Supplemental Digital Content is available in the text
Keywords: meta-analysis, postoperative radiotherapy, surgery
Abstract
Background:
This meta-analysis was conducted to evaluate the effect of postoperative radiotherapy for patients having esophagus squamous cell carcinoma after radical surgery.
Methods:
A comprehensive research was performed in Pubmed, Embase and Cochrane Library electronic databases from inception until December 10, 2017. We collected all published full articles about comparison of surgery plus postoperative radiotherapy with surgery alone.
Results:
Four randomized-controlled trials (RCTs) with 1050 participants and 8 non-randomized-controlled trials with 3248 participants were included and evaluated separately. The risk ratio rate and its 95% confidence interval (CI) were calculated. Both RCTs and non-randomized-controlled trials (NRCTs) groups showed a significant increase in 3-year overall survival (OS) rate (RRRCT = 0.89, 95% CI: 0.80–0.99; RRNRCT = 0.82, 95% CI: 0.76–0.88) and decrease locoregional recurrence rate (RRRCT = 0.53, 95% CI: 0.43–0.66; RRNRCT = 0.47, 95% CI: 0.32–0.69) after postoperative radiotherapy compared with surgery alone. The 5-year OS rate in the group of NRCTs was markedly enhanced (RR = 0.87, 95% CI: 0.82–0.92), while that of the RCTs group was not enhanced in a significant way (RR = 0.84, 95% CI: 0.70–1.02). Subgroup analysis based on pathological lymph node status revealed that postoperative radiotherapy could improve OS regardless of pathological lymph node status (pathological lymph node positive patients: RR5-year os-RCT = 0.81, 95% CI: 0.70–0.93; RR5-year os-NRCT = 0.87, 95% CI: 0.80–0.94; Pathological lymph node negative patients: RR3-year os-RCT = 0.76, 95% CI: 0.59–0.96; RR3-year os-NRCT = 0.52, 95% CI: 0.30–0.89). No effect on distant recurrence rate was detected. Adverse effects induced by postoperative radiotherapy were comparatively modest and tolerable.
Conclusion:
Polled results yielded that postoperative radiotherapy was promising in improving OS and reducing the locoregional recurrence rate. More large-scale up-to-date RCTs are needed to further validate the use of postoperative radiotherapy in modern practice.
1. Introduction
Esophageal cancer is the eighth most common malignancy worldwide and the sixth most common cause of death from cancer.[1] Esophageal squamous cell carcinoma (ESCC), one of the main subtypes of esophageal cancer and predominantly found in Asia, Africa, and South America, accounts for 90% of cases of esophageal cancer worldwide.[2]
Esophagectomy with extended lymph node dissection is an essential treatment for ESCC patients with curative intention. However, the prognosis for ESCC patients receiving surgery alone is poor, for example, the 5-year overall survival (OS) rate is less than 30%[3] while the local-regional recurrence rate is as high as 33%, and the distant recurrence rate is approximately up to 20% depending on the stage.[4] These data clearly indicate that surgery alone may not be sufficient.
For resectable ESCC, neoadjuvant chemoradiotherapy followed by surgery has been regarded as a standard primary treatment, which was recommended by the National Comprehensive Cancer Network and European Society for Medical Oncology guidelines.[5,6] However, there remained a large number of patients receiving upfront surgery, which is especially prevalent in China.[7] For these patients, adjuvant therapy may be necessary although no study of its impact has been well established.[8–10]
Since the 1990s, postoperative radiation for esophageal cancer has been attempted, with some critical advanced techniques in radiotherapy treatment emerging in recent years, including 3-dimensional conformal radiotherapy, intensity-modulated radiotherapy, image-guided radiotherapy, proton radiation, and more, where postoperative radiotherapy has been used more often in esophageal cancer. However, the efficacy of postoperative radiotherapy in treating ESCC is controversial, because some randomized-controlled trials (RCT) conducted earlier failed to confer a survival difference between surgery with and without postoperative radiotherapy.[11,12] Whereas recently, some RCTs and a series of retrospective studies have reported that postoperative radiotherapy with advanced radiation techniques such as intensity-modulated radiotherapy can dramatically improve OS and locoregional control.[13–16]
Until now, there has been no systematic review quantitatively assessing the benefit of additional postoperative radiotherapy alone for ESCC patients receiving upfront surgery. To further examine the role of postoperative radiotherapy, this meta-analysis was performed according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis. Our primary outcomes were OS rates for 3 and 5 years, and data regarding disease-free survival (DFS), recurrence pattern, and adverse effects were also collected if available.
2. Materials and methods
2.1. Study design
This systematic review and meta-analysis were carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines.[17] No ethical approval and patient written informed consent is needed because all processes of the whole study were performed on the basis of previous information.
2.2. Search strategy
A literature search was performed in Pubmed, Embase, and Cochrane Library electronic databases from inception until December 10, 2017, where we used the following search terms: ESCC, radiotherapy, postoperative. MeSH terms and text-words searches were used. Some references were included manually by retrieving original articles to identify other potentially eligible studies.
2.3. Criteria for inclusion and exclusion
The studies met the following criteria were considered to be eligible:
-
1.
All studies that compare postoperative radiotherapy versus surgery alone in ESCC patients, where no exclusion was set based on the type of study design.
-
2.
Surgery was conducted as an esophagectomy with curative intent, and no limitation was set for these procedures.
-
3.
ESCC was diagnosed according to pathological report.
-
4.
No preoperative treatment was conducted and postoperative radiotherapy was used as the major adjuvant treatment.
-
5.
The associated outcomes could be extracted.
-
6.
Only published full papers were considered for inclusion.
-
7.
Publication language was limited to English.
-
8.
For studies reporting the results from duplicate patients, only those with the most comprehensive data were included.
The exclusion criteria are:
-
1.
Duplicate data, reviews, case reports, conferences abstracts, and single-armed studies.
-
2.
Surgery was performed with endoscopic resection or with palliative intent,
-
3.
Presence of confounding factors such as preoperative treatment modalities or utilization of postoperative chemotherapy.
2.4. Assessment of methodological quality
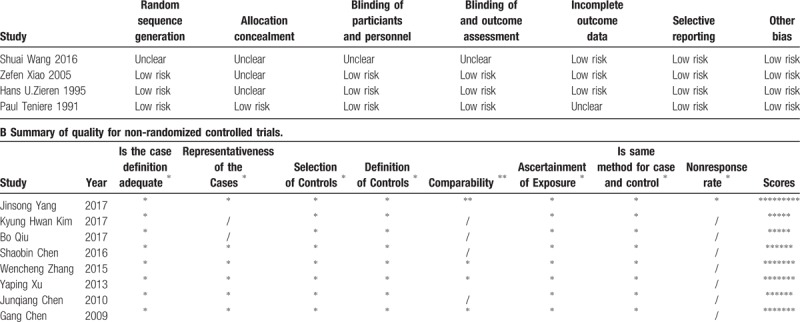
Qualities of the RCTs were evaluated by the Risk of Bias Table in accordance with the Cochrane Handbook for Systematic Reviews of Interventions.[18] There are 3 levels of bias: high, unclear, or low risk of bias to assess the following items: generation of allocation sequences, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete date addressed, presence of biases in reports, and other sources of bias that could influence the study's validity. Non-randomized-controlled trails (NRCTs) were assessed according to the Newcastle–Ottawa Scale, which contains 3 parameters of quality: selection, comparability, and exposure assessment. The Newcastle–Ottawa Scale assigns maximum scores of 4 for selection, 2 for comparability, and 3 for exposure.
2.5. Data extraction and statistical analysis
The following data was extracted from the selected articles by 2 reviewers independently: the name of first author, year of publication, sample size, baselines of patients, recruitment period, country, radiotherapy dose and schedule, site of tumor, OS rates for 3 and 5 years, DFS rates for 1 year and 3 years, locoregional recurrence rate and distant recurrence rate, adverse effects. Any disagreement was discussed with a third reviewer. Statistical analyses were performed with Review Manager 5.3 (Cochrane Collaboration, Oxford, UK), and the primary outcomes were 3-year OS and 5-year OS rates. Subgroup analysis according to pathological lymph node status was also prepared. Other outcomes such as DFS, locoregional recurrence rate, and distant recurrence rate were also evaluated if available. All the outcomes were analyzed using an RR with a 95% confidence interval (CI), which were extracted from article directly if available. Otherwise, Engauge Digitizer version 4.1 (http://sourceforge.net) was used to extract the associated data to calculate the RR and its 95% CI. Heterogeneity was evaluated by Cochran's Q statistic and I2 test, The I2 yielded results ranging from 0 to 100% (I2 = 0–40%, low heterogeneity; I2 = 40–60%, moderate heterogeneity; I2 = 50–90%, large heterogeneity; and I2 = 75–100%, extreme heterogeneity). The random-effects model was used if I2 ≥ 50%, otherwise, the fix-effects model was utilized. Sensitivity analysis was performed by omitting each study to test the robustness of results and to discover sources of heterogeneity. Publication bias was assessed by funnels plots. P values were considered significant at the .05 level.
3. Results
3.1. Study characteristics
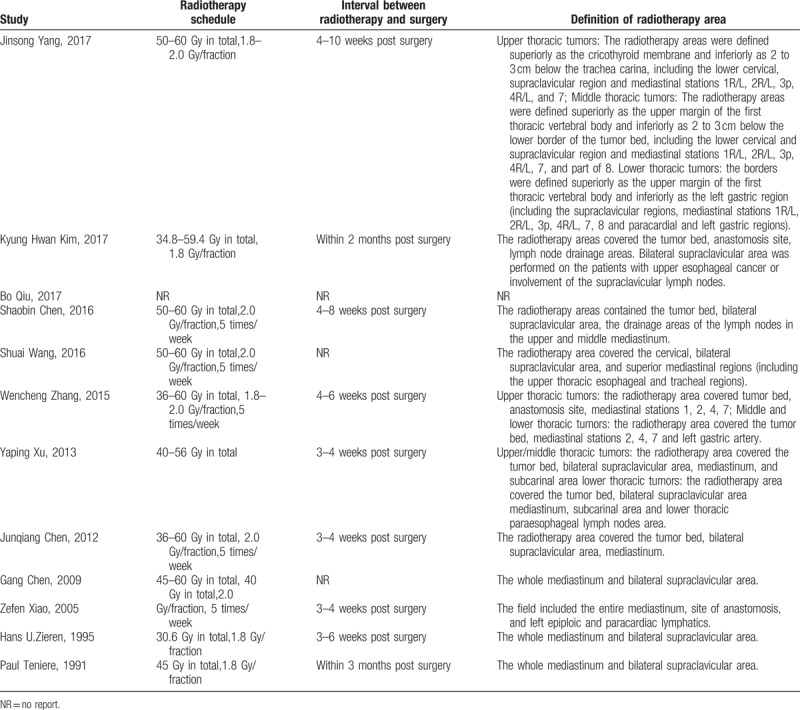
A total of 12 studies[11–16,19–24] (4 RCTs and 8 NRCTs) involving 4298 participants were eligible for this review, which included 1728 patients in the postoperative radiotherapy group and 2570 patients in the surgery alone group. The flowchart of the retrieved studies was presented in Figure 1, and details of the eligible articles were summarized in Table 1. The recruitment period ranged from 1979 to 2015. R0 resections were performed for nearly all patients if reported except in the study from Bo Qiu, which specifically focused on 70 patients with R1 resection. The postoperative TNM stages of participants ranged from stage I to stage IV, and stage II and stage III were the most common. Postoperative chemoradiotherapy cohorts were excluded from the calculation, and only patients receiving postoperative radiotherapy as the main adjuvant treatment were included in this review. Although studies by Wen-cheng Zhang and Gang Chen included a small portion of patients (30 of 190, 79 of 181, respectively) receiving chemotherapy in the postoperative radiotherapy group, these studies were included in this review after a sensitivity analysis (see Supplemental Digital contents) showed no significant effect on outcome and heterogeneity. Two-dimensional radiotherapy technique was the most frequently introduced technique in studies published in the 20th century, whereas 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy were used extensively since the 21st century. Most radiotherapy targets included tumor bed, anastomosis site, and associated lymph node drainage areas. The total radiation dose ranged from 36 Gy to 60 Gy whereas radiation schedule was similar with an arrangement of 1.8 to 2.0 Gy/fraction per day, 5 times/week. The details for the radiotherapy arrangement were shown in Table 2. Most of these studies were performed in Asia.
Figure 1.
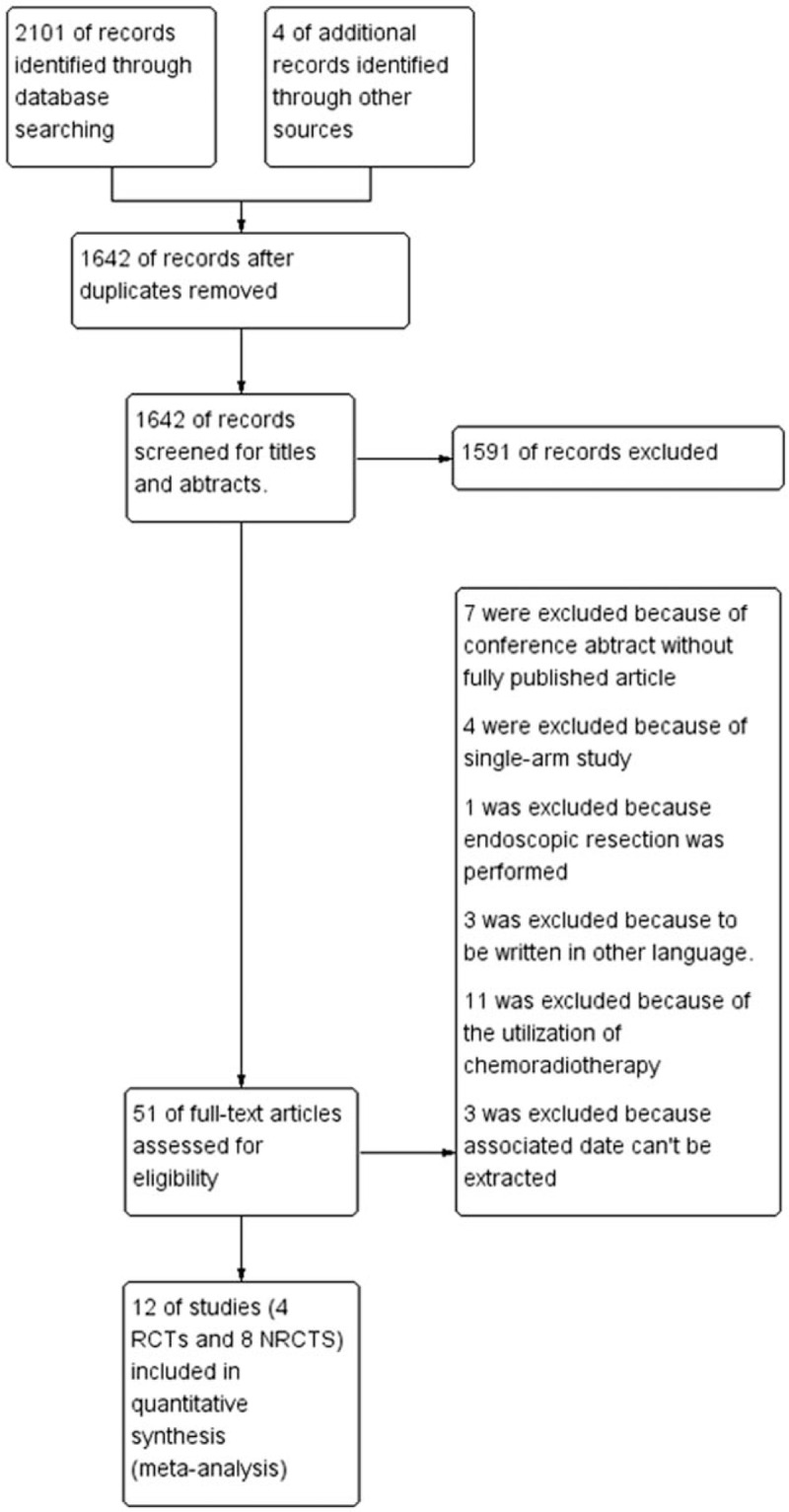
Flow diagram for studies retrieving and inclusion.
Table 1.
Basic characteristics for all of the pooled studies in the meta-analysis.
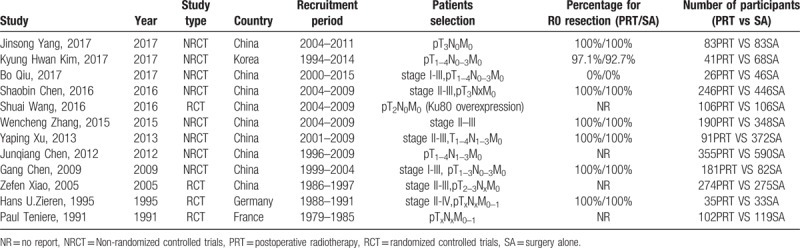
Table 2.
Details for radiotherapy arrangements.
3.2. Quality of the included studies
The selected RCTs had a high overall quality, and most of them had a low risk of bias despite allocation concealments being unclear in 3 studies. For NRCTs, the most common selection biases were in the selection of controls from hospital controls, and no study reported a nonresponse rate. As for comparability, most studies enrolled comparable patients whereas in some studies, the postoperative radiotherapy group had a relatively worse baseline level of health than that in the surgery alone group. Details of quality assessment were shown in Table 3. The overall methodological quality of included studies was high.
Table 3.
A Summary of quality for randomized controlled trials.
3.3. 3-year OS rate
Data for 3-year OS was available in 12 studies, among which 4 were RCTs[11,12,15,16] and 8 were NRCTs.[13,14,19–24] The results from the RCTs and NRCTs were analyzed separately, and a consistent conclusion showed that the 3-year OS was higher in postoperative radiotherapy group than in the surgery alone group (RRrct = 0.89, 95% CI: 0.80–0.99; RRnrct = 0.82, 95% CI: 0.76–0.88). There was a modest heterogeneity in the RCTs group (I2 = 47%, P = .13), and the heterogeneity was very low in NRCTs group (I2 = 6%, P = .38) (Fig. 2).
Figure 2.
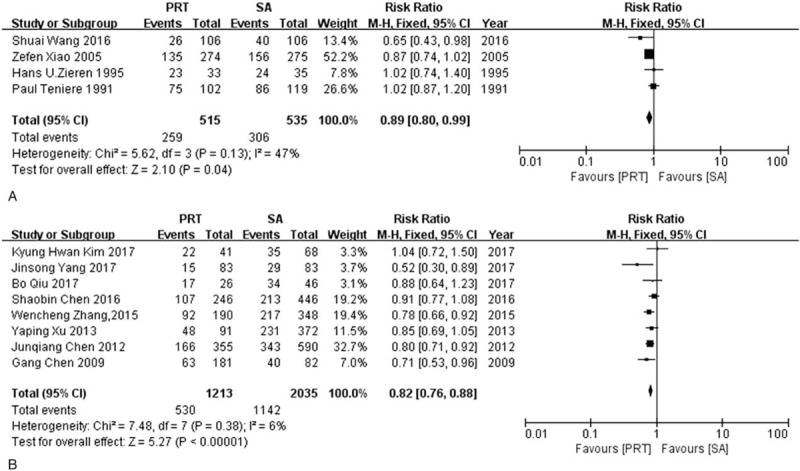
Forest plot of risk ratio for 3-year overall survival. (A) The polled results from randomized-controlled trials; (B) the polled results from non-randomized-controlled trials. PRT = postoperative radiotherapy, SA = surgery alone.
3.4. 5-year OS rate
Five-year OS was extracted from 11 studies including 4 RCTs[11,12,15,16] and 7 NRCTs[13,14,19–21,23,24], There was a tendency favoring postoperative radiotherapy in RCTs group although a significant difference was not found (RR = 0.84, 95% CI: 0.70–1.02) and a large heterogeneity was detected (I2 = 78%, P = .004). Postoperative radiotherapy was shown to be beneficial in improving 5-year OS in the NRCTs group (RR = 0.87, 95%CI: 0.82–0.92) with low heterogeneity (I2 = 5%, P = .39) (Fig. 3).
Figure 3.
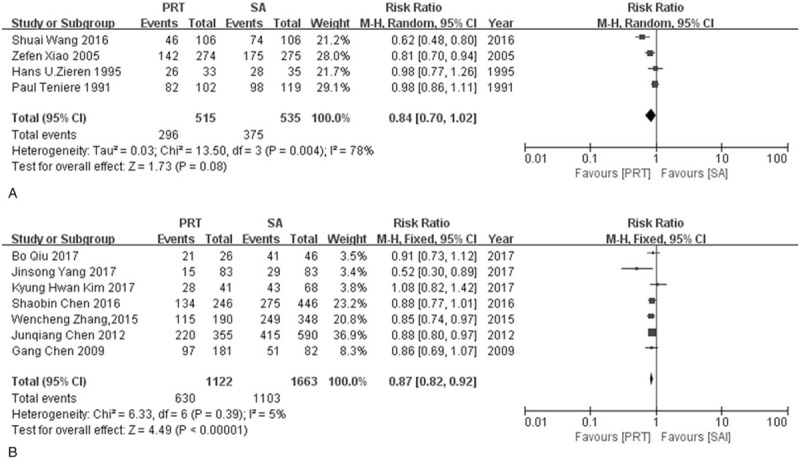
Forest plot of risk ratio for 5-year overall survival. (A) The polled results from randomized-controlled trials, (B) the polled results from non-randomized-controlled trials. PRT = postoperative radiotherapy, SA = surgery alone.
3.5. Subgroup analysis of pathological lymph node status for OS
In order to investigate the relationship between the effectiveness of postoperative radiotherapy and pathological lymph node status for ESCC, subgroup analysis was performed. For pathological lymph node positive (pN+) patients, 1 RCT and 2 NRCTs were available. A tendency towards a better 3-year OS (RR = 0.85, 95%CI: 0.72–1.01) and a significant 5-year OS (RR = 0.81, 95% CI: 0.70–0.93) were observed in postoperative radiotherapy group in RCT study. NRCTs showed consistent favor of postoperative radiotherapy in 3-year OS (I2 = 0%, P = .76; RR = 0.79, 95% CI: 0.72–0.88) and 5-year OS (I2 = 0%, P = .67; RR = 0.87, 95% CI: 0.80–0.94). For pathological lymph node negative (pN-) patients, the results from 2 RCTs studies and 1 NRCT study were assessed separately. Survival advantage was obvious in RCTs group for 3-year OS (RR = 0.76, 95% CI: 0.59–0.96) with a low heterogeneity (I2 = 0%, P = .35) while no significant difference was seen for 5-year OS and heterogeneity existed (I2 = 87%, P = .005; RR = 0.80, 95% CI: 0.49–1.32). NRCTs indicated postoperative radiotherapy can improve 3-year and 5-year OS (RR3-year = 0.52, 95% CI: 0.30–0.89; RR5-year = 0.59, 95% CI: 0.37–0.93) (Fig. 4, Fig. 5).
Figure 4.
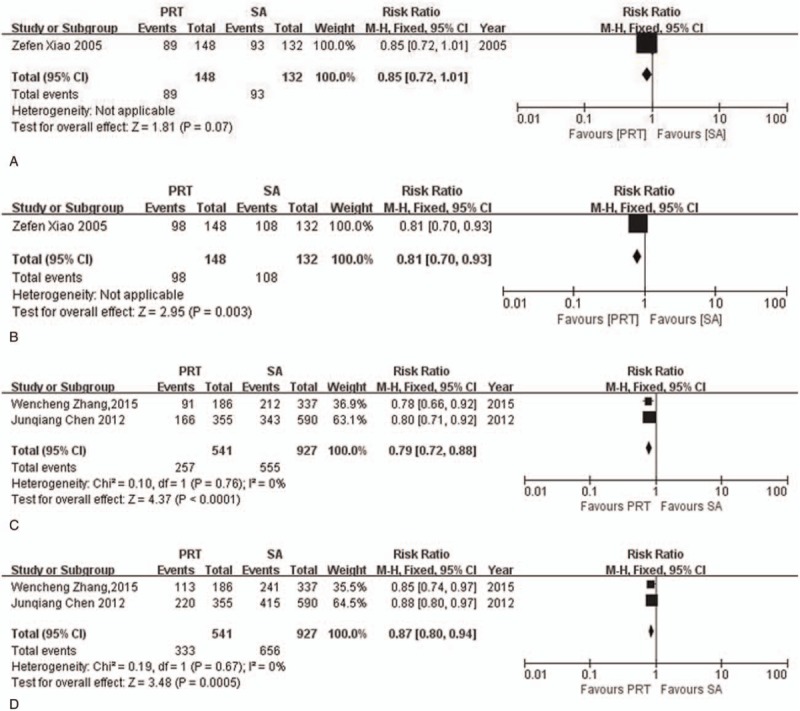
Forest plot of risk ratio for overall survival rate in pathological lymph node positive (pN+) patients. (A) The polled results for 3-year overall survival rate from randomized-controlled trial; (B) the polled results for 5-year overall survival rate from randomized-controlled trial; (C) the polled results for 3-year overall survival rate from non-randomized-controlled trials; (D) the polled results for 5-year overall survival rate from non-randomized-controlled trials. PRT = postoperative radiotherapy, SA = surgery alone.
Figure 5.
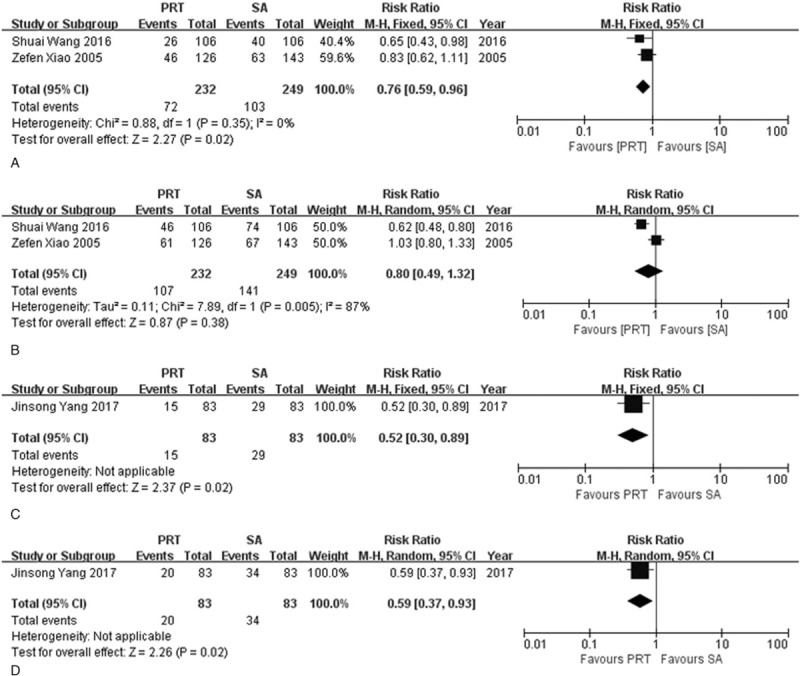
Forest plot of risk ratio for overall survival rate in pathological lymph node negative (pN-) patients. (A) The polled results for 3-year overall survival rate from randomized-controlled trials; (B) the polled results for 5-year overall survival rate from randomized-controlled trials; (C) the polled results for 3-year overall survival rate from non-randomized-controlled trial; (D) the polled results for 5-year overall survival rate from non-randomized-controlled trial. PRT = postoperative radiotherapy, SA = surgery alone.
3.6. DFS rate
5 studies were used to calculate DFS for 1 year and 3 years including 2 RCTs and 3 NRCTs. Neither RCTs nor NRCTs showed any difference in 1-year or 3-year DFS. The RR rates for 1-year DFS were 0.79 (95% CI: 0.54–1.18) in RCTs group and 0.65 (95% CI: 0.38–1.09) in NRCTs group respectively. The RR rates for 3-year DFS were 0.83 (95%CI: 0.63–1.09) in RCTs group and 0.82 (95% CI: 0.58–1.17) in NRCTs group respectively. The heterogeneity for 1-year DFS was small in the RCTs group (I2 = 0%, P = .38), and higher in NRCTs group (I2 = 73%, P = .02). There was a heterogeneity in both RCTs and NRCTs group for 3-year DFS (I2 = 57%, P = .13; I2 = 81%, P = .006, respectively) (Fig. 6, Fig. 7).
Figure 6.
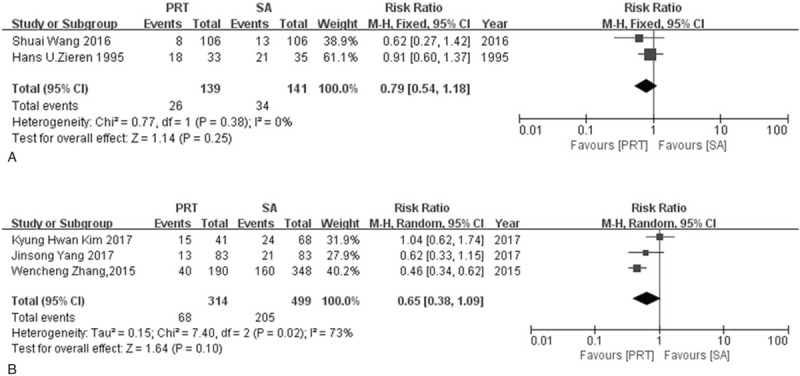
Forest plot of risk ratio for 1-year disease free survival rate. (A) The polled results from randomized-controlled trials; (B) the polled results from non-randomized-controlled trials. PRT = postoperative radiotherapy, SA = surgery alone.
Figure 7.
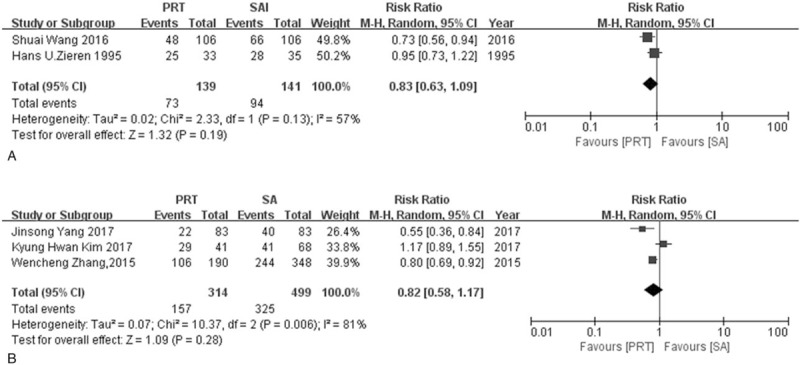
Forest plot of risk ratio for 3-year disease free survival rate. (A) The polled results from randomized-controlled trials; (B) the polled results from non-randomized-controlled trials. PRT = postoperative radiotherapy, SA = surgery alone.
3.7. Locoregional recurrence rate and distant recurrence rate
Locoregional recurrence rate and distant recurrence rate were evaluated separately by 2 RCTs and 5 NRCTs. Both RCTs and NRCTs groups suggested that postoperative radiotherapy can reduce the likelihood of locoregional recurrence rate (RRRCT = 0.53, 95% CI: 0.43–0.66; RRNRCT = 0.47, 95% CI: 0.32–0.69). Modest heterogeneity was found in the RCTs group (I2 = 49%, P = .16) and significant heterogeneity was seen in NRCTs group (I2 = 84%, P <.0001). For distant recurrence rate, Neither the RCTs group nor NRCTs group found a significant difference between postoperative radiotherapy and surgery alone (RRRCT = 1.29, 95% CI: 0.94–1.77, RRNRCT = 0.91, 95% CI: 0.72–1.15). Heterogeneity was considered to be low in RCTs group whereas modest in NRCTs group (I2RCT = 0%, P = .84, I2NRCT = 63%, P = .03) (Fig. 8, Fig. 9).
Figure 8.
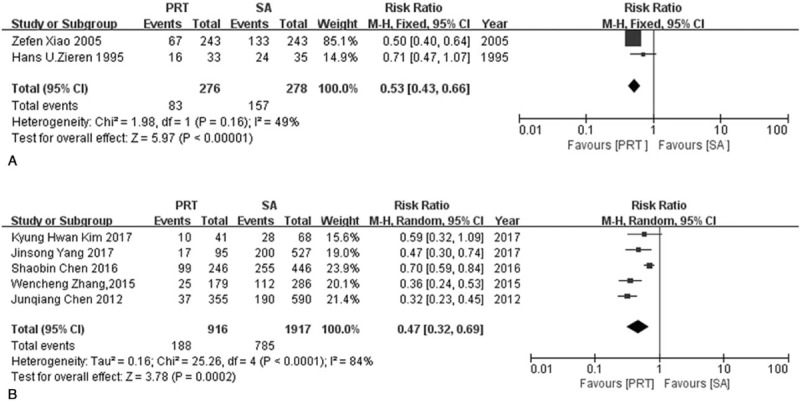
Forest plot of risk ratio for locoregional recurrence rate. (A) The polled results from randomized-controlled trials; (B) the polled results from non-randomized-controlled trials. PRT = postoperative radiotherapy, SA = surgery alone.
Figure 9.
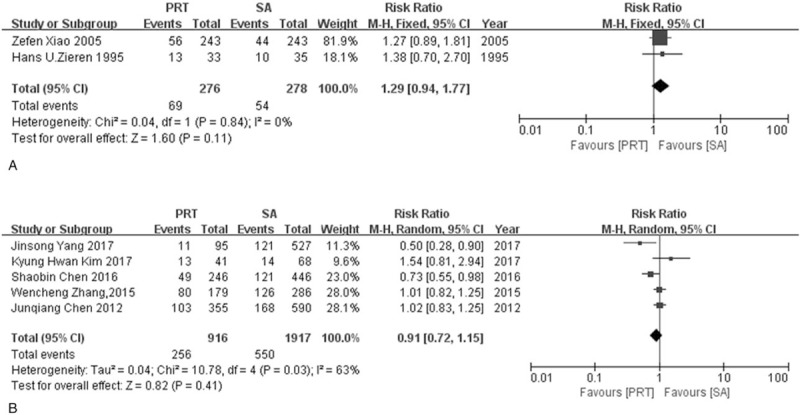
Forest plot of risk ratio for distant recurrence rate. (A) The polled results from randomized-controlled trials; (B) the polled results from non-randomized-controlled trials. PRT = postoperative radiotherapy, SA = surgery alone.
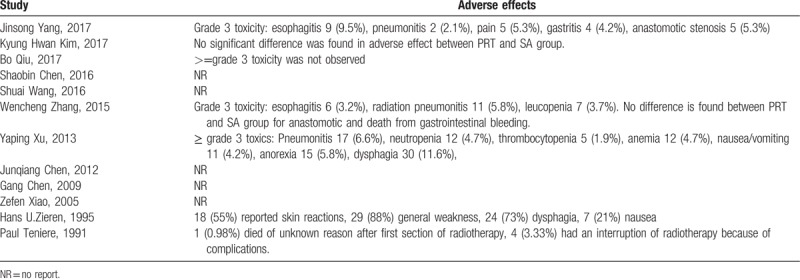
3.8. Adverse effects
There were a total of 7 studies reporting adverse effects associated with postoperative radiotherapy. Common Terminology Criteria for Adverse Events was commonly used to evaluate adverse effects. The most common side effects were radiation esophagitis, radiation pneumonitis, leucopenia, vomiting, and nausea. The incidence of grade 4 or 5 adverse effects was rare, and the probability for grade 3 adverse effects ranged from 1.9% to 9.5%. The likelihood for anastomotic stenosis was similar between postoperative radiotherapy and surgery alone. The details were shown in Table 4. The radiation after surgery was tolerated by most patients.
Table 4.
Adverse effects from postoperative radiotherapy cohorts.
3.9. Sensitivity analysis and publication bias
The sensitivity analysis showed robust conclusions for NRCTs group and less sound results for RCTs group. The overall result for sensitivity analysis was relatively credible. More details for sensitivity analysis were shown in supplementary digital contents (Tables 1A-P). Funnel plots were presented in Figure 10, which showed no significant publication bias among the included studies.
Figure 10.
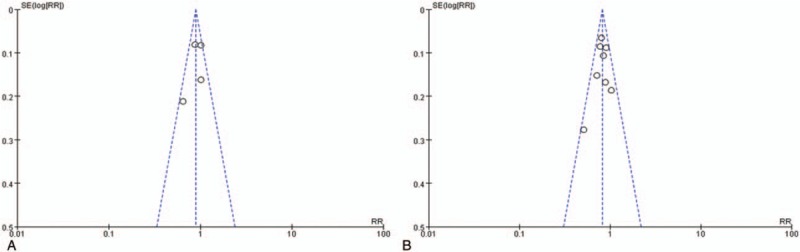
Funnel plots. (A) Funnel plot from randomized-controlled trials; (B) Funnel plot from non-randomized-controlled trials.
4. Discussion
As one of the major subsets of esophageal cancer, ESCC has many unique features. According to genomic analyses, ESCC shares some common pathogenic mechanisms with head and neck squamous cell carcinoma,[25] where postoperative radiotherapy always takes a vital part of the treatment regimen. The location of ESCC is always in the upper third of the thoracic esophageal,[26] making it difficult to perform a surgery with comprehensive lymph node resection and likely to leave behind residual lesions. In addition, locoregional recurrence rate is likely to be a cause of treatment failure in ESCC[4,27] and postoperative radiotherapy is regarded as a method for locoregional control. Based on these characteristics, postoperative radiotherapy may bring with some benefits theoretically. Although some clinical trials have already verified the advantage of postoperative radiotherapy, there existed other studies demonstrating that postoperative radiotherapy did not improve survival outcomes and suggesting surgery alone for ESCC patients without prior neoadjuvant treatment.[10] Until now, no consistent conclusion has been reached about this issue. Our study, based on 4 RCTs and 8 NRCTs, including 4298 participants, is the first large-scale meta-analysis to compile clinical data and quantitatively assess the value of postoperative radiotherapy as the major adjuvant treatment for ESCC patients.
OS was considered as the primary outcome and calculated according to RCTs and NRCTs separately. In NRCTs group, postoperative radiotherapy was demonstrated to show a clear advantage in improving both 3-year OS and 5-year OS as compared with surgery alone. Results from the RCTs group indicated a significant improvement in 3-year OS rate and a tendency towards better 5-year OS rate in postoperative radiotherapy cohorts. These results illustrated that postoperative radiotherapy is promising method to improve surgery outcome, though heterogeneity was present in the data, suggesting that more RCTs are needed for further validation.
The recommendation for postoperative radiotherapy in RCTs group was not as well established as in the NRCTs group, which may be a result of an insufficient number of participants in RCTs group, besides, the majority of RCTs studies were published 10 to 20 years earlier than NRCTs studies, traditional 2-dimensional radiotherapy method was utilized as the major treatment modality in these RCTs studies, which was observed to be inferior to modern radiation methods in conformal degree of the target area and organ protection, an OS disadvantage was also shown.[28–32] Thus the effect of radiotherapy might be underestimated in RCTs group.
Earlier published RCTs using traditional 2-dimensional radiotherapy radiation techniques as postoperative treatment initially showed a detrimental effect in many malignancies such as non-small cell lung cancer and breast cancer, until recent RCTs were conducted and a clear OS advantage was show with modern radiation techniques.[33–36] This improvement may also be present in the treatment of ESCC. Notably, with increasing of publication year, the tendency in favor of postoperative radiotherapy became more and more evident in RCTs group, and we thereby hypothesize that the 5-year OS rate of postoperative radiotherapy will be clearly demonstrated and the heterogeneity in 3-year OS rate will be less if more modern RCTs are conducted in the future.
Current National Comprehensive Cancer Network guideline indicates that the status of pathological lymph nodes is an important factor in determining the necessity of adjuvant treatment for adenocarcinoma of esophagus cancer, and thus a subgroup analysis of OS according to lymph node status was performed to explore the prognostic value of lymph node status. There were robust conclusions in NRCTs group that postoperative radiotherapy was beneficial to improve 3-year and 5-year OS rate for both pN+ and pN− patients. RCTs group demonstrated a clearly superior 3-year OS rate for pN+ patients and 5-year OS rate for pN− patients. The other outcomes showed a tendency in favor of postoperative radiotherapy, although had no significant difference. We speculated that the reason for this may result from the small number of participants. The baseline difference might be the source of heterogeneity. In conclusion, regardless of pathological lymph node status, postoperative radiotherapy is worthy of trying to increase survival rate for ESCC patients. On the other hand, more underlying factors affecting the prognosis of postoperative radiotherapy are needed to be explored. Notably, among lymph node negative patients, most participants in postoperative radiotherapy group had a comparatively late T stage (stage T3) or negative prognosis factors (Ku80 positive), and pT1–2N0M0 ESCC patients were only a small part of the sample. Thus more clinical trials are needed to verify the value of postoperative radiotherapy for early stage (pT1–2N0M0) ESCC with a comparatively good prognosis.
There was a tendency favoring postoperative radiotherapy in 1 and 3-year DFS, whereas neither RCTs nor NRCTs found any difference, the relatively small sample size (2RCTs and 3NRCTs) may decrease the ability to detect a significant difference and eliminate heterogeneity. The result from Kyung Hwan Kim was the biggest source of heterogeneity, and in this study, the T classification in postoperative radiotherapy group was worse than in the surgery alone group (percentage for T3-T4:75.6% vs 39.7%). Thus the potential benefit from radiation may be hidden. After sensitivity analysis supporting deletion of the result from Kyung Hwan Kim, a DFS advantage was shown in NRCTs group although the heterogeneity persisted (RR = 0.70, 95% CI: 0.49–0.99, I2 = 63%).
As for recurrence rate, both RCTs and NRCTs group demonstrated a consistent conclusion that postoperative radiotherapy was effective in reducing locoregional recurrence rate. Heterogeneity was modest in RCTs group and may be attributed to the relatively small number of studies included. In NRCTs group, the results from Shaobin Chen was the main source of heterogeneity, and after a sensitivity analysis indicated deletion of the report from Shaobin Chen, the heterogeneity was low (I2 = 25%, P = 0.26) and postoperative radiotherapy was still shown to be effective for locoregional control (RR = 0.39, 95% CI: 0.31–0.50), indicating the robustness of the conclusion. Baseline differences in participants from Shaobin Chen's study maybe the source for the heterogeneity. On the other hand, no difference in distant metastases was detected between postoperative radiotherapy and surgery alone, indicating that postoperative radiotherapy may eradicate residual lesions left behind after surgery. In cases where it does not fully prevent systematic spread of tumor cells, supplementary chemotherapy for patients with high risk of distant metastases may be considered.
There were some limitations within this study. First, recently published randomized-controlled studies were limited until the last literature retrieval, and retrospective studies constituted the majority. Therefore some biases were inevitable because of the retrospective nature of the included studies. Second, there were variations in surgical procedures, radiotherapy target delineation, and treatment schedules. Third, most included studies did not perform sufficient subgroup analysis. Only subgroup analysis for lymph node status was feasible, and the sample size was small. Finally, the majority of the included studies come from Asia, so further evidence is needed to conclude if the results will be consistent for ESCC patients worldwide.
Despite the drawbacks mentioned above, this meta-analysis is still informative. In this article, postoperative radiation was first thoroughly evaluated using several outcomes such as 3-year and 5-year OS rate, 1-year and 3-year DFS rate, locoregional recurrence rate, distant recurrence rate and adverse effects, subgroup analysis according to lymph node status was also performed. Results from RCTs and NRCTs were calculated separately to remain objective in this study, and basically conclusive results were reached in 2 groups. Though NRCTs constituted the most considerable portion, the overall quality of included studies was high and most researches were conducted recently and well-designed, reflecting contemporary radiation techniques. All studies were published with full articles and details for our detailed evaluation. The comparatively large sample size decreased potential bias from confounding factors. Finally, the baselines of the postoperative radiotherapy group in NRCTs were comparable or even worse than surgery alone group, which strengthens the results favoring postoperative radiotherapy.
Taken together, postoperative radiotherapy can improve the OS rate and reduce locoregional recurrence rate for ESCC patients. With advanced radiation techniques and the improvements of associated treatments, surgery alone might not be the optimal treatment. Postoperative radiotherapy is a promising choice for ESCC patients with upfront surgery. More large-scale up-to-date RCTs are needed to validate the use of postoperative radiotherapy in modern practice further.
Acknowledgments
We thank all anonymous reviewers and editors for taking the time to review our paper and for providing clinically, statistically, and methodologically thoughtful comments.
Author contributions
The literature search was conducted by Xiao-han Zhao and Duo Wang independently. Discrepancies were resolved through discussion with Shu-chai Zhu until a final consensus was reached. Two reviewers (Xiao-han Zhao, Duo Wang) independently performed the methodological quality assessment, and discrepancies were discussed with a third reviewer (Fang Wang) until a consensus was reached.
Data curation: Xiao-han Zhao, Duo Wang, Shu-chai Zhu.
Formal analysis: Xiao-han Zhao, Duo Wang.
Funding acquisition: Shu-chai Zhu.
Methodology: Xiao-han Zhao, Duo Wang, Fang Wang.
Software: Xiao-han Zhao, Duo Wang, Fang Wang.
Supervision: Shu-chai Zhu.
Writing – original draft: Xiao-han Zhao.
Writing – review & editing: Xiao-han Zhao, Duo Wang.
Supplementary Material
Footnotes
Abbreviations: DFS = disease free survival, ESCC = esophageal squamous cell carcinoma, NRCT = non-randomized-controlled studies, OS = overall survival, RCTs = randomized-controlled trials, RR = risk ratio.
This work is supported by Natural Science Foundation of Hebei Province [H2014206330, H2017206170], Youth Foundation of Hebei Province Department of Education [QN2015256].
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- [2].Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499–509. [DOI] [PubMed] [Google Scholar]
- [3].Hirst J, Smithers BM, Thomas J, et al. Defining cure for esophageal cancer: analysis of actual 5-year survivors following esophagectomy. Ann Surg Oncol 2011;18:1766–74. [DOI] [PubMed] [Google Scholar]
- [4].Mariette C, Balon JM, Piessen G, et al. Pattern of recurrence following complete resection of esophageal carcinoma and factors predictive of recurrent disease. Cancer 2003;97:1616–23. [DOI] [PubMed] [Google Scholar]
- [5].National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology. Esophageal and Esophagogastric Junction Cancers, Version 2. 2017. Available at: https://www.nccn.org/profes--sionals/physician_gls/f_guidelines.asp Accessed December 24, 2017. [Google Scholar]
- [6].Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27suppl 5:v50–7. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Y, Liu J, Zhang W, et al. Treatment of esophageal cancer with radiation therapy—a pan-Chinese survey of radiation oncologists. Oncotarget 2017;8:34946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang SS, Yang H, Xie X, et al. Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studies. Dis Esophagus 2014;27:574–84. [DOI] [PubMed] [Google Scholar]
- [9].Luo H, Cui YY, Zhang JG, et al. Meta-analysis of survival benefit with postoperative chemoradiotherapy in patients of lymph node positive esophageal carcinoma. Clin Transl Oncol 2018;20:889–98. [DOI] [PubMed] [Google Scholar]
- [10].Malthaner RA, Wong RK, Rumble RB, et al. Neoadjuvant or adjuvant therapy for resectable esophageal cancer: a systematic review and meta-analysis. BMC Med 2004;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Teniere P, Hay JM, Fingerhut A, et al. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. Surg Gynecol Obstet 1991;173:123–30. [PubMed] [Google Scholar]
- [12].Zieren HU, Muller JM, Jacobi CA, et al. Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg 1995;19:444–9. [DOI] [PubMed] [Google Scholar]
- [13].Yang J, Zhang W, Xiao Z, et al. The impact of postoperative conformal radiotherapy after radical surgery on survival and recurrence in pathologic T3N0M0 esophageal carcinoma: a propensity score-matched analysis. Exp Hematol Oncol 2017;12:1143–51. [DOI] [PubMed] [Google Scholar]
- [14].Chen SB, Weng HR, Wang G, et al. The impact of adjuvant radiotherapy on radically resected T3 esophageal squamous cell carcinoma. J Cancer Res Clin Oncol 2016;142:277–86. [DOI] [PubMed] [Google Scholar]
- [15].Wang S, Wang Z, Yang Z, et al. Postoperative radiotherapy improves survival in stage pT2N0M0 esophageal squamous cell carcinoma with high risk of poor prognosis. Ann Surg Oncol 2016;23:265–72. [DOI] [PubMed] [Google Scholar]
- [16].Xiao ZF, Yang ZY, Miao YJ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radial Oncol 2005;62:82–90. [DOI] [PubMed] [Google Scholar]
- [17].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- [18].Higgins JPT, Green S. Cochrane Handbook for Systematic Review of Intervention Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at: http://www.Cochrane-hand.book.org December 29, 2017. [Google Scholar]
- [19].Qiu B, Li J, Wang B, et al. Adjuvant therapy for a microscopically incomplete resection margin after an esophagectomy for esophageal squamous cell carcinoma. J Cancer 2017;8:249–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kim KH, Chang JS, Cha JH, et al. Optimal adjuvant treatment for curatively resected thoracic esophageal squamous cell carcinoma: a radiotherapy perspective. Cancer Res Treat 2017;49:168–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang W, Liu X, Xiao Z, et al. Postoperative intensity-modulated radiotherapy improved survival in lymph node-positive or stage III thoracic esophageal squamous cell carcinoma. Oncol Res Treat 2015;38:97–102. [DOI] [PubMed] [Google Scholar]
- [22].Xu Y, Liu J, Du X, et al. Prognostic impact of postoperative radiation in patients undergoing radical esophagectomy for pathologic lymph node positive esophageal cancer. Radiat Oncol 2013;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen J, Pan J, Zheng X, et al. Number and location of positive nodes, postoperative radiotherapy, and survival after esophagectomy with three-field lymph node dissection for thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys 2012;82:475–82. [DOI] [PubMed] [Google Scholar]
- [24].Chen G, Wang Z, Liu XY, et al. Adjuvant radiotherapy after modified Ivor-Lewis esophagectomy: can it prevent lymph node recurrence of the mid-thoracic esophageal carcinoma. Ann Thorac Surg 2009;87:1697–702. [DOI] [PubMed] [Google Scholar]
- [25].Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 2014;509:91–5. [DOI] [PubMed] [Google Scholar]
- [26].Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400–12. [DOI] [PubMed] [Google Scholar]
- [27].Saigi M, Oliva M, Aliste L, et al. Clinical relevance of histologic subtypes in locally advanced esophageal carcinoma treated with pre-operative chemoradiotherapy: experience of a monographic oncologic centre. PLoS One 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Diwanji TP, Mohindra P, Vyfhuis M, et al. Advances in radiotherapy techniques and delivery for non-small cell lung cancer: benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy. Transl Lung Cancer Res 2017;6:131–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Deng JY, Wang C, Shi XH, et al. Reduced toxicity with three-dimensional conformal radiotherapy or intensity-modulated radiotherapy compared with conventional two-dimensional radiotherapy for esophageal squamous cell carcinoma: a secondary analysis of data from four prospective clinical trials. Dis Esophagus 2016;29:1121–7. [DOI] [PubMed] [Google Scholar]
- [30].Zhang MX, Li J, Shen GP, et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. Eur J Cancer 2015;51:2587–95. [DOI] [PubMed] [Google Scholar]
- [31].Shi A, Liao Z, Allen PK, et al. Long-term survival and toxicity outcomes of intensity modulated radiation therapy for the treatment of esophageal cancer: a large single-institutional cohort study. Adv Radiat Oncol 2017;2:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xu D, Li G, Li H, et al. Comparison of IMRT versus 3D-CRT in the treatment of esophagus cancer: a systematic review and meta-analysis. Medicine 2017;96:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Billiet C, Decaluwe H, Peeters S, et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: a meta-analysis. Radiother Oncol 2014;110:3–8. [DOI] [PubMed] [Google Scholar]
- [34].PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet 1998;352:257–63. [PubMed] [Google Scholar]
- [35].Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish breast cancer cooperative group 82b trial. N Engl J Med 1997;337:949–55. [DOI] [PubMed] [Google Scholar]
- [36].Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med 1997;337:956–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


