Supplemental Digital Content is available in the text
Keywords: appendicitis, children, culture, microbiome, sequencing
Abstract
Intestinal microbiota is involved in metabolic processes and the pathophysiology of various gastrointestinal disorders. We aimed to characterize the microbiome of the appendix in acute pediatric appendicitis comparing extraluminal and intraluminal samples.
Between January and June 2015, 29 children (3–17 years, mean age 10.7 ± 3.4 years, sex M:F = 2.6:1) undergoing laparoscopic appendectomy for acute appendicitis were prospectively included in the study. Samples for bacterial cultures (n = 29) and 16S ribosomal desoxyribonucleic acid (rDNA) sequencing (randomly chosen n = 16/29) were taken intracorporeally from the appendiceal surface before preparation (“extraluminal”) and from the appendiceal lumen after removal (“intraluminal”). The degree of inflammation was histologically classified into catarrhal, phlegmonous, and gangrenous appendicitis.
Seventeen bacterial species were cultivated in 28 of 29 intraluminal samples and 4 species were cultivated in 2 of 29 extraluminal samples. Using 16S rDNA sequencing, 267 species were detected in intraluminal but none in extraluminal samples. Abundance and diversity of detected species differed significantly between histological groups of acute appendicitis in bacterial cultures (P = .001), but not after 16S rDNA sequencing.
The appendiceal microbiome showed a high diversity in acute pediatric appendicitis. The intraluminal microbial composition differed significantly depending on the degree of inflammation. As bacteria were rarely found extraluminally by culture and not at all by sequencing, the inflammation in acute appendicitis may start inside the appendix and spread transmurally.
1. Introduction
Acute appendicitis is one of the main causes for acute abdominal pain in children and adolescents with a lifetime risk of 7%.[1] The etiopathogenesis is still unknown. It has been suggested that an intraluminal obstruction by lymphoid hyperplasia, foreign bodies, parasites, tumors, or fecaliths is responsible for the onset of inflammation. Here, the accumulation of bowel secretions and distension of the appendiceal lumen, which compromises the capillary blood flow and weakens the epithelial mucosal barrier, potentially allow a bacterial invasion into the appendiceal wall.[2,3]
In recent years, the (im)balance of the appendiceal flora has gained increasing interest.[4–6] Several studies reported a higher abundance of Fusobacteria in acute appendicitis that penetrate the appendiceal wall as assessed by ribosomal ribonucleic acid (rRNA)-based fluorescence in situ hybridization with increasing degree of inflammation.[7,8] In addition, the abundance of other bacteria like Peptostreptococcus, Bilophila, and Bulleidia were augmented, while others like Paenibacillaceae, Acidobacteriaceae, and Bacteroides spp. were decreased.[5,6] Others have investigated the intraluminal microbiome in phlegmonous, gangrenous, and perforated appendicitis by 16S ribosomal desoxyribonucleic acid (rDNA) sequencing before, but did not find any significant difference.[9] In clinical practice, however, traditional culture-based approaches are still the gold standard to detect bacteria, for example, to guide antibiotic treatment in complicated appendicitis.
The aim of our study was to characterize the microbial composition at the intraluminal and extraluminal site of the inflamed appendix in different histopathologic stages of acute pediatric appendicitis using bacterial cultures and 16S rDNA sequencing.
2. Materials and methods
2.1. Study design
The present study was approved by the research ethics committee of the University of Leipzig (reference number: 401-14-15122014). All participants’ parents signed written informed consent at the time of enrollment. Pathological examination was performed at the Institute of Pathology, University Hospital Leipzig; cultivation of collected samples was carried out at the Institute for Medical Microbiology and Epidemiology of Infectious Diseases, University Hospital Leipzig. Amplicon generation, sequencing, and microbiome profiling were conducted at Eurofins MWG Operon (Ebersberg, Germany).
2.2. Participants
Patients affected by acute appendicitis who subsequently underwent laparoscopic appendectomy between January and June 2015 were prospectively included in the study. Children with perforated appendicitis, incidental appendectomy and appendectomy for chronic abdominal pain as well as patients with postoperative complications were excluded. The following clinical data were collected prior to surgery: age, sex, weight, body mass index (BMI), laboratory tests (leukocytes, neutrophil count, and C-reactive protein [CRP]), clinical signs for appendicitis (e.g., right lower quadrant tenderness) and antibiotic treatment. Patients only received preoperative antibiotics intravenously depending on the severity of the disease and time frame until surgery. Either a combination of cefotaxime (30 mg/kg body weight) and metronidazole (10 mg/kg body weight) or piperacillin/tazobactam (100 mg/kg body weight) was administered. Finally, the Pediatric Appendicitis Score (PAS, 0–10) was calculated, indicating an acute appendicitis if PAS ≥ 6.[10]
2.3. Sample acquisition
Laparoscopic appendectomy was performed in a 3-trocar technique in all patients as described previously.[11] After visualizing the appendix and prior to dissection, 2 sterile extraluminal swabs of the appendix (eSwab; Hain Lifesience GmbH, Nehren, Germany) were taken to ensure “untouched” sample assessment. The appendix was dissected out by electrocautery (BiClamp; ERBE Elektromedizin GmbH, Tübingen, Germany), stapled over its base (Endopath, ETS Endoscopic Linear Cutter; Ethicon, Norderstedt, Germany) and removed by a specimen bag. Under sterile conditions, the appendix was immediately opened longitudinally and 2 swabs from the intraluminal side of the appendix were taken (eSwab; Hain Lifesience GmbH). Of each sample pair, one was placed in a medium-free sterile Eppendorf tube (Eppendorf, Wesseling-Berzdorf, Germany) and cryoconserved at −80°C for bacterial DNA extraction, the other one was stored in an anaerobic manner using 1 mL Amies medium at room temperature for immediate transfer and subsequent bacterial culture.[12] Appendices were stored in formalin 4% over night for histology by 1 pathologist, who was blinded to the study.
2.4. Pathological diagnosis
Hematoxylin–eosin staining was performed and the grade of inflammation was assessed according to Carr[13]—catarrhal appendicitis: local inflammation with few intraepithelial segmented neutrophils and reactive intraepithelial changes; phlegmonous appendicitis: neutrophilic invasion of mucosa, submucosa, and muscularis propria, mucosal ulcera, intramural abscesses and invasion in surrounding tissue, for example, thrombophlebitis; and gangrenous appendicitis: additional intramural necrosis or perforation to the features of phlegmonous appendicitis without free perforation into the abdominal cavity.
2.5. Bacterial cultures
Intra- and extraluminal samples of all patients were plated on Columbia blood agar (Thermo Fischer Scientific, Oxoid Microbiology Products, Hampshire, UK), chocolate blood agar (Thermo Fischer Scientific), Endo agar (Sigma–Aldrich, Steinheim, Germany), bile esculin agar (Thermo Fischer Scientific), Sabouraud agar (Thermo Fischer Scientific), and brain–heart infusion broth (Thermo Fischer Scientific), which were incubated aerobically at 37°C for 48 h, and Columbia blood agar, supplemented with hemin (0.005/L) and vitamin K (0.01/L), Bilophila medium (Thermo Fischer Scientific), and thioglycolate broth, which were incubated in an anaerobic atmosphere (Whitley MG 1000, anaerobic workstation, Meintrup Laborgeräte, Lähden-Holte, Germany) at 37°C for 4 days. All growing colonies were further identified using Matrix Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF-MS; VITEK-MS, bioMerieux, Lyon, France). In case of no growth on solid media and presence of turbidity of the fluid media, subcultures were performed and the growing colonies were identified using MALDI-TOF-MS.
2.6. 16S rDNA sequencing
For 16S rDNA sequencing of bacterial DNA, samples were processed according to a modified protocol: Swabs were defrosted on crushed ice and treated with 360 μL lysozyme solution for 30 min at 37°C (20 mg/mL lysozyme, 20 mM Tris–HCl, 2 mM ethylenediaminetetraacetic acid, 1.2% Triton X100, pH 8.00) followed by adding 40 μL proteinase K for 30 min at 56°C in 400 μL buffer AL (lysis buffer) for protein digestion. Enzyme inactivation was performed by heating to 95°C for 15 min; 500 mg of sterile 0.5 mm glass beads (Carl Roth GmbH, Karlsruhe, Germany) were added and bacterial cell walls were disrupted by shaking with a Tissue Lyser bead mill (Qiagen, Hilden, Germany). DNA purification was performed using the QIAamp DNA Mini/Micro Kit (Qiagen) according to the manufacturer's protocol. Nanodrop (Thermo Scientific, Waltham, MA) was used to measure DNA quantity and quality (aim: A260/A280 ratio: 1.7–1.9). Purified DNA was stored in 20 to 50 μL buffer AL at −20°C.
Extracted DNA was quantified fluorometrically using the PicoGreen Assay for dsDNA (Life Technologies, Darmstadt, Germany). Amplicon generation of 10 ng DNA/reaction was conducted by polymerase chain reaction (PCR) for the bacteria-specific 16S ribosomal V1–V3 region using the FastStart High Fidelity PCR System (Roche Diagnostics, Mannheim, Germany) and the template-specific barcoded fusion-primers: 27F: GAGTTTGATCATGGCTCAG and 530R: GTATTACCGCGGCTGCTG. The amplicon libraries were pooled and sequenced on MiSeq (Illumina, San Diego, CA) with 2 × 300 base pairs (bp) paired-end read modus, chemistry v3. Prior to the microbiome analysis, raw reads were de-barcoded and reads with ambiguous bases (“N”) were removed. To preserve only high-quality reads, sequencing errors in the inline barcodes and primer sequences were not tolerated. The remaining set of high-quality reads was processed into Operational Taxonomic Units (OTUs) using minimum entropy decomposition.[14,15] To assign taxonomic information to each OTU, Basic Local Alignment Search Tool alignments of cluster representative sequences to the National Center for Biotechnology Information sequence database were performed. A most specific taxonomic assignment for each OTU was then transferred from the set of best-matching reference sequences. Hereby, a sequence identity of 80% across at least 80% of the representative sequence was a minimal requirement for considering reference sequences. Further processing of OTUs and taxonomic assignments was performed using the QIIME software package. Abundances of bacteria taxonomic units were normalized using lineage specific copy numbers of the relevant marker genes to improve estimates (QIIME software package, Version 1.8.0).[16]
2.7. Statistical analysis
Statistical analysis was performed using Graphpad Prism (V7) and Statistical Package for the Social Science (V24). After testing continuous data for Gaussian contribution by Shapiro–Wilk normality test, either Kruskal–Wallis test or Mann–Whitney test for nonparametric samples was applied. Parametric data were conducted using analysis of variance (ANOVA) and post hoc analysis. For nominal data, contingency tables and Fisher exact test analyses were used. Regarding sequencing samples, differences in α-diversity were calculated as species richness and in terms of the inverse Simpson index, followed by ANOVA with a Tukey post hoc test. Differences in community variation between groups (β-diversity) were calculated using Pielou Evenness index as well as Chord distance with subsequent analysis of similarity. Significance level was set as P < .05. Data are presented as means ± standard deviation (SD) if not indicated differently.
3. Results
3.1. Study population
Thirty-four patients who underwent laparoscopic appendectomy for acute appendicitis were recruited. Three children were excluded for perforated appendicitis, 1 for a complicated postoperative course and 1 for a missing clinical history. Thus, 29 participants (21 male and 8 female patients) with a mean age of 10.7 ± 3.4 years (range: 3.7–17.9) were analyzed. Five severely ill patients at presentation (2 with phlegmonous and 3 with gangrenous appendicitis) received preoperative antibiotics intravenously 4.4 ± 1.55 h (range: 1.7–5.4) prior to surgery, of which 2 were included for 16S rDNA sequencing. The administration of antibiotics did not impact on the assessed outcome variables (data not shown).
Subgrouping according to the histological grade was as follows: catarrhal (n = 4), phlegmonous (n = 21), and gangrenous appendicitis (n = 4) (Fig. 1). The groups did not differ significantly in age, sex, BMI, leukocytes, and neutrophil count (Supplemental Table 1). However, PAS and CRP levels were significantly higher in gangrenous as well as in phlegmonous compared with catarrhal appendicitis (P < .05).
Figure 1.
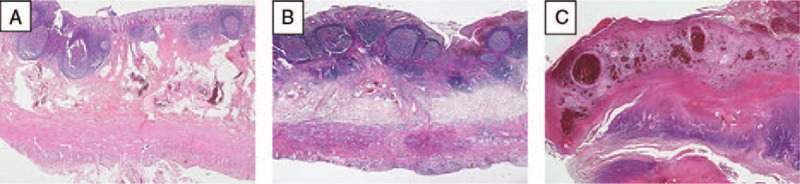
Histological differentiation into (A) catarrhal, (B) phlegmonous, and (C) gangrenous appendicitis using hematoxylin–eosin staining of paraffin-embedded tissue sections. (A) Minimal appendicitis with focal erosion of mucosa and an inflammatory infiltrate only in the submucosal layer. (B) Acute appendicitis with focal ulceration of mucosa, hemorrhage, and an inflammatory infiltrate in the submucosal, muscular, and serosal layer. (C) Extensive ulceration of the mucosa with a loss of mucosa, a massive inflammation of all layers of the wall next to necrotic areas. Magnification: ×25.
3.2. Bacterial cultures
Bacterial cultures showed positive results from 28 intraluminal and 2 extraluminal samples. Intraluminally, a total of 17 different species of 11 genera and 4 phyla were detected. At phylum level, Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria were identified. In catarrhal and phlegmonous appendicitis Proteobacteria and Bacteroidetes (catarrhal: 50% and 33%, phlegmonous: 54% and 32%) and in gangrenous appendicitis Proteobacteria and Firmicutes were most frequently detected (57% and 29%, Fig. 2). At genus level 11 genera were found. Escherichia dominated in all groups (catarrhal: 43%, phlegmonous: 41%, gangrenous: 50%), followed by Bacteroides in catarrhal and phlegmonous appendicitis (29% and 24%). In gangrenous appendicitis, Bacteroides, Enterococcus, Streptococcus, and Pseudomonas were equally present (13% each) (Fig. 3). At species level, Escherichia coli was found predominantly in all groups (catarrhal: 43%, phlegmonous: 41%, gangrenous: 50%) followed by Bacteroides thetaiotaomicron (29%) in catarrhal, Bacteroides fragilis (15%) in phlegmonous, and Pseudomonas aeruginosa (13%) in gangrenous appendicitis (Fig. 4). Microbial composition at genus and species level was significantly different in all 3 groups (P < .001).
Figure 2.
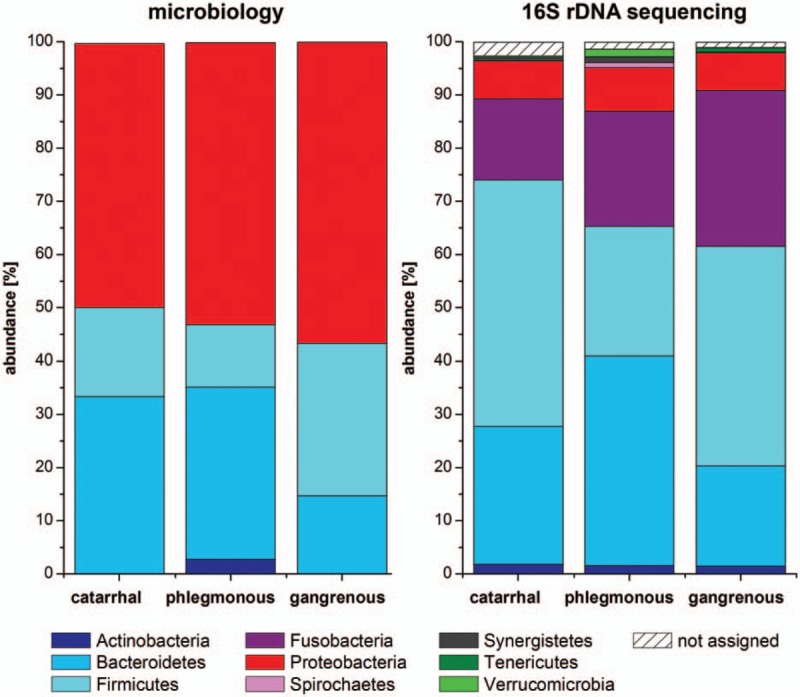
Microbiome analyses at phylum level in acute appendicitis. Four different phyla were detected by cultivation and 9 different phyla by 16S rDNA sequencing. No significant differences in abundance were found between the different groups for both methods. DNA reads that could not be assigned to a phylum are represented as “not assigned.”
Figure 3.
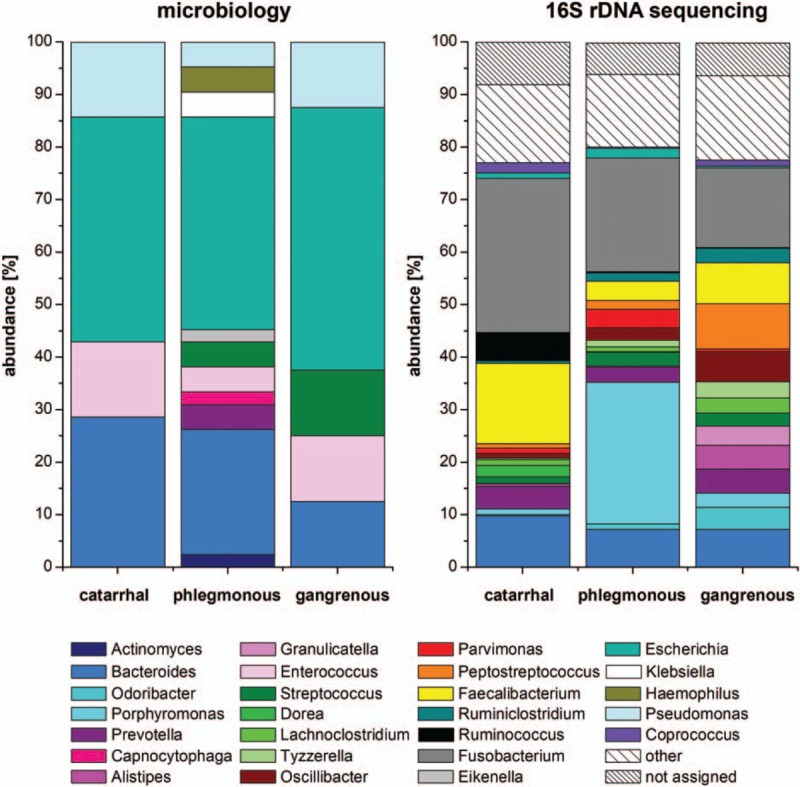
Microbiome analyses at genus level in acute appendicitis. Eleven genera could be identified by cultivation with the highest abundance of Escherichia in all 3 stages of inflammation, which differed significantly from each other in their compositions (P < .05); 109 genera were found by 16S rDNA sequencing, with highest abundance of Fusobacterium. No significant differences could be detected between the different stages after sequencing. Nine of 11 cultivated genera were also found by 16S rDNA sequencing. Bacterial genera with an abundance ≥2% in at least 1 group were included in the figure. Bacterial genera showing abundance <2% are summarized in “other.” Nonallocable sequences as well as bacterial genera showing an abundance <0.1% are indicated “not assigned.”
Figure 4.
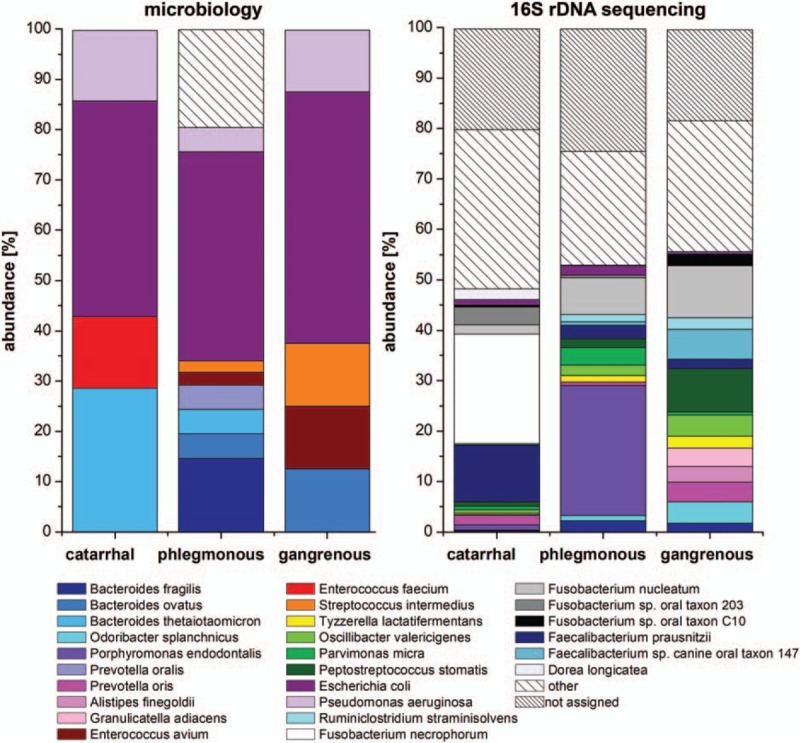
Microbiome analyses at species level in acute appendicitis. Seventeen species were found by bacterial culturing with the highest abundance of Escherichia coli in all 3 stages of inflammation, which differed significantly from each other in their composition (P < .05). By 16S rDNA sequencing, 267 species were identified, which did not differ between the histological groups. Species with an abundance of ≥2% in at least 1 group were included in the figure. Bacterial species showing an abundance <2% or detected only once in bacterial culture are summarized in “other.” Nonallocable sequences are indicated “not assigned.”
Extraluminally, 4 different species of 2 phyla (Bacteroidetes and Proteobacteria) were detected in 2 samples (both phlegmonous appendicitis). At genus level Escherichia and Bacteroides were found in both samples and Prevotella in one. At species level E coli was found in both samples while B fragilis, Bacteroides ovatus, and Prevotella oralis were present in only one of both.
3.3. Bacterial sequencing
Of 29 patients, 16 were randomly selected for 16S rDNA sequencing (catarrhal: n = 4, phlegmonous: n = 8, gangrenous: n = 4). Clinical and demographic data of the subgroup did not differ significantly from the main group (Supplemental Table 2); 4,478,219 reads were included for downstream analyses and an average of 218,717 sequences (SD 134,680) were assigned to each sample (range: 11,634–547,512 sequences).
The analysis of intraluminal but not extraluminal samples revealed sufficient DNA reads for further comparison: A total of 9 different phyla were found. Four phyla (Bacteroidetes, Firmicutes, Fusobacteria, and Proteobacteria) were present in all groups with a relative abundance of ≥2% and 5 phyla with an abundance of <2% (Actinobacteria, Spirochaetes, Synergistetes, Tenericutes, and Verrucomicrobia). In catarrhal appendicitis, Firmicutes and Fusobacteria (41% and 29%) and in phlegmonous and gangrenous appendicitis, Bacteroidetes and Firmicutes (phlegmonous: 39% and 24%; gangrenous: 26% and 46%) were mainly present (Fig. 2). A total of 109 genera were identified, 18 being present with a relative abundance of ≥2% in at least 1 group. Fusobacterium (29%) and Faecalibacterium (15%) dominated in catarrhal appendicitis, Porphyromonas (27%) and Fusobacterium (22%) in phlegmonous, and Fusobacterium (15%) as well as Peptostreptococcus (9%) in gangrenous appendicitis (Fig. 3). At species level, 267 species were found. Fusobacterium necrophorum (22%) and Faecalibacterium prausnitzii (11%) were mainly found in catarrhal appendicitis, Porphyromonas endodontalis (26%) and Fusobacterium nucleatum (7%) in phlegmonous, and F nucleatum (10%) as well as Peptostreptococcus stomatis (9%) in gangrenous appendicitis (Fig. 4). No statistically significant differences were found between the different appendicitis groups at all levels. We found no significant difference between intraluminal samples in terms of α-diversity or β-diversity (data not shown).
3.4. Bacterial cultures versus microbiome profiling
The assessed microbial composition differed in diversity and abundance between bacterial cultures and 16S rDNA sequencing. At genus level, 11 different genera were detected by bacterial cultures and 109 genera by 16S rDNA sequencing. Nine of 11 genera found in cultures were also found by 16S rDNA sequencing (except: Capnocytophaga and Pseudomonas) (Fig. 3). At species level, microbiological culture identified 17 different species and 16S rDNA sequencing 267 species. However, only 6 of 17 cultivated species were also detected by 16S rDNA sequencing (Fig. 4). In catarrhal appendicitis, Escherichia and Bacteroides dominated in culture but Fusobacterium and Faecalibacterium in 16S rDNA sequencing. In phlegmonous appendicitis, Escherichia and Bacteroides were prevalent in culture but Porphyromonas and Fusobacterium in 16S rDNA sequencing. Likewise, in gangrenous appendicitis, Escherichia, Bacteroides, Enterococcus, Streptococcus, and Pseudomonas were present in culture but 16S rDNA sequencing yielded high proportions of Fusobacterium and Peptostreptococcus.
4. Discussion
In the present study, we performed a comprehensive comparison of extra- and intraluminal appendiceal samples of acute pediatric appendicitis in different stages of inflammation. Intraluminally, different microbial compositions, in terms of abundance and diversity, were detected by comparing standard culturing to 16S rDNA sequencing. This microbial composition differed significantly at phylum and species level depending on the degree of inflammation in bacterial cultures but not after 16S rDNA sequencing. Finally, even in advanced appendicitis bacteria were rarely found extraluminally (and then only in cultures).
4.1. Bacterial cultures
Overall, bacterial cultures revealed 17 species of 11 genera and 4 phyla. Intraluminally, E coli, Bacteroides spp., and P aeruginosa were the dominant species. Escherichia coli is part of the physiologic flora of the gut. Nonpathogenic E coli strains are harmless and their presence prevents pathogenic bacteria from colonizing the intestinal tract.[17] However, they may cause severe peritonitis in case of a ruptured appendix. As a member of the normal intestinal flora, E coli showed highest abundances in all 3 stages of inflammation, potentially due to its fast growth and survival under different culture conditions.[18]Bacteroides spp., also part of the normal flora of the human intestine, can produce intramural and extraluminal abscess formation in acute appendicitis.[19] Enterotoxin-producing Bacteroides spp. may cause transmural inflammation by destroying tight junctions in intestinal epithelial cells and damaging the mucosa.[20] However, our study found no difference in the presence of Bacteroides spp. within the 3 histological groups. Pseudomonas aeruginosa, as a biofilm forming opportunistic pathogen, was only found in gangrenous appendicitis, which corresponds to previous results showing P aeruginosa in 16.7% of gangrenous and 27.8% of perforated appendicitis, indicating advanced appendicitis.[21,22] These results are in line with earlier studies, which reported an abundance of E coli, Bacteroides spp., and P aeruginosa in inflamed appendices.[23,24] However, microbiology cultures may lead to false-negative results due to a small sample volume, slow-growing bacteria, previously administered antibiotics or inefficient transport and storage. Thus, several studies suggest other methods, including real-time PCR and sequencing to detect pathogens effectively.[25,26]
Extraluminally, B fragilis, B ovatus, P oralis, and E coli could be cultivated in only 2 of 29 samples (both phlegmonous appendices). However, both patients did not differ significantly in clinical and laboratory findings as compared to all other patients. All species found extraluminally corresponded to the intraluminal bacteria of the same patient. In 1 patient, all species cultured intraluminally were also found extraluminally (E coli, P oralis, and B ovatus). In the other patient, 3 species (E coli, Enterococcus faecalis, and B fragilis) were detected intraluminally and 2 of them were also found extraluminally (E coli and B fragilis). This is particularly interesting, as all extraluminal samples were taken prior to dissection of the appendix. At that point, the appendix had not been exposed to manipulation by surgical instruments and ischemia due to ligation of the appendicular artery. Thus, translocation of intraluminal bacteria to the outer lumen, revealing bacterial abundances extraluminally was not caused by the surgical procedure but may already occur in the absence of a visible perforation. Moreover, future studies should exactly describe the localization of harvesting any culture relevant samples for exact interpretation.
4.2. Bacterial sequencing
16S rDNA sequencing identified 267 species of 109 genera and 9 phyla intraluminally. Fusobacterium necrophorum was mainly found in catarrhal appendicitis, P endodontalis in phlegmonous appendicitis and F nucleatum in gangrenous appendicitis. Several studies suggest that Fusobacterium spp. play a key role in the pathogenesis of acute appendicitis.[4–7]Fusobacterium spp. is an anaerobic oral pathogen that can cause periodontitis but also contribute to extra-oral inflammation such as acute appendicitis.[26,27] Its presence in the appendiceal mucosa was reported to correlate to the severity of acute appendicitis.[8] In our study, Fusobacterium spp. was detected in all appendicitis groups. Although we did not see an increasing abundance depending on the stage of inflammation as demonstrated in other studies, our data underline the importance of Fusobacterium spp. in the pathogenesis of appendicitis. Porphyromonas endodontalis, which usually causes oral infections, has been also associated with acute appendicitis before.[4–6,27] In our study, P endodontalis was found in catarrhal and phlegmonous but not gangrenous appendicitis, suggesting a contribution of P endodontalis to the onset of acute appendicitis.
No significant differences could be found for all sequencing data between the 3 histological groups, most likely due to the wide variety of abundances. This is supported by the most recent study on the appendiceal microbiome by Salö et al.[9] The authors compared intraluminal samples of the proximal and distal site of the appendix by 16S rDNA sequencing and found varying microbial compositions depending on the patient but also on the sample site. However, a correlation between specific species and different degrees of inflammation could not be determined. Likewise, in our study increased abundances of bacteria in the individual groups were seen, but specific bacterial species representing the stage of inflammation could not be identified.
Surprisingly, no bacteria were detected by 16S rDNA sequencing at the extraluminal site of the appendix due to the very low number of DNA reads. Even in gangrenous appendicitis with areas of massive inflammation of all layers next to necrotic regions, the intestinal barrier function seems to be maintained. Thus, an imbalance of the intraluminal microbial composition may play the superior role in acute appendicitis.
4.3. Bacterial cultures versus microbiome profiling
We detected a huge discrepancy in the identified species assessed by bacterial culturing and 16S rDNA sequencing. Bacterial cultures are routinely used to detect bacteria in daily clinical routine to test their susceptibility to antibiotics, either from the peritoneal cavity or the appendix fossa during appendectomy.[23,26,28]
In our study, special media and appropriate growth conditions were used in order to detect all cultivatable bacteria (including obligate anaerobic bacteria such as Fusobacterium and Porphyromonas) from the collected samples. The discrepancies between culture and 16S rDNA sequencing can be explained by the fact that molecular techniques do not distinguish between viable (microbiology) and nonviable (sequencing) cells.
16S rDNA sequencing provides more information on bacterial composition by detecting and identifying species based on the 16S rRNA gene of the small subunit of the prokaryote ribosome, a gene that plays an important role in cellular function. 16S rRNA gene sequences are compared to a 16S ribosomal database for identification of species. However, in our study, 2 genera (Capnocytophaga and Pseudomonas) detected by culture could not be found by 16S rDNA sequencing. This may be explained by the method of 16S rDNA sequencing, which amplifies only variable regions, for instance V1 to V3 but not the entire ribosomal gene. Species with characteristic features outside those regions cannot be differentiated.[29] Furthermore, some bacterial species only differ in a few bps and exact assignment of species by bioinformatics is difficult. In our study, P aeruginosa is one of the species that was not sequenced but was detected in culture. Likewise, other studies using 16S rDNA sequencing did not find Pseudomonas either.[4–6,9]
We are aware of several limitations of this study. When examining the bacterial microbiome, there are a huge number of (un)controllable confounding factors such as medication, diet, and lifestyle.[30] Antibiotic treatment with bactericidal or bacteriostatic agents can alter the microbial composition, but is sometimes inevitable in gastrointestinal surgery. This is especially relevant for culture, which detects only vital bacteria, but less in 16S rDNA sequencing. The latter also detects bacterial fragments of extinguished bacteria and is therefore less influenced by antibiotic treatment. Moreover, David et al demonstrated a prompt modification of the intestinal microbiome within a single day after rearrangement of individual eating habits. In our study, all children were reported to be on a balanced diet; however, acute changes in eating habits due to abdominal pain, nausea, and preoperative fasting cannot be excluded.[31]
5. Conclusion
To the best of our knowledge, this is the first study comparing bacterial cultures and 16S rDNA sequencing of extraluminal and intraluminal bacterial samples in relation to histopathological stages of acute pediatric appendicitis. Although a key pathogen could not be identified, we found a significant microbial diversity between different stages of inflammation. Moreover, our data suggest that appendicitis starts from the intraluminal site of the appendix and proceeds transmurally. The mucosal barrier remains intact, even in advanced inflammation. Our results question the role of postoperative antibiotic treatment in uncomplicated appendicitis.
Acknowledgments
The authors are grateful to Nicole Peukert and Marco Ginzel for their excellent theoretical and technical assistance and Arne Rodloff for careful reading of the manuscript. Further, we acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing.
Supplementary Material
Footnotes
Abbreviations: ANOVA = analysis of variance, BMI = body mass index, bp = base pair, CRP = C-reactive protein, MALDI-TOF-MS = Matrix Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry, OTU = Operational Taxonomic Unit, PAS = Pediatric Appendicitis Score, PCR = polymerase chain reaction, rDNA = ribosomal desoxyribonucleic acid, rRNA = ribosomal ribonucleic acid, SD = standard deviation.
SS and NS contributed equally to the work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Kim JS. Acute abdominal pain in children. Pediatr Gastroenterol Hepatol Nutr 2013;16:219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Birnbaum BA, Wilson SR. Appendicitis at the millennium. Radiology 2000;215:337–48. [DOI] [PubMed] [Google Scholar]
- [3].Jones BA, Demetriades D, Segal I, et al. The prevalence of appendiceal fecaliths in patients with and without appendicitis. A comparative study from Canada and South Africa. Ann Surg 1985;202:80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guinane CM, Tadrous A, Fouhy F, et al. Microbial composition of human appendices from patients following appendectomy. MBio 2013;4:pii: e00366-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jackson HT, Mongodin EF, Davenport KP, et al. Culture-independent evaluation of the appendix and rectum microbiomes in children with and without appendicitis. PLoS ONE 2014;9:e95414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhong D, Brower-Sinning R, Firek B, et al. Acute appendicitis in children is associated with an abundance of bacteria from the phylum Fusobacteria. J Pediatr Surg 2014;49:441–6. [DOI] [PubMed] [Google Scholar]
- [7].Swidsinski A, Dorffel Y, Loening-Baucke V, et al. Mucosal invasion by fusobacteria is a common feature of acute appendicitis in Germany, Russia, and China. Saudi J Gastroenterol 2012;18:55–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Swidsinski A, Dorffel Y, Loening-Baucke V, et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 2011;60:34–40. [DOI] [PubMed] [Google Scholar]
- [9].Salö M, Marungruang N, Roth B, et al. Evaluation of the microbiome in children's appendicitis. Int J Colorectal Dis 2017;32:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Samuel M. Pediatric appendicitis score. J Pediatr Surg 2002;37:877–81. [DOI] [PubMed] [Google Scholar]
- [11].Lacher M, Muensterer OJ, Yannam GR, et al. Feasibility of single-incision pediatric endosurgery for treatment of appendicitis in 415 children. J Laparoendosc Adv Surg Tech A 2012;22:604–8. [DOI] [PubMed] [Google Scholar]
- [12].Van Horn KG, Audette CD, Sebeck D, et al. Comparison of the Copan ESwab system with two Amies agar swab transport systems for maintenance of microorganism viability. J Clin Microbiol 2008;46:1655–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Carr NJ. The pathology of acute appendicitis. Ann Diagn Pathol 2000;4:46–58. [DOI] [PubMed] [Google Scholar]
- [14].Eren AM, Maignien L, Sul WJ, et al. Oligotyping: differentiating between closely related microbial taxa using 16S rRNA gene data. Methods Ecol Evol 2013;4:1111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eren AM, Morrison HG, Lescault PJ, et al. Minimum entropy decomposition: unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J 2015;9:968–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 2010;7:335–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hudault S, Guignot J, Servin AL. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut 2001;49:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clark DP, Pazdernik NJ. Molecular Biology. Cambridge, MA, USA: Academic Cell; 2012. [Google Scholar]
- [19].Wexler HM. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 2007;20:593–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wu S, Lim KC, Huang J, et al. Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin. Proc Natl Acad Sci USA 1998;95:14979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Stover CK, Pham XQ, Erwin AL, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000;406:959–64. [DOI] [PubMed] [Google Scholar]
- [22].Bennion RS, Thompson JE, Baron EJ, et al. Gangrenous and perforated appendicitis with peritonitis: treatment and bacteriology. Clin Ther 1990;12(suppl C):31–44. [PubMed] [Google Scholar]
- [23].Leigh DA, Simmons K, Norman E. Bacterial flora of the appendix fossa in appendicitis and postoperative wound infection. J Clin Pathol 1974;27:997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rautio M, Saxen H, Siitonen A, et al. Bacteriology of histopathologically defined appendicitis in children. Pediatr Infect Dis J 2000;19:1078–83. [DOI] [PubMed] [Google Scholar]
- [25].Kenig J, Richter P. The need for culture swabs in laparoscopically treated appendicitis. Wideochir Inne Tech Maloinwazyjne 2013;8:310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Foo FJ, Beckingham IJ, Ahmed I. Intra-operative culture swabs in acute appendicitis: a waste of resources. Surgeon 2008;6:278–81. [DOI] [PubMed] [Google Scholar]
- [27].Lombardo Bedran TB, Marcantonio RA, Spin Neto R, et al. Porphyromonas endodontalis in chronic periodontitis: a clinical and microbiological cross-sectional study. J Oral Microbiol 2012;4: doi: 10.3402/jom.v4i0.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gladman MA, Knowles CH, Gladman LJ, et al. Intra-operative culture in appendicitis: traditional practice challenged. Ann R Coll Surg Engl 2004;86:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chakravorty S, Helb D, Burday M, et al. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J Microbiol Methods 2007;69:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016;375:2369–79. [DOI] [PubMed] [Google Scholar]
- [31].David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014;505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


