysate. Body weight was determined to the nearest 0.1 kg using a calibrated digital scale. Body mass index (BMI) was calculated using the formula [weight (kg)/height (m2)].
2.5. Laboratory measurements
Blood were collected in the morning after an overnight fasting (at least 12 hours). In all patients, the levels of fasting plasma glucose (mg/dL), total serum cholesterol (mg/dL), triglycerides (mg/dL), high-density lipoprotein (HDL) (mg/dL), low-density lipoprotein (LDL) (mg/dL), serum nitrogen (mmol/L), serum calcium (mg/dL), phosphate (mg/dL), sodium (mEq/L), potassium (mEq/L), serum uric acid (mg/dL), C-reactive protein (CRP) (mg/dL), and erythrocyte sedimentation velocity (ESV) (mm/H), were measured using standard automated techniques. Serum albumin (g/dL) was determined by bromcresol purple method. Parathormone (iPTH) (pg/mL) was measured using a 2-site assay that measures “intact” hormone. LDL-cholesterol was calculated using the Friedewald equation: LDL (mg/dL) = total cholesterol − HDL − (triglicerydes/5). Arterial blood gas analysis was performed using a blood gas analyzer (Nova Biomedical Corporation Waltham MA, USA).
2.6. Blood pressure measurements
Systolic and diastolic blood pressure (BP) levels were measured in patients on PD during routine visits. Clinic BP measurements were made 3 times after 10 minutes of rest in a seated position using a standard automated sphygmomanometer and cuffs adapted to the arm circumference, according to the British Hypertension Society guidelines. The mean values for systolic BP (SBP) and diastolic BP (DBP) were calculated for all participants.[22] The systolic and diastolic BP levels were taken as the points of appearance and disappearance of Korotkoff sounds, respectively. Hypertension was defined as SBP >140 mmHg or DBP >90 mmHg on repeated measurements.
2.7. Bioelectrical impedance analysis
Multifrequency bioelectrical impedance analysis (MF-BIA) (Akern, Florence, Italy) was performed using an impedance plethysmograph that emits an 800-μA, 50-kHz alternating current. Measurements were taken with patients being in a supine position for 5 minutes, according to the manufacturer's guidelines. The analysis of the entire body involves the placement of 2 electrohydraulic injectors at the back of the hands and feet at the distal ends of metacarpals and metatarsals, and 2 measuring electrodes were placed on the dorsal surfaces of the wrists and ankles. We proceeded to the recording of impedance, resistance, reactance, and phase angles and the subsequent transformation into estimates of lean body mass, body fat, cell mass, total body water, and intracellular water.[23,24]
2.8. Common carotid intima-media thickness (IMT)
At T0 and T1, right and left carotid ultrasound was blindly performed by an experienced sonographer who was unaware of the characteristics of the patients under examination. Participants were studied with the high-resolution B-mode ultrasound machine Toshiba Aplio XV (Toshiba AplioxV, Toshiba American Medical Systems, Inc., Tustin, CA) equipped with a 5- to 12-MHz linear transducer with a 0.01-mm resolution, following a standardized vascular protocol. Three different longitudinal views (anterior oblique, lateral, and posterior oblique) and a transverse view were obtained. IMT was measured at three points on the far walls of both left and right distal common carotid arteries, carotid bulb, and the proximal portion of the internal carotid arteries. Images were captured in end diastole triggered by electrocardiographic recording. The mean IMT was computed as the average IMT on both sides. The value of IMT was considered normal when between 0.55 and 1 mm.[25,26]
2.9. Echocardiography
Transthoracic echocardiography after emptying the peritoneal cavity was performed. M-mode 2D echocardiographic examinations by a single experienced sonographer in the echocardiography laboratory and using a standard institutional protocol were completed.[27,28] Commercially available instruments (Toshiba AplioxV, Toshiba American Medical Systems, Inc., Tustin, CA) equipped with 2.25- to 7.5-MHz imaging transducers were used; the patients were in the left decubitus position, and the sonographer was blinded to all clinical details of the patients. All echocardiographic data according to the guidelines of the American Society of Echocardiography were recorded.[29] The end-diastolic and end-systolic left ventricular internal diameter, interventricular septum thickness, and posterior wall thickness were measured. The left ventricular mass (LVM) was estimated following Devereux's formula normalized by body surface area and height.[30]
2.10. Kidney Disease Quality of Life Short Form
The KDQOL-SF is a self-report measure developed for individuals with kidney disease and those on dialysis. It is a shorter version of a measure developed by the same authors. It includes 43 kidney disease-targeted items, such as the effects of the disease of activities of daily living, work status, and social interaction, and 36 items that provide a measure of physical and mental health. The 80 items take about 16 minutes to complete. The item role physical, emotional well-being, and weight of kidney disease is assigned a score from 0 to 4; emotional role instead corresponds to the item a score from 0 to 3. The total score of the questionnaire ranges from 0 to 15, associating at the greater value the lower perceived well-being.[31]
2.11. Statistical analysis
All continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as numbers (percentage). Student t-tests and ANOVA were performed to determine differences between groups, as appropriate. Binomial test or χ2 test was used for the comparison of categorical data. A probability value of P < .05 was considered to be statistically significant. Data management and analysis were performed using IBM SPSS Statistics 17 for Windows software (IBM Corporation, New Orchard Road Armonk, New York, NY).
3. Results
A total of 51 patients (28 males) were enrolled. Group A included 22 patients (mean age: 48.7 ± 8.3 years), 21% were on automated PD. Group B consisted of 29 patients (mean age of 74.1 ± 6.4 years), 72% are on continuous ambulatory PD. The etiology of end-stage renal disease (ESRD) was autosomal dominant polycystic kidney disease in 4 patients (18%) in Group A and 2 patients (7%) in Group B; chronic glomerulonephritis in 4 patients (18%) in Group A and 6 patients (21%) in Group B; diabetic nephropathy in 2 patients (9%) in Group A and 6 patients (21%) in Group B; chronic pyelonephritis in 2 patients (9%) in Group A and 1 patient (3%) in Group B; hypertensive nephrosclerosis in 6 patients (27%) in Group A and 12 patients (41%) in Group B; and unknown in 4 patients (18%) in Group A and 2 patients (7%) in Group B.
3.1. Baseline characteristics
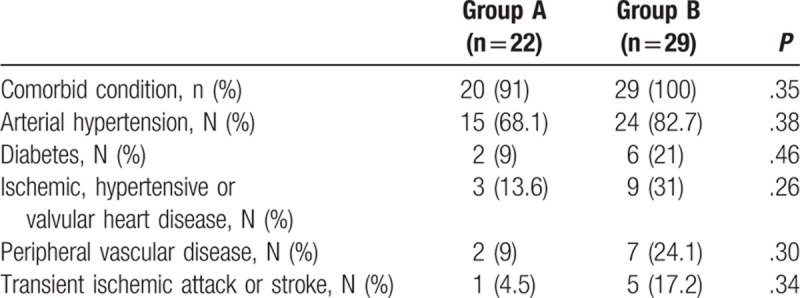
Patient's characteristics at baseline (T0) are shown in Table 1. In summary, there were no significant differences between the 2 groups, except for higher values in Group A with respect to Group B of total cholesterol (mg/dL) (195.4 ± 43.3 vs 172.2 ± 32.3; P = .03), LDL (mg/dL) (125.2 ± 34.3 vs 103.9 ± 31.1; P = .03), and triglycerides (mg/dL) (137.8 ± 51 vs 110.1 ± 35.5; P = .03), while Group A, with respect to Group B, had a significant lower BMI (kg/m2) (23.45 ± 3.08 vs 26.05 ± 3.50; P = .02), and carotid IMT (mm) (0.97 ± 0.32 vs 1.54 ± 0.31; P < .0001). The prevalence of comorbidities, including diabetes and cardiovascular diseases, was not statistically different between the 2 groups, as shown in Table 2.
Table 1.
Patient's characteristics at baseline (T0).
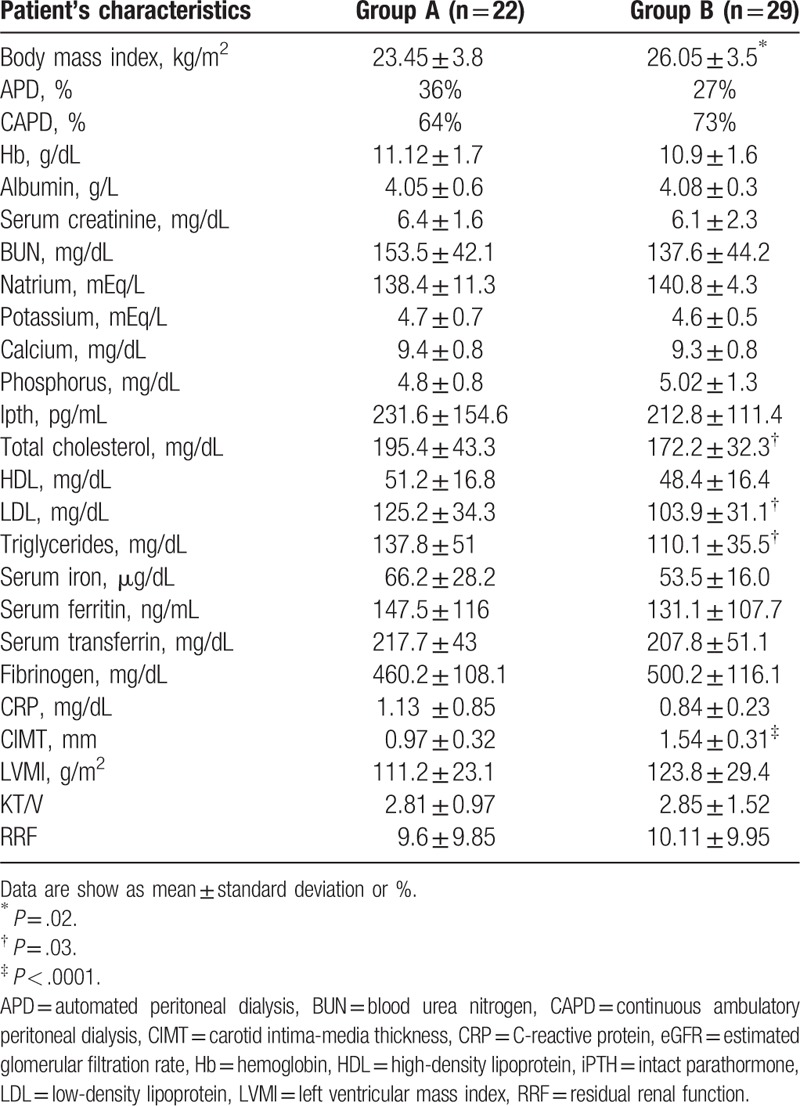
Table 2.
Comorbid conditions in Group A and Group B.
At T0 the 2 groups appeared relatively homogeneous on the items General Health, Role Physical, Role Emotional, and Social Function of the KDQOL-SF test. Also, the items cognitive function (87.18 ± 11.42 vs 89.67 ± 14.72; P = .51) and Physical Function (63.24 ± 24.30 vs 53.75 ± 21.49; P = .15) did not show statistically significant differences between the 2 groups at T0.
3.2. Follow-up results
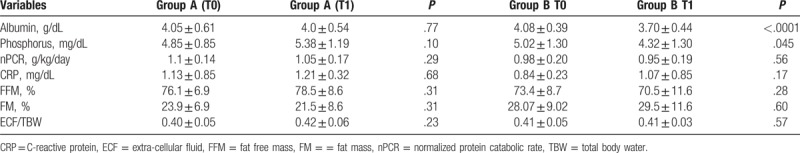
At T1 in Group B, a significant reduction in albumin (P < .0001) and phosphorus levels was observed (P = .045) (Table 3). KT/V, RRF and nPCR were not different between the 2 groups (3.0 ± 1.2 vs 2.5 ± 0.80, P = .08; 7.53 ± 4.21 vs 10.21 ± 5.59, P = .28; 1.05 ± 0.17 vs 0.95 ± 0.19, P = 0.29, respectively). No significant changes in body composition parameters were shown (Table 3) and no differences in the index of peritonitis (episodes of peritonitis/month) were registered (Group A: 0.03 ± 0.001 vs Group B: 0.03 ± 0.002; P = .99) In addition, the survival of the technique during the follow-up was of 77.2% (n = 17) and 72.4% (n = 21) (P = .54) and the patient’ s survival rate at the end of the follow-up was 95.4% (n = 21) in Group A and 86.2% (n = 25) in Group B (P = .05). We reported 9 hospitalizations in Group A and 11 in Group B (P = .94). The reasons for hospitalization were comparable between the 2 groups (including cardiovascular and cerebrovascular events). Both groups did not differ regarding the item cognitive functions of the KDQOL-SF (Group A 86.27 ± 11.42 vs Group B 91.25 ± 9.34; P = .09). And they appeared relatively homogeneous on the items General Health, Role Physical, Role Emotional, Social and Physical Function and Satisfaction care. Also, Group A had a higher score for Energy /Fatigue (Group A 59.4 ± 13.1 vs Group B 54.1 ± 23.9; P = .35) and Sleep Quality (Group A 67.7 ± 13.3 vs Group B 60 ± 23.2; P = .17), although not statistically significant.
Table 3.
Biochemical, inflammatory and body composition variables in Group A and Group B at T0 and T1.
4. Discussion
Chronic kidney disease is a common condition among older adults and only a few patients with ESRD are treated with PD.[32–34] The Broadening Options for Long-Term Dialysis in the Elderly (BOLDE) study has shown that older patients can successfully manage PD, and that in 2 closely matched demographic groups of older dialysis patients on PD and HD, quality of life was similar, but with significantly less perception of intrusion of the disease in their lives in the PD group.[12,35,36] In our study, there were also no statistically significant differences in the index of peritonitis and in term of mean survival of the technique between younger and older patients. In HD, complications of the vascular access, intradialytic hypotension, and impaired autonomic function with hemodynamic instability, bleeding, and amyloidotic arthropathy represent the most relevant limitations, especially in older adults.[7,8,37] Authors suggested that patients on PD perceived lower distress and higher psychological well-being compared with patients undergoing other replacement therapies.[38,39] Moreover, several studies indicated that the psychological impact of a disease can affect compliance with medical treatments.[40,41] A study showed that the non-adherence to medical prescriptions is a consistent concern in patients on PD causing major difficulties to manage their treatments,[41] that may include the adherence to the dietetic regimen. This aspect should be taken into consideration to effectively improve patient's outcomes. In fact, previous studies showed that patients on renal replacement therapy presented lower adherence to therapy, contributing to a greater morbidity and earlier mortality.[42] Our study showed significant modifications in serum phosphorus levels during the follow-up, suggesting a possible lower adherence to the therapy and to the dietetic regimen in younger patients and better acceptance of the specific food restriction in older adults. Patients with ESRD present a markedly increased cardiovascular risk and increased serum phosphate levels can have several negative effects on organ functions being associated with increased cardiovascular events and mortality.[43,44] The relationship between serum phosphorus and cardiovascular mortality has been also evaluated in patients in the REIN and NANHES III study,[45,46] suggesting that phosphorus is an independent predictor of cardiovascular mortality. Moreover, the phosphate-regulating hormone fibroblast growth factor (FGF)-23 is directly associated with left ventricular hypertrophy. Yamamoto et al[47] have shown that higher serum phosphorus intake is associated with higher left ventricular mass. Short-term studies have also shown that dietary phosphate reduction effectively decreases FGF-23 levels.[48] Moreover, our study showed a significant reduction of albumin levels in older patients, suggesting an increased risk of protein-energy wasting in this population, although albumin is a poor marker of nutrition, since it also represents an indirect measure of inflammation. Indeed, PD patients lose albumin in the dialysate, which could also cause hypoalbuminemia, and presumably older patients are not able to replace the losses, due to reduced liver function.[49] Other clinical and instrumental data, including body composition, showed similar results in both groups during the 24-month follow-up, suggesting an equally effective maintenance of most of the clinical and metabolic parameters during PD. Although Group B showed higher BMI with respect to Group A, we did not find differences in term of body composition, in particular regarding lean body mass. In fact, we were expecting a significant reduction of muscle compartment, which is a common feature among older patients. Interestingly, we observed a stable hydration status in patients of both groups. Also, echocardiographic indexes, including LVM index, which represents a clinical parameter to monitor cardiac hypertrophy [50] and possibly skeletal muscle mass modifications,[51] remained unchanged during the follow-up in the 2 groups. We did not observe modifications in atherosclerosis indexes (carotid IMT), although they are age-related and no significant differences in the number of hospitalizations. The 2 groups were comparable regarding specific items of the KDQOL-SF and group B showed no physical or cognitive disabilities that may justify the lack of use of PD.
We acknowledge the limitations of our study. In particular, we enrolled a relatively small, selective cohort of PD patients and a larger population appears necessary to confirm our results. Furthermore, as shown in our results, a significant proportion of patients were on several medications with potential impact on different metabolic indices that may have possibly confounded the results.
Our study showed similar results in term of clinical outcomes between younger and older patients treated with PD. We did not observe increased risk of peritonitis between groups and we found a good control of different clinical parameters and perception of general health during the 24-month follow-up. Our data support the expansion of PD procedure in older adults. Patients’ preferences, after a precise information regarding dialysis techniques, should be elicited and carefully considered by healthcare professionals, considering the lower costs of PD compared to HD.[8,9] Larger randomized clinical trials are needed to confirm the results here presented.
4.1. Compliance with ethical standards
The authors declare that they have no conflict of interest.
This study was supported by institutional funds of the Department of Clinical Medicine, Sapienza University of Rome, Rome, Italy.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
The authors alone are responsible for the content and writing of the article.
The article has been seen and approved by all the authors.
The article is not under consideration for publication elsewhere.
The results presented in this article have not been published previously in whole or part, except in abstract format.
Author contributions
Conceptualization: Silvia Lai, Massimo Testorio, Daniela Mastroluca.
Data curation: Silvia Lai, Maria I. Amabile, Matteo B. Bargagli, Tania G. Musto, Andrea Martinez, Daniela Mastroluca, Alessio Molfino.
Formal analysis: Silvia Lai, Maria I. Amabile, Matteo B. Bargagli, Tania G. Musto, Andrea Martinez, Daniela Mastroluca, Carlo Lai, Paola Aceto, Alessio Molfino.
Investigation: Silvia Lai, Matteo B. Bargagli, Tania G. Musto, Andrea Martinez, Paola Aceto.
Methodology: Silvia Lai, Andrea Martinez, Carlo Lai, Paola Aceto, Alessio Molfino.
Software: Andrea Martinez, Carlo Lai, Paola Aceto.
Supervision: Silvia Lai, Massimo Testorio, Daniela Mastroluca, Paola Aceto, Alessio Molfino.
Validation: Silvia Lai, Massimo Testorio, Daniela Mastroluca, Alessio Molfino.
Visualization: Silvia Lai, Massimo Testorio, Daniela Mastroluca.
Writing – original draft: Silvia Lai, Daniela Mastroluca, Alessio Molfino.
Writing – review & editing: Silvia Lai, Maria I. Amabile, Alessio Molfino.
Footnotes
Abbreviations: BMI = body mass index, BOLDE = Broadening Options for Long-Term Dialysis in the Elderly, CKD = chronic kidney disease, CRP = C-reactive protein, DBP = diastolic blood pressure, ESV = erythrocyte sedimentation velocity, FGF = fibroblast growth factor, HD = hemodialysis, HDL = high-density lipoprotein, IMT = intima-media thickness, iPTH = parathormone, KDQOL-SF = Kidney Disease Quality of Life Short Form, LDL = low-density lipoprotein, LVM = left ventricular mass, MF-BIA = multifrequency bioelectrical impedance analysis, nPCR = normalized protein catabolic rate, PD = peritoneal dialysis, RRF = residual renal function, RRT = renal replacement therapy, SBP = systolic blood pressure.
This study was supported by institutional funds of the Department of Clinical Medicine, Sapienza University of Rome, Rome, Italy.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study. The authors alone are responsible for the content and writing of the article.
The article has been seen and approved by all the authors.
The article is not under consideration for publication elsewhere.
The authors declare no conflicts of interest.
Contributor Information
Collaborators: Study Group on Geriatric Nephrology of the Italian Society of Nephrology (SIN)
References
- [1].Ansell D. Summary of findings in the 2006 UK Renal Registry report (chapter 1). Nephrol Dial Transplant 2007;22: [DOI] [PubMed] [Google Scholar]
- [2].Byrne C, Steenkamp R, Castledine C, Ansell D, Feehally J. UK Renal Registry 12th Annual Report (December 2009): chapter 4: UK ESRD prevalent rates in 2008: national and centre-specific analyses. Nephron Clin Pract 2010;115:c41–67. [DOI] [PubMed] [Google Scholar]
- [3].McDonald S, Excell L, Dent H. McDonald S, Excell L, Livingston B. New patients commencing treatment in 2008. The Thirty-Second Report: Australia and New Zealand Dialysis and Transplant Registry, 2009. Adelaide, Australia: ANZDATA Registry; 2009. 1–2. [Google Scholar]
- [4].Fenton SS, Desmeules M, Jeffery JR, et al. Dialysis therapy among elderly patients; data from the Canadian Organ Replacement Register, 1981-1991. Adv Perit Dial 1993;9:124–9. [PubMed] [Google Scholar]
- [5].Grun RP, Constantinovici N, Normand C, et al. North Thames Dialysis Study Group. Costs of dialysis for elderly people in the UK. Nephrol Dial Transplant 2003;18:2122–7. [DOI] [PubMed] [Google Scholar]
- [6].Burkart J. The future of peritoneal dialysis in the United States: optimizing its use. Clin J Am Soc Nephrol 2009;4:125–31. [DOI] [PubMed] [Google Scholar]
- [7].Lim WH, Dogra GK, McDonald SP, et al. Compared with younger peritoneal dialysis patients, elderly patients have similar peritonitis-free survival and lower risk of technique failure, but higher risk of peritonitis-related mortality. Perit Dial Int 2011;31:663–71. [DOI] [PubMed] [Google Scholar]
- [8].Brown EA. Peritoneal dialysis in elderly patients: clinical experience. Perit Dial Int 2005;25:88–91. [PubMed] [Google Scholar]
- [9].Griva K, Yu Z, Chan S, et al. Age is not a contraindication to home-based dialysis: quality-of-life outcomes favour older patients on peritoneal dialysis regimes relative to younger patients. J Adv Nurs 2014;70:1902–14. [DOI] [PubMed] [Google Scholar]
- [10].Griva K, Kang AW, Yu ZL, et al. Quality of life and emotional distress between patients on peritoneal dialysis versus community-based hemodialysis. Qual Life Res 2014;23:57–66. [DOI] [PubMed] [Google Scholar]
- [11].Harris SA, Lamping DL, Brown EA, et al. North Thames Dialysis Study (NTDS) Group. Clinical outcomes and quality of life in elderly patients on peritoneal dialysis versus hemodialysis. Perit Dial Int 2002;22:463–70. [PubMed] [Google Scholar]
- [12].Brown EA. Should older patients be offered peritoneal dialysis? Perit Dial Int 2008;28:444–8. [PubMed] [Google Scholar]
- [13].Otowa T, Sakurada T, Nagasawa M, et al. Clinical outcomes in elderly (more than 80 years of age) peritoneal dialysis patients: five years’ experience at two centers. Adv Perit Dial 2013;29:43–5. [PubMed] [Google Scholar]
- [14].Prakash S, Coffin R, Schold J, et al. Travel distance and home dialysis rates in the United States. Perit Dial Int 2014;34:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cheng CH, Shu KH, Chuang YW, et al. Clinical outcome of elderly peritoneal dialysis patients with assisted care in a single medical centre: a 25 year experience. Nephrology (Carlton) 2013;18:468–73. [DOI] [PubMed] [Google Scholar]
- [16].Vikrant S. Long-term clinical outcomes of peritoneal dialysis patients: 9-year experience of a single center from north India. Perit Dial Int 2014;34:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Oliver MJ, Quinn RR, Richardson EP, et al. Home care assistance and the utilization of peritoneal dialysis. Kidney Int 2007;71:673–8. [DOI] [PubMed] [Google Scholar]
- [18].De Vecchi AF, Dratwa M, Wiedemann ME. Healthcare systems and end-stage renal disease (ESRD) therapies-an international review: costs and reimbursement/funding of ESRD therapies. Nephrol Dial Transplant 1999;14:31–41. [DOI] [PubMed] [Google Scholar]
- [19].Molfino A, Heymsfield SB, Zhu F, et al. Prealbumin is associated with visceral fat mass in patients receiving hemodialysis. J Ren Nutr 2013;23:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Twardowski ZJ, Nolph KD, Khanna R. Peritoneal equilibration test. Perit Dial Bull 1987;7:138–47. [PubMed] [Google Scholar]
- [21].Misra M, Khanna R. The clinical interpretation of peritoneal equilibration test. Semin Dial 2014;27:598–602. [DOI] [PubMed] [Google Scholar]
- [22].Williams B, Poulter NR, Brown MJ, et al. British Hypertension Society guidelines for hypertension management 2004 (BHS-IV): summary. BMJ 2004;328:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu F, Wystrychowski G, Kitzler T, et al. Application of bioimpedance techniques to peritoneal dialysis. Contrib Nephrol 2006;150:119–28. [DOI] [PubMed] [Google Scholar]
- [24].Martínez Fernández G, Ortega Cerrato A, Masiá Mondéjar J, et al. Efficacy of dialysis in peritoneal dialysis: utility of bioimpedance to calculate Kt/V and the search for a target Kt. Clin Exp Nephrol 2013;17:261–7. [DOI] [PubMed] [Google Scholar]
- [25].Ho CY, Solomon SD. A clinician's guide to tissue Doppler imaging. Circulation 2006;113:396–8. [DOI] [PubMed] [Google Scholar]
- [26].Lai S, Mariotti A, Coppola B, et al. Uricemia and homocysteinemia: nontraditional risk factors in the early stages of chronic kidney disease-preliminary data. Eur Rev Med Pharmacol Sci 2014;18:1010–7. [PubMed] [Google Scholar]
- [27].Lai S, Coppola B, Dimko M, et al. Vitamin D deficiency, insulin resistance, and ventricular hypertrophy in the early stages of chronic kidney disease. Ren Fail 2014;36:58–64. [DOI] [PubMed] [Google Scholar]
- [28].Lai S, Dimko M, Galani A, et al. Early markers of cardiovascular risk in chronic kidney disease. Ren Fail 2014;14:1–8. [DOI] [PubMed] [Google Scholar]
- [29].Lang RM, Bierig M, Devereux RB, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- [30].Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–67. [DOI] [PubMed] [Google Scholar]
- [31].Goller JL, McMahon JM, Rutledge C, et al. Dialysis adequacy and self-reported health status in a group of CAPD patients. Adv Perit Dial 1997;13:128–33. [PubMed] [Google Scholar]
- [32].Laplante S, Krepel H, Simons B, et al. Offering assisted peritoneal dialysis is a cost-effective alternative to the current care pathway in frail elderly Dutch patients. Int J Healthc Manag 2013;6:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Power A, Brown E. Optimising treatment of end-stage renal disease in the elderly. Nephron Clin Pract 2014;124:202–8. [DOI] [PubMed] [Google Scholar]
- [34].Yang X, Fang W, Kothari J, et al. Clinical outcomes of elderly patients undergoing chronic peritoneal dialysis: experiences from one center and a review of the literature. Int Urol Nephrol 2007;39:1295–302. [DOI] [PubMed] [Google Scholar]
- [35].Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis Study of elderly people on dialysis: a prospective cohort study. Lancet 2000;356:1543–50. [DOI] [PubMed] [Google Scholar]
- [36].Brown EA, Johansson L, Farrington K, et al. Broadening options for long-term dialysis in the elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant 2010;25:3755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ulutas O, Farragher J, Chiu E, et al. Functional disability in older adults maintained on peritoneal dialysis therapy. Perit Dial Int 2016;36:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ginieri-Coccossis M, Theofilou P, Synodinou C, et al. Quality of life, mental health and health beliefs in haemodialysis and peritoneal dialysis patients: investigating differences in early and later years of current treatment. BMC Nephrol 2008;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cameron JI, Whiteside C, Katz J, et al. Differences in quality of life across renal replacement therapies: a meta-analytic comparison. Am J Kidney Dis 2000;35:629–37. [DOI] [PubMed] [Google Scholar]
- [40].Fukunishi I, Maeda K, Kubota M, et al. Association of alexithymia with low utilization and perception on a measure of social support in patients on peritoneal dialysis. Psychol Rep 1997;80:127–30. [DOI] [PubMed] [Google Scholar]
- [41].Wu AW, Fink NE, Marsh-Manzi JV, et al. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol 2004;15:743–53. [DOI] [PubMed] [Google Scholar]
- [42].DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101–7. [DOI] [PubMed] [Google Scholar]
- [43].Griva K, Lai AY, Lim HA, et al. Non-adherence in patients on peritoneal dialysis: a systematic review. PLoS One 2014;9:890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Christensen AJ, Ehlers SL. Psychological factors in end-stage renal disease: an emerging context for behavioral medicine research. Review. J Consult Clin Psychol 2002;70:712–24. [PubMed] [Google Scholar]
- [45].Zoccali C, Ruggenenti P, Perna A, et al. Phosphate may promote CKD progression and attenuate renoprotective effect of ACE inhibition. J Am Soc Nephrol 2011;22:1923–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chang AR, Grams ME. Serum Phosphorus and Mortality in the Third National Health and Nutrition Examination Survey (NHANES III): effect modification by fasting. Am J Kidney Dis 2014;64:567–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yamamoto KT, Robinson-Cohen C, de Oliveira MC, et al. Dietary phosphorus is associated with left ventricular mass: the Multi-Ethnic Study of Atherosclerosis. Kidney Int 2013;83:707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Newsome B, Ix JH, Tighiouart H, et al. Effect of protein restriction on serum and urine phosphate in the modification of diet in renal disease (MDRD) study. Am J Kidney Dis 2013;61:1045–6. [DOI] [PubMed] [Google Scholar]
- [49].Zyga S, Christopoulou G, Malliarou M. Malnutrition-inflammation-atherosclerosis syndrome in patients with end-stage renal disease. J Ren Care 2011;37:12–5. [DOI] [PubMed] [Google Scholar]
- [50].Lai S, Molfino A, Russo GE, et al. Cardiac, inflammatory and metabolic parameters: hemodialysis versus peritoneal dialysis. Cardiorenal Med 2015;5:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Molfino A, Papa A, Gasperini-Zacco ML, et al. Left ventricular mass correlates with lean body mass in patients with disease-associated wasting. J Cachexia Sarcopenia Muscle 2014;5:251–2. [DOI] [PMC free article] [PubMed] [Google Scholar]


