Abstract
The “super-healing” Murphy Roths Large (MRL/MpJ) mouse possesses a superior regenerative capacity for repair of many tissues, which makes it an excellent animal model for studying molecular and cellular mechanisms during tissue regeneration. Since the role of muscle progenitor cells (MPCs) in muscle healing capacity of MRL/MpJ mice has not been previously studied, we investigated the muscle regenerative capacity of MRL/MpJ mice following muscle injury, and the results were compared to results from C57BL/6J (B6) age-matched control mice. Our results show that muscle healing upon cardiotoxin injury was accelerated in MRL/MpJ mice and characterized by reduced necrotic muscle area, reduced macrophage infiltration, and more regenerated myofibers (eMyHC+/centronucleated fibers) at 3, 5, and 12 days post-injury, when compared to B6 age-matched control mice. These observations were associated with enhanced function of MPCs, including improved cell proliferation, differentiation, and resistance to stress, as well as increased muscle regenerative potential when compared to B6 MPCs. Mass spectrometry of serum proteins revealed higher levels of circulating antioxidants in MRL/MpJ mice when compared to B6 mice. Indeed, we found relatively higher gene expression of superoxide dismutase 1 (Sod1) and catalase (Cat) in MRL/MpJ MPCs. Depletion of Sod1 or Cat by siRNA impaired myogenic potential of MRL/MpJ MPCs, indicating a role for these antioxidants in muscle repair. Taken together, these findings provide evidence that improved function of MPCs and higher levels of circulating antioxidants play important roles in accelerating muscle healing capacity of MRL/MpJ mice.
Significance statement
Skeletal muscle injuries account for 30–50% of all sports-related injuries. Often, impaired angiogenesis and regeneration lead to incomplete functional recovery. Using a mouse model (MRL/MpJ mice) with superior healing capacity for repair of several tissues, we show that muscle healing was accelerated at 3, 5, and 12 days post-injury in these mice compared to control B6 mice. This improvement of muscle repair was associated with enhanced function of muscle progenitor cells (MPCs) and higher levels of antioxidants, allowing them to withstand various stresses. This study suggests that a combination of MPCs and modulating antioxidative stress can represent a novel therapy to improve muscle healing after injury, disease, and aging.
Keywords: Stem cell, paracrine factors, tissue regeneration, antioxidant, skeletal muscle
Graphical Abstract
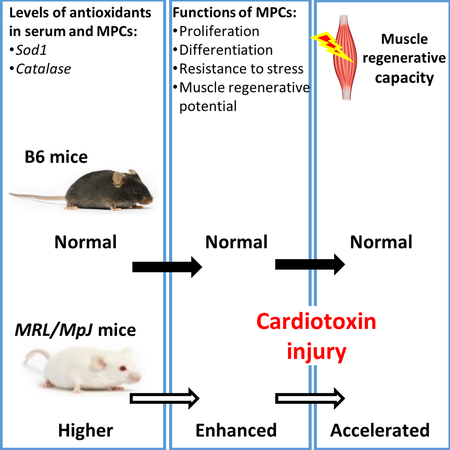
Overview of factors contributing to accelerated muscle regeneration in MRL/MpJ mice. Higher levels of circulating antioxidants (superoxide dismutase 1 and catalase) and improved function of MPCs play key roles in accelerating the muscle regenerative capacity in MRL/MpJ mice following cardiotoxin injury. MPCs, muscle progenitor cells; WT, wild-type, MRL/MpJ, Murphy Roths Large.
Introduction
Tissue regeneration and repair are important to ensure the restoration of functional tissues after injuries. Following injury, inflammatory cells are recruited at wound site to remove foreign antigens and cell debris [1]. Subsequently, native uninjured cells including fibroblasts, endothelial cells, and progenitor cells, migrate to the wound site, proliferate and differentiate to form new tissue [2]. In regeneration process, the injured tissue is restored to its normal state and architecture, often by replacement of the damaged tissue mass [3]. Skeletal muscle is among one of the most studied tissues capable of regenerating after injuries [4, 5]. Robust skeletal muscle regeneration is orchestrated by involvement of numerous cell populations at the injured muscle site. Muscle progenitor cells (MPCs), including satellite cells, are the major cell population involved in postnatal muscle repair [6, 7]. In adult muscle, satellite cells expressing the transcription factor paired box 7 (Pax7) are mitotically quiescent. In response to muscle injury, the MPCs residing beneath the surrounding basal lamina are activated and undergo cell expansion. Subsequently, MPCs differentiate and fuse to repair damaged muscle fibers. Therefore, the functional integrity of MPCs is crucial in modulating muscle regeneration [8, 9].
Multiple tools and techniques have been employed to isolate MPCs, including cell sorting using flow cytometry, bead enrichment, and cell culture selection [10–12]. Previously, our group successfully isolated the slow adhering MPCs by exploiting their differential cell adhesive characteristic using a preplate technique [12, 13]. These cells have a bona fide property of stem cell-like behavior, display high self-renewal capacity, and are multipotent with the ability to differentiate into bone, cartilage, and muscle lineages [11, 14]. More importantly, these MPCs show robust muscle regenerative capacity in vivo when transplanted into various musculoskeletal tissues [11, 15].
The Murphy Roths Large (MRL/MpJ) mouse, also known as the “super-healer” mouse strain, was established from crosses between C57BL/6 (0.3%), C3H/HeDi (12.1%), AKR/J (12.6%), and LG/J (75%) mice [16]. The superior wound healing capacity of MRL/MpJ mice was first discovered when investigators observed their ability to spontaneously close a through-and-through ear punch wound without scar formation [17]. Further studies extended the increased wound repair/tissue regeneration capacity of MRL/MpJ mice to a variety of tissues and injuries, including alkali-burned cornea injury [18], full-thickness articular cartilage injury [19], intra-articular fracture [20], and digit tip regrowth [21]. However, whether there are differences in muscle healing capacity between MRL/MpJ and B6 mice remains unclear. Studies have shown that dorsal skin wounds in MRL/MpJ mice heal with scarring, similarly to the C57BL/6 control mice [22–24]. Also, confounding results of enhanced or no differences in healing were reported for cardiac [25, 26] and nervous system injuries [27–29]. Nevertheless, the unique tissue regenerative capacity of MRJ/MpJ mice is an invaluable tool for studying tissue-specific regulators during regeneration processes.
The mechanism by which MRL/MpJ mice effectively repair and regenerate tissue is still unclear. Previous studies have suggested that alteration in immune response [30], cellular metabolism [16], and extracellular matrix remodeling are involved in the enhanced tissue repair abilities of MRL/MPJ mice [31, 32]. Further, it has been reported that reduced cell apoptosis [33], increased cell proliferation [31] and differentiation, improved remodeling, and enhanced stem cell function [34, 35] may play important roles in the enhanced repair process. Increased gene expression for anti-inflammatory processes, tissue remodeling, and cell cycle regulators has been reported in the MRL/MpJ mice after injury [16, 35], which may account for enhanced tissue repair capacity of these mice.
A previous study using dystrophic mice bred with MRL/MpJ mice showed alleviation of muscle fibrosis after injury [16, 36]; however, the mechanisms and role of MRL/MpJ MPCs in dictating muscle regeneration remain unclear. In the present study, we demonstrate that repair of a toxin-induced muscle injury is accelerated in MRL/MpJ mice when compared to C57BL/6J (B6) control mice. We observed that the number of Pax7-expressing MPCs and vessel density were higher in skeletal muscles of MRL/MpJ than B6 mice. We also observed that MPCs from MRL/MpJ mice had higher proliferation and multi-differentiation capacities than B6 MPCs, and maintained their differentiation potentials under different stress conditions. Moreover, a tandem mass spectrometry-based proteomic analysis of sera from B6 and MRL/MpJ mice revealed higher levels of antioxidants, including superoxide dismutase 1(Sod1) and catalase (Cat), in MRL/MpJ mouse serum. Depletion of Sod1 and Cat by siRNAs in MRL/MpJ MPCs impaired their myogenic capacity. Taken together, these findings support our hypothesis that MPCs and antioxidants play key roles in accelerating the muscle regeneration process in MRL/MpJ mice.
Materials and Methods
Animals, muscle and ear punch injuries
C57BL/6J (B6) and MRL/MpJ mice at 7 weeks old were purchased from Jackson Laboratories (Bar Harbor, ME). All animal studies and procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health Science Center at Houston (UTHealth). For muscle injury, isofluorane-anesthetized mice were intramuscularly injected with 30 μl of cardiotoxin (CTX) (Naja Pallida, 4 μM, Sigma-Aldrich, St.Louis, MO) into the gastrocnemius (GAS) muscle. GAS muscles were harvested at 3, 5, 12 and 30 days post-CTX injection. Mice were euthanized according to UTHealth IACUC-approved protocols prior to tissue extraction. For ear punch injury, a standard 2-mm diameter stainless steel thumb punch tool was used to create a crude wound at the center of each ear [17, 37]. Images of ear wounds were taken at day 0 and 5 weeks post-injury. The area of each ear wound was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Cell isolation and culture
Hindlimb muscles from B6 and MRL/MpJ mice were resected, minced, and digested with collagenase, dispase, and trypsin as previously described [38] Preparations were passed through a 70-μm strainer and centrifuged at 2630Xg for 5 min. MPCs were isolated using a modified preplate technique that separates cells based on differential adhering capacity of cells to collagen-coated plates [38]. The MPCs were subjected to immunostaining and Western blotting. MPCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 10% horse serum (Invitrogen, Carlsbad, CA), 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA), and 1% chicken embryo extract (Accurate Chemical Co. Westbury, NY).
Cell proliferation
Cell proliferation assays were performed using a live-cell imaging (LCI) system (Kairos Instruments LLC, Pittsburgh, PA) as previously described [15]. Briefly, 1–3×103 MPCs from B6 or MRL/MpJ mice were plated onto 12-well plates in triplicate. Cells were incubated in the biobox incubator and live-cell images were captured using a Nikon Eclipse TE 2000 U microscope at 30-min intervals over a period of 72 h. Images from 10 random fields per well at 10X magnification were taken. The cell proliferation rates were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Oxidative stress, myogenic differentiation, and MPC transplantation
B6 and MRL/MpJ MPCs were plated onto 12-well or 6-well plates in triplicate. For oxidative stress assays, MPCs were treated with 400 μM of hydrogen peroxide for 6 h. The percentage of viable cells was determined by the Trypan blue exclusion method in which live cells are negative for Trypan blue dye. For MPC proliferation during stress, MPCs were treated with a sub-optimal dose of hydrogen peroxide (50 μM) for 48 h, and cell proliferation was measured using an LCI system. B6 and MRL/MpJ MPCs were treated with 40 nM of CTX for 24 h or 10 μM of eeyarestatin for 24 h, followed by myogenic induction. The effect of stress on the myogenic potential of MPCs was determined by quantitative RT-PCR. For in vitro myogenic differentiation assays, MPCs from 7-week-old B6 and MRL/MpJ mice were isolated and cultured in myogenic differentiation medium (2% FBS serum in DMEM supplemented with 1% penicillin/streptomycin, Invitrogen, Carlsbad, CA). At indicated time points, myotubes were stained with fast Myosin Heavy Chain (f-MyHC, Sigma-Aldrich, St.Louis, MO) for identification of differentiated myotubes. The number of myotubes was counted from 10 random fields. The percentage of differentiated myotubes was quantified as the number of nuclei in f-MyHC-positive myotubes relative to the total number of nuclei. For muscle regenerative capacity, 4×105 MPCs from B6 or MRL/MpJ mice were intramuscularly injected into the GAS muscle of dystrophin-deficient mdx/SCID mice. At 2 weeks post-cell transplantation, GAS muscle was harvested and immunostained for dystrophin.
Histochemical analysis and immunostaining
GAS muscle samples were harvested and flash frozen in liquid nitrogen-cooled 2-methylbutane solution. Tissues were cryosectioned to a 10-μm thickness, followed by fixation of tissue sections with 4% paraformaldehyde for 10 min at room temperature. Sections were washed with PBS containing 0.01% Triton X-100, permeabilized with 0.3% Triton X-100 for 5 min, and then blocked with Seablock (Thermo scientific, Waltham, MA) for 1 h at room temperature. Cells or frozen sections were incubated at 4oC overnight with primary antibodies as follows: rabbit anti-dystrophin (1:300, Abcam, Cambridge, MA), rat anti-F4/80 (1:200, AbD Serotec, Raleigh, NC), and rabbit anti-CD31 (1:300, Abcam, Cambridge, MA). Mouse IgG (1:300, Vector, Burlingame, CA), Pax7 (1:100, DSHB, Iowa), and embryonic myosin heavy chain (1:50, eMyHC, DSHB, Iowa) were stained using a MOM kit (Vector, Burlingame, CA) according to the manufacturer’s protocols. Following three washes, frozen sections were incubated with secondary antibody at room temperature for 1 h in PBS/0.01% Triton X-100. Secondary antibodies used were donkey Alexa 488-conjugated IgG (Invitrogen, Carlsbad, CA, 1:500) and Cy3-conjugated IgG (Jackson ImmunoResearch, West Grove, PA, 1:300). Nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI, 1:1000, Invitrogen, Carlsbad, CA). Image acquisition was performed with a Leica Microsystems Inc. (Buffalo Grove, IL) camera at 4–40× magnification. For quantification of eMyHC, IgG, F4/80, Pax7, CD31, and MyHC: at least 10 random 10x or 20x magnification fields were blindly scored and/or measured with Image J or Adobe Photoshop CC.
Signal threshold was set with cells or tissue sections stained with secondary antibodies alone to exclude non-specific signal. The percentage of myotube fusion was quantified as the number of nuclei in MyHC-positive myotubes relative to the total number of nuclei.
Western blot analysis
Tissues or cell lysates were prepared with radio-immuno-protein assay (RIPA) buffer supplemented with a protease inhibitor cocktail (Sigma-aldrich, St Louis, MO). Protein concentrations were determined using the BCA protein assay (Thermo scientific, Waltham, MA). Equal amounts of protein were loaded onto 10% Biorad stain-free polyacrylamide sodium dodecyl sulfate Tris–Glycine gels and transferred onto PVDF membranes (Millipore, USA). Membranes were blocked (5% nonfat dry milk) and then incubated with primary antibody overnight at 4oC, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 1 h. Blots were developed using enhanced chemiluminescence (ThermoFisher Scientific, Waltham, MA) and images were captured with a ChemiDoc (Bio-Rad Laboratories, Hercules, CA). Primary antibodies used were mouse anti-vinculin (1:2000, Sigma-aldrich, St Louis, MO), and anti-cat and anti-Sod1 (1:1000, GeneTex, Irvine, CA). Sod1- and Cat-specific siRNAs were obtained from Sigma-Aldrich, St. Louis, MO (Cat# EMU200961 for mouse Sod1, and EMU052541 for mouse Cat). MPCs were transfected with specific siRNA at a concentration of 20 nM per well using Viromer blue, according to the manufacturer instructions (Origene, Rockville, MD, USA) for 48 h, and then induced with myogenic media. Immunostaining was performed to analyze myotube formation as described for Supplemental Figure 2).
Gene expression analsyis by quantitative RT-PCR
Total RNA from GAS muscle or cells was extracted with TRIZOL (Life Technologies, Carlsbad, CA). The cDNA was synthesized using the iScript Reverse Transcription Kit (Bio-Rad Laboratories, Hercules, CA). Quantitative real-time PCR was performed using an iCycler thermal cycler (Bio-Rad Laboratories, Hercules, CA.). The gene-specific primer sequences used are listed in Supplemental Table 1 for Atf4, Caveolin-3 (Cav3) [39], Pax7, MyoD, Mor23, Runx2, Alp, Acan, Il6, Klotho, Col2a1, Myog, Myf5, Sod1, Sox9, Cat, and Gapdh, which was used as an internal control to normalize gene expression. All results are expressed as means ± SEM.
Tandem mass spectrometry
Serum proteins from B6 and MRL/MpJ mice were identified by mass spectrometry. Briefly, serum processed from whole blood withdrawn from B6 and MRL/MpJ mice (n=5 each) was pooled for B6 and MRL/MpJ, respectively. Equal amounts of total protein from pooled samples were digested
with trypsin. The sample digest, labeling, and mass spectrometry (LC-MS/MS) were carried out in the UT MD Anderson Cancer Center Proteomics Core Facility. Datasets were analyzed using Ingenuity Pathway Analysis (IPA) software (Qiagen, Germany).
Statistics
Microsoft Excel or Prism software (GraphPad) was used to plot graphs as mean ± SEM and to calculate P-values using a Two-tailed heteroscedastic Student’s t-test. P<0.05 was considered significant.
Results
Enhanced ear wound healing in MRL/MpJ mice
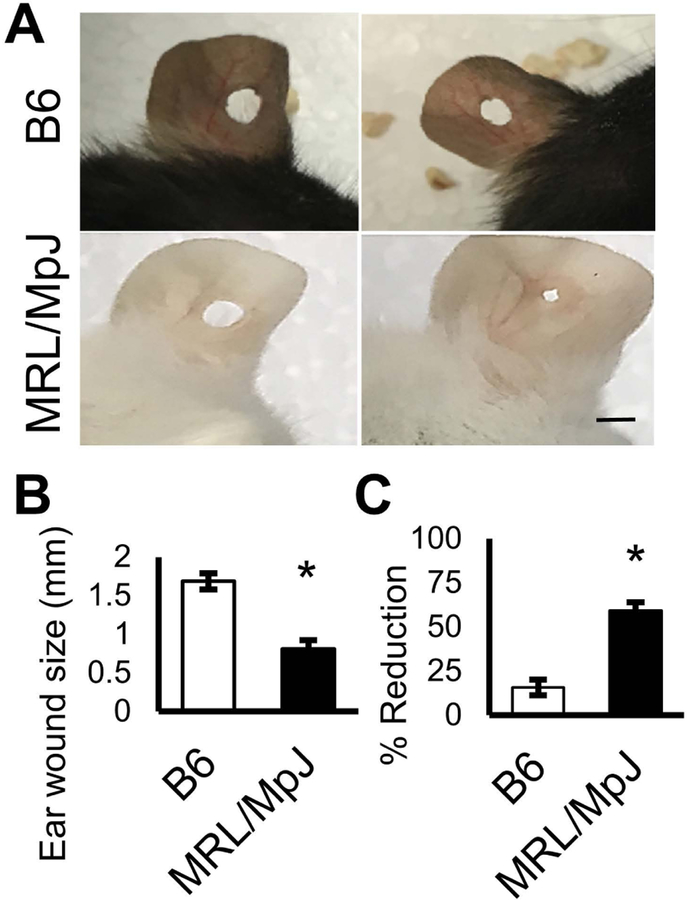
To confirm enhanced healing capacity in our MRL/MpJ mouse model, we performed a standard 2-mm through-and-through ear wound by thumb punch in these mice. We observed that MRL/MpJ mice healed the ear punch faster than did B6 mice at 5 weeks post-ear punch (Fig. 1A, B). These results are consistent with previous reports that have demonstrated enhanced tissue repair for ear punch wounds in MRL/MpJ mice [17, 30, 37, 40]. Quantitation analysis indicated the mean ear wound size was reduced by 59 ± 5% in MRL/MpJ mice and 16 ± 5% in B6 mice (Fig. 1C).
Figure 1. MRL/MpJ mice show enhanced ear wound healing.
(A) Ear wound size of B6 and MRL/MpJ mice after through-and-through ear punch injury. Left image indicates original ear wound size and right image indicates healed wound size. (B) Graph shows the quantification of ear wound size at 5 weeks post-injury. (C) Quantification of reduction in ear wound size. Error bars indicate mean ± SEM (n=6); P<0.05. Scale bar=2 mm.
Accelerated muscle healing after CTX-induced injury in MRL/MpJ mice
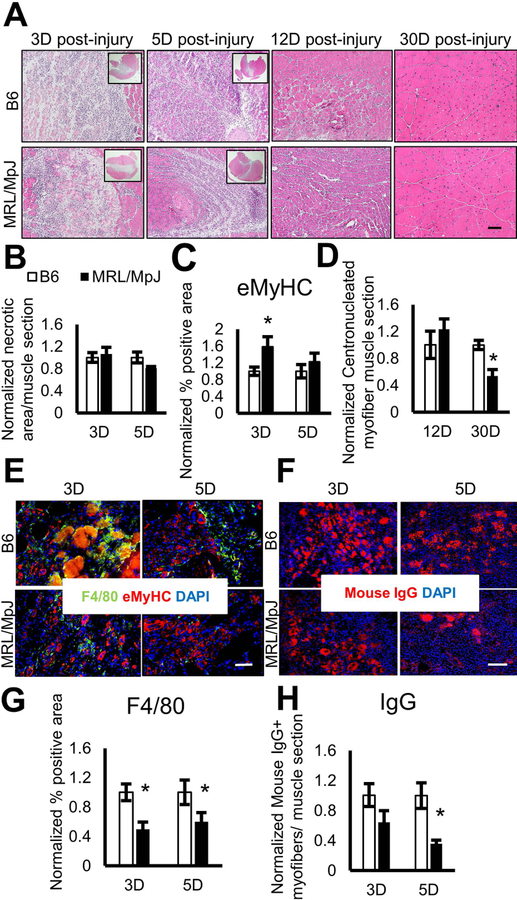
To examine whether muscle healing is also improved in MRL/MpJ mice, muscle injury was induced in 4-month-old B6 or MRL/MpJ mice by intramuscular injection of 4 μM of CTX into the GAS muscle. At 3, 5, 12, and 30 days post-injury, GAS muscles were harvested, flash frozen, and sectioned for histochemical analysis. Haemotoxylin and eosin (H&E) staining revealed comparable skeletal muscle damage in both the MRL/MpJ and B6 mice at 3 days post-injury (Fig. 2A, B). At 5 days post-injury, we found that the damaged muscle areas of MRL/MpJ mice were reduced compared to B6 mice (Fig. 2A, B). To test if muscle repair in MRL/MpJ mice is accelerated, we evaluated the number of newly regenerated muscle fibers by immunostaining the skeletal muscle with eMyHC antibody. We observed a higher number of eMyHC+ myofibers in the skeletal muscle of MRL/MpJ than in B6 mice at 3 and 5 days post-injury (Fig. 2C, E), indicating skeletal muscle in the MRL/MpJ mouse regenerates faster than in the B6 mouse. At 12 days post-CTX injury, the damaged skeletal muscles in both B6 and MRL/MpJ mice were healed with the necrotic region predominately replaced by centronucleated muscle fibers (Fig. 2A). Since no eMyHC+ myofibers were detectable by immunofluorescence staining at 12 and 30 days post-injury, centronucleated myofibers were quantified to determine the number of newly regenerated muscle fibers. At 12 days post-injury, we observed a higher mean number of centronucleated myofibers in MRL/MpJ muscle, when compared to B6 muscle (Fig. 2D), confirming an accelerated muscle healing in MRL/MpJ mice. Interestingly, our results show that the centronucleated myofiber number was significantly lower in skeletal muscle of MRL/MpJ mice by 30 days’ post-injury (Fig. 2D). The reduction in centronucleated myofiber in MRL/MpJ mice is consistent with faster myofiber maturation, in which the nuclei are organized and placed regularly at the periphery of the plasma membrane of a fully matured myofiber [41].
Figure 2. Accelerated muscle injury healing in MRL/MpJ mice.
(A) H&E staining analysis of the CTX-injected GAS from 4-month-old B6 and MRL/MpJ mice. Skeletal muscles were analyzed at 3, 5, 12, and 30 days post-CTX injury. Inset showing 1X magnification of muscle sections. (B-C) Graphs show normalized necrotic area and normalized eMyHC+ myofibers within muscle at 3 and 5 days post-injury. (D) Quantification of centronucleated fibers from skeletal muscle analyzed at 12 and 30 days post-injury. (E) Immunofluorescence staining for macrophage marker F4/80 (green) and embryonic myosin heavy chain (eMyHC, red), and DAPI staining for nuclei (blue). (F) Immunofluorescence staining of the CTX-injected GAS from 4-month-old B6 and MRL/MpJ mice for necrotic myofibers (IgG, red) and DAPI staining for nuclei (blue). (G) Quantification of F4/80 staining. (H) Quantification of mouse IgG. Error bars indicate mean ± SEM; P<0.05. (n=5 for 3 and 30 days post-injury and n=8 for 5 and 12 days post-injury). Scale bar=50 μm (A, E). Scale bar=100 μm (F).
Lastly, we evaluated the level of inflammation in injured muscles during the regeneration process by immunostaining muscle tissue sections with antibodies specific for mouse IgG (necrotic fibers) and F4/80 (macrophage marker). We observed that, in MRL/MpJ mice, there were significant decreases in necrotic fibers (Fig. 2F, H) in the injured skeletal muscle, when compared to B6 mice at 5 days post-injury. The necrotic fibers were not detected in skeletal muscle of both B6 and MRL/MpJ at 12 and 30 days post-injury (data not shown).
Immunofluorescence analysis of skeletal muscle sections at 3, 5, 12, and 30 days post-injury demonstrated presence of F4/80+ infiltrating macrophages. The F4/80+ macrophages were most abundant at 3 days post-injury and declined with time in both B6 and MRL/MpJ mice (Fig. 2A, E, G). We found that the F4/80+ infiltrating macrophages were significantly lower at 3 and 5 days post-muscle injury in MRL/MpJ mice compared to B6 mice (Fig. 2A, E, G). And even though the overall architecture of muscle was restored at 12 days post-injury, a higher amount of F4/80+ macrophages was observed in B6 than MRL/MpJ skeletal muscle (Fig 2A, Sup. 1B), which is consistent with ongoing tissue remodeling [42, 43]. At 30 days post-injury, the infiltrating macrophages were returned to basal levels and no differences were observed between B6 and MRL/MpJ muscle (Fig. 2A).
Increased numbers of Pax7- and CD31-expressing cells in muscles of MRL/MpJ mice
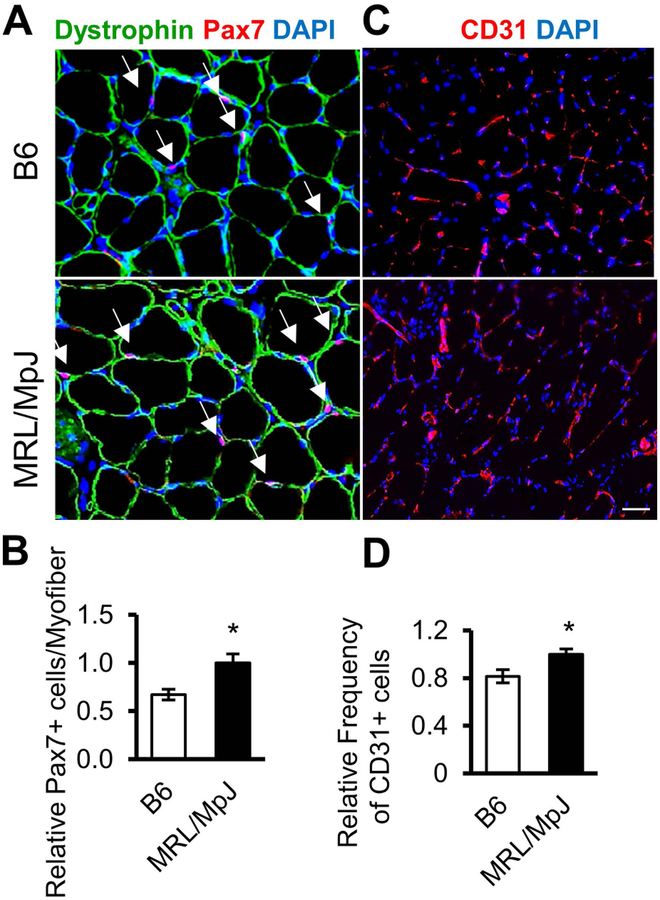
Tissue regenerative capacity is often dependent on the number and function of tissue-specific adult stem cells [44–46]. Therefore, we investigated the number of Pax7-expressing MPCs in skeletal muscle of MRL/MpJ mice and results were compared to that of B6 mice. We observed that skeletal muscles of MRL/MpJ mice contain significantly more Pax7+ MPCs than muscles of B6 mice (Fig. 3A, B). Since angiogenesis plays an important role during tissue repair and, more importantly, perivascular cells have been shown to be the origin of adult stem cells [13], we also evaluated the number of blood vessels in muscles of MRL/MpJ and B6 mice by staining for CD31 (marker for endothelial cells). Our results show that CD31+ cells were significantly more abundant in muscles of MRL/MpJ mice than in B6 mice (Fig. 3C, D). These results suggest that numbers of Pax7+ muscle stem cells and CD31+ endothelial cells are higher in skeletal muscle of MRL/MpJ mice than in skeletal muscle of B6 mice.
Figure 3. MRL/MpJ skeletal muscle contains relatively higher MPCs and blood vessel cells compared to B6 muscle.
(A) Immunostaining for muscle stem cell marker Pax7 (red, arrow), dystrophin (green), and DAPI staining for nuclei (blue) in the GAS muscle of B6 and MRL/MpJ mice. (B) Quantification of Pax7-positive cells per myofiber. (C) Immunostaining for blood vessel marker CD31 in skeletal muscle of B6 and MRL/MpJ mice. (D) Quantification of CD31-positive cells. Error bars indicate mean ± SEM (n=4); P<0.05. Scale bar=50 μm.
Improved function of MPCs isolated from MRL/MpJ mice
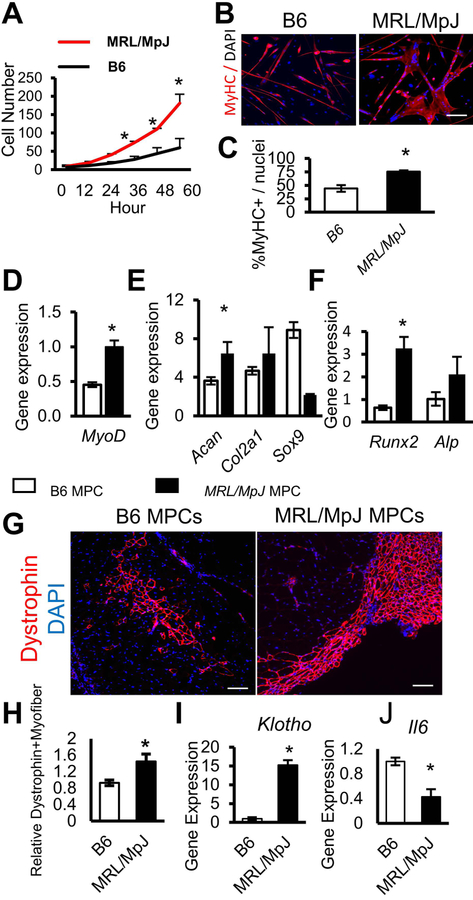
Next, we examined the proliferation and differentiation capacities of MPCs isolated by the preplate technique from MRL/MpJ and B6 mice at 8 weeks of age [13]. The proliferation capacities of the MPCs were analyzed in vitro with a robotic time-lapse microscopic LCI system as previously described [15]. Our results indicate that the proliferation rate of MRL/MpJ MPCs was significantly higher at 36, 48, and 60 h (*P<0.05) when compared to B6 MPCs (Fig. 4A). We also observed that MRL/MpJ MPCs formed significantly more multi-nucleated MyHC-positive myotubes compared to B6 MPCs (Fig. 4B, C). These results were further supported by quantitative RT-PCR analysis which showed higher expression levels of the MyoD gene (an early myogenic marker) in MRL/MpJ than in B6 MPCs (Fig. 4D). Then, we evaluated the chondrogenic and osteogenic potentials of MRL/MpJ MPCs and B6 MPCs by measuring levels of respective marker gene expression when cultured in appropriate differentiation medium. Our results show that expression levels of the chondrogenic markers Acan and Col2a1 were higher in MRL/MpJ MPCs than in B6 MPCs (Fig. 4E). Similarly, levels of expression of osteogenic markers Runx2 and Alp were also higher in MRL/MpJ MPCs than in B6 MPCs (Fig. 4F). Taken together, these data indicate an improved function of MRL/MpJ MPCs when compared to MPCs isolated from B6 mice.
Figure 4. MRL/MpJ MPCs show improved proliferation and myogenic differentiation potential.
(A) Cell proliferation rates of B6 and MRL/MpJ MPCs over a period of 60 h. (B) Immunostaining for MyHC in B6 and MRL/MpJ MPCs after myogenic differentiation. Data are representative of three independent experiments. (C) Quantification of myofiber fusion. (D) Quantitative RT-PCR shows levels of gene expression for myogenic gene expression of MPCs. (E) Quantitative RT-PCR shows levels of gene expression for chondrogenic differentiation of MPCs. (F) Quantitative RT-PCR shows levels of osteogenic differentiation of MPCs (G) Immunostaining for dystrophin in skeletal muscle sections of dystrophin-deficient mdx/SCID mice transplanted with B6 or MRL/MpJ MPCs. (H) Quantitation of regenerated dystrophin-positive myofibers was performed (n=5). (I, J) Quantitative RT-PCR shows levels of gene expression for anti-inflammatory Klotho and pro-inflammatory IL-6 in B6 and MRL/MpJ mice. Error bars indicate mean ± SEM (n=3); P<0.05. Scale bar=50 μm (B). Scale bar=100 μm (G).
MRL/MpJ MPCs have greater muscle regenerative capacity in vivo
To further support our hypothesis that an enhanced potency of the MPCs accelerates muscle regenerative capacity in MRL/MpJ mice, we tested the muscle regenerative capacity of MRL/MpJ and B6 MPCs after their transplantation in mdx/SCID mice. A total of 4×105 MPCs from either MRL/MpJ or B6 mice were injected into the GAS muscle of 10-week-old dystrophin-deficient mdx/SCID mice, which are commonly used as a mouse model for studying muscle pathologies in Duchenne muscular dystrophy [47–49]. At 2 weeks post-injection of MPCs, GAS muscles were harvested, sectioned, and stained for dystrophin. Our data indicated that there were significantly higher numbers of dystrophin-positive myofibers in the muscles injected with MRL/MpJ MPCs than in the muscles injected with B6 MPCs (Fig. 4G, H). Although MRL/MpJ MPCs had higher expression levels of the anti-inflammatory Klotho gene than did B6 MPCs (Fig. 4I), expression levels of the pro-inflammatory cytokine Il-6 gene were reduced in the MRL/MpJ MPCs when compared to B6 MPCs (Fig. 4J).
MRL/MpJ mouse serum contains circulating factors important for MPC function
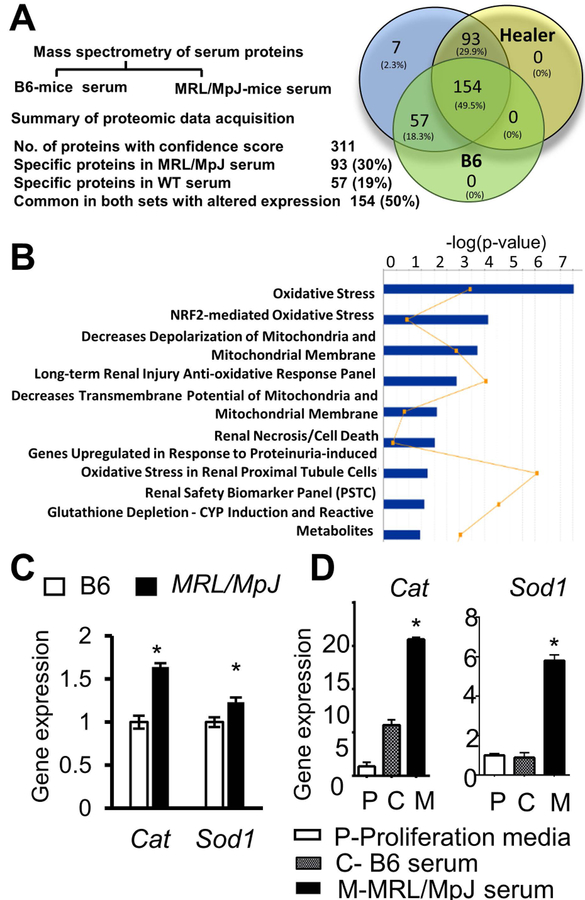
We and others have shown that in addition to adult stem cells, rejuvenating circulating factors play important roles in regulation of tissue-specific stem cell behavior and subsequent tissue regeneration processes [50–52]. These studies prompted us to identify factors that might support the enhanced superior regenerative capacity of MRL/MpJ mice, including muscle repair and MPC function. By using tandem mass spectrometry (MS/MS) on equal amounts of sera from B6 and MRL/MpJ mice, we identified the 311 highest-ranked proteins for which peptide scores (not an absolute quantitative score, but an estimation of relative abundance) were either higher or lower in MRL/MpJ serum than in B6 serum (Fig. 5A). Among those proteins, 93 proteins (~30%) were exclusively detected in MRL/MpJ serum and the majority of these proteins are known to play a role in oxidative stress, glycogenesis, and superoxide radical scavenger pathways. These proteins are superoxide dismutases, peroxiredoxin 1, catalase, and glutathione peroxidase (Fig. 5B). We also observed that, in cultured MPCs, mRNA levels of Cat and Sod1 genes were higher in MRL/MpJ than B6 MPCs (Fig. 5C). Because circulating factors, through paracrine actions, influence cellular behavior and capacity by regulating gene expression, we cultured B6 MPCs in the presence of MRL/MpJ or B6 serum and measured the mRNA levels of Cat and Sod1 genes. Our results indicate that treating B6 MPCs with MRL/MpJ serum induced a higher level of expression of Cat and Sod1 genes compared to B6 serum-treated MPCs (Fig. 5D). Consistent with other reports [53, 54], these findings suggest that circulating serum factors of MRL/MpJ mice may provide stress resistance to MPCs, an important feature to enhance tissue regeneration.
Figure 5.
Mass spectrometry of serum proteins from MRL/MpJ and B6 mice. (A) Summary of proteomic data analysis. (B) Ingenuity pathway analysis (IPA) of circulating factors enriched in MRL/MpJ serum. (C) Quantitative RT-PCR for expression levels of Cat and Sod1 mRNA in B6 and MRL/MpJ MPCs, and (D) B6 MPCs cultured in proliferating media (white bars), B6 serum only (grey bars), and MRL/MpJ serum only (black bars). Error bars indicate mean ± SEM (n=5); P<0.05.
MRL/MpJ MPCs maintain cell survival and multipotency potentials under various stresses
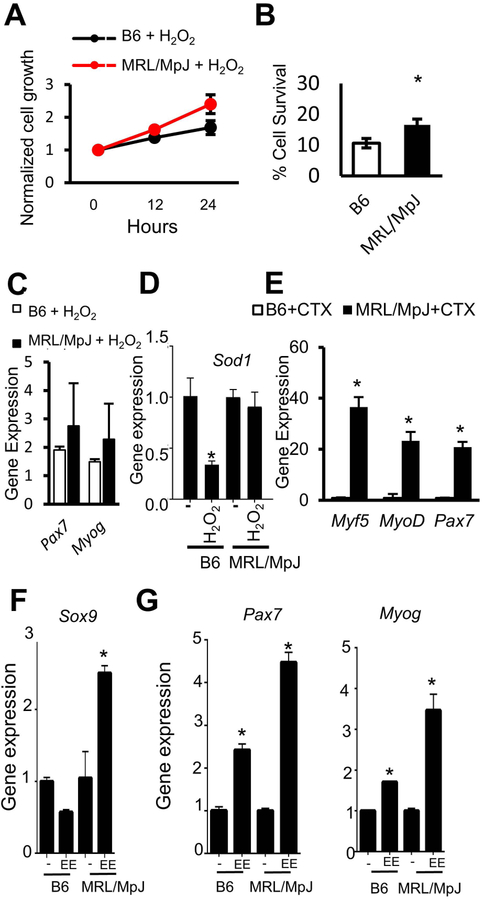
Based on our above observations that MRL/MpJ mouse serum contains increased levels of antioxidative enzymes and can stimulate expression of antioxidant genes (Sod1 and Cat) in B6 MPCs, we further hypothesized that MRL/MpJ MPCs might be resistant to various stress conditions. We treated MRL/MpJ and B6 MPCs with hydrogen peroxide to induce accumulation of free radical O2, an oxidative stress, and then analyzed their proliferation and differentiation potentials. Our results indicate that, in the presence of hydrogen peroxide (H2O2), the proliferative capacity of MRL/MpJ MPCs was higher than that of B6 MPCs (Fig. 6A). Also, MRL/MpJ MPCs were more resistant to oxidative stress, because significantly more MRL/MpJ MPCs survived in media containing 400 μM hydrogen peroxide than did B6 MPCs (Fig. 6B). To test whether MRL/MpJ MPCs maintain their myogenic potential in stress conditions, we treated B6 and MRL/MpJ MPCs with H2O2 or 4 nM of CTX for 24 h, and then cultured these cells in myogenic differentiation media. Gene expression analysis indicated that higher levels of Pax7 and Myog were induced in H2O2-treated MRL/MpJ MPCs when compared to H2O2-treated B6 MPCs (Fig. 6C). Interestingly, Sod1 mRNA decreased in H2O2-treated relative to untreated B6 MPCs, and no change was observed in MRL/MpJ MPCs (Fig. 6D); thus, indicating that proliferation and survival of MRL/MpJ MPCs could be, at least in part, due to expression of Sod1 upon H2O2 exposure. Upon exposure to CTX, we also found that levels of myogenic markers, including Myf5, MyoDitalic, and Pax7, were significantly increased in MRL/MpJ MPCs compared to B6 MPCs (Fig. 6E). In addition, after treatment with eeyarestatin (EE, 10 μM) to induce ER stress, we found that levels of the chondrocyte marker Sox9 (Fig. 6F) and myogenic markers Myog and Pax7 (Fig. 6G) were significantly higher in MRL/MpJ MPCs than in B6 MPCs. These results suggest that MRL/MpJ MPCs retain their differential potential under stressful conditions.
Figure 6. MRL/MpJ MPCs maintain proliferation capacity and potency under stress conditions.
(A) Proliferation rate of B6 MPCs and MRL/MpJ MPCs after treatment with 50 μM hydrogen peroxide (H2O2). (B) Graph bars show the survival ability of B6 MPCs and MRL/MpJ MPCs after treatment with 400 μM of H2O2 for 6 h. (C, D) Quantitative RT-PCR analysis shows levels of gene expression in B6 MPCs and MRL/MpJ MPCs treated with H2O2 for Pax7 and Myog (C) and for Sod1 (D). (E) Quantitative RT-PCR analysis for myogenic gene expression upon CTX-treatment. (F) Quantitative RT-PCR analysis for Sox9 and (G) for myogenic genes after eeyarestatin treatment of B6 and MRL/MpJ MPCs. Error bars indicate mean ± SEM (n=3); P<0.05.
Sod1 and Cat are required for MRL/MpJ MPC function
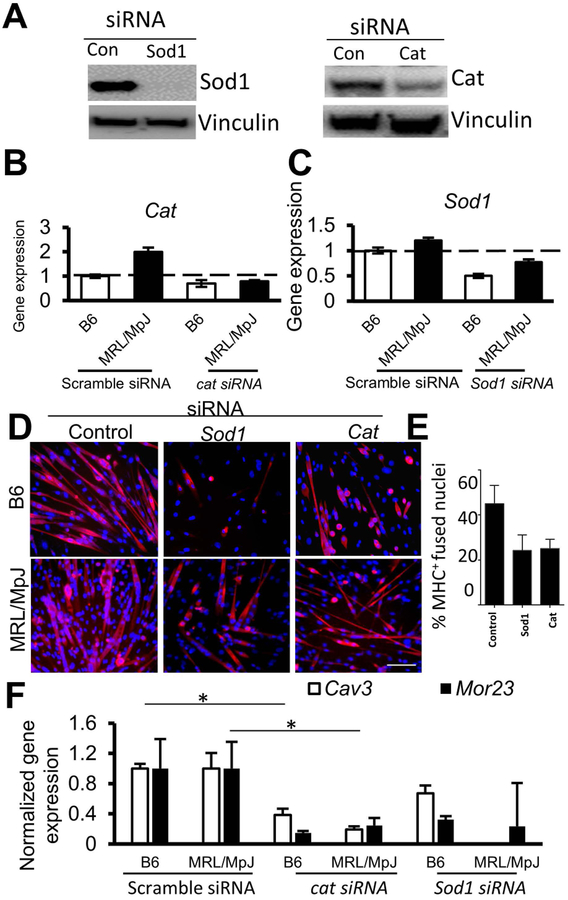
To provide additional evidence for the role of antioxidants in the high regenerative capacity of MRL/MpJ mice, MPCs isolated from MRL/MpJ and B6 mice were transfected with control (scrambled) siRNA or Sod1- or Cat-specific siRNA. Western blot analysis showed an effective knock-down of Sod1 and Cat proteins by their respective siRNAs in MRL/MpJ MPCs (Fig. 7A). The mRNA levels of Sod1 and Cat also decreased in siRNA-transfected MPCs, with a modest decrease of Cat mRNA in siRNA-transfected B6 MPCs (Fig. 7B, C). B6 and MRL/MpJ MPCs transfected with siRNAs were cultured with myogenic media and then immunostained with MyHC-specific antibody for myotube formation. Our results showed that myogenic differentiation of both B6 and MRL/MpJ MPCs was reduced in Sod1- or Cat-depleted cells compared to control (scrambled siRNA-treated) cells based on decreased expression and number of MyHC+ cells in siRNA-treated B6 and MRL/MpJ MPCs (Fig. 7D, E). However, overexpression of Sod1 in MRL/MpJ and B6 MPCs, unexpectedly, did not improve the myogenic differentiation before and after H2O2 treatment (Suppl. Fig. 2A). Levels of myogenic gene expression and myotube formation were inhibited by Sod1 expression, though expression of the cell proliferation marker gene Ccnd1 was induced (Suppl. Fig. 2B). Consistent with other reports, Sod1 may improve proliferation and potency of MPCs during oxidative stress and maintain their multipotent abilities in an undifferentiated stage [55–57] Furthermore, expression levels of Caveolin-3 (Cav3) and mouse odorant receptor 23 (Mor23) genes, both being critical markers for myoblast fusion, migration, and adhesion [58, 59], were significantly reduced in siRNA-treated MPCs, when compared to scrambled siRNA treated MPCs (Fig. 7F).
Figure 7. Depletion of Sod1 and Cat by siRNAs in MPCs impairs their myogenic capacity.
(A) Western blot shows levels of Sod1 and Cat in siRNA-transfected MRL/MPJ MPCs. (B, C) Quantitative RT-PCR shows levels of Cat and Sod1 mRNAs in B6 MPCs and MRL/MpJ MPCs transfected with control and specific siRNAs. (D) Immunostaining of B6 and MRL/MpJ MPCs induced for myogenesis shows staining of MyHC+ myotubes (red) after delivery of siRNAs specific to Cat or Sod1. Data are representative of three independent experiments. (E) Graph shows decreased percentage of fused nuclei in Sod1- or Cat-depleted MRL/MpJ MPCs. (F) Gene expression of myoblast fusion marker Cav3 and Mor23 in B6 and MRL/MpJ MPCs treated with control (scrambled siRNA) and specific siRNA-treated cells. Error bars indicate mean ± SEM (n=3); P<0.05. Scale bar=50 μm.
Discussion
Impaired wound healing, a major complication in a wide variety of diseases, may lead to poor health conditions and affects a large population [1]. Therefore, it is pertinent to identify novel methods that can improve wound healing under different disease conditions. MRL/MpJ mice have been shown to have enhanced wound healing capacity in studies of alkali-burned cornea [18], intra-articular fracture [19], and digit tip regrowth following amputation [21] when compared to B6 control mice. The caveat of using MRL/MpJ is that the enhanced healing phenotype in MRL/MpJ is a multigenic trait and there is no obvious transgenic reference strain available to compare. Therefore, better healing in MRL/MpJ mice remains debatable due to the possibility of worse healing in B6 reference mice. Nevertheless, these mice provide an invaluable animal model system and have been extensively studied to gain insights into the cellular and molecular mechanisms involved in enhanced wound healing. However, the muscle repair capacity as well as regenerative capacity of adult stem cells and circulating factors in MRL/MpJ mice have not been previously described. Although, Heydemann et al. (2012) reported that MRL/MpJ mice bred with sgcg mice (having muscular dystrophy and cardiomyopathy) resulted with reduced fibrosis and improved muscle regeneration in the sgcg mice, suggesting a beneficial effect of the unique genetic beackground of MRL/MpJ mice in muscle regeneration and reducing fibrosis [16, 36].
Our findings show that skeletal muscle healing capacity of MRL/MpJ mice increased following CTX injury when compared to B6 mice. The accelerated muscle healing is most likely due to an increased number, proliferation, and potency of MPCs, as well as an increased expression of antioxidative stress enzymes, all of which improves MPC function under stressful conditions. Our findings are in agreement with the widely perceived view that stem cells help in wound healing by not only serving as the cell source for repair, but also by providing necessary paracrine factors that are beneficial to wound healing by promoting cell survival, angiogenesis, and reduction of fibrosis and inflammation [50, 51, 60]. Consistently, we found a decreased number of infiltrating immune cells in the damaged region within MRL/MpJ muscle at 3, 5 and 12 days post-CTX injury, when compared to B6 mice.
It is well-known that, in age-related diseases (such as sarcopenia, muscle atrophy, and osteoarthritis), various cellular stresses (such as free radical accumulation, oxidative stress, and endoplasmic reticulum stresses) are accumulated [61, 62], which then induce cellular apoptosis and tissue degeneration. Superoxide radicals, the primary component of reactive oxidative species (ROS), are produced in skeletal muscle during energy production to sustain cell functions and for muscle contraction [56]. Accumulation of excessive ROS leads to oxidative stress, thereby damaging cellular components, including cells and macromolecules (lipids, DNA, and proteins) that are critical for tissue regeneration, myogenesis, and muscle cell function [63]. Therefore, antioxidant enzymes are crucial to ensure proper maintenance of redox homeostasis [54, 56]. Interestingly, our proteomic data, and protein and gene expression analyses consistently showed that MRL/MpJ MPCs contain higher levels of antioxidative enzymes, including Cat and Sod1, rendering them more resistant to oxidative stress damage. Reduction of Cat and Sod1 levels reduced myogenic potential of MRL/MpJ MPCs to levels comparable to those of B6 MPCs. However, Sod1 overexpression in MRL/MpJ and B6 MPCs stimulated cyclin D1 expression (Ccnd1), a marker of cell proliferation, but inhibited the expression of Myod1 and Myog (Sup 2A,B), suggesting that antioxidants are important determinants of survival and proliferation of MRL/MpJ MPC during stress. Finally, our findings suggest that a direct application of skeletal muscle stem cells along with modulating antioxidants may prove useful in future therapeutic applications to improve tissue regeneration.
Conclusion
In this study, we found that increased levels of circulating antioxidants and potency of MPCs are distinguishing features of the MRL/MpJ mouse, and these features could be associated with accelerated muscle regeneration capacity of MRL/MpJ mice. This, thereby, suggests that modulating MPCs and their antioxidative capacity may represent a potential future therapeutic application to improve tissue regeneration.
Supplementary Material
Acknowledgments
This research was supported in part by NIH grants awarded to Dr. Johnny Huard (PO1AG043376 and RO1AR065445) and institutional start-up funding by the Department of Orthopaedic Surgery, McGovern Medical School, The University of Texas Health Science Center at Houston. This work was also supported in part by NIH grants awarded to Dr. Holger Eltzschig (R01 DK097075, P01-HL114457, R01-HL109233, R01-DK109574, R01-HL119837, and R01-HL133900). We thank Dr. Mary A. Hall for editorial assistance.
Footnotes
Conflicts of Interest Statement: J.H. declare Consultant/Advisory role with Steadman Philippon Research Institute. H.E. declare Consultant/Advisory role with Novartis Pharma AG. All other authors declared no conflicts of interest.
REFERENCES:
- 1.Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37:1528–1542. [DOI] [PubMed] [Google Scholar]
- 2.Atala A, Irvine DJ, Moses M et al. Wound Healing Versus Regeneration: Role of the Tissue Environment in Regenerative Medicine. MRS Bull. 2010;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6:265sr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Studitsky AN. Free Auto- and Homografts of Muscle Tissue in Experiments on Animals. Ann N Y Acad Sci. 1964;120:789–801. [DOI] [PubMed] [Google Scholar]
- 5.Lash JW, Holtzer H, Swift H. Regeneration of mature skeletal muscle. Anat Rec. 1957;128:679–697. [DOI] [PubMed] [Google Scholar]
- 6.Scharner J, Zammit PS. The muscle satellite cell at 50: the formative years. Skelet Muscle. 2011;1:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Relaix F, Montarras D, Zaffran S et al. Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J Cell Biol. 2006;172:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambasivan R, Yao R, Kissenpfennig A et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki T, Okada Y, Uchiyama Y et al. Skeletal muscle-derived CD34+/45- and CD34-/45- stem cells are situated hierarchically upstream of Pax7+ cells. Stem Cells Dev. 2008;17:653–667. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Qu-Petersen Z, Cao B et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu-Petersen Z, Deasy B, Jankowski R et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharaibeh B, Lu A, Tebbets J et al. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–1509. [DOI] [PubMed] [Google Scholar]
- 14.Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1:333–342. [DOI] [PubMed] [Google Scholar]
- 15.Deasy BM, Lu A, Tebbets JC et al. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heydemann A The super super-healing MRL mouse strain. Front Biol (Beijing). 2012;7:522–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. [DOI] [PubMed] [Google Scholar]
- 18.Ueno M, Lyons BL, Burzenski LM et al. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest Ophthalmol Vis Sci. 2005;46:4097–4106. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald J, Rich C, Burkhardt D et al. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage. 2008;16:1319–1326. [DOI] [PubMed] [Google Scholar]
- 20.Ward BD, Furman BD, Huebner JL et al. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58:744–753. [DOI] [PubMed] [Google Scholar]
- 21.Chadwick RB, Bu L, Yu H et al. Digit tip regrowth and differential gene expression in MRL/Mpj, DBA/2, and C57BL/6 mice. Wound Repair Regen. 2007;15:275–284. [DOI] [PubMed] [Google Scholar]
- 22.Colwell AS, Krummel TM, Kong W et al. Skin wounds in the MRL/MPJ mouse heal with scar. Wound Repair Regen. 2006;14:81–90. [DOI] [PubMed] [Google Scholar]
- 23.Beare AH, Metcalfe AD, Ferguson MW. Location of injury influences the mechanisms of both regeneration and repair within the MRL/MpJ mouse. J Anat. 2006;209:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metcalfe AD, Willis H, Beare A et al. Characterizing regeneration in the vertebrate ear. J Anat. 2006;209:439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grisel P, Meinhardt A, Lehr HA et al. The MRL mouse repairs both cryogenic and ischemic myocardial infarcts with scar. Cardiovasc Pathol. 2008;17:14–22. [DOI] [PubMed] [Google Scholar]
- 26.Leferovich JM, Bedelbaeva K, Samulewicz S et al. Heart regeneration in adult MRL mice. Proc Natl Acad Sci U S A. 2001;98:9830–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley G, Metcalfe AD, Ferguson MW. Peripheral nerve regeneration in the MRL/MpJ ear wound model. J Anat. 2011;218:163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thuret S, Thallmair M, Horky LL et al. Enhanced functional recovery in MRL/MpJ mice after spinal cord dorsal hemisection. PLoS One. 2012;7:e30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kostyk SK, Popovich PG, Stokes BT et al. Robust axonal growth and a blunted macrophage response are associated with impaired functional recovery after spinal cord injury in the MRL/MpJ mouse. Neuroscience. 2008;156:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Mohan S, Gu W et al. Analysis of gene expression in the wound repair/regeneration process. Mamm Genome. 2001;12:52–59. [DOI] [PubMed] [Google Scholar]
- 31.Heber-Katz E, Leferovich J, Bedelbaeva K et al. The scarless heart and the MRL mouse. Philos Trans R Soc Lond B Biol Sci. 2004;359:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vorotnikova E, McIntosh D, Dewilde A et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol. 2010;29:690–700. [DOI] [PubMed] [Google Scholar]
- 33.Naseem RH, Meeson AP, Michael Dimaio J et al. Reparative myocardial mechanisms in adult C57BL/6 and MRL mice following injury. Physiol Genomics. 2007;30:44–52. [DOI] [PubMed] [Google Scholar]
- 34.Baker KL, Daniels SB, Lennington JB et al. Neuroblast protuberances in the subventricular zone of the regenerative MRL/MpJ mouse. J Comp Neurol. 2006;498:747–761. [DOI] [PubMed] [Google Scholar]
- 35.Naviaux RK, Le TP, Bedelbaeva K et al. Retained features of embryonic metabolism in the adult MRL mouse. Mol Genet Metab. 2009;96:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heydemann A, Swaggart KA, Kim GH et al. The superhealing MRL background improves muscular dystrophy. Skelet Muscle. 2012;2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajnoch C, Ferguson S, Metcalfe AD et al. Regeneration of the ear after wounding in different mouse strains is dependent on the severity of wound trauma. Dev Dyn. 2003;226:388–397. [DOI] [PubMed] [Google Scholar]
- 38.Lavasani M, Lu A, Thompson SD et al. Isolation of muscle-derived stem/progenitor cells based on adhesion characteristics to collagen-coated surfaces. Methods Mol Biol. 2013;976:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaghy PL, Fang J, Wu W et al. Increased caveolin-3 levels in mdx mouse muscles. FEBS Lett. 1998;431:125–127. [DOI] [PubMed] [Google Scholar]
- 40.Gourevitch D, Clark L, Chen P et al. Matrix metalloproteinase activity correlates with blastema formation in the regenerating MRL mouse ear hole model. Dev Dyn. 2003;226:377–387. [DOI] [PubMed] [Google Scholar]
- 41.Cadot B, Gache V, Gomes ER. Moving and positioning the nucleus in skeletal muscle - one step at a time. Nucleus. 2015;6:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnold L, Henry A, Poron F et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chazaud B, Brigitte M, Yacoub-Youssef H et al. Dual and beneficial roles of macrophages during skeletal muscle regeneration. Exerc Sport Sci Rev. 2009;37:18–22. [DOI] [PubMed] [Google Scholar]
- 44.Usas A, Maciulaitis J, Maciulaitis R et al. Skeletal muscle-derived stem cells: implications for cell-mediated therapies. Medicina (Kaunas). 2011;47:469–479. [PubMed] [Google Scholar]
- 45.Gates CB, Karthikeyan T, Fu F et al. Regenerative medicine for the musculoskeletal system based on muscle-derived stem cells. J Am Acad Orthop Surg. 2008;16:68–76. [DOI] [PubMed] [Google Scholar]
- 46.Almada AE, Wagers AJ. Molecular circuitry of stem cell fate in skeletal muscle regeneration, ageing and disease. Nat Rev Mol Cell Biol. 2016;17:267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hudecki MS, Pollina CM. Mdx mouse as therapeutic model system: development and implementation of phenotypic monitoring. Adv Exp Med Biol. 1990;280:251–263; discussion 263–255. [DOI] [PubMed] [Google Scholar]
- 48.Partridge TA. The mdx mouse model as a surrogate for Duchenne muscular dystrophy. FEBS J. 2013;280:4177–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu A, Poddar M, Tang Y et al. Rapid depletion of muscle progenitor cells in dystrophic mdx/utrophin−/− mice. Hum Mol Genet. 2014;23:4786–4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Best TM, Gharaibeh B, Huard J. Stem cells, angiogenesis and muscle healing: a potential role in massage therapies? Postgrad Med J. 2013;89:666–670. [DOI] [PubMed] [Google Scholar]
- 51.Caplan AI. MSCs: The Sentinel and Safe-Guards of Injury. J Cell Physiol. 2016;231:1413–1416. [DOI] [PubMed] [Google Scholar]
- 52.Conboy IM, Rando TA. Aging, stem cells and tissue regeneration: lessons from muscle. Cell Cycle. 2005;4:407–410. [DOI] [PubMed] [Google Scholar]
- 53.Selsby JT. Increased catalase expression improves muscle function in mdx mice. Exp Physiol. 2011;96:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Moal E, Pialoux V, Juban G et al. Redox Control of Skeletal Muscle Regeneration. Antioxid Redox Signal. 2017;27:276–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaccagnini G, Martelli F, Magenta A et al. p66(ShcA) and oxidative stress modulate myogenic differentiation and skeletal muscle regeneration after hind limb ischemia. J Biol Chem. 2007;282:31453–31459. [DOI] [PubMed] [Google Scholar]
- 56.Kozakowska M, Pietraszek-Gremplewicz K, Jozkowicz A et al. The role of oxidative stress in skeletal muscle injury and regeneration: focus on antioxidant enzymes. J Muscle Res Cell Motil. 2015;36:377–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franco AA, Odom RS, Rando TA. Regulation of antioxidant enzyme gene expression in response to oxidative stress and during differentiation of mouse skeletal muscle. Free Radic Biol Med. 1999;27:1122–1132. [DOI] [PubMed] [Google Scholar]
- 58.Rochlin K, Yu S, Roy S et al. Myoblast fusion: when it takes more to make one. Dev Biol. 2010;341:66–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell. 2009;17:649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sacitharan PK, Vincent TL. Cellular ageing mechanisms in osteoarthritis. Mamm Genome. 2016;27:421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doria E, Buonocore D, Focarelli A et al. Relationship between human aging muscle and oxidative system pathway. Oxid Med Cell Longev. 2012;2012:830257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sullivan-Gunn MJ, Lewandowski PA. Elevated hydrogen peroxide and decreased catalase and glutathione peroxidase protection are associated with aging sarcopenia. BMC Geriatr. 2013;13:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.