Abstract
Approved treatment for hepatitis C virus (HCV) with all‐oral direct‐acting antivirals (DAA) therapy is now entering into its fourth year; however, little has been reported on the real‐world clinical (decompensated cirrhosis [DCC] and hepatocellular carcinoma [HCC]) and economic outcomes. A retrospective cohort analysis of the Truven Health MarketScan Database (2012‐2016) was conducted. In a cohort of 26,105 patients with newly diagnosed HCV, 30% received all‐oral DAA therapy (DAA group) and 70% were not treated (untreated group). Multivariate Cox proportional hazards models were used to compare the risk of developing HCC and DCC, stratified by cirrhosis status. Among patients with cirrhosis (n = 2157), DAA therapy was associated with a 72% and a 62% lower incidence of HCC (hazard ratio [HR], 0.28; 95% confidence interval [CI], 0.15‐0.52) and DCC (HR, 0.38; 95% CI, 0.26‐0.56). Similarly, DAA therapy was associated with a 57% and a 58% lower incidence of HCC (HR, 0.43; 95% CI, 0.26‐0.71) and DCC (HR, 0.42; 95% CI, 0.30‐0.58) in patients with noncirrhotic HCV (n = 23,948). A propensity score–matched cohort of 8064 HCV‐infected patients who had at least a 12‐month follow‐up after HCV treatment was included for economic analysis. For patients with cirrhosis in the DAA group, the mean adjusted liver‐related costs ($1749 vs. $4575; P < 0.001) and all‐cause medical costs ($19,300 vs. $33,039; P < 0.001) were significantly lower compared with those in the untreated group. The mean adjusted costs were not statistically different between the two groups among patients without cirrhosis. Conclusion: In the short term, all‐oral DAA treatment for HCV infection was associated with a decreased risk of developing HCC and DCC, resulting in decreased health care costs, especially in patients with cirrhosis. A longitudinal study is necessary to confirm our findings.
Abbreviations
- ALD
alcoholic liver disease
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- COPD
chronic obstructive pulmonary disease
- CI
confidence interval
- DAA
all‐oral direct‐acting antivirals
- DCC
decompensated cirrhosis
- HBV
hepatitis B infection
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ICD‐9‐CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- ICD‐10‐CM
International Classification of Diseases, Tenth Revision, Clinical Modification
- OR
odds ratio
- PEG‐IFN
peg‐interferon
- PPI
proton‐pump inhibitor
- PPPY
per person per year
- PS
propensity score
- RBV
ribavirin
- SVR
sustained virologic response
Hepatitis C virus (HCV) infection is the most common chronic bloodborne infection in the United States and a substantial cause of morbidity and mortality.1, 2 Many patients with chronic hepatitis C progress to advanced liver disease such as decompensated cirrhosis (DCC) and hepatocellular carcinoma (HCC).3, 4 Furthermore, HCV is currently the leading indication for liver transplantation in the United States, suggesting that the burden of fatal liver disease is increasing in the estimated 2.7 million adults chronically infected with HCV in the United States.5
Several studies reported that patients with HCV who received treatment and/or achieved a sustained virologic response (SVR, the surrogate for cure) experienced significantly reduced cumulative rates of HCC, liver transplantation, and liver‐related death in the United States.6, 7, 8 Furthermore, an economic study reported that HCV therapy with peg‐interferon (PEG‐IFN) and ribavirin (RBV) was associated with lower follow‐up health care costs.9
However, PEG‐IFN therapy was plagued with significant side effects, leading to premature treatment stoppage decreasing the number of HCV‐infected patients who achieved SVR rate. Fortunately, in recent years, HCV treatment has taken a major step forward with the introduction of highly efficacious direct‐acting antiviral (DAA) therapy, which has demonstrated therapeutic efficacy, limited adverse effects, and a shorter treatment period compared with interferon‐based regimens.10 Despite guideline recommendations, access to HCV treatment has been frequently restricted because of the high DAA drug costs and prior authorization policies in which only the sicker get treated, slowing the expected rise in treatment rates.11, 12 This delay in potentially curative treatment until development of advanced liver disease may have costly consequences.9, 13, 14
Several economic modeling studies using data from the DAA clinical trials and the literature have forecasted an economic benefit with the DAA use due to lower disease complications. However, none of these studies used real‐world clinical and economic outcomes data.15, 16, 17, 18 Therefore, the aims of this study were to determine the clinical outcomes (incidents of HCC and DCC) as well as the economic impact of all‐oral DAA treatment in chronically HCV‐infected patients in the United States using real‐world data obtained from a large national insurance database.
Materials and Methods
Data Source
We conducted a retrospective cohort study using the Truven Health Analytic MarketScan Commercial and Medicare Supplemental databases (January 2012 to December 2016). This nationwide administrative claims database contains deidentified person‐level information of diagnoses, procedures, and prescriptions for over 80 million individuals in the commercial data set and 6 million individuals in the Medicare Supplement database, which captures health care use and enrollment records across all settings including physician outpatient office visits, hospital stays, and pharmacy claims. The study population consisted of employees, dependents, and retirees with employer‐sponsored or Medicare Supplemental insurance plans. Institutional review board approval was obtained from the University of Florida.
Study Population
We used 2013‐2016 Truven Health Analytic MarketScan Commercial and Medicare supplemental files to establish the new chronic HCV cohort, and the 2012 data were used to ensure at least 1 year of claims prior to HCV diagnosis. The new patients with chronic HCV, defined as those who did not have an HCV diagnosis in the previous 12 months, were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes [070.44, 070.54, 070.70, 070.71, V02.62] and ICD‐10‐CM [B18.2, B19.20, B19.21, Z22.52]. A patient was determined to be infected with chronic HCV if they had one inpatient chronic HCV diagnosis or two outpatient diagnoses of HCV on separate days within 1 year.
Based on the receipt of treatment received, we classified patients into two exposure statuses: 1) patients treated with all‐oral DAA therapy (DAA group), or 2) patients who did not receive any HCV treatments (untreated group). The all‐oral DAA therapy group was defined as any patient who received one of the following DAA treatments: sofosbuvir/ledipasvir +/‐ RBV, sofosbuvir + simeprevir +/‐ RBV, sofosbuvir + RBV, sofosbuvir + daclatasvir +/‐ RBV, paritaprevir/ritonavir/ombitasvir +/‐ dasabuvir +/‐ RBV, elbasvir/grazoprevir +/‐ RBV, and sofosbuvir/velpatasvir +/‐ RBV.
For patients in the DAA group, the first DAA prescription date was assigned as the index date. To assign the index date for untreated patients, we used a prescription time‐distribution matching using the number of days from the first HCV diagnosis to the dispensing time of the first DAA prescription for treated patients.19 For each untreated patient, a hypothetical index date was selected at random based on the distribution for treated patients and assigned to him/her. Therefore, the overall distribution of the index date of the untreated patients was matched to that of the treated patients’ index date.
Patients were included if they were 18 years old and continuously enrolled in the health plan 1 year before the index date. We excluded patients who had a history of DCC or HCC before the index date or received treatment with PEG‐IFN‐containing regimens. Furthermore, we determined the presence of cirrhosis prior to their index date using ICD‐9‐CM codes [571.5] and ICD‐10‐CM codes [K74.0, K74.60, K74.69].
Study Outcomes
Clinical Outcomes
The primary clinical outcomes were incident rate of HCC and liver decompensation. All outcomes were then stratified by baseline cirrhosis status. HCC was defined as presence of 1 inpatient or 2 outpatients ICD‐9 or ICD‐10 diagnosis of HCC (Supporting Table S1 for ICD9‐CM/ICD10 CM codes).20 The earliest date for diagnosis of HCC was considered the incident date of HCC. Follow‐up started from the index date of HCV treatment and continued until the incidence of HCC, end of enrollment, or the end of the study (December 31, 2016), whichever came first (Supporting Fig. S1). DCC was defined by presence of at least 1 inpatient or 2 outpatients ICD‐9 diagnosis of ascites, esophageal varices, or spontaneous bacterial peritonitis.21, 22 Based on the results of the previous validation study, we did not include the diagnosis for hepatic encephalopathy, as this diagnosis was frequently linked to unrelated conditions.21 The incident date of liver decompensation was defined as the appearance of the first episode of DCC. Follow‐up for DCC started from the index date of HCV treatment and continued until the incidence of DCC, end of enrollment, or the end of the study (December 31, 2016), whichever came first. If a patient had a liver decompensation event after an HCC diagnosis, we presumed this patient had HCC and was censored at that date for HCC and was not included in DCC events.
In a sensitivity analysis, we excluded patients who had any type of cancers before the index date to avoid the possibility of erroneously including metastatic cancer to the liver. We also conducted another sensitivity analysis to define HCC as the presence of at least 1 inpatient or 2 outpatients ICD‐9 or ICD‐10 codes for HCC made ≥3 months after baseline. For incidence of DCC, we performed a sensitivity analysis without censoring patients at their date of HCC. In a subgroup analysis, we included minimum effectively treated patients who received either the all‐oral DAA regimen of sofosbuvir/ledipasvir for at least 8 weeks or a 12‐week treatment of the other all‐oral therapy.
Economic Outcomes
To determine at least 1‐year posttreatment costs, we excluded patients with HCV who had less than 1‐year follow‐up after their index date (Supporting Fig. 2). Medical costs were defined as the estimated costs associated with medical services including ambulatory care, inpatient hospitalizations, and emergency department obtained from a third‐party payer’s perspective. Both liver‐related and all‐cause costs were obtained. Medical costs per person per year (PPPY) were defined as the amount paid to the provider plus the member’s cost sharing for liver‐related and all‐cause services before and after initiation of HCV treatments. Liver‐related costs were identified by any liver‐related ICD‐9 code (i.e., HCV, cirrhosis, DCC, HCC, and liver transplant) as a primary diagnosis on the claim. All‐cause costs were identified as medical services received for any reason. The mean unadjusted and adjusted posttreatment liver‐related and all‐cause medical costs were compared between the DAA and untreated groups. We also estimated the DAA treatment costs using pharmacy claims for minimum effectively treated patients who received either the all‐oral DAA regimen of sofosbuvir/ledipasvir for at least 8 weeks or a 12‐week treatment of the other all‐oral therapy. Costs were adjusted to 2016 U.S. dollars using an annual 3% inflation rate.
Statistical Analysis
Baseline characteristics were compared between the DAA and untreated groups using Student t test for continuous variables and chi‐square tests for categorical variables. We used logistic regression to identify factors associated with receiving DAA therapy. The number of HCC and DCC events and person‐time of observation were determined for each group and used to calculate the incidence rates of HCC and DCC (number of events/1000 person‐years). A multivariate Cox proportional hazards regression model was employed to compare the risk of developing HCC and DCC between the DAA‐treated and untreated HCV groups. The covariates adjusted for the model included the following: age, gender, region, insurance type, HCV disease duration, alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), alcohol use disorder, injection/noninjection drug use disorder, hypertension, dyslipidemia, diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), cardiovascular disease (CVD), human immunodeficiency virus (HIV) infection, hepatitis B infection (HBV), hepatitis A infection (HAV), depression, schizophrenia/bipolar disorder, pregnancy, epilepsy, cancer history, and use of proton‐pump inhibitors (PPIs) and statins (Model 1) (Supporting Table S2).
For economic analysis, for each treated patient, 1 untreated patient was matched using the propensity score (PS) that was calculated to adjust for the baseline differences in demographics, comorbidity conditions, and pretreatment costs between the DAA‐treated and untreated groups. The PS was estimated using logistic regression to create a PS of the probability of receiving DAA therapy based on baseline demographic variables including covariates age and gender, alcohol/drug use disorders, medical conditions including hypertension, dyslipidemia, diabetes mellitus, COPD, CKD, CVD, HIV infection, HBV, HAV, depression, epilepsy, and schizophrenia/bipolar disorder, identified by ICD‐9‐CM and ICD‐10‐CM codes, as well as disease‐modifying medications including statins and PPIs, and pretreatment costs (Model 2) (Supporting Table S2). We choose these comorbidities because they are associated with, but not caused by, HCV and may have a high impact on costs.
Posttreatment PPPY costs between the DAA‐treated and untreated groups were compared using the Mann‐Whitney U test, a nonparametric test for independent sample t test. Annual posttreatment liver‐related and all‐cause direct medical costs were estimated using a two‐part model consisting of logistic regression to predict the likelihood of having health care costs greater than zero, and a generalized linear model with a gamma distribution and log‐link function.23 Mean adjusted posttreatment costs were estimated after adjusting for all covariates mentioned in PS matching analysis. A bootstrap resampling method was used to estimate the 95% confidence intervals (CI) of the health care costs. All the analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and STATA 13 (Stata Corp, College Station, TX).
Results
Patient Characteristics
We identified 26,105 patients with newly diagnosed HCV who met our inclusion/exclusion criteria between January 2013 and December 2016 (Fig. 1). Of 26,105 HCV‐infected patients, 18,177 patients (70%) did not receive any HCV treatment whereas 7,928 patients (30%) received the all‐oral DAA treatments. Table 1 summarizes the baseline demographic characteristics between the DAA‐treated and untreated groups. Patients who received the all‐oral DAA regimens were older (mean age 54.4 vs. 52.7; P < 0.001) and had significantly more cirrhosis (15.2% vs. 5.2%; P < 0.001) and NAFLD (12.9% vs. 6.7%; P < 0.01). Patients in the untreated group had similar comorbidities (e.g., hypertension, diabetes) compared with patients receiving the all‐oral DAA therapy, but they were more likely to have alcohol use disorder (9.1% vs. 6.5%; P < 0.001), drug use disorder (25.2% vs. 16.5%; P < 0.001), depression (21.8% vs. 15.0%; P < 0.001), and schizophrenia/bipolar disorder (4.5% vs. 2.5%; P < 0.001) and be pregnant (2.1% vs. 0.4%; P < 0.001). The mean follow‐up time was 14.7 months and 11 months for the DAA‐treated and untreated groups, respectively.
Figure 1.
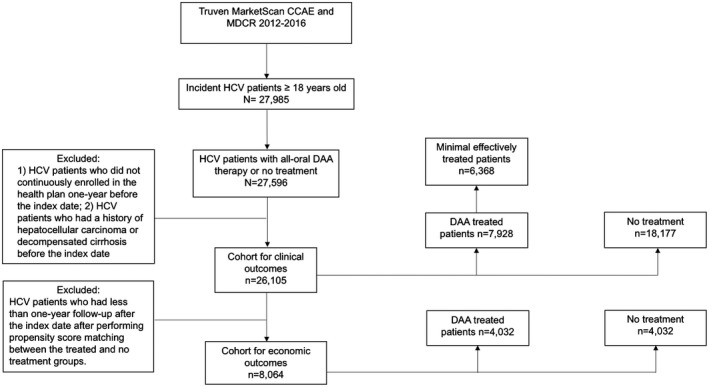
Diagram for the patient selection. Abbreviations: CCAE, Commercial Claims and Encounters; DAA, direct‐acting antivirals; HCV, hepatitis C virus; MDCR, Medicare Supplemental and Coordination of Benefits.
Table 1.
Baseline Characteristics of HCV‐infected Patients by Treatment Status
| Baseline Characteristics | DAA Treatment (n = 7928) | No Treatment (n = 18,177) | P Value | ||
|---|---|---|---|---|---|
| Age | |||||
| Mean (SD), years | 54.4 | (11.1) | 52.7 | (14.2) | <0.001 |
| Age, n (%) | <0.001 | ||||
| 18‐35 | 3324 | (8.02) | 2667 | (14.67) | |
| 36‐50 | 3961 | (13.90) | 2857 | (15.72) | |
| 51‐65 | 16,091 | (68.66) | 10,588 | (58.25) | |
| >65 | 2729 | (9.42) | 2065 | (11.36) | |
| Male, n (%) | 4859 | (61.76) | 10,580 | (58.21) | <0.001 |
| Insurance type, n (%) | <0.001 | ||||
| Comprehensive | 587 | (8.40) | 1897 | (10.44) | |
| HMO | 914 | (10.41) | 2823 | (15.53) | |
| POS | 668 | (8.76) | 1180 | (6.49) | |
| PPO | 4554 | (58.51) | 9842 | (54.15) | |
| Others | 1205 | (13.91) | 2435 | (13.40) | |
| Comorbidities, n(%) | |||||
| HBV | 786 | (2.36) | 609 | (3.35) | <0.001 |
| HAV | 168 | (0.79) | 110 | (0.61) | 0.240 |
| HIV | 696 | (2.80) | 475 | (2.61) | 0.421 |
| Cirrhosis | 2157 | (15.15) | 939 | (5.17) | <0.001 |
| NALFD | 2218 | (12.86) | 1213 | (6.67) | <0.001 |
| ALD | 356 | (1.40) | 249 | (1.37) | 0.897 |
| Alcohol use disorder | 2170 | (6.47) | 1650 | (9.08) | <0.001 |
| Drug use disorder | 5904 | (16.49) | 4572 | (25.15) | <0.001 |
| Hypertension | 12,526 | (48.38) | 8696 | (47.84) | 0.485 |
| Dyslipidemia | 8762 | (30.17) | 6374 | (35.07) | 0.049 |
| Diabetes | 5147 | (18.95) | 3642 | (20.04) | <0.001 |
| COPD | 3995 | (12.45) | 3005 | (16.53) | <0.001 |
| CVD | 2563 | (7.63) | 1941 | (10.68) | <0.001 |
| CKD | 1585 | (4.11) | 1235 | (6.79) | <0.001 |
| Depression | 5169 | (15.01) | 3961 | (21.79) | <0.001 |
| Schizophrenia/Bipolar disorder | 1020 | (2.50) | 818 | (4.50) | <0.001 |
| Pregnancy | 412 | (0.42) | 372 | (2.05) | <0.001 |
| Cancer history | 1938 | (6.85) | 1417 | (7.80) | 0.001 |
| Drug use, n (%) | |||||
| PPIs | 5126 | (19.21) | 3599 | (19.80) | 0.314 |
| Statins | 5087 | (15.25) | 3865 | (21.26) | <0.001 |
| Follow‐up time | |||||
| Mean (SD), months | 14.7 | (8.8) | 11.0 | (3.1) | <0.001 |
Abbreviations: ALD, alcoholic liver disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; DAA, direct‐acting antivirals; HAV, hepatitis A virus; HBV, hepatitis B virus; HIV, human immunodeficiency virus; HMO, health maintenance organization; NALFD, nonalcoholic fatty liver disease; POS, point of service plan; PPIs, proton‐pump inhibitors; PPO, preferred provider organization; SD, standard deviation.
Factors Associated with not Receiving DAA Therapy
As shown in Supporting Table S3, we identified factors associated with a significantly lower probability of initiating DAAs including the following: female (odds ratio [OR], 0.91; 95% CI, 0.85‐0.95), alcohol use disorder (OR, 0.82; 95% CI, 0.72‐0.93), drug use disorder (OR, 0.72; 95% CI, 0.67‐0.78), HBV (OR, 0.58; 95% CI, 0.49‐0.70), ALD (OR, 0.50; 95% CI, 0.38‐0.66), COPD (OR, 0.83; 95% CI, 0.76‐0.90), dyslipidemia (OR, 0.82; 95% CI, 0.77‐0.88), CVD (OR, 0.86; 95% CI, 0.78‐0.96), CKD (OR, 0.70; 95% CI, 0.62‐0.90), depression (OR, 0.79; 95% CI, 0.73‐0.85), cancer history (OR, 0.85; 95% CI, 0.76‐0.96), being pregnant (OR, 0.33; 95% CI, 0.24‐0.47), and use of statins (OR, 0.74; 95% CI, 0.68‐0.81).
Effect of All‐Oral DAA Regimen on Incident HCC
Table 2 displays the risk of developing HCC among the patients with HCV, stratified by cirrhosis status and receipt of HCV treatment. Among patients with cirrhosis (n = 2157), there were 57 (2.6%) HCC events. The majority of HCC events (70%) occurred in patients who received no treatment, providing an HCC incidence rate of 48.9 per 1000 person‐years compared with the HCC incidence rate in the DAA group (11.7 per 1000 person‐years). After adjusting for covariates, patients with cirrhosis in the DAA‐treated group had a 72% decreased risk of developing HCC compared with those who received no treatment (hazard ratio [HR], 0.28; 95% CI, 0.15‐0.52). There were 114 HCC events observed among patients without cirrhosis (n = 23,948). In patients without cirrhosis with HCV, the crude incidence rate of HCC was 5.8 per 1000 person‐years in the untreated group and 3.1 per 1000 person‐years in patients in the DAA‐treated group. After adjusting for covariates, we found patients without cirrhosis in the DAA‐treated group had a 57% decreased risk of developing HCC compared with those who received no treatment (HR, 0.43; 95% CI, 0.26‐0.72).
Table 2.
Incidence Rate of Hepatocellular Carcinoma in Patients with HCV with and without Cirrhosis, by Treatment Status
| Incidence Rate of Hepatocellular Carcinoma | Person‐years | No. of Events | Crude Incidence/1000 Person‐years | Multivariate Cox Regression* | |
|---|---|---|---|---|---|
| HR | 95% CI | ||||
| Patients with cirrhosis (n = 2157) | |||||
| DAA treatment (n = 1218) | 1399 | 17 | 11.7 | 0.28 | 0.15‐0.52 |
| No treatment (n = 939) | 819 | 40 | 48.9 | 1 | Reference |
| Patients without cirrhosis (n = 23,948) | |||||
| DAA treatment (n = 6710) | 8076 | 25 | 3.1 | 0.43 | 0.26‐0.71 |
| No treatment (n = 17,238) | 15,389 | 89 | 5.8 | 1 | Reference |
Abbreviations: CI, confidence interval; DAA, direct‐acting antivirals; HR, hazard ratio.
Cox proportional hazards model was used to adjust for age, gender, region, insurance type, HCV disease duration, alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), alcohol use disorder, injection/noninjection drug use disorder, hypertension, dyslipidemia, diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), cardiovascular disease (CVD), HIV infection, hepatitis B virus (HBV), hepatitis A virus (HAV), depression, schizophrenia/bipolar disorder, pregnancy, cancer history, and use of proton‐pump inhibitors (PPIs) and statins.
In the sensitivity analyses for patients who did not have cancer at baseline (HR, 0.28; 95% CI, 0.14‐0.54 in patients with cirrhosis; HR, 0.37; 95% CI, 0.21‐0.65 in patients without cirrhosis) and patients with HCC diagnosis ≥3 months after baseline (HR, 0.29; 95% CI, 0.16‐0.55 in patients with cirrhosis; HR, 0.47; 95% CI, 0.27‐0.80 in patients without cirrhosis), study results remained consistent with base case analysis (Table 3). In a subgroup analysis in which we assessed HCC incidence among the minimum effectively treated patients who received either the all‐oral DAA regimen of sofosbuvir/ledipasvir for at least 8 weeks or a 12‐week treatment of the other all‐oral DAA therapy, study results remained consistent (HR, 0.31; 95% CI, 0.16‐0.59 in patients with cirrhosis; HR, 0.43; 95% CI, 0.26‐0.74 in patients without cirrhosis) (Supporting Table S4). Factors associated with an increased risk of developing HCC included being older age (HR, 1.05; 95% CI, 1.04‐1.07), male (HR, 2.62; 95% CI,1.76‐3.89), having cirrhosis (HR, 4.52; 95% CI, 3.17‐6.43), diabetes (HR, 1.58; 95% CI, 1.12‐2.23), and cancer history at baseline (HR, 4.83; 95% CI, 2.41‐9.70), whereas DAA treatment significantly decreased the risk of HCC (HR, 0.38; 95% CI, 0.26‐0.57) (Supporting Table S5).
Table 3.
Incidence Rate of Hepatocellular Carcinoma in Patients with HCV with and without Cirrhosis by Treatment Status in Sensitive Analyses
| Incidence Rate of Hepatocellular Carcinoma | Person‐years | No. of Events | Crude Incidence/1000 Person‐years | Multivariate Cox Regression* | |
|---|---|---|---|---|---|
| HR | 95% CI | ||||
| Patients with HCV without any cancer history at baseline | |||||
| Patients with cirrhosis (n = 1954) | |||||
| DAA treatment (n = 1127) | 1325 | 15 | 11.3 | 0.28 | 0.14‐0.54 |
| No treatment (n = 827) | 721 | 33 | 45.7 | 1 | Reference |
| Patients without cirrhosis (n = 21,994) | |||||
| DAA treatment (n = 6270) | 7477 | 21 | 2.8 | 0.37 | 0.21‐0.65 |
| No treatment (n = 15,724) | 14,162 | 79 | 5.6 | 1 | Reference |
| Patients with HCV with HCC diagnosis ≥3 months after baseline | |||||
| Patients with cirrhosis (n = 2155) | |||||
| DAA treatment (n = 1218) | 1449 | 17 | 11.7 | 0.29 | 0.16‐0.55 |
| No treatment (n = 937) | 819 | 38 | 46.4 | 1 | Reference |
| Patients without cirrhosis (n = 23,735) | |||||
| DAA treatment (n = 6704) | 8076 | 23 | 2.8 | 0.47 | 0.27‐0.80 |
| No treatment (n = 17,031) | 15,388 | 77 | 5.0 | 1 | Reference |
Abbreviations: CI, confidence interval; DAA, direct‐acting antivirals; HR, hazard ratio.
Cox proportional hazards model was used to adjust for age, gender, region, insurance type, HCV disease duration, alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), alcohol use disorder and injection/noninjection drug use disorder, hypertension, dyslipidemia, diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), cardiovascular disease (CVD), HIV infection, hepatitis B virus (HBV), hepatitis A virus (HAV), depression, schizophrenia/bipolar disorder, pregnancy, cancer history, and use of proton‐pump inhibitor (PPI) and statins.
Effect of All‐Oral DAA Regimen on Incident Liver Decompensation
Among patients with cirrhosis (n = 2157), there were 131 (6.1%) DCC events. The majority of DCC events (66%) occurred in patients in the untreated group, providing a DCC incidence rate of 108.7 per 1000 person‐years compared with that of 32.0 per 1000 person‐years among patients in the DAA‐treated group (Table 4). After adjusting for covariates, we found patients with cirrhosis in the DAA‐treated group had a 62% decreased risk of developing DCC compared with those in the untreated group (HR, 0.38; 95% CI, 0.26‐0.56). Among patients without cirrhosis (n = 23,948), 312 DCC events (1.3%) occurred. In patients without cirrhosis, the crude DCC incidence rate was 16.2 per 1000 person‐years in the untreated group and 8.0 per 1000 person‐years for the DAA‐treated group (Table 4). After adjusting for covariates, patients without cirrhosis in the DAA group had a 58% decreased risk of developing DCC compared with those in the untreated group (HR, 0.42; 95% CI, 0.30‐0.58). In a sensitivity analysis in which we assessed DCC incidence without censoring patients at their date of HCC, study results were similar (HR, 0.35; 95% CI, 0.24‐0.51 in patients with cirrhosis; HR, 0.42; 95% CI, 0.30‐0.57 in patients without cirrhosis). We also performed a subgroup analysis in which we assessed DCC incidence in patients who received DAA therapy of at least 8 weeks of sofosbuvir/ledipasvir or 12 weeks of the other DAA regimens and found no significant change in the results (HR, 0.38; 95% CI, 0.25‐0.57 in patients with cirrhosis; HR, 0.39; 95% CI, 0.27‐0.56 in patients without cirrhosis) (Supporting Table S4).
Table 4.
Incidence Rate of Decompensated Cirrhosis in Patients with HCV with and without Cirrhosis, by Treatment Status
| Incidence Rate of Decompensated Cirrhosis | Person‐years | No. of Events | Crude Incidence/1000 Person‐years | Multivariate Cox regression* | |
|---|---|---|---|---|---|
| HR | 95% CI | ||||
| Base case analysis | |||||
| Patients with cirrhosis (n = 2157) | |||||
| DAA treatment (n = 1218) | 1406 | 45 | 32.0 | 0.38 | 0.26‐0.56 |
| No treatment (n = 939) | 791 | 86 | 108.7 | 1 | Reference |
| Patients without cirrhosis (n = 23,948) | |||||
| DAA treatment (n = 6710) | 8022 | 64 | 8.0 | 0.42 | 0.30‐0.58 |
| No treatment (n = 17,238) | 15,315 | 248 | 16.2 | 1 | Reference |
| Sensitivity analysis: Patients with HCV without censoring at their date of hepatocellular carcinoma | |||||
| Patients with cirrhosis (n = 2157) | |||||
| DAA treatment (n = 1218) | 1416 | 45 | 31.8 | 0.35 | 0.24‐0.51 |
| No treatment (n = 939) | 801 | 92 | 114.9 | 1 | Reference |
| Patients without cirrhosis (n = 23,948) | |||||
| DAA treatment (n = 6710) | 8036 | 69 | 8.5 | 0.42 | 0.30‐0.57 |
| No treatment (n = 17,238) | 15,336 | 262 | 17.0 | 1 | Reference |
Abbreviations: CI, confidence interval; DAA, direct‐acting antivirals; HR, hazard ratio.
Cox proportional hazards model was used to adjust for age, gender, region, insurance type, HCV disease duration, alcoholic liver disease (ALD), nonalcoholic fatty liver disease (NAFLD), Alcohol dependence/abuse and injection/noninjection drug abuse, hypertension, dyslipidemia, diabetes mellitus, chronic obstructive pulmonary disease (COPD), chronic kidney disease(CKD), cardiovascular disease (CVD), HIV infection, hepatitis B virus (HBV), hepatitis A virus (HAV), depression, schizophrenia/bipolar disorder, pregnancy, and use of proton‐pump inhibitor (PPI) and statins.
Effect of All‐Oral DAA Regimen on Health Care Costs
For the economic analysis, we further excluded patients who had less than a 12‐month follow‐up period in order to evaluate at least 1 year of medical costs after HCV treatment (mean follow‐up, 17 months). After PS matching, a total of 8064 patients (682 with cirrhosis and 7382 without cirrhosis) were included.
Unadjusted Medical Costs
Unadjusted medical costs for the PPPY pre‐ and posttreatment periods in patients with HCV with and without cirrhosis are shown in Table 5. In patients with cirrhosis (n = 682), liver‐related total medical costs were significantly lower in the DAA group ($1863) compared with the untreated group ($4079; P < 0.001). All‐cause costs were lower in the DAA group ($18,601) compared with the untreated group ($34,359), although the difference was not statistically significant (P = 0.158). In patients without cirrhosis, (n = 7382), liver‐related costs were significantly higher in the DAA group ($737) compared with the untreated group ($436; P < 0.001), which was driven by a higher rate of outpatient visits in the DAA group compared with the untreated group (3.2 vs. 0.7; P < 0.001; data not shown). However, all‐cause costs were lower in the DAA group ($13,721) compared with the untreated group ($14,157), but this difference was not statistically significant (P = 0.093).
Table 5.
Unadjusted Liver‐related and All‐cause Costs per Person per Year Before and After Treatment in the Propensity Score–Matched Patients with HCV with and without Cirrhosis, by Treatment Status
| Health care costs* | Pretreatment | Posttreatment | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P Value† | |
| Liver‐related costs, $ | |||||
| Patients with cirrhosis (n = 682) | |||||
| DAA treatment (n = 341) | 1742 | 2291 | 1863 | 8157 | <0.001 |
| No treatment (n = 341) | 1450 | 2420 | 4079 | 23,623 | |
| Patients without cirrhosis (n = 7382) | |||||
| DAA treatment (n = 3691) | 1051 | 1506 | 737 | 3819 | <0.001 |
| No treatment (n = 3691) | 315 | 825 | 436 | 6727 | |
| All‐cause costs, $ | |||||
| Patients with cirrhosis (n = 682) | |||||
| DAA treatment (n = 341) | 17,537 | 31,178 | 18,601 | 34,704 | 0.158 |
| No treatment (n = 341) | 23,798 | 45,683 | 34,359 | 75,676 | |
| Patients without cirrhosis (n = 7382) | |||||
| DAA treatment (n = 3691) | 12,370 | 30,557 | 13,721 | 38,151 | 0.093 |
| No treatment (n = 3691) | 13,334 | 32,493 | 14,157 | 39,727 | |
Abbreviations: DAA, direct‐acting antivirals.
Costs were adjusted to 2016 U.S. dollars using an annual 3% inflation rate.
P values compare differences in posttreatment costs between the treated and untreated groups using Wilcoxon signed rank tests.
DAA Treatment Costs
The mean all‐oral DAA treatment costs for patients with HCV with cirrhosis were $123,086, which was $22,619 higher than the mean for patients with HCV without cirrhosis ($100,467) because of a longer duration of therapy (98 vs. 85 days) (Supporting Table S6).
Adjusted Medical Costs
After adjustment for demographic characteristics, comorbidities, and pretreatment medical costs, patients with cirrhosis in the DAA group had significantly lower liver‐related and all‐cause health care costs compared with those in the untreated group (Fig. 2). Patients with cirrhosis in the DAA group were estimated to have mean liver‐related costs of $1749, which was 62% lower than that for the untreated group ($4575; P < 0.001). All‐cause medical costs were estimated at $19,300 in the treated group, which was 42% lower compared with patients in the untreated group ($33,039; P < 0.001). In contrast, patients without cirrhosis who received all‐oral DAA treatment were estimated to have slightly higher liver‐related costs ($726) compared with those of untreated patients ($441). All‐cause medical costs were also slightly higher in the DAA‐treated group ($14,071) compared with the untreated group ($13,828). However, these differences were not statistically significant.
Figure 2.
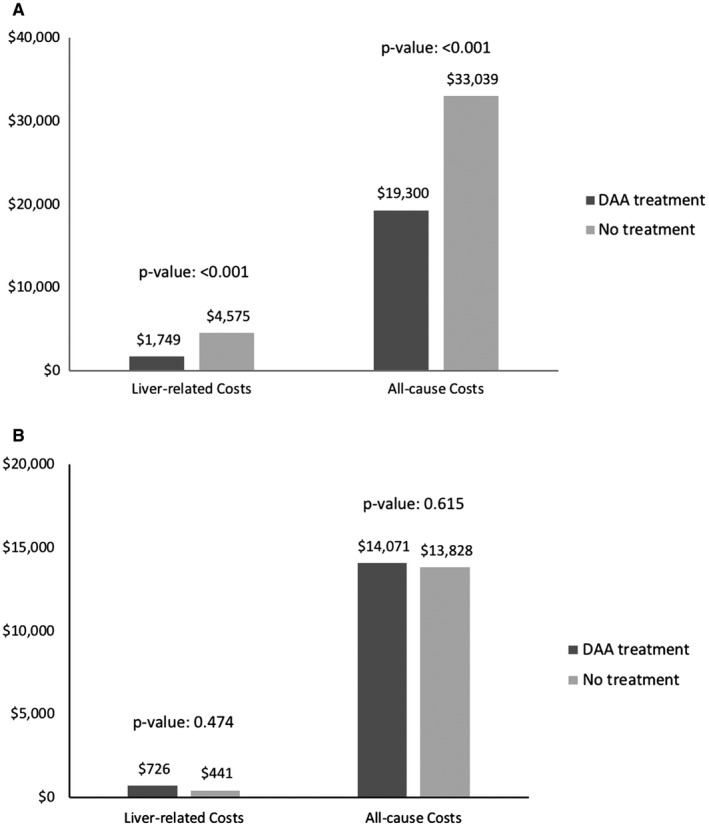
Adjusted liver‐related and all‐cause costs per person per year by treatment status in propensity score–matched cohorts. (A) Medical costs in patients with cirrhosis. (B) Medical costs in patients without cirrhosis. Abbreviation: DAA, direct‐acting antivirals.
Discussion
This retrospective cohort study provides real‐world evidence from the U.S. general population on the effects of all‐oral DAA therapy for clinical and economic outcomes covering the first 3 years after the approval of the second generation of DAAs in the United States. In patients with cirrhosis, the crude incidence rates of HCC (11.7 vs. 48.9 per 1000 person‐years) and DCC (32.0 vs. 108.7 per 1000 person‐years) were three to four times lower in patients in the DAA‐treated group compared with those in the untreated group. We observed a similar trend among patients without cirrhosis, in which the crude incidence rates for HCC (3.1 vs. 5.8 per 1000 person years) and DCC (8.0 vs. 16.2 per 1000 person‐years) were approximately two times lower for the DAA group compared with the untreated group. These findings were further confirmed in our Cox proportional hazards regression models, which indicated DAA therapy was associated with a 72% and a 62% decreased risk of developing HCC and DCC in patients with cirrhosis, respectively. Similarly, the DAA therapy significantly reduced the risk of HCC (HR, 0.43, 95% CI, 0.26‐0.71) and DCC (HR, 0.42, 95% CI, 0.30‐0.58) in patients without cirrhosis.
Our results are very encouraging, as DAA therapy is associated with high cure rates, few side effects, and a decrease in adverse liver‐related outcomes, unlike earlier HCV interferon‐based therapies, which carried lower cure rates probably as a result of the high side‐effect profile of interferon leading to high discontinuance of treatment prior to cure.10, 24 Interestingly, unlike our results, a recent report indicated that DAA treatment was associated with an unexpected high rate of HCC.25, 26, 27 Investigators suggest that the high rate of HCC may actually be the results of the “warehousing effect”—a state where curative treatment was offered to those who were the sickest and were unable to be cured with the prior IFN treatment.28 A recent study of U.S. veterans found that DAA treatment was not associated with a higher risk of HCC in patients with HCV with cirrhosis.28 Our findings are more in line with the U.S. veterans, as we determined that being male, of older age, and having cirrhosis, diabetes, and any type of cancer, in addition to no treatment, were the predictors for HCC.
Moreover, in our study, we also determined that the risk of incident decompensation was significantly lower in the DAA‐treated group regardless of cirrhosis status, although more DCC events occurred in the group with cirrhosis. Our results are different than previous studies, which reported the possibility of an increased risk for hepatic decompensation related to DAA therapy.29, 30 One reason for these reported differences may come about as a result of the type of data and populations used in the analyses. Our analysis compared the incident liver decompensation of DAA therapy with untreated patients with HCV without DCC at baseline whereas the previous studies did not have a control group and included patients with DCC prior to initiation of DAA therapy.29, 30 Consistent with our study, the previous studies reported that the risk of hepatic decompensation was observed in patients with pretreatment cirrhosis receiving DAAs.29, 30
A disturbing study finding was that the majority of patients with HCV (70%) within our study were not treated. During the study period, 45% of patients with cirrhosis and 72% of patients without cirrhosis did not receive any HCV treatment. It is noteworthy that DCC and HCC incidence rates were significantly higher in patients with cirrhosis receiving DAA therapy compared with patients without cirrhosis who did not receive any treatment, suggesting that delaying this curative treatment until patients develop cirrhosis can substantially increase the risk of liver complications.
Nonetheless, the high percentage of patients not receiving treatment suggests that patients are still encountering barriers to treatment even within a group with access to health insurance. In our study, we determined that being female, having alcohol/drug use disorder, HBV, COPD, CKD, CVD, depression, cancer history, and ALD as well as being pregnant were predictors for no treatment. Therefore, identifying and then overcoming the barriers to treatment remains a significant issue in eradicating HCV and its clinical burden.
Besides reporting the reduction in the clinical burden of HCV with DAA treatment, we also investigated the economic burden of HCV and found that HCV DAA treatment was associated with a decline in health expenditures for both liver‐related (by $2826) and all‐cause‐related ($13,739) expenditures, after controlling for comorbidities and pretreatment costs, especially in patients with cirrhosis. When we monetized PPPY data to all patients with cirrhosis in the United States (n = 223,000 patients with cirrhosis [8.3% among 2.7 million patients with HCV in the United States]), assuming 100% of patients were treated with DAA therapy, liver‐related and all‐cause cost savings from treatment with DAA therapy were estimated to result in savings of $0.6 billion ($2826 PPPY) and $3 billion per year ($13,739 PPPY), relative to no treatment, respectively.
Although we did not observe decreased costs in the DAA group among patients without cirrhosis, we believe that over time the savings associated with the avoidance of advanced liver disease and other complications of HCV will be offset, as predicted by several economic investigations in which DAA therapy was found to be cost‐effective in the long term.16, 17, 18, 31 Furthermore, although we did not study HCV‐associated extrahepatic manifestations in this study, previous investigators found that a large proportion of all‐cause medical costs were attributable to extrahepatic manifestation–related costs in patients with HCV, adding strength to our findings that treatment with DAAs can reduce the high medical costs in patients with HCV by saving in both liver complications and extrahepatic manifestations avoided with cure.14, 32, 33
It is important to note that in our economic analysis, we estimated that the costs for at least an 8‐week DAA treatment course ranged between approximately $100,467 and $123,086. However, the actual cost paid for the DAA therapy may be significantly less than our estimation considering the high rebates and discounts negotiated between pharmaceutical companies and payers.34 Nonetheless, despite the competition and negotiated pricing, cost still remains a financial burden for payers and continues to limit the public health impact of new DAA therapies. Therefore, our findings provide policy makers and stakeholders with information they need when determining policies that affect the accessibility of these highly effective drugs and the impact on the long‐term public health as well as the economic benefits of curing HCV, especially during the opioid crisis when HCV infection is actually increasing.35
There are several strengths to our study. First, this study has methodological strength as a result of using a prescription time‐distribution matching to adjust time to initiate HCV treatment after diagnosis that add the same duration to the time between the first HCV diagnosis date in each untreated patient. In addition, we used regression‐adjusted matching; matching was followed by regression adjustment to the matched data, which is a relatively robust method for estimating treatment effects in health economic evaluation compared with matching or regression adjustment alone.36 These statistical methods have not been performed in many of the previous economic studies for patients with HCV.
Second, this study included a number of strongly associated covariates that were controlled for in the Cox models (e.g., diabetes, PPIs, statins), stratified by the presence of cirrhosis. For the economic model, we further controlled for pretreatment costs in addition to demographics and comorbidities to account for skewness and effects of confounders to isolate the estimated treatment effects on medical costs. Third, this study has a large sample size that is representative of the general insured populations in the United States. We also conducted several sensitivity analyses to assess the robustness of our results and found that our results were consistent.
Our study also had a few limitations. The study lacked laboratory results (e.g., SVR, biopsy) to corroborate ICD coding. The Truven claims databases contain deidentified patient‐level health data, where individual records cannot be linked back to an original health record system and/or other databases for ICD code validations. However, a recent validation study reported that a coding algorithm including 1 inpatient or 2 outpatients ICD‐9 codes for HCV and cirrhosis had high accuracy, although using ICD‐10 codes has not yet been validated.37 Previous studies also validated that the use of billing ICD‐9 codes for the diagnosis of DCC and HCC had positive predictive value of 88%‐91%.20 It is possible that incomplete, missing, or miscoded claims may impact the study findings; however, coding errors are likely distributed evenly between the treated and untreated groups. This study is also limited by its design as a retrospective cohort study; however, prospective studies comparing DAA therapy with no treatment would be infeasible and unethical, as DAAs have been shown to be highly efficacious and significantly associated with a decreased risk of advanced liver diseases (i.e., HCC and DCC). In addition, we have a relatively short follow‐up, which did not allow us to fully explore the long‐term effects of all‐oral DAAs.
In conclusion, DAA therapy was found to be associated with significant reductions in the incidence of HCC and DCC among patients with and without cirrhosis. In addition, the use of DAA treatments was found to be economically sound for both liver‐related and all‐cause health care resource use. Yet, despite these positive findings, and the availability of these highly effective all‐oral DAA therapies, the majority of patients with HCV within our study were not treated. Future studies must continue to investigate ways to improve access to treatment for all patients with HCV.
Supporting information
Supported in part by the National Institute on Drug Abuse (NIDA) of the National Institutes of Health under Award Number K01DA045618 (to H.P). D.R.N. was supported by the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflict of interest: Dr. Nelson owns stock in Target Pharma Solutions and receives research grant support from AbbVie, BMS, Gilead and Merck.
References
Author names in bold designate shared co‐first authorship.
- 1. Denniston MM, Jiles RB, Drobeniuc J, Klevens RM, Ward JW, McQuillan GM, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med 2014;160:293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012;156:271‐278. [DOI] [PubMed] [Google Scholar]
- 3. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck‐Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta‐analysis of observational studies. Ann Intern Med 2013;158:329‐337. [DOI] [PubMed] [Google Scholar]
- 4. Veldt BJ, Heathcote EJ, Wedemeyer H, Reichen J, Hofmann WP, Zeuzem S, et al. Sustained virologic response and clinical outcomes in patients with chronic hepatitis C and advanced fibrosis. Ann Intern Med 2007;147:677‐684. [DOI] [PubMed] [Google Scholar]
- 5. Bhamidimarri KR, Satapathy SK, Martin P. Hepatitis C virus and liver transplantation. Gastroenterol Hepatol 2017;13:214‐220. [PMC free article] [PubMed] [Google Scholar]
- 6. Kimer N, Dahl EK, Gluud LL, Krag A. Antiviral therapy for prevention of hepatocellular carcinoma in chronic hepatitis C: systematic review and meta‐analysis of randomised controlled trials. BMJ Open 2012;2:e001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010;52:833‐844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all‐cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012;308:2584‐2593. [DOI] [PubMed] [Google Scholar]
- 9. Gordon SC, Hamzeh FM, Pockros PJ, Hoop RS, Buikema AR, Korner EJ, et al. Hepatitis C virus therapy is associated with lower health care costs not only in noncirrhotic patients but also in patients with end‐stage liver disease. Aliment Pharmacol Ther 2013;38:784‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Webster DP, Klenerman P, Dusheiko GM. Hepatitis C. Lancet 2015;385:1124‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stepanova M, Younossi ZM. Interferon‐free regimens for chronic hepatitis C: barriers due to treatment candidacy and insurance coverage. Dig Dis Sci 2015;60:3248‐3251. [DOI] [PubMed] [Google Scholar]
- 12. Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for medicaid reimbursement of sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med 2015;163:215‐223. [DOI] [PubMed] [Google Scholar]
- 13. Gordon SC, Pockros PJ, Terrault NA, Hoop RS, Buikema A, Nerenz D, et al. Impact of disease severity on healthcare costs in patients with chronic hepatitis C (CHC) virus infection. Hepatology 2012;56:1651‐1660. [DOI] [PubMed] [Google Scholar]
- 14. Reau N, Vekeman F, Wu E, Bao Y, Gonzalez YS. Prevalence and economic burden of extrahepatic manifestations of hepatitis C virus are underestimated but can be improved with therapy. Hepatol Commun 2017;1:439‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All‐oral, interferon‐free treatment for chronic hepatitis C: cost‐effectiveness analyses. J Viral Hepat 2013;20:847‐857. [DOI] [PubMed] [Google Scholar]
- 16. Chhatwal J, He T, Hur C, Lopez‐Olivo MA. Direct‐acting antiviral agents for patients with hepatitis C virus genotype 1 infection are cost‐saving. Clin Gastroenterol Hepatol 2017;15:827‐837. [DOI] [PubMed] [Google Scholar]
- 17. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost‐effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med 2015;162:397‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Younossi ZM, Park H, Saab S, Ahmed A, Dieterich D, Gordon SC. Cost‐effectiveness of all‐oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther 2015;41:544‐563. [DOI] [PubMed] [Google Scholar]
- 19. Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time‐to‐treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol 2005;162:1016‐1023. [DOI] [PubMed] [Google Scholar]
- 20. Goldberg DS, Lewis JD, Halpern SD, Weiner MG, Lo Re V 3rd. Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database. Pharmacoepidemiol Drug Saf 2013;22:103‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lo Re V 3rd, Lim JK, Goetz MB, Tate J, Bathulapalli H, Klein MB, et al. Validity of diagnostic codes and liver‐related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf 2011;20:689‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lu M, Chacra W, Rabin D, Rupp LB, Trudeau S, Li J, et al. Validity of an automated algorithm using diagnosis and procedure codes to identify decompensated cirrhosis using electronic health records. Clin Epidemiol 2017;9:369‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mullahy J. Specification and testing of some modified count data models. J Econom 1986;33:341‐365. [Google Scholar]
- 24. LaFleur J, Hoop R, Morgan T, DuVall SL, Pandya P, Korner E, et al. High rates of early treatment discontinuation in hepatitis C‐infected US veterans. BMC Res Notes 2014;7:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ravi S, Axley P, Jones D, Kodali S, Simpson H, McGuire BM, et al. Unusually high rates of hepatocellular carcinoma after treatment with direct‐acting antiviral therapy for hepatitis C related cirrhosis. Gastroenterology 2017;152:911‐912. [DOI] [PubMed] [Google Scholar]
- 26. Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV‐related cirrhosis treated with direct‐acting antivirals. J Hepatol 2016;65:727‐733. [DOI] [PubMed] [Google Scholar]
- 27. Kozbial K, Moser S, Schwarzer R, Laferl H, Al‐Zoairy R, Stauber R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferon‐free direct‐acting antiviral treatment. J Hepatol 2016;65:856‐858. [DOI] [PubMed] [Google Scholar]
- 28. Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V 3rd, et al. The short‐term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct‐acting antivirals: An ERCHIVES study. Hepatology 2018;67:2244‐2253. [DOI] [PubMed] [Google Scholar]
- 29. Fernandez Carrillo C, Lens S, Llop E, Pascasio JM, Crespo J, Arenas J, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end‐stage liver disease: Analysis of data from the Hepa‐C registry. Hepatology 2017;65:1810‐1822. [DOI] [PubMed] [Google Scholar]
- 30. Butt AA, Ren Y, Marks K, Shaikh OS, Sherman KE, ERCHIVES study . Do directly acting antiviral agents for HCV increase the risk of hepatic decompensation and decline in renal function? Results from ERCHIVES. Aliment Pharmacol Ther 2017;45:150‐159. [DOI] [PubMed] [Google Scholar]
- 31. Younossi ZM, Tanaka A, Eguchi Y, Henry L, Beckerman R, Mizokami M. Treatment of hepatitis C virus leads to economic gains related to reduction in cases of hepatocellular carcinoma and decompensated cirrhosis in Japan. J Viral Hepat 2018;25:945‐951. [DOI] [PubMed] [Google Scholar]
- 32. Cacoub P, Vautier M, Desbois AC, Saadoun D, Younossi Z. Direct medical costs associated with the extrahepatic manifestations of hepatitis C virus infection in France. Aliment Pharmacol Ther 2018;47:123‐128. [DOI] [PubMed] [Google Scholar]
- 33. Cacoub P, Buggisch P, Carrion JA, Cooke GS, Zignego AL, Beckerman R, et al. Direct medical costs associated with the extrahepatic manifestations of hepatitis C infection in Europe. J Viral Hepat 2018;25:811‐817. [DOI] [PubMed] [Google Scholar]
- 34. American Association for the Study of Liver Disease (AASLD)/Infectious Disease Society of America (IDSA)/International Antiviral Society‐USA (IAS‐USA) . HCV Guidance: Recommendations for testing, managing, and treating Hepatitis C 2017. https://www.hcvguidelines.org/. Accessed June 10, 2018.
- 35. Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018;108:175‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kreif N, Grieve R, Radice R, Sekhon JS. Regression‐adjusted matching and double‐robust methods for estimating average treatment effects in health economic evlauation. Health Serv Outcomes Res Methodol 2013;13:174‐202. [Google Scholar]
- 37. Niu B, Forde KA, Goldberg DS. Coding algorithms for identifying patients with cirrhosis and hepatitis B or C virus using administrative data. Pharmacoepidemiol Drug Saf 2015;24:107‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


