Abstract
Research regarding sex or gender difference in chronic pain proliferated in this decade. This study was to analyze gender difference in Taiwan patients receiving long-term opioids for chronic noncancer pain.
An observational cross-sectional survey was conducted among the registered outpatients by the Taiwan Food and Drug Administration. Participants completed a self-report questionnaire, including the Taiwanese version of Brief Pain Inventory and enquiry regarding sexual activities, depressive symptoms, and misuse behaviors.
In total, 68 female and 142 male patients were analyzed. Both pain intensity and daily function interference reduced comparably (around 50%) between women and men after taking opioids in the past 1 week. The opioid-related adverse effects, including constipation, decreased sexual desire and satisfaction, and misuse behaviors were not significantly different. Women were exceedingly diagnosed with depression (67.7% vs 49.3%, P = .012) and had a higher mean depressive symptom score in the past 1 month, especially among those age <40 years (23.3 vs 11.9, P = .009), as compared with men. In addition, women had a lower mean self-rated health score (37.9 vs 44.3, P = .047). The mean morphine equivalent dose was significantly lower in women (131.6 vs 198.2 mg/day, P = .008), which was not correlated with their depressive scores.
Gender differences in the effectiveness and adverse effects of long-term opioids were not found among Taiwan registered outpatients with chronic noncancer pain. However, more female patients inclined to have a coexisting depression diagnosis, depressive symptoms, and a lower perceived health score, needing regular screening and closer monitoring.
Keywords: chronic pain, difference, gender, noncancer, opioid
1. Introduction
This decade has witnessed substantial literatures regarding sex or gender differences in their responses to experimental pain perception,[1] biopsychosocial factors,[2] and pharmacological or nonpharmacological pain interventions.[3] The emerging evidence implicates biological sex hormones as key factors influencing pain sensitivity, while psychosocial and environmental processes may explain gender differences in pain expression,[2] such as increased pain sensitivity and risk for clinical pain among women.[3]
By now, several population-based epidemiological studies have demonstrated greater pain prevalence among women relative to men.[4–9] Women are more likely to experience a variety of chronic pain syndromes and tend to report more severe pain, at a higher frequency, and in a greater number of body regions,[2,9] resulting in a lower self-report health and functioning status.[8] However, men with chronic noncancer pain (CNCP) are more likely to receive opioids than women [6,10] and are at higher risk than women for escalation to high-dose opioid therapy and death from opioid-related causes.[11]
Long-term use of opioids has been strictly regulated in Taiwan since 1996.[12] Each CNCP patient should be assessed by the hospital's opioid committee and finally approved by the Taiwan Food and Drug Administration. The duration of each opioid prescription is limited and the long-term opioid therapy should be re-evaluated and recorded for surveillance at least every 4 months. The physicians must report the patients with aberrant behaviors to the hospital's opioid committee for discontinuing the opioid treatment.[12]
Consistent with a 55% increase of opioid consumption in Taiwan from 2002 to 2007,[13] the registered CNCP outpatients escalated from 114 in 2001 [14] to 328 in 2010.[15] We had interviewed 210 CNCP patients in 2010 [15] and this study further analyzed the gender difference among them regarding the concurrent perceptions of pain relief and adverse effects by chronic opioid therapy, including daily function, depression, sexual activity, and drug misuse behaviors.
2. Methods
2.1. Participants
With the approval of the Taiwan Food and Drug Administration in January 2010, all of the registered CNCP patients were included.[15] According to the official patient list with omitted Chinese first names, the study interviewers visited the outpatient departments and requested the treating physicians to identify the patients and their conditions. A total of 210 (64.0%) of the 328 registered CNCP patients signed the written informed consent approved by the hospital institutional review board (TSGHIRB-098-05-254) and completed the Chinese language questionnaire by themselves or with verbal help from the interviewer.[15] The questionnaire included the Taiwanese version of Brief Pain Inventory,[16] the Chinese version of Beck Depression Inventory,[14] aberrant behaviors associated with prescription opioid misuse, self-reported impacts on sexual function (desire, frequency, capability, and satisfaction), and use of complementary and alternative medicine (CAM).[15] The opioid prescriptions were verified by the treating physicians and converted to a daily oral morphine equivalent dose (MED), with a so-called watchful dose as 200 mg per day which suggests a careful reassessment.[17] The secondary analysis of gender difference among these 210 patients was conducted in this study.
2.2. Statistical analysis
The results of the questionnaire were entered into SPSS version 17 (SPSS, Chicago, IL). The demographic data were presented as patient number (%) or mean ± SD. Gender difference in pain severity (0–10), daily function interference scores by pain (0–10), total depressive scores (0–63), and self-rated health score (0–100) were analyzed by using t-test or Mann–Whitney U test. Gender impact on opioid adverse effects, depression diagnosis, misuse behaviors, and use of CAM were examined as categorical variables by using the χ2 test or Fisher's exact test. In addition, t-test and Mann–Whitney U test were used again to compare the MEDs between different genders among patients with side effects, sexual interference, and depression. In all cases, a P value < .05 was considered statistically significant.
3. Results
Among 210 (64.0%) of 328 registered outpatients, 68 (54.8%) of 124 women and 142 (69.6%) of 204 men completed the questionnaires. Table 1 presents comparable demographic data between women and men, including work status, pain treatment experience, suicidal ideation, and mean durations of chronic pain (median 84 vs 108 months) and opioid treatment (median 48 vs 60 months). Up to 72.1% of women and 79.6% of men became workless due to chronic pain, however, without significance. More women ever had a diagnosis of depression (67.6 vs 49.3%, P = .012), with an odds ratio 2.15 (95% confidential interval 1.17–3.94), as compared with men. Women reported significantly higher depressive scores summed by more depressive symptoms, especially among those aged under 40 (mean 23.3 vs 11.9, P = .009, maximal score 63). Consistently, women perceived a lower mean health score than men (37.9 vs 44.3, P = .047, maximal score 100).
Table 1.
Demographic data and depressive status.
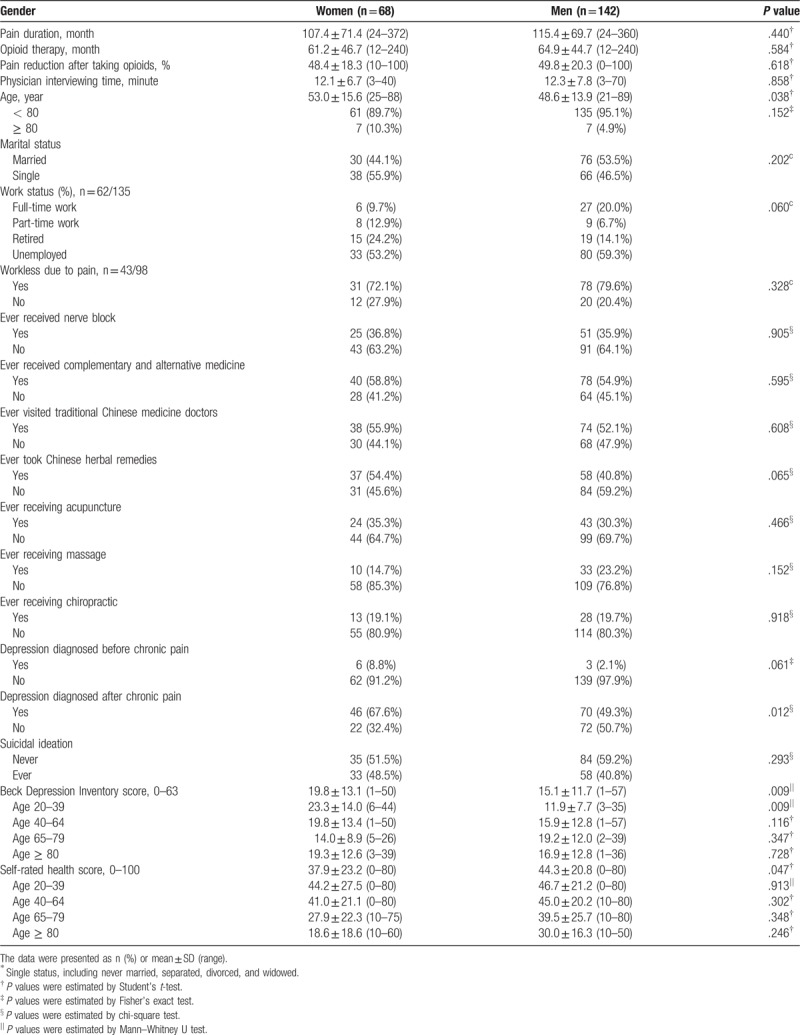
As shown in Table 2, women had a lower mean MED than men did (131.6 vs 198.2 mg/day, P = .008), and less women received high-dose opioids that surpassed the watchful dose of 200 mg/day (19.7 vs 37.5%, P = .011). The leading 3 diagnoses of chronic pain were chronic pancreatitis, spinal cord injury and neuralgia. All 7 patients diagnosed with fibromyalgia were female. There were no significant differences in daily MEDs for each diagnosis among women and men. The highest 3 MEDs were 1100, 910, and 740 mg/day in 3 men with chronic pancreatitis, neuralgia, and spinal cord injury, respectively. The high doses came mainly from fentanyl patch (300 μg/h) plus sustained-release oral morphine 30 and 60 mg.
Table 2.
Opioid dosage and diagnosis of chronic noncancer pain.
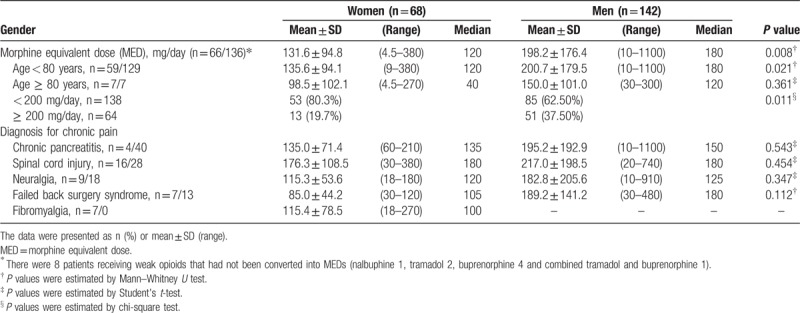
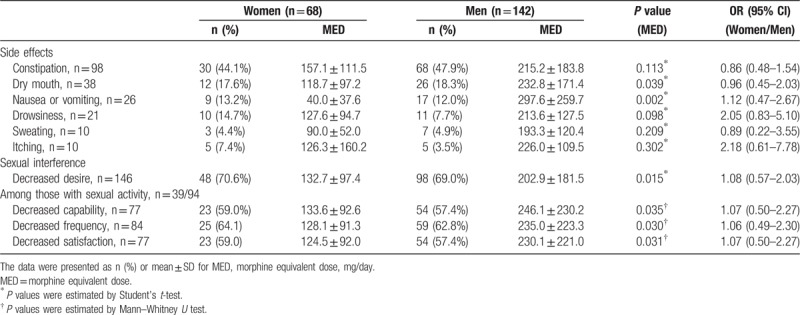
Figure 1 depicts comparable pain scores and interference with daily function after taking opioids in the past week. In addition, the opioid-related side effects and depressed sexual issues were compared in Table 3. Constipation, dry mouth, and nausea-vomiting were the leading side effects. Despite taking lower opioid doses, women were not less or more likely to suffer the side effects and decreases of sexual capability, frequency, and satisfaction. All 95% confidential intervals overstrode 1.0.
Figure 1.
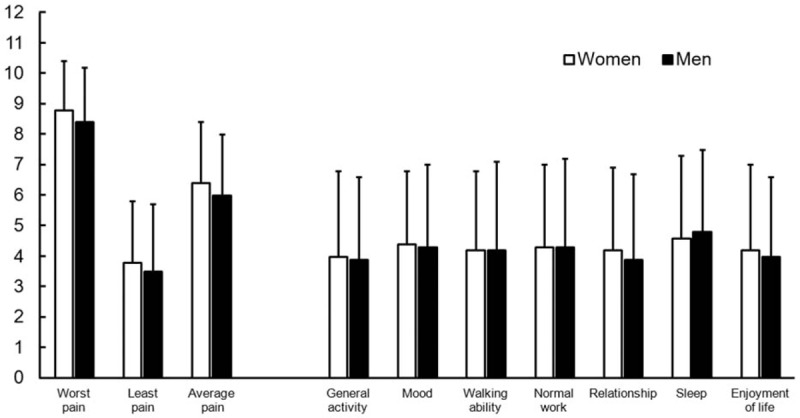
No observed gender differences in pain intensity and interference with daily function after taking opioids in the past 1 week.
Table 3.
Side effects and sexual interference after taking opioids in the past 1 week.
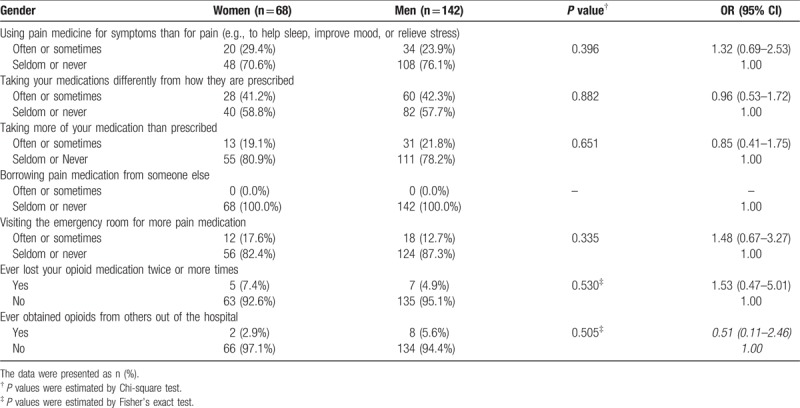
As demonstrated in Table 4, opioid misuse and aberrant behaviors were not significantly different between women and men. Only 2 (1.0%) men reported that they had ever, but seldom, borrowed pain medication from someone else. Relative to men, women did not have a greater risk to visit an emergency department for a pain-related complaint (odds ratio 1.48, 95% confidential interval 0.67–3.27, P = .335).
Table 4.
Prescription opioid misuse behaviors.
4. Discussion
This country-wide survey first demonstrated gender difference among the Taiwan registered patients receiving long-term opioids for chronic noncancer pain. The experience of the other pain management and the impact of long-term opioids on either effectiveness or adverse effects were comparable between women and men. However, more female patients had a coexisting depression diagnosis, more depressive symptoms, and a lower perceived health score.
Women are more likely to report severe pain and chronic pain in multiple geographic regions,[4–9] in addition to Taiwan [18] and China.[19] After receiving chronic opioid therapy, women perceived more unfavorable pain and a lower function status than did men (59% vs 42%).[5] However, women had lower odds of being prescribed chronic opioid therapy (odds ratio 0.67, 95% CI 0.58–0.78),[10] but greater odds of receiving physical therapy (OR 1.40, 95% CI 1.18–1.65) [10] and guideline-recommended psychotherapy, rehabilitation therapy, and pharmacy reconciliation.[4] The Taiwan Food and Drug Administration summarized all of the registered patients between 2003 and 2012,[20] with a ratio of women/men as 0.62 (246/398). In this study, the women/men ratio was 0.61 (124/204) from 328 registered patients in 2010. Generally, women and man in this study had comparable demographic data, experience of pain treatment, opioid effectiveness, and side effects. Therefore, to facilitate multidisciplinary pain treatment for Taiwan women, or to eliminate the factors deterring women from receiving long-term opioids would provide more adequate pain management for the under-estimated women suffering chronic pain in Taiwan.
Depression is prevalent in people living with chronic pain.[21] The patients with opioid analgesic use of more than 90 days had an increased risk of a new depression diagnosis (hazard ratio from 1.35 to 2.05 in 3 patient populations), as compared with those with opioid use of less than 30 days in a retrospective survey.[22] However, another prospective observational cohort study did not observe an increase of depressive symptoms following the 12-month opioid use for chronic pain.[23] It is noteworthy that two-thirds of our female patients had a diagnosis of depression after chronic pain, especially among those age < 40. Among chronic opioid users, young and middle-aged women (< 65 years) were at particularly higher risk of unfavorable global pain status (66% of women vs 40% of men among those aged 21 to 44 years; 59% vs 39% in those aged 45 to 64).[5] Consequently, suicidal ideation is a risk factor in this population that it must be assumed some proportion of drug overdose by intended suicide, not just by temporarily relieving their pain.[21] When possible, clinicians should avoid rapid dose escalation, which may be a proxy for loss of control or undetected abuse known to be associated with depression.[24] Over 40% of our patients ever had suicidal ideation, without gender difference. In Taiwan, prescription opioid abuse and overdose-related death have not yet become public health issues. According to the Taiwan official opioid regulation,[12] physicians should refer each CNCP patient to an initial psychiatric consultation, which should encompass the patient's psychiatric status, any comorbid psychiatric disorders, history of drug abuse, and social psychological function, followed by psychiatric re-assessment at least every 6 months.[25] Further prospective and longitudinal surveys in Taiwan are needed to determine the opioid prescription-related mortality that we did not obtain in this cross-sectional survey.
Opioids can suppress gonadal hormone production, resulting in low testosterone levels, reduced libido, decreased sexual capability, and even infertility in both men and women.[26,27] Long-term use of opioids in women exhibited clinically associated reproductive dysfunction, including decreased libido (61%–100%) and altered menstrual cycle (amenorrhea, 23%–71%).[28] A cross-sectional survey using the National Health and Nutrition Examination Survey program in the United States revealed both men and women with opioid exposure in the past 30 days had a higher odds ratio (1.40) of low testosterone levels than those unexposed,[29] especially among those over 70 years (OR 1.70), as compared with those between 17 and 45 years. Meanwhile, men with chronic opioid therapy are at higher risk (hazard ratio 1.44) than women for escalation to high-dose opioid therapy in a Canadian cohort study.[11] Similarly, our male patients had a higher mean daily dose and were more likely to receive high-dose opioids that surpassed the watchful dose of 200 mg per day, despite comparable sexual function interference than women. Further prospective surveys are needed to discover the suppression of testosterone and estrogen levels and its relationship with opioid doses in the Taiwan CNCP patients.
This uncontrolled and nonblinded study has several limitations, some of which have been discussed in the earlier publication.[15] Some more limitations should be considered in this gender study. First, the ratio of women/men respondents is only 48%. The higher rates of depression diagnosis in these women may predispose them to a more unfavorable opioid effectiveness and more adverse effects. Second, the secondary analysis in this study was limited to the variables contained in the original questionnaires, which did not enquire about the psychosocial factors that correlate with the diagnosis of depression in women and the details of medication adherence. Third, these self-report data were subject to recall bias as well. The effectiveness might be overrepresented by high utilizers of care, while the intolerable side effects and aberrant behaviors might have been under-reported on account of a total of non-respondent rate 36% of all CNCP patients. Consequently, those with severe depressive symptoms would be unrevealed among the nonrespondent women (45.2%) and men (30.4%). From a clinical point of view, the statistically significant difference in depression diagnosis did not influence their effectiveness and major side effects of opioid therapy between genders.
This cross-sectional survey analyzed the gender difference among the Taiwan officially registered and surveilled outpatients with chronic noncancer pain and revealed comparable effectiveness and adverse effects of long-term opioids. Notably, more female patients inclined to have a coexisting depression diagnosis, more depressive symptoms, and a lower perceived health score. Routine screening and closer monitoring for depression are needed.
Acknowledgments
We gratefully acknowledge the research assistant Mr. Cheng-Shien Yang for questionnaire collection and data processing.
Author contributions
Conceptualization: Tso-Chou Lin, Shung-Tai Ho, Shu-Ling Hwang.
Data curation: Tso-Chou Lin, Luo-Ping Ger.
Formal analysis: Tso-Chou Lin, Shung-Tai Ho, Luo-Ping Ger, Huei-Han Liou.
Funding acquisition: Shung-Tai Ho.
Investigation: Tso-Chou Lin, Shung-Tai Ho.
Methodology: Tso-Chou Lin, Shung-Tai Ho, Luo-Ping Ger.
Resources: Shung-Tai Ho.
Software: Huei-Han Liou.
Validation: Tso-Chou Lin, Shung-Tai Ho, Luo-Ping Ger, Shu-Ling Hwang.
Visualization: Huei-Han Liou.
Writing – original draft: Tso-Chou Lin.
Writing – review & editing: Tso-Chou Lin, Shung-Tai Ho, Luo-Ping Ger, Shu-Ling Hwang.
Footnotes
Abbreviations: CNCP = chronic noncancer pain, MED = morphine equivalent dose.
This work was supported by Taiwan Food and Drug Administration (DOH99-FDA-61404).
The authors have no conflicts of interest to disclose.
References
- [1].Racine M, Tousignant-Laflamme Y, Kloda LA, et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception—part 1: are there really differences between women and men? Pain 2012;153:602–18. [DOI] [PubMed] [Google Scholar]
- [2].Racine M, Tousignant-Laflamme Y, Kloda LA, et al. A systematic literature review of 10 years of research on sex/gender and pain perception—part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 2012;153:619–35. [DOI] [PubMed] [Google Scholar]
- [3].Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Oliva EM, Midboe AM, Lewis ET, et al. Sex differences in chronic pain management practices for patients receiving opioids from the Veterans Health Administration. Pain Med 2015;16:112–8. [DOI] [PubMed] [Google Scholar]
- [5].LeResche L, Saunders K, Dublin S, et al. Sex and age differences in global pain status among patients using opioids long term for chronic noncancer pain. J Womens Health (Larchmt) 2015;24:629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ringwalt C, Gugelmann H, Garrettson M, et al. Differential prescribing of opioid analgesics according to physician specialty for Medicaid patients with chronic noncancer pain diagnoses. Pain Res Manag 2014;19:179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ruau D, Liu LY, Clark JD, et al. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain 2012;13:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Boerma T, Hosseinpoor AR, Verdes E, et al. A global assessment of the gender gap in self-reported health with survey data from 59 countries. BMC Public Health 2016;16:675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wranker LS, Rennemark M, Berglund J. Pain among older adults from a gender perspective: findings from the Swedish National Study on Aging and Care (SNAC-Blekinge). Scand J Public Health 2016;44:258–63. [DOI] [PubMed] [Google Scholar]
- [10].Weimer MB, Macey TA, Nicolaidis C, et al. Sex differences in the medical care of VA patients with chronic non-cancer pain. Pain Med 2013;14:1839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kaplovitch E, Gomes T, Camacho X, et al. Sex differences in dose escalation and overdose death during chronic opioid therapy: a population-based cohort study. PLoS One 2015;10:e0134550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Taiwan Food and Drug Administration. Physician guidelines on clinical use of narcotics in chronic non-cancer pain. (in Chinese, revised on May 6, 2015). Available at: https://www.fda.gov.tw/TC/law.aspx?cid=183&cchk=9bb4ade3-dd48-4fbf-9bf2-b2c2ebd5d1b4&pn=7 Accessed December 31, 2017. [Google Scholar]
- [13].Pan HH, Ho ST, Lu CC, et al. Trends in the consumption of opioid analgesics in Taiwan from 2002 to 2007: a population-based study. J Pain Symptom Manage 2013;45:272–8. [DOI] [PubMed] [Google Scholar]
- [14].Lin TC, Hsu CH, Lu CC, et al. Chronic opioid therapy in patients with chronic noncancer pain in Taiwan. J Anesth 2010;24:882–7. [DOI] [PubMed] [Google Scholar]
- [15].Lin TC, Ger LP, Pergolizzi JV, Jr, et al. Long-term use of opioids in 210 officially registered patients with chronic noncancer pain in Taiwan: a cross-sectional study. J Formos Med Assoc 2017;116:257–65. [DOI] [PubMed] [Google Scholar]
- [16].Ger LP, Ho ST, Sun WZ, et al. Validation of the brief pain inventory in a Taiwanese population. J Pain Symptom Manage 1999;18:316–22. [DOI] [PubMed] [Google Scholar]
- [17].Kahan M, Mailis-Gagnon A, Wilson L, et al. Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians. Part 1: general population. Can Fam Physician 2011;57:1257–66. e1407-218. [PMC free article] [PubMed] [Google Scholar]
- [18].Tsai YF, Liu LL, Chung SC. Pain prevalence, experiences, and self-care management strategies among the community-dwelling elderly in Taiwan. J Pain Symptom Manage 2010;40:575–81. [DOI] [PubMed] [Google Scholar]
- [19].Chen B, Li L, Donovan C, et al. Prevalence and characteristics of chronic body pain in China: a national study. Springerplus 2016;5:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheng IC, Chang CS, Tsay WI. Long-term usage of narcotic analgesics by chronic intractable noncancer pain patients in Taiwan from 2003 to 2012. J Formos Med Assoc 2016;115:773–8. [DOI] [PubMed] [Google Scholar]
- [21].Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med 2011;12(suppl 2):S43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Scherrer JF, Salas J, Copeland LA, et al. Prescription opioid duration, dose, and increased risk of depression in 3 large patient populations. Ann Fam Med 2016;14:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Von Korff M, Shortreed SM, LeResche L, et al. A longitudinal study of depression among middle-aged and senior patients initiating chronic opioid therapy. J Affect Disord 2017;211:136–43. [DOI] [PubMed] [Google Scholar]
- [24].Salas J, Scherrer JF, Schneider FD, et al. New-onset depression following stable, slow, and rapid rate of prescription opioid dose escalation. Pain 2017;158:306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lin TC, Ger LP, Pergolizzi JV, Jr, et al. Knowledge, attitude and practice survey of prescribing opioids for chronic noncancer pain in Taiwan—Comparison of pain and non-pain physicians. Pain Med 2016; 10.1093/pm/pnw189 10.1093/pm/pnw189 Epub 13 August, 2016. [DOI] [PubMed] [Google Scholar]
- [26].Smith HS, Elliott JA. Opioid-induced androgen deficiency (OPIAD). Pain Physician 2012;15(3 suppl):ES145–56. [PubMed] [Google Scholar]
- [27].De Maddalena C, Bellini M, Berra M, et al. Opioid-induced hypogonadism: why and how to treat it. Pain Physician 2012;15(3 suppl):ES111–8. [PubMed] [Google Scholar]
- [28].Wersocki E, Bedson J, Chen Y, et al. Comprehensive systematic review of long-term opioids in women with chronic noncancer pain and associated reproductive dysfunction (hypothalamic-pituitary-gonadal axis disruption). Pain 2017;158:8–16. [DOI] [PubMed] [Google Scholar]
- [29].Cepeda MS, Zhu V, Vorsanger G, et al. Effect of opioids on testosterone levels: cross-sectional study using NHANES. Pain Med 2015;16:2235–42. [DOI] [PubMed] [Google Scholar]


