Abstract
Background:
The use of contrast agents during coronary angiography can result in contrast-induced nephropathy (CIN), particularly in patients with renal dysfunction. On the contrary, different contrast agents can induce different degree of changes in cardiac function. The objective of our meta-analysis was to compare the clinical safety of iso-osmolar contrast medium iodixanol to low-osmolar contrast medium iopromide in patients with renal insufficiency undergoing coronary angiography with or without percutaneous coronary intervention (PCI).
Methods:
We searched Medline, Embase, Cochrane Library, and reference lists to identify randomized controlled trials that compared iodixanol to iopromide with the incidence of CIN as an endpoint in renal impaired patients undergoing coronary angiography. Outcome measures were the incidence of CIN, absolute peak serum creatinine (Scr) increase from baseline and a composite of cardiovascular adverse events.
Results:
A total of 8 trials with 3532 patients were pooled; 1759 patients received iodixanol and 1773 patients received iopromide. There was no significant difference in the incidence of CIN (summary odds ratio [OR] 0.50, 95% confidence interval [CI] 0.19–1.35, P = .17) and peak Scr increase (mean difference −0.01 mg/dL, 95% CI −0.08 to 0.05, P = .69) when iodixanol was compared with iopromide. But iodixanol was associated with a statistically significant reduction in cardiovascular adverse events when compared with iopromide (OR 0.47, 95% CI 0.30–0.73, P = .0009).
Conclusions:
Analysis of pooled data from 8 randomized controlled trials of iodixanol against iopromide in patients with renal insufficiency undergoing coronary angiography with or without PCI showed that iodixanol nonsignificantly reduced the incidence of CIN, but was associated with a significantly reduced risk of cardiovascular adverse events when compared with iopromide.
Keywords: angiography, iodixanol, iopromide, meta-analysis
1. Introduction
Contrast-induced nephropathy (CIN; also referred to as contrast-induced acute kidney injury [CI-AKI]) is 1 of the most clinically important complications of interventional coronary procedure.[1] It is associated with considerably increased morbidity and mortality,[2–4] and also prolonged hospitalization.[5] CIN typically occurs within the first 2 to 3 days after contrast administration, usually characterized by an absolute increase in serum creatinine concentration of at least 0.5 mg/dL or by a relative increase of at least 25% from baseline.[6] Generally, CIN is reversible, but it can occasionally lead to chronic renal failure, increasing long-term morbidity and mortality.[3,7–9]
Although patients with preserved renal function are at low risk for developing CIN,[10] the proportion may be much higher in patients with chronic kidney disease (CKD).[11] The choice of contrast media may also influence the risk of CIN. In mixed study populations, high-osmolar contrast media has been found to result in more CIN than the more contemporary low-osmolar contrast media (LOCM) or the iso-osmolar contrast media iodixanol.[12,13] Controversy remains whether iodixanol has a lower risk of CIN than LOCM. Previous several relevant meta-analyses showed inconsistent results.[14–17] This may be attributed to the great difference between diverse LOCMs and between patients with different risk stratifications. A previous review focusing on CIN suggested that low-osmolar contrast agents are reasonable for moderate-risk patients and iso-osmolar contrast is indicated for the highest-risk patients (estimated glomerular filtration rate [eGFR] <30 mL/min/1.73 m2 with diabetes, heart failure, or urgent percutaneous coronary intervention [PCI] for acute coronary syndrome).[18] However, the validity of this standpoint remains unknown. Pooling data of trials comparing iodixanol to certain specific LOCM may be a better choice. Iopromide, as a nonionic, monomeric LOCM, has been compared with iodixanol in clinical safety in many trials, but the results are controversial. Although a previous meta-analysis[15] comparing iodixanol to LOCMs in renal safety contained an iopromide subgroup, no restriction such as type of radiographic procedure or baseline renal function was imposed on the patients included, making the result more generalizable, but with lack of precision. In addition, since the publication of this study, several new randomized trials comparing iodixanol and iopromide in renal impaired patients receiving coronary angiography have been performed,[19–24] calling for a need to do an update. On the contrary, there remains conflicting results and raised doubts whether iodixanol and iopromide have different impacts on cardiac function during coronary angiography.[19,25–27] In view of the above, the aim of this meta-analysis was to provide a comprehensive comparison of clinical safety between iodixanol and iopromide in patients with renal insufficiency undergoing interventional coronary procedures.
2. Methods
2.1. Search strategy
This systematic review and meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.[28] Because our meta-analysis was based on previously published studies, the ethic approval and patient consent were not required.
An extensive search of literature was performed in Pubmed, Embase, and the Cochrane library for relevant articles from 1995 through March, 2017. The complete search used for Pubmed was: ((Iopromide[Text Word]) OR lopromid[Text Word]) OR iopromid[Text Word]) OR Ultravist[Text Word])) AND ((Iodixanol[Text Word]) OR Visipaque[Text Word]). A similar search was performed in EMBASE and the Cochrane library. We considered all potential eligible articles published in English, irrespective of the primary outcome. Reference lists of the studies selected by searching were also used to identify additional articles meeting the inclusion in this meta-analysis by manual search.
2.2. Selection criteria and quality evaluation
The inclusion criteria were as follows: study design—randomized controlled trials (RCTs); study population—patients referred for coronary angiography with or without PCI were eligible if they had a calculated creatinine clearance (CrCl) ≤60 mL/min according to the Cockcroft and Gault formula,[29] or eGFR ≤60 mL/min/1.73 m2; comparison—to compare clinical safety between iodixanol and iopromide; outcome measurements—reported the incidence of CIN. We treated CI-AKI as equal to CIN. No restriction was imposed on the definition of CIN or on the time elapsed before CIN occurred. Studies which did not meet the above criteria were excluded from selection. Two independent investigators reviewed study titles and abstracts for possible inclusion. If discrepancies existed between them, the opinion of a third reviewer was adopted. The risk for bias was assessed using the risk of bias assessment tools made by the Cochrane Collaboration.[30] The quality of trials was assessed using the quality score proposed by the grading of recommendations assessment, development and evaluation working group.[31]
2.3. Data extraction and outcomes
We extracted baseline demographic, clinical, and procedural characteristics from each selected study, including concentration of iodixanol and iopromide, total number of participants, mean age, race, baseline serum creatinine, mean estimated eGFR, average contrast volume, average iodine dose, percentage of patients undergoing PCI, information about sex, diabetes, prophylactic hydration, use of N-acetylcysteine (NAC), the definition of CIN used, and the inclusion criteria of renal function. The primary outcomes were the incidence of CIN defined as a postdose serum creatinine (Scr) increase ≥25% or ≥0.5 mg/dL within 3 days. Secondary outcomes were absolute peak Scr increase from baseline to day 7 after contrast administration and a composite of cardiovascular adverse events as defined by each study.
2.4. Statistical analysis
A systematic review and meta-analysis of RCTs was performed to compare the renal safety between iodixanol and iopromide. For analyses of the proportion of CIN and adverse events, we calculated the overall odds ratio (OR) with 95% confidence intervals (CIs). We analyzed peak Scr increase as a continuous variable, and the pooled estimates was presented as mean difference (MD) with 95% CI. Heterogeneity was assessed using the Cochrane Q test and quantified by calculating I2 statistic, with P < .1 and I2 >50% regarded as being statistically significant. Random-effects or fixed-effects models were used depending on the heterogeneity of the studies included. For outcomes with moderate-to-high heterogeneity, we performed a sensitivity analysis, in which the pooled estimates were recalculated by omitting 1 study at a time to detect which study is the main source of heterogeneity. In addition, meta-regressions and subgroup analyses were performed when needed. Publication bias was assessed by constructing a funnel plot. We tested funnel plot asymmetry using Egger test, in which P value <.1 was indicated of significant publication bias. The trim-and-fill method was used to assess the influence of publication bias on the interpretation of results. All statistical analyses were performed with Review Manager (Version 5.1, the Cochrane Collaboration) and Stata (Version 12.0).
3. Results
According to the searching strategy as depicted above, a total of 649 articles were found, of which 139 were found in Pubmed, 411 in Embase, 96 in the Cochrane Library, and 3 by manual search. Among these articles, 8 trials fulfilled the criteria for inclusion in the meta-analysis. The specific screening flow is shown in Fig. 1.
Figure 1.
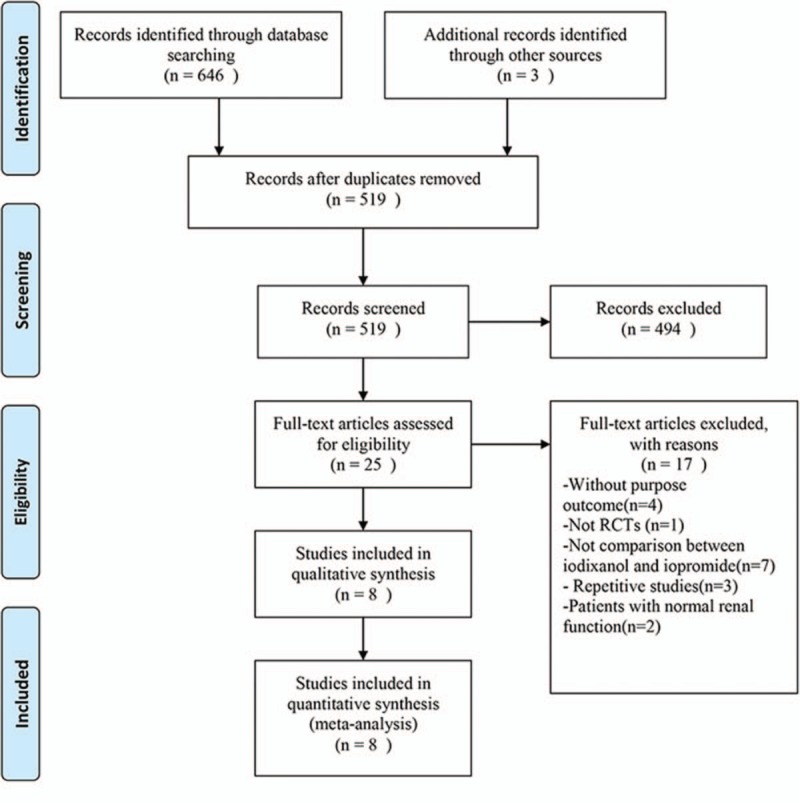
Results of search in Pubmed, Embase, and the Cochrane Library.
Of the 8 studies finally identified, 2 studies had no full text, but the data we needed were available in their abstracts (both of them reported the incidence of CIN as its primary outcome).[21,24] One trial had no restriction on patients’ renal function, but a subgroup for patients with CKD was set, which provided the data we needed.[19] In addition to it, other 7 trials exclusively included patients with renal insufficiency defined as either CrCl ≤60 mL/min or eGFR ≤60 mL/min/1.73 m2. On the basis of renal insufficiency, 1 trial only included patients with congestive heart failure and another only patients with ST-segment elevation myocardial infarction (STEMI),[19,20] of which all the patients received PCI <12 hours after the onset of symptoms were considered eligible.[19] That is to say, patients of these 2 studies were of highest risk according to the criteria referred by the review by McCullough et al.[18] The proportion of patients receiving PCI differed between trials. Except for 1 study comparing iodixanol 320 mg I/mL to iopromide 300 mg I/mL,[22] all other 7 studies compared iodixanol 320 mg I/mL to iopromide 370 mg I/mL. In terms of prevention measures, all patients in 8 studies received hydration. However, the use of NAC was identified in only 3 trials.[19,22,32] Each of the 8 trials used CIN as a primary or secondary outcome. Most studies defined CIN as a relative increase in creatinine by at least 25% or an absolute increase by at least 0.5 mg/dL. Only 1 study defined CIN as a relative increase in Scr of ≥50% from baseline,[23] but it also reported the proportion of a postdose Scr increase of ≥25% as its secondary endpoint, which was extracted by us as the incidence of CIN in our meta-analysis. Overall, 3532 patients were included in our meta-analysis, with 1759 in iodixanol group and 1773 in iopromide group. All of them were patients with renal insufficiency undergoing coronary angiography with or without PCI. Characteristics of the 8 trials were summarized in Table 1. No relevant differences were noted between the baseline characteristics of the iodixanol and iopromide groups of patients in each of the individual trials.
Table 1.
Basic characteristics of included studies.
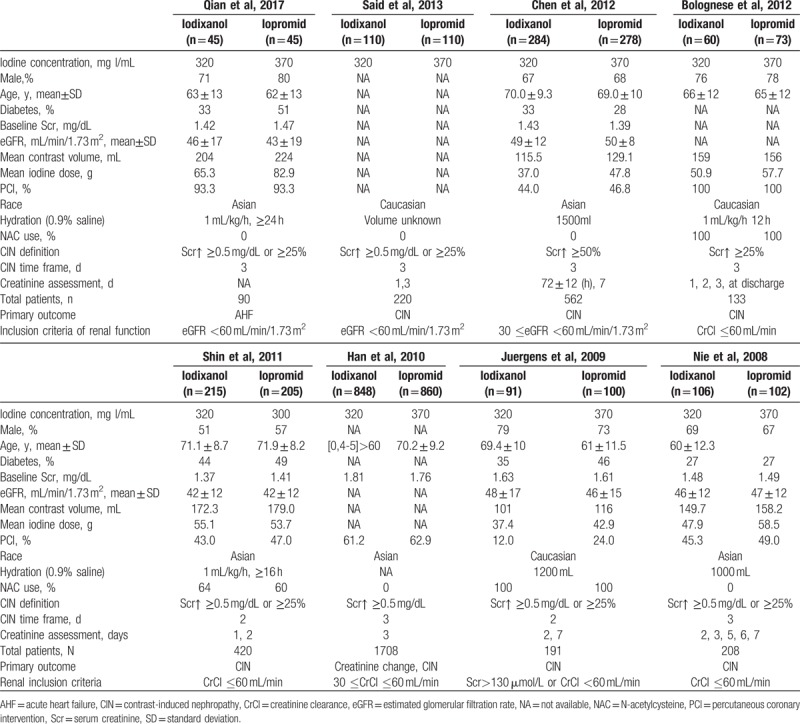
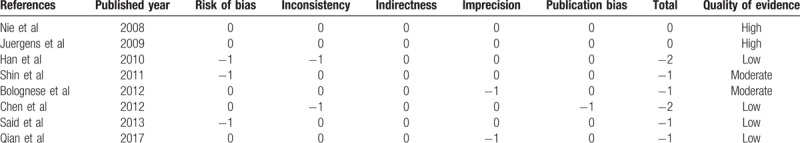
Table 2 shows the assessment of evidence quality of the trials. All studies included were RCTs with relatively high quality. Other than the 2 trials without full-text remaining unknown in risk of bias,[21,24] all other 6 RCTs reported adequate randomization, none was stopped early, and 3 were multicenter. Three trials did not specify whether data collectors and outcome assessors were masked to treatment allocation.[21,22,24] Only 1 trial was funded by industry.[23]
Table 2.
Grading of quality of studies according to GRADE.
Except that one study lacked baseline Scr value, other 7 studies all showed a baseline Scr of below 2 mg/dL. There is a notion that a relative increase of Scr ≥25% is more sensitive than an absolute increase of Scr ≥0.5 mg/dL from baseline in patients with minor degrees of renal insufficiency (serum creatinine level of less than 2 mg/dL).[33] Accordingly, if a study reported outcomes of CIN of both the 2 definitions, data of the relative definition (ΔScr ≥25%) would be adopted. As a result, because all the 8 studies (n = 3532 participants) reported the incidence of increase in Scr ≥25% from baseline, the definition of CIN in our meta-analysis was actually ΔScr ≥25%. As shown in Fig. 2, although there was an overall trend in favor of iodixanol, no significant difference was found in the risk of CIN between the iodixanol and iopromide group (OR 0.50, 95% CI 0.19–1.35, P = .17). Because a very high between-study heterogeneity was detected (I2 = 94%, Q = 110.20, P < .00001), a sensitivity analysis was performed by recalculating the OR value after omitting 1 study at a time. As a result, the heterogeneity became much smaller (I2 = 56%, Q = 13.52, P = .04) (Fig. 3) when the study performed by Han et al[24] was omitted, indicating it was the main source of high heterogeneity. The remaining high heterogeneity (I2 = 56%) arouse mainly from the 2 studies by Bolognese et al[19] and Shin et al,[22] respectively (I2 = 0% without including these 2 studies) (Fig. 4). Different from other 6 studies, these 2 trials both demonstrated a nonsignificantly higher risk of CIN with the use of iodixanol compared with iopromide. In addition, we recalculated the OR value by omitting the 2 studies without full text (Han et al[24] and Said et al[21]), and the result remained insignificant (OR 0.80, 95% CI 0.49–1.28, P = .35) (Fig. 5). Meta-regressions did not suggest any significant interaction between the OR of CIN for comparison of iodixanol with iopromide and variables such as average age (slope 0.100, standard error [SE] 0.051, P = .143), proportion of diabetes (slope 6.320, SE 2.75, P = .105), mean baseline eGFR (slope −0.145, SE 0.071, P = .135), use of NAC (slope 0.921, SE 0.399, P = .104) or any of the other variables studied (data not shown). We therefore performed a subgroup analysis according to the use of NAC. Still, there was no significant difference in each subgroup, but the advantage of iodixanol over iopromide in the incidence of CIN seemed to be reversed in patients using NAC, with a very low heterogeneity (I2 = 0). The between-subgroup differences was relatively apparent but without statistical significance (P = .07) (Fig. 6). We also performed a subgroup analysis by whether patients are in moderate risk or highest risk, referring to the criterion by the review mentioned above.[18] However, iopromide seemed to show no advantage in either moderate-risk subgroup or highest-risk subgroup (Fig. 7). Last, but not the least, because diabetes is known to be a modulator of CIN,[34] we also performed a subgroup analysis in patients with and without diabetes. A total of 3 studies[23,32,35] offered detailed data on CIN, respectively, in patients with and without diabetes. As a result, there was still no significant difference between iodixanol and iopromide, both in patients with and without diabetes mellitus (P = .99 and P = .69). The between-subgroup difference was also statistically insignificant (P = .78) (Fig. 8). However, a further analysis demonstrated that on the base of renal insufficiency, the occurrence of CIN in the smaller subset of patients with diabetes was significantly more frequent than that in patients without diabetes (9.95% vs 5.93%, OR 1.58, 95% CI 1.02–2.46, P = .04), as was shown in Fig. 9. In this analysis, there was publication bias on Egger test (P = .017), but further analysis using trim-and-fill test indicated this publication bias did not impact the estimates (ie, no trimming performed because data unchanged).
Figure 2.
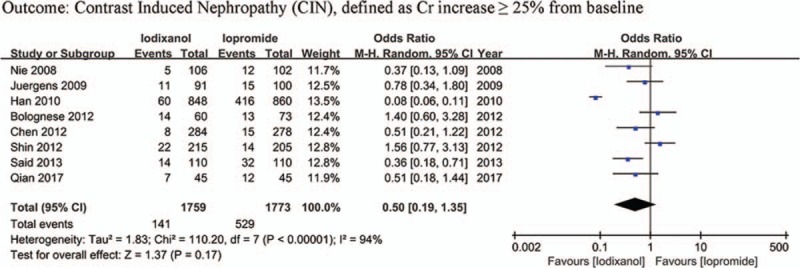
Forest plot of odds ratio (OR) of contrast-induced nephropathy (CIN) when iodixanol is compared with iopromide.
Figure 3.
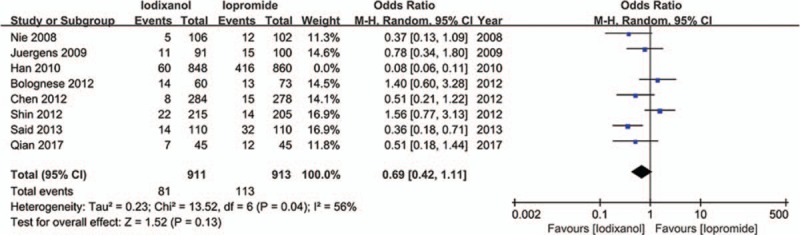
Forest plot of meta-analysis of the risk of CIN when iodixanol is compared with iopromide after the study by Han et al[24] was omitted.
Figure 4.
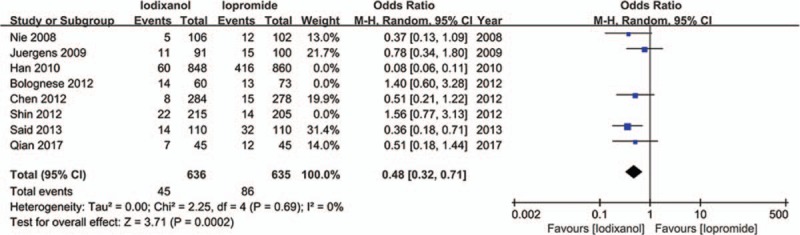
Odds ratio (OR) of CIN for comparison of iodixanol with iopromide after removing the studies by Han et al[24], Shin et al,[22] and Bolognese et al[19]. CIN = contrast-induced nephropathy.
Figure 5.
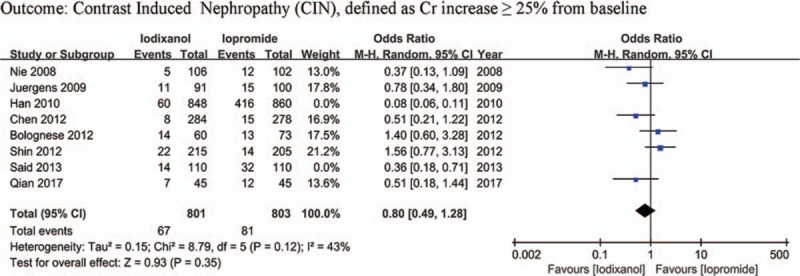
Odds ratio (OR) of CIN for comparison of iodixanol with iopromide after removing the studies by Han et al[24] and Said et al[21]. CIN = contrast-induced nephropathy.
Figure 6.
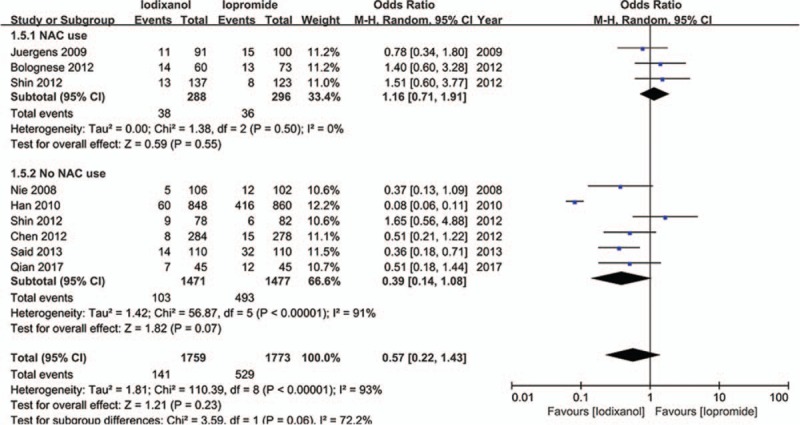
Odds ratio (OR) of CIN for comparison of iodixanol with iopromide (subgroup analysis: NAC use vs no NAC use). CIN = contrast-induced nephropathy, NAC = N-acetylcysteine.
Figure 7.
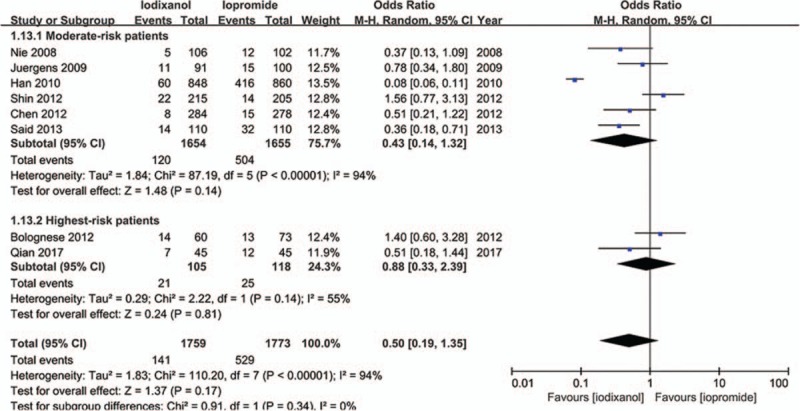
Odds ratio (OR) of CIN for comparison of iodixanol with iopromide (subgroup analysis: moderate-risk patients vs highest-risk patients). CIN = contrast-induced nephropathy.
Figure 8.
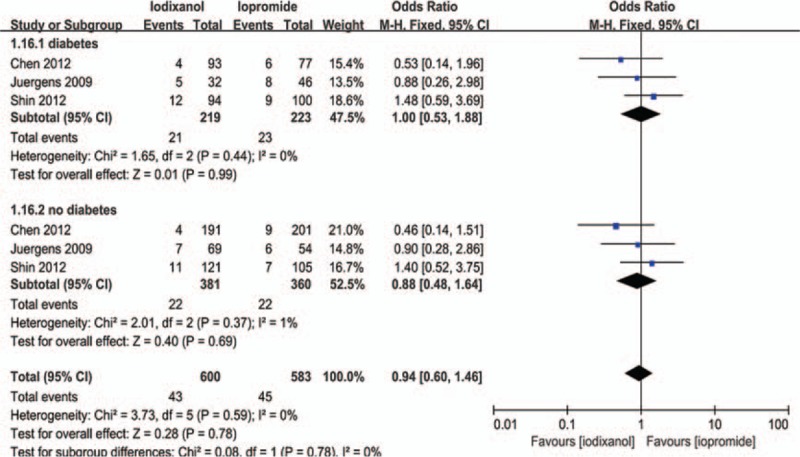
Odds ratio (OR) of CIN for comparison of iodixanol with iopromide in patients with and without diabetes. CIN = contrast-induced nephropathy.
Figure 9.
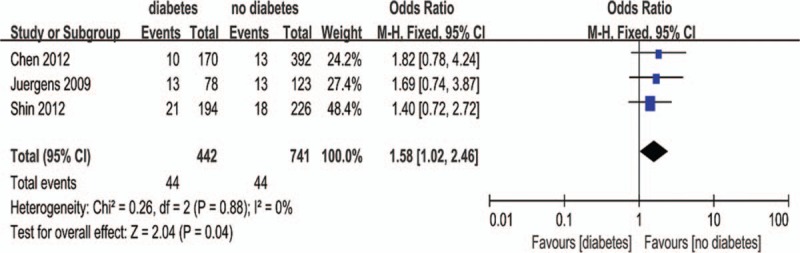
Meta-analysis of the risk of CIN in patients with diabetes versus patients without diabetes. CIN = contrast-induced nephropathy.
Four studies (n = 1381 participants) reported the peak increase in Scr after contrast administration. Pooling the data of these studies showed no significant difference in the MD of the iodixanol and iopromide group (MD −0.01 mg/dL, 95% CI −0.08 to 0.05, P = .69) (Fig. 10), with statistically significant between-study heterogeneity (I2 = 51%, Q = 6.17, P = .10). Sensitivity analysis showed this heterogeneity mainly resulted from the trial by Nie et al[36] (I2 = 0% after this trial was omitted). However, the result was not changed by exclusion of this study. There was no significant publication bias in this analysis (P = .628).
Figure 10.
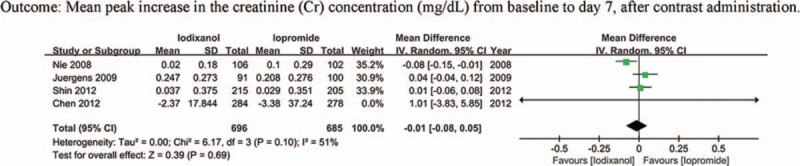
Weighted mean difference of mean peak Scr increase for comparison of iodixanol with iopromide.
Extractable data of cardiovascular adverse events were available in 4 studies (n = 1280 participants), and a total of 104 cardiovascular adverse events were recorded, including all-cause death, stroke, myocardial infarction, angina pectoris, new arrhythmias, acute heart failure, and repeat revascularization. Follow-up time for investigation of adverse events was 90 days in the trial performed by Qian et al[20] and 30 days in other 3 trials.[22,23,36] The iodixanol group had a significantly lower rate of cardiovascular adverse events than the iopromide group (OR 0.47, 95% CI 0.30–0.73, P = .0009) (Fig. 11). No statistically significant between-study heterogeneity was detected (I2 = 0%, Q = 2.53, P = .47), and thus a fixed-effects model was used. No significant publication bias was evident (P = .575).
Figure 11.
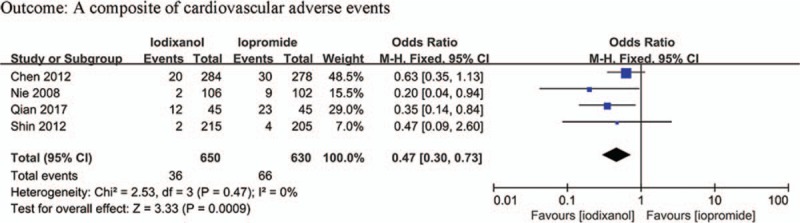
Odds ratio (OR) of total cardiovascular adverse events for comparison of iodixanol with iopromide.
4. Discussion
The main finding of this meta-analysis of RCTs was that in the population of patients with renal insufficiency undergoing coronary angiography with or without PCI. The iso-osmolar, nonionic dimer iodixanol was associated with a lower risk in the incidence of CIN that was not statistically significant when compared with the low-osmolar, nonionic monomeric iopromide. There was no significant difference in the maximum increase in Scr after contrast exposure between iodixanol and iopromide. Iodixanol had a significant lower risk of adverse events after contrast media (CM) administration compared with iopromide.
In general, our findings for the incidence of CIN between iodixanol and iopromide are consistent with other meta-analyses performed by Reed et al,[15] Heinrich et al,[16] and From et al.[17] These 3 studies drew a similar conclusion that no significant difference in the risk of CIN could be found between iodixanol and LOCM other than iohexol, of which iopromide was included. However, the results of our meta-analysis seem to conflict with a much earlier meta-analysis by McCullough et al,[14] both in the outcome of CIN and the maximum increase in Cr. In that study iodixanol was demonstrated to had a lower risk for CIN than LOCM among patients with CKD, and the maximum increase in Cr was significantly less in patients treated with iodixanol than with LOCM, both in all patients (P < .001) and in patients with CKD (P = .004). We must point out, although each of the meta-analyses mentioned above had subgroup analysis comparing iodixanol to iopromide, they had no further restriction on either reason for contrast or renal function on the patients included, weakening the comparability between these meta-analyses and ours. With respect to the notion introduced by McCullough et al[18] that low-osmolar contrast agents are reasonable for moderate-risk patients and iso-osmolar contrast is indicated for the highest-risk patients, we did verification by performing subgroup analysis according to the risk stratification criterion in that review. However, iopromide as a low-osmolar contrast showed no advantage in the reduction of CIN in moderate-risk patients. On the contrary, there was no indication that the advantage of iodixanol over iopromide in highest-risk patients was more remarkable than that in moderate-risk patients. Thus, the conclusion by McCullough et al seemed equivocal from our meta-analysis, probably because of the limitation of the sample size of the population included.
Our subgroup analysis on diabetes verified the notion that diabetes is a risk factor for CIN, which was proved by many previous studies.[34,37,38] However, it seemed that the lack of significant difference between iodixanol and iopromide in the risk of CIN in renal impaired patients would not be changed by whether the patient was combined with diabetes or not. This finding was consistent with several original researches[22,23,32] and a meta-analysis by From et al.[17]
Our findings that iodixanol may be associated with a lower occurrence of cardiovascular adverse events seem to contradict another meta-analysis performed by Zhang et al,[39] which shows no significant reduction in cardiovascular events with iodixanol when compared with LOCM overall in all patients. However, it should be noted that in that analysis, 7 of 13 were trials comparing iodixanol with ioxaglate. In contrast, only 2 were trials comparing iodixanol with iopromide. Ioxaglate, as an ionic dimer, has great difference in chemical with nonionic monomer iopromide.[40] Therefore, conclusions from that analysis may, at most, be drawn for the comparative cardiovascular safety of iodixanol versus ioxaglate, but not for iodixanol versus iopromide. The benefit of iodixanol over iopromide in cardiac safety may be explained by the difference in electrophysiological and hemodynamic effects of the 2 agents.[26,41] Iodixanol, a nonionic dimer, affects hemodynamic and ECG parameters to a lesser degree than nonionic monomeric CM iopromide, in part, due to the iso-osmolarity. In a cardioangiographic study,[42] systolic pressure decreased and heart rate increased with iopromide, whereas iodixanol did not significantly influence these hemodynamic parameters. Furthermore, the mean increases in femoral blood flow measured after injection of iodixanol or ioprmoide in the aorta were significantly lower with iodixanol.[43] From the above, iodixanol induces minor changes in cardiac function than iopromide, thereby may lead to the lower occurrence of cardiovascular adverse events.
Finally, given the relative absence of difference in CIN with use of iodixanol and iopromide, cost emerged as an important factor in the choice of which contrast to use. One study comparing the cost-effectiveness of various contrast medium suggested that iodixanol is less costly when compared with iopromide,[44] whereas another study drew an opposite conclusion.[45] This could be due to the variation of pricing for agents among institutions depending on the cost per vial and the packaging of materials.
A major strength of this meta-analysis is the precision of the comparison between iodixanol and iopromide. It is the first meta-analysis that specifically compared iodixanol to iopromide in patients with renal insufficiency undergoing coronary angiography with or without PCI. Previous several meta-analyses have suggested that one must be cautious when interpreting results of analyses that are based on the pooling of all LOCM, because the risk of CIN may not be explained by osmolarity alone.[15–17] Ionicity, viscosity, molecular structure, and direct molecular toxicity may also play important roles in the pathogenesis of CIN,[46–49] stressing the necessity of comparing iodixanol to certain specific LOCM.
In previous studies, the population studied was heterogeneous.[14–17,39] On one hand, high-risk and healthy patients were all included. On the other hand, reasons for contrast were various, amplifying the population heterogeneity. As is widely accepted, there is a close relationship between baseline renal function and the risk of CIN.[6,10,11] It has even been suggested that renal dysfunction may amplify the difference between iodixanol and LOCM in the incidence of CIN.[14,50] Therefore, we think reducing the population heterogeneity should be highlighted. However, authors of previous studies attributed heterogeneity mainly to confounding factors such as the use of NAC and the amount or type of intravenous hydration given before and after the contrast exposure.[14–16] It is true that the use of vigorous hydration in combination with NAC could reduce the risk of CIN, thereby weakening the validity of comparison between 2 contrast media. However, as our subgroup analysis showed, even in studies in which NAC was used, there was no significant difference between iodixanol and iopromide in the risk of CIN, and the difference between the NAC arm and the no NAC arm was statistically nonsignificant. Similar conclusion can be drawn in hydration. Differences between the contrast media and the population seem to play a major role in risk reduction, rather than differences in hydration and the use of NAC.[51–53]
A potential limitation of our meta-analysis is the inclusion of 2 abstracts (studies by Said et al[21] and Han et al[24]). Considering the need to minimize publication bias and to guarantee validity, we did not exclude them. Most importantly, we also performed our analyses without including data from abstracts; again, our results were not changed by exclusion of those data. Even if the study by Han et al led to a huge heterogeneity, given its large sample and the unchanged results after its exclusion, we ultimately preserved it.
The comparative small number of included studies seems to be a weakness of our meta-analysis, which should be mainly ascribed to our rigorous inclusion criterion. However, the number of total participants and the incidences of CIN and cardiovascular events were substantial, which significantly increase the statistical power of the analysis.
Another potential limitation of this study is that although we performed a subgroup analysis of CKD ± diabetes mellitus in the outcome of CIN, we failed to perform a similar subgroup analysis in the outcome of cardiovascular adverse events, mainly because data of this outcome were not uniformly available among patients with baseline diabetes.
5. Conclusions
In conclusion, results of this meta-analysis demonstrated that iso-osmolar iodixanol, as compared with low-osmolar iopromide, is associated with a nonsignificantly lower risk of CIN, but a significantly reduced risk of cardiovascular adverse events after coronary angiography in patients with renal insufficiency. Despite an overall trend in favor of iodixanol, there is no significant difference between iodixanol and iopromide in renal safety in both moderate-risk and highest-risk patients. No significant difference could be found between iodixaol and iopromide in the incidence of CIN in renal insufficiency patients with and without diabetes. Nonetheless, considering the overall renal and cardiovascular security, our analysis still lends support to the use of iodixanol for the contrast application in coronary angiography in patients with CKD when compared with iopromide. Further studies are needed to confirm whether the use of iodixanol or LOCM should be dependent on the patients’ risk stratification.
Author contributions
Conceptualization: Min Chen, Ya-Feng Zhou.
Data curation: Nan-Nan Zhang.
Formal analysis: Qing Rui.
Funding acquisition: Ya-Feng Zhou.
Methodology: Hua-Jia Yang.
Resources: Ya-Feng Zhou.
Writing – original draft: Jun-Yi Zhang.
Writing – review & editing: Yu-Feng Jiang.
Footnotes
Abbreviations: CI = confidence interval, CI-AKI = contrast-induced acute kidney injury, CIN = contrast-induced nephropathy, CKD = coronary kidney disease, CrCl = creatinine clearance, eGFR = estimated glomerular filtration rate, LOCM = low-osmolar contrast medium, MD = mean difference, NAC = N-acetylcysteine, OR = odds ratio, PCI = percutaneous coronary intervention, Scr = serum creatinine, SE = standard error.
JZ and YJ contributed equally to this work.
Funding: This work was supported by grants from National Natural Science Foundation of China (81170174), Natural Scientific Fund of Jiangsu Province's Key Provincial Talents program (ZDRCA2016043), Jiangsu Province's 333 High-Level Talents Project (BRA2017539). The funders had no roles in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that they have no conflicts of interest.
References
- [1].Seeliger E, Sendeski M, Rihal CS, et al. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 2012;33:2007–15. [DOI] [PubMed] [Google Scholar]
- [2].From AM, Bartholmai BJ, Williams AW, et al. Mortality associated with nephropathy after radiographic contrast exposure. Mayo Clin Proc 2008;83:1095–100. [DOI] [PubMed] [Google Scholar]
- [3].McCullough PA, Wolyn R, Rocher LL, et al. Acute renal failure after coronary intervention. Am J Med 1997;103:368–75. [DOI] [PubMed] [Google Scholar]
- [4].Rihal CS. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation 2002;105:2259–64. [DOI] [PubMed] [Google Scholar]
- [5].Mehran R, Brar S, Dangas G. Contrast-induced acute kidney injury. Underappreciated or a new marker of cardiovascular mortality? J Am Coll Cardiol 2010;55:2210–1. [DOI] [PubMed] [Google Scholar]
- [6].Morcos SK. Contrast media-induced nephrotoxicity–questions and answers. Br J Radiol 1998;71:357–65. [DOI] [PubMed] [Google Scholar]
- [7].Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 2004;44:1780–5. [DOI] [PubMed] [Google Scholar]
- [8].Liss P, Persson PB, Hansell P, et al. Renal failure in 57 925 patients undergoing coronary procedures using iso-osmolar or low-osmolar contrast media. Kidney Int 2006;70:1811–7. [DOI] [PubMed] [Google Scholar]
- [9].Gupta R, Gurm HS, Bhatt DL, et al. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv 2005;64:442–8. [DOI] [PubMed] [Google Scholar]
- [10].Barrett BJ, Parfrey PS, Vavasour HM, et al. Contrast nephropathy in patients with impaired renal function: high versus low osmolar media. Kidney Int 1992;41:1274–9. [DOI] [PubMed] [Google Scholar]
- [11].Morcos SK, Thomsen HS, Webb JAW. Contrast-media-induced nephrotoxicity: a consensus report. Eur Radiol 1999;9:1602–13. [DOI] [PubMed] [Google Scholar]
- [12].Barrett BJ, Carlisle EJ. Metaanalysis of the relative nephrotoxicity of high- and low-osmolality iodinated contrast media. Radiology 1993;188:171–8. [DOI] [PubMed] [Google Scholar]
- [13].Rudnick MR, Goldfarb S, Wexler L, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. Kidney Int 1995;47:254–61. [DOI] [PubMed] [Google Scholar]
- [14].McCullough PA, Bertrand ME, Brinker JA, et al. A meta-analysis of the renal safety of isosmolar iodixanol compared with low-osmolar contrast media. J Am Coll Cardiol 2006;48:692–9. [DOI] [PubMed] [Google Scholar]
- [15].Reed M, Meier P, Tamhane UU, et al. The relative renal safety of iodixanol compared with low-osmolar contrast media: a meta-analysis of randomized controlled trials. JACC Cardiovasc Interv 2009;2:645–54. [DOI] [PubMed] [Google Scholar]
- [16].Heinrich MC, Haberle L, Muller V, et al. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology 2009;250:68–86. [DOI] [PubMed] [Google Scholar]
- [17].From AM, Al Badarin FJ, McDonald FS, et al. Iodixanol versus low-osmolar contrast media for prevention of contrast induced nephropathy: meta-analysis of randomized, controlled trials. Circ Cardiovasc Interv 2010;3:351–8. [DOI] [PubMed] [Google Scholar]
- [18].McCullough PA, Choi JP, Feghali GA, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol 2016;68:1465–73. [DOI] [PubMed] [Google Scholar]
- [19].Bolognese L, Falsini G, Schwenke C, et al. Impact of iso-osmolar versus low-osmolar contrast agents on contrast-induced nephropathy and tissue reperfusion in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention (from the Contrast Media and Nephrotoxicity Following Primary Angioplasty for Acute Myocardial Infarction [CONTRAST-AMI] Trial). Am J Cardiol 2012;109:67–74. [DOI] [PubMed] [Google Scholar]
- [20].Qian G, Yang YQ, Dong W, et al. Comparison of iodixanol and iopromide in patients with renal insufficiency and congestive heart failure undergoing coronary angiography by hemodynamic monitoring. Angiology 2017;68:907–13. [DOI] [PubMed] [Google Scholar]
- [21].Said K, Elgabail E, Adel A, et al. Contrast-induced acute kidney injury in patients with chronic kidney disease undergoing coronary catheterization: a comparative randomized study between iodixanol versus iopromide contrast media. Eur Heart J 2013;34:5490–15490. [Google Scholar]
- [22].Shin DH, Choi DJ, Youn TJ, et al. Comparison of contrast-induced nephrotoxicity of iodixanol and iopromide in patients with renal insufficiency undergoing coronary angiography. Am J Cardiol 2011;108:189–94. [DOI] [PubMed] [Google Scholar]
- [23].Chen Y, Hu S, Liu Y, et al. Renal tolerability of iopromide and iodixanol in 562 renally impaired patients undergoing cardiac catheterisation: the DIRECT study. EuroIntervention 2012;8:830–8. [DOI] [PubMed] [Google Scholar]
- [24].Han Y, Wang S, Wang X, et al. Contrast-induced nephropathy following coronary intervention in elderly, renally impaired patients: a randomised comparison of the renal safety of iodixanol and iopromide. EuroIntervention 2010;6: [Google Scholar]
- [25].Le Feuvre C, Batisse A, Collet JP, et al. Cardiac events after low osmolar ionic or isosmolar nonionic contrast media utilization in the current era of coronary angioplasty. Catheter Cardiovasc Interv 2006;67:852–8. [DOI] [PubMed] [Google Scholar]
- [26].Grynne BH, Nossen JØ, Bolstad B, et al. Main results of the first comparative clinical studies on Visipaque. Acta Radiol 2016;36:265–70. [DOI] [PubMed] [Google Scholar]
- [27].Davidson CJ, Laskey WK, Hermiller JB, et al. Randomized trial of contrast media utilization in high-risk PTCA: the COURT trial. Circulation 2000;101:2172–7. [DOI] [PubMed] [Google Scholar]
- [28].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- [30].van Tulder M, Furlan A, Bombardier C, et al. Editorial Board, Cochrane Back Review Group. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976) 2003;28:1290–9. [DOI] [PubMed] [Google Scholar]
- [31].Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- [32].Juergens CP, Winter JP, Nguyen-Do P, et al. Nephrotoxic effects of iodixanol and iopromide in patients with abnormal renal function receiving N-acetylcysteine and hydration before coronary angiography and intervention: a randomized trial. Intern Med J 2009;39:25–31. [DOI] [PubMed] [Google Scholar]
- [33].Solomon R, Barrett B. Follow-up of patients with contrast-induced nephropathy. Kidney Int Suppl 2006;S46–50. [DOI] [PubMed] [Google Scholar]
- [34].Heyman SN, Rosenberger C, Rosen S, et al. Why is diabetes mellitus a risk factor for contrast-induced nephropathy? Biomed Res Int 2013;2013:123589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shin D-H, Choi DJ, Youn TJ, et al. Comparison of contrast-induced nephrotoxicity of iodixanol and iopromide in patients with renal insufficiency undergoing coronary angiography. Am J Cardiol 2011;108:189–94. [DOI] [PubMed] [Google Scholar]
- [36].Nie B, Cheng WJ, Li YF, et al. A prospective, double-blind, randomized, controlled trial on the efficacy and cardiorenal safety of iodixanol vs. iopromide in patients with chronic kidney disease undergoing coronary angiography with or without percutaneous coronary intervention. Catheter Cardiovasc Interv 2008;72:958–65. [DOI] [PubMed] [Google Scholar]
- [37].Zaytseva NV, Shamkhalova MS, Shestakova MV, et al. Contrast-induced nephropathy in patients with type 2 diabetes during coronary angiography: risk-factors and prognostic value. Diabetes Res Clin Pract 2009;86:S63–9. [DOI] [PubMed] [Google Scholar]
- [38].Solomon R. Contrast-induced nephropathy: update with special emphasis on patients with diabetes. Curr Diab Rep 2008;7:454–8. [DOI] [PubMed] [Google Scholar]
- [39].Zhang BC, Wu Q, Wang C, et al. A meta-analysis of the risk of total cardiovascular events of isosmolar iodixanol compared with low-osmolar contrast media. J Cardiol 2014;63:260–8. [DOI] [PubMed] [Google Scholar]
- [40].Almén T. Contrast media: the relation of chemical structure, animal toxicity and adverse clinical effects. Am J Cardiol 1990;66:F2–8. [DOI] [PubMed] [Google Scholar]
- [41].Dunkel JA, Bøkenes J, Karlsson JOG, et al. Cardiac effects of iodixanol compared to those of other nonionic and ionic contrast media in the isolated rat heart. Acta Radiol 1995;36:142–54. doi:10.1177/0284185195036S39917. [DOI] [PubMed] [Google Scholar]
- [42].Manninen H, Tahvanainen K, Borch K, et al. Iodixanol, a new non-ionic, dimeric contrast medium in cardioangiography: a double-masked, parallel comparison with iopromide. Eur Radiol 1995;5:364–70. [Google Scholar]
- [43].Pugh ND, Sissons GR, Ruttley MS, et al. Iodixanol in femoral arteriography (phase III): a comparative double-blind parallel trial between iodixanol and iopromide. Clin Radiol 1993;47:96–9. [DOI] [PubMed] [Google Scholar]
- [44].Chicaiza-Becerra LA, Garcia-Molina M, Gamboa O. Cost-effectiveness of iso- versus low-osmolality contrast media in outpatients with high risk of contrast medium-induced nephropathy. Biomedica 2012;32:182–8. [DOI] [PubMed] [Google Scholar]
- [45].Schonefeld E, Howler S, Osada N, et al. [Effectiveness of nephroprotection by the selection of contrast media used during vascular interventions in patients with chronic renal failure?]. Zentralbl Chir 2011;136:426–30. [DOI] [PubMed] [Google Scholar]
- [46].Heinrich M, Scheer M, Heckmann M, et al. Reversibility and time-dependency of contrast medium induced inhibition of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) conversion in renal proximal tubular cells in vitro: comparison of a monomeric and a dimeric nonionic iodinated contrast medium. Invest Radiol 2007;42:732–8. [DOI] [PubMed] [Google Scholar]
- [47].Seeliger E, Flemming B, Wronski T, et al. Viscosity of contrast media perturbs renal hemodynamics. J Am Soc Nephrol 2007;18:2912–20. [DOI] [PubMed] [Google Scholar]
- [48].Heinrich MC, Kuhlmann MK, Grgic A, et al. Cytotoxic effects of ionic high-osmolar, nonionic monomeric, and nonionic iso-osmolar dimeric iodinated contrast media on renal tubular cells in vitro. Radiology 2005;235:843–9. [DOI] [PubMed] [Google Scholar]
- [49].Heinrich MC, Kuhlmann MK, Kohlbacher S, et al. Cytotoxicity of iodinated and gadolinium-based contrast agents in renal tubular cells at angiographic concentrations: in vitro study. Radiology 2007;242:425–34. [DOI] [PubMed] [Google Scholar]
- [50].Aspelin P, Aubry P, Fransson SG, et al. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med 2003;348:491–9. [DOI] [PubMed] [Google Scholar]
- [51].Thomsen HS, Morcos SK, Erley CM, et al. The ACTIVE trial: comparison of the effects on renal function of iomeprol-400 and iodixanol-320 in patients with chronic kidney disease undergoing abdominal computed tomography. Invest Radiol 2008;43:170–8. [DOI] [PubMed] [Google Scholar]
- [52].Hardiek KJ, Katholi RE, Robbs RS, et al. Renal effects of contrast media in diabetic patients undergoing diagnostic or interventional coronary angiography. J Diabetes Complications 2008;22:171–7. [DOI] [PubMed] [Google Scholar]
- [53].Barrett BJ, Katzberg RW, Thomsen HS, et al. Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed tomography: a double-blind comparison of iodixanol and iopamidol. Invest Radiol 2006;41:815–21. [DOI] [PubMed] [Google Scholar]


