Abstract
This study aimed to explore the association between the percentage of reticulated platelets (RP%) and infection, and analyze the value of combined measurement of RP% with other inflammatory indicators in diagnosing infection. A total of 190 patients with signs and symptoms suspicious of infection were included in the infection group, and 70 healthy subjects with comparable percentages of gender and age were included in the control group. Peripheral white blood cell (WBC) count, percentage of neutrophils (N%), platelet count, C-reactive protein (CRP), procalcitonin (PCT), RP%, and axillary temperature were recorded. Dynamic changes in RP% with infection were measured to analyze the association between RP% and infection. The receiver operating characteristic curve was used to evaluate the value of each inflammatory indicator in diagnosing infection and analyze the diagnostic value of the combined adoption of multiple inflammatory indicators. RP% was significantly higher in the infection group than in the noninfection and control groups. The sensitivity and specificity for diagnosing infection were, respectively, 91.78% and 93.18% when RP% and CRP were used in combination, 90.41% and 90.90% when RP% and PCT were used in combination, and 100% and 100% when RP%, CRP, and PCT were used in combination. RP% changed dynamically with the progression of infection and recovered to lower than 5.5% at 2 to 7 days before the body temperature recovered to a normal level. The diagnostic value of RP% was the highest. A combined use with CRP/PCT could improve the sensitivity and specificity in the early diagnosis of infectious diseases.
Keywords: diagnostic value, infection, reticulated platelet
1. Introduction
Infection, especially serious infection, has been a major cause of high mortality and morbidity rates in patients in the intensive care unit (ICU). Currently, the early diagnosis of infection mainly depends on clinical presentations and measurement of inflammatory indicators. However, the early clinical manifestations of infection are similar to systemic inflammatory response syndrome (SIRS) and lack specificity. Therefore, the infection cannot be diagnosed early according to the presentations of SIRS.[1] Measuring biomarkers of infection, such as procalcitonin (PCT), C-reactive protein (CRP), lactic acid (LA), and interleukin (IL)-6, could reflect the progression of infection. However, these indicators lack specificity, and some of them are extremely expensive to measure and thus not suitable for the dynamic measurement of infection. In recent years, clinical studies have shown that the percentage of reticulated platelets (RP%) is more sensitive for diagnosing infectious diseases than conventional inflammatory indicators, and the diagnostic value is also higher.[2–4] RP is the immature stage in thrombocytopoiesis, with small amounts of mRNA and rough endoplasmic reticulum in the cytoplasm. RP also can synthesize a small amount of proteins.[5] With the advancement of RP detection methods, some researchers found that RP% could not only improve the early diagnostic rate of infection but also help distinguish serious and nonserious infections. However, different results have been reported by previous studies. The aims of this study were to further investigate the association between RP% and infectious diseases, explore the diagnostic value of RP%, and analyze the dynamic changes in RP% with infection.
2. Methods
2.1. Subjects
Between December 2015 and August 2016, 190 patients with a body temperature > 37.3°C or < 36°C and suspicious of infection, who were hospitalized in the First Affiliated Hospital of Anhui Medical University, were included in the infection group. Seventy healthy subjects, with comparable percentages of age and gender, who received physical examinations in the hospital, were included in the control group. Among the 190 patients in the infection group, 104 were males and 86 were females (age range: 18–91 years). A total of 128 patients were from regular inpatient wards, and 62 were from the ICU. According to the results of pathogen culture (including various bacteria and fungi) and imaging results, the patients were divided into infection (n = 146) and noninfection groups (n = 44). According to the diagnostic criteria of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference,[6] 89 patients in the infection group had sepsis, of whom 57 were in the severe sepsis group/complicated sepsis group (39 had severe sepsis and 18 had septic shock). No clear infectious lesion was found in 44 patients in the noninfection group; however, all of them met the diagnostic criteria of SIRS.[7] Patients meeting the diagnostic criteria of SIRS, and with clear infectious lesions or positive results of pathogen culture, were diagnosed with sepsis. Patients with infection accompanied by dysfunction of one or multiple organs were diagnosed with severe sepsis. Patients with infection accompanied by persistent hypotension, which could not be explained by other reasons and could not be corrected by sufficient fluid infusion, and tissue and/or organ hypoperfusion (such as oliguria, anuria, and lactic acidosis) were diagnosed with septic shock. The detailed information of the patients in different groups is summarized in Table 1. The inclusion criteria of the patients were as follows: hospitalized patients suspicious of infection with a body temperature > 37.3°C or < 36°C and age > 18 years. The exclusion criteria of the patients were as follows: pregnant women or children; patients with malignant tumor and receiving chemotherapy; and patients who recently received platelets, plasma, and blood coagulation factor, or used drugs that could affect the platelet and coagulation function.
Table 1.
General characteristics of the subjects in each group.

The exclusion criteria were as follows: pregnant women, age < 18 years; patients with acute coronary disease and stroke, cerebral infarction, and cerebral hemorrhage; noninfected patients with autoimmune diseases, or platelet or coagulation abnormalities; patients with malignant tumors who were receiving chemotherapy or radiotherapy, and who had malignancies that affected the coagulation system or platelets; patients who were taking medications that modified coagulation or platelet behavior (such as aspirin, heparin, warfarin, etc.); patients who had received platelet transfusions, or fresh-frozen plasma, or concentrated blood coagulation products in the previous 1 to 2 days; patients with acute or chronic hepatic failure, chronic renal failure, or a known hematologic disease affecting platelets and coagulation; and RP% detected with difficulty in patients.
2.2. Methods
Fasting peripheral venous blood (2 mL) was collected into anticoagulative tubes in the morning from the 190 patients in the infection group and 70 healthy controls. The blood was mixed by inversion and sent for examination within 1 hour. The peripheral blood collection time of patients with suspected infection was controlled within 2 days of body temperature, without antibiotics, hormones, and so forth, or before administering the drug that changed the body temperature and blood clotting function. The Sysmex XN-9000 automated blood cell analyzer (Sysmex Corporation, Sysmex Medical Electronic Co. Ltd, Shanghai) and reagents provided by the manufacturers were used to measure the white blood cell (WBC) count, percentage of neutrophils (N%), platelet count (PLT), and RP%. CRP and PCT, 2 inflammatory indicators, were measured uniformly in the laboratory. The data were collected and analyzed.
2.3. Statistical analysis
The SPSS 20.0 software (SPSS Inc, Chicago, IL) was used for statistical analysis. Quantitative data in normal distribution were described by means and standard divisions (X ± S) and compared using the t test, while quantitative data in nonnormal distribution were described by medians and interquartile ranges (M[Q]) and compared using the rank-sum test. A P value < .05 was considered as statistically significant. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the clinical value of each inflammatory indicator in diagnosing infection. The Youden index (sensitivity + specificity–1) was used to identify the cutoff value from the curve.
3. Results
3.1. Relationship between RP% and age
According to the age division of the World Health Organization (WHO), the healthy group was divided into the following 3 groups: youth group (aged less than 44 years), 18 people; middle-age group (aged 45–59 years), 28 people; and old-age group (aged more than 60 years), 24 people. The Levene test results showed that the F value was 0.969, the P value was .385, and the difference was not statistically significant.
3.2. Comparison of inflammatory indicators among the infection, noninfection, and control groups
The inflammatory indicators, including WBC, N%, CRP, PCT, RP%, and axillary temperature (T), were significantly different between the infection and noninfection groups (P < .05). However, the PLT was not significantly different between the infection and noninfection groups (P = .544). The levels of WBC, N%, PLT, and RP% were also significantly different between the infection and control groups (P < .001).
3.3. Comparisons of inflammatory indicators between the serious infection and nonserious infection groups
The levels of WBC, N%, PLT, CRP, PCT, and RP% were significantly different between the serious infection and nonserious infection groups (P < .05). However, the PLT was not significantly different between these 2 groups (P = .544).
3.4. ROC curves of each inflammatory indicator in diagnosing serious and nonserious infections
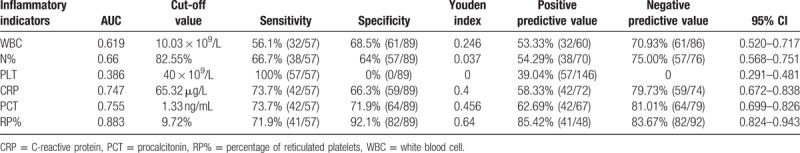
As summarized in Tables 2 and 3, the AUC of WBC, N%, CRP, PCT, T, and RP% in diagnosing nonserious infection was 0.707, 0.688, 0.712, 0.739, 0.647, and 0.868, respectively. The AUC of WBC, N%, PLT, CRP, PCT, and RP% in diagnosing serious infection was 0.619, 0.660, 0.386, 0.747, 0.755, and 0.883, respectively. The AUCs of RP% in diagnosing serious and nonserious infections were the highest.
Table 2.
AUC and diagnostic value of each inflammatory biomarker in diagnosing infection.
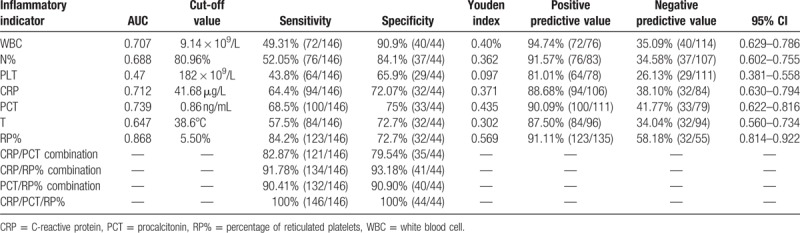
Table 3.
AUC and diagnostic value of each inflammatory markers for serious infection.
3.5. Optimal cutoff value of RP% in diagnosing serious and nonserious infections
The points most close to the upper left corner of the ROC curve in Fig. 1 were selected. According to the sensitivity and specificity of each point, the highest Youden index of RP% was 5.5%, and the corresponding sensitivity and specificity were 84.2% and 72.2%, respectively. Therefore, RP% = 5.5% was selected as the optimal cutoff value in Fig. 1, suggesting that the patients with RP% ≥5.5% had infection. The same method was adopted in Fig. 2, and it was found that the optimal cutoff value of RP% was 9.72%, suggesting that the patients with RP% ≥9.72% had a serious infection.
Figure 1.
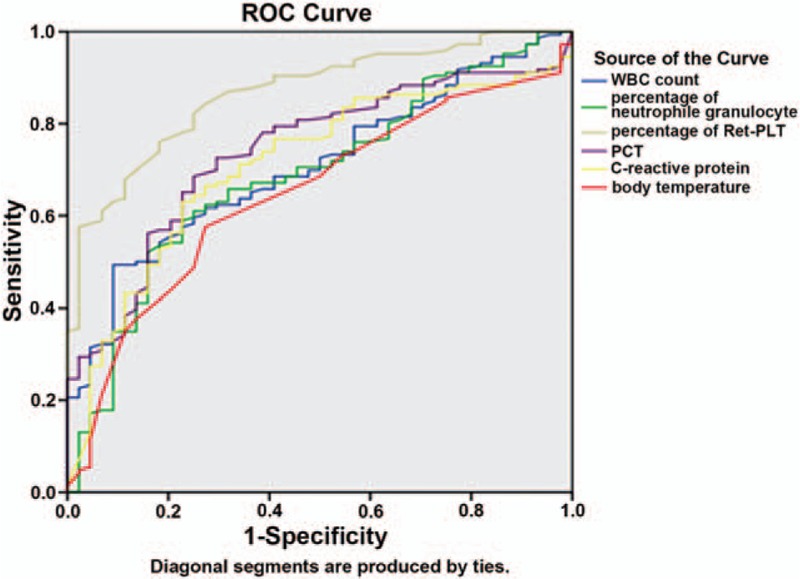
ROC curves of the inflammatory indicators in diagnosing nonserious infection.
Figure 2.
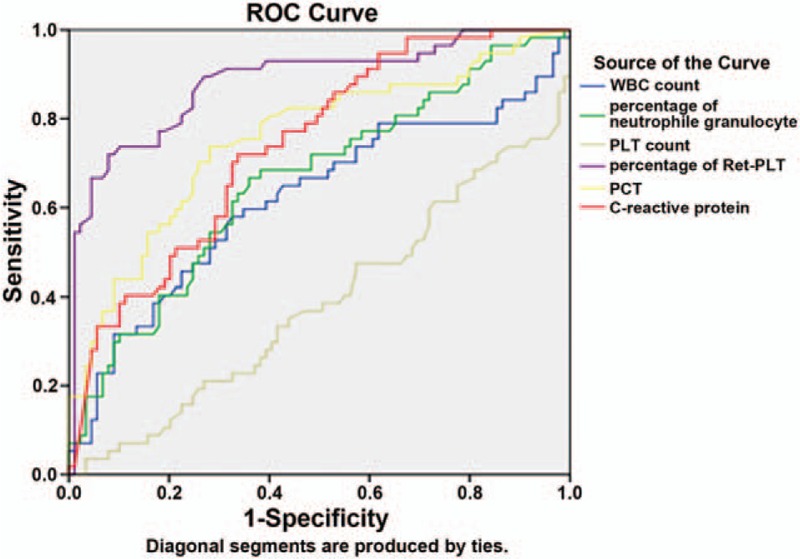
ROC curves of the inflammatory indicators in diagnosing serious infection.
3.6. Value of the combined use of RP% and inflammatory indicators
As summarized in Table 2, the sensitivity and specificity were 90.41% and 90.90% when RP% and PCT (RP% ≥5.5%; PCT ≥0.86 ng/mL) were used in combination, and were 91.78% and 93.18% when RP% and CRP (RP% ≥5.5%, CRP ≥41.68 μg/L) were used in combination for diagnosing infection, respectively. In contrast, the sensitivity and specificity both reached 100% when RP%, PCT, and CRP were used in combination for diagnosing infection.
3.7. Value of dynamically measured RP% in diagnosing infection
The dynamic measurement of RP% in 12 patients showed that RP% changed dynamically with the progression of infection and recovered to lower than 5.5% at 2 to 7 days before the body temperature recovered to a normal level (Table 4). One patient was randomly selected from the 12 patients, and the line–bar chart was used to analyze the association between the body temperature and RP% (Fig. 3), showing that the RP% of patient 2 started to recover on the day 12 of infection, while the recovery of body temperature, PCT, and CRP was later than the recovery of RP%.
Table 4.
Changes in T, RP%, CRP, and PCT with the progression of infection.
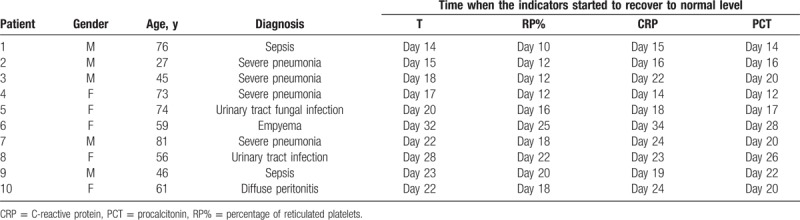
Figure 3.

Dynamic changes in RP% and T with the progression of infection (patient 2).
4. Discussion
Although the pathogenesis of infection has been elucidated, and the treatment methods are continuously improving and updating, the abuse of immunosuppressants and antibiotics has resulted in a high incidence of infection, even under the best medical care conditions.[8] Currently, the most commonly used inflammatory indicators include PCT, CRP, LA, and interleukin (IL)-6. The sensitivities of CRP and IL-6 are extremely high in diagnosing infection, but the specificities are relatively low. Therefore, the percentage of noninfectious diseases increases. PCT has relatively high specificity but low sensitivity, and the medical cost of measuring PCT is relatively high. Thus, it is not suitable for the dynamic monitoring of infection. LA is the product of anaerobic glycolysis. Any disease inducing insufficient oxygen supply and blood perfusion of the tissues and organs can induce increased LA. However, the specificity of LA is relatively low, and is clinically significant only in detecting patients with infectious shock. Therefore, an inflammatory indicator with high sensitivity and specificity and acceptable price is urgently needed to improve the early diagnostic rate of infectious diseases.
Previous studies have shown that the expression of adenosine diphosphate, arachidonic acid, collagen, and GPIIb/IIIa was significantly higher in blood samples rich in RP than in mature platelets. Compared with mature platelets, RP is more reactive to thrombin receptor–activating peptide and has higher hemostatic activity. Thus, it could help in thrombus formation and induce changes in microcirculation and coagulation function.[9] However, the changes in microcirculation and coagulation dysfunction play important roles in sepsis.[10,11] Therefore, it is speculated that RP plays an important role in the pathogenesis of infection. Di Mario et al[12] found that RP% was significantly higher in patients with positive blood culture results than in those with negative blood culture results, suggesting that RP could be an indicator for the screening of bacterial infection. The results of a clinical study performed by De Blasi et al[13] showed that during the diagnosis of sepsis, RP increased significantly at 3 days before the appearance of clinical manifestations. With the introduction of laboratory instruments, such as Sysmex XE-2100 and XN-9000, the measurement of RP has become simpler, more accurate, and more rapid in recent years, and the association between RP% and infection has also been further investigated. A clinical study performed by Wu et al[14] in May 2015 prospectively evaluated the potential value of RP in predicting the serious infection-related mortality rate. It was found that the increase in RP was closely associated with adverse clinical outcomes, and suggested for the first time that RP could be used as an important indicator to predict the risk of death.[14] Enz Hubert et al[3] found that RP% was positively associated with the SOFA score in the infection risk assessing system. Compared with other conventional inflammatory indicators, only RP%/LA could be used to distinguish between serious and nonserious infections. However, a later clinical study performed by Park Sang Hyuk yielded different results. They found that although using RP% could increase the early diagnostic rate of infectious diseases, it could not be used to distinguish serious and nonserious infections, similar to other conventional inflammatory indicators.[4] The present study mainly investigated the value of RP% in distinguishing patients with infectious or noninfectious diseases from healthy subjects, hence further clarifying the different conclusions of previous studies.
In this study, the sample size was large and the age range of the sample selection was wider to analyze the influence of age on the value of RP%. According to the age division of the WHO, the healthy control group was divided into 3 groups: youth group, middle-age group, and old-age group. The variance homogeneity Levene test was used to compare the mean value of RP% in the three groups, and the difference was not statistically significant. Therefore, the effect of age on the results was not significant. This study found that compared with other conventional inflammatory indicators, RP% had the highest AUC in diagnosing serious and nonserious infections, while its combined use with other inflammatory indicators, such as CRP and PCT, could help improve the sensitivity and specificity of early diagnosis of infectious diseases. These results were similar to the findings of most previous clinical studies. This study also analyzed the dynamic changes in RP% in 10 patients with infectious diseases and found that RP% changed dynamically with the progression of infection. Moreover, RP% recovered to the normal level 2 to 7 days before the body temperature recovered to normal, and therefore could be used to guide the clinical application of antibiotics.
The sample size of this study was larger compared with previous clinical studies on investigating whether RP% could help distinguish between serious and nonserious infections. The scope of sample selection was also expanded, and the subjects were not just selected from the ICU. The findings of this study showed that RP%, PCT, and CRP could distinguish between serious and nonserious infections, but the diagnostic value of RP% was the highest. Enz Hubert et al[3] found that only RP%/LA could distinguish serious and nonserious infections, which was not in agreement with the present findings. However, the study performed by Enz Hubert et al[3] only included 23 patients, and therefore, the sampling error was relatively high. In addition, the study did not analyze the association between PCT and serious infection. In contrast, the present study did not investigate the association between LA and serious infection, which could account for the differences in the findings of the studies. The clinical study performed by Park Sang Hyuk showed that none of the inflammatory indicators including RP% could be used to distinguish serious and nonserious infections, which was completely different from the present findings. These differences could be associated with the regions of sample selection, sample size, sample measuring time, equipment of the measurement, range of the ages of the sample, and percentage of the gender of the sample. The sample in the study performed by Park Sang Hyuk was mainly from European and American countries, the sample size was relatively high, the samples were immediately sent for measurement, and the equipment used was the XE-2100 automatic blood cell analyzer. However, the sample in the present study was mainly from Eastern Asian countries, the sample size was smaller than the sample size in the study performed by Park Sang Hyuk, the samples were sent for measurement 1 hour later instead of immediately, and the equipment used for the measurement was the XN-9000 automatic blood cell analyzer. In this study, the RP count detection is used PLT-F channel of XN-9000 automated blood cell analyzer, which can avoid the influence of small red cells and red cell fragments on platelet count and RP count, being superior to the previous detection methods, so this research on RP count is credible.
It was speculated that the differences in the results could be associated with these factors. However, more clinical studies are still needed to further investigate the association between RP% and serious infection.
5. Conclusion
The detection of RP% is to use the automatic blood cell analyzer in blood routine detection and analysis of the data obtained at the same time, without the need to draw blood inflammatory indexes. Therefore, compared with the traditional inflammation index, RP% detection reduces not only the blood volume but also the cost of testing. The value of RP% in diagnosing infection and serious infection was higher than conventional inflammatory indicators, while its combined use with CRP/PCT could further increase the early diagnostic rate of infectious diseases. The dynamic measurement of RP% could be used to evaluate the progression of infection and assist inflammatory indicators, such as CRP and PCT, in guiding the application of antibiotics in clinical practice.
Footnotes
Abbreviations: AUC = receiver operating characteristic curve, CRP = C-reactive protein, ICU = intensive care unit, LA = lactic acid, M[Q] = medians and interquartile ranges, N% = percentage of neutrophils, PCT = procalcitonin, PLT = platelet count, REC = rough endoplasmic reticulum, ROC = receiver operating characteristic, RP% = percentage of reticulated platelets, SIRS = systemic inflammatory response syndrome, T = axillary temperature, WBC = white blood cell.
The study was approved by the ethics committee.
All data in this study are in publicly available resources and can be used.
Funding/support: This study was funded by the Natural Foundation of Anhui Province (No. 1308050MH157).
The authors report no conflicts of interest.
References
- [1].Otero RM, Nguyen HB, Huang DT, et al. Early goal-directed therapy in severe sepsis and septic shock revisited: concepts, controversies, and contemporary findings. Chest 2006;130:1579–95. [DOI] [PubMed] [Google Scholar]
- [2].Zhang HM, Lu ZX, Yu J. Diagnostic value of reticulated platelet percentage in early diagnosing infectious diseases. Shan Dong Yi Yao 2010;11:66–7. [Google Scholar]
- [3].Enz Hubert RM, Rodrigues MV, Andreguetto BD, et al. Association of the immature platelet fraction with sepsis diagnosis and severity. Sci Rep 2015;5:8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Park SH, Ha SO, Cho YU, et al. Immature platelet fraction in septic patients: clinical relevance of immature platelet fraction is limited to the sensitive and accurate discrimination of septic patients from non-septic patients, not to the discrimination of sepsis severity. Ann Lab Med 2016;36:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Briggs C, Kunka S, Hart D, et al. Assessment of an immature platelet fraction (IPF) in peripheral thrombocytopenia. Br J Haematol 2004;126:93–9. [DOI] [PubMed] [Google Scholar]
- [6].American College of Chest Physicians. Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–74. [PubMed] [Google Scholar]
- [7].Chen XP. Surgery. 8th editionBeijing: People's Medical Publishing House; 2013. [Google Scholar]
- [8].Gaieski DF, Edwards JM, Kallan MJ, et al. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013;41:1167–74. [DOI] [PubMed] [Google Scholar]
- [9].Secor D, Li F, Ellis CG, et al. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med 2010;36:1928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Macchi I, Chamlian V, Sadoun A, et al. Comparison of reticulated platelet count and mean platelet volume determination in the evaluation of bone marrow recovery after aplastic chemotherapy. Eur J Haematol 2002;69:152–7. [DOI] [PubMed] [Google Scholar]
- [11].Hernandez G, Bruhn A, Ince C. Microcirculation in sepsis: new perspectives. Curr Vasc Pharmacol 2013;11:161–9. [PubMed] [Google Scholar]
- [12].Di Mario A, Garzia M, Leone F, et al. Immature platelet fraction (IPF) in hospitalized patients with neutrophilia and suspected bacterial infection. J Infect 2009;59:201–6. [DOI] [PubMed] [Google Scholar]
- [13].De Blasi RA, Cardelli P, Costante A, et al. Immature platelet fraction in predicting sepsis in critically ill patients. Intensive Care Med 2013;39:636–43. [DOI] [PubMed] [Google Scholar]
- [14].Wu Q, Ren J, Hu D, et al. An elevated percentage of reticulated platelet is associated with increased mortality in septic shock patients. Medicine (Baltimore) 2015;94:e814. [DOI] [PMC free article] [PubMed] [Google Scholar]


