Abstract
For the first time, we present an analytical method to simultaneously extract, fractionate, and quantify four groups of semi-volatile organic compounds (SVOCs) in silicone wristbands, including 35 polybrominated diphenyl ethers (PBDEs), 10 novel flame retardants (NFRs), 19 organophosphate esters (OPEs), and 13 polycyclic aromatic hydrocarbons (PAHs). Wristbands were extracted using ultrasonication, and cleaned and fractionated on two multi-layer columns: one consisting of neutral alumina, neutral silica and Florisil, and the other consisting of neutral alumina, neutral silica, and acidic silica. Method accuracy and precision were validated using spiked wristband samples (n = 8) and procedural blanks (n = 7). Average matrix spike percent recoveries for all target analytes were within 57 - 107% with relative standard errors < 20%, with a few exceptions. This method was applied to analyze thirteen wristbands worn by ten participants for seven days; three participants wore two wristbands to evaluate duplicate samples. Percent recoveries of surrogate standards for all four groups of analytes in these wristbands were all within the 80 - 120% range with a few exceptions: recoveries for 13C12BDE-209 and for 13C12-triphenyl phosphate ranged from 35 - 62% and 69 - 176%, respectively. The majority of target analytes were detected in at least half of worn wristbands. The levels of total PBDEs, NFRs, OPEs and PAHs in deployed wristbands ranged from 28.4 to 412 ng, 40.7 to 625 ng, 2,440 to 9,580 ng, and 76.2 to 1,240 ng, respectively.
Keywords: Silicone wristband, personal exposure, semi-volatile organic compounds, novel brominated flame retardants, organophosphate esters, polycyclic aromatic hydrocarbons
1. Introduction
Passive samplers are increasingly employed in investigations of outdoor and indoor exposure to semi-volatile organic compounds (SVOCs) due to their ease of use [1-3]. Silicone wristbands are a new tool for assessing personal exposure to anthropogenic chemicals. As passive samplers, silicone wristbands work by chemical diffusion (absorption) of an environmental contaminant into the polymer of the silicone over time [4]. They were first introduced by O’Connell et al. [5] in 2014 to assess exposure in an occupational setting, but have since been used in several studies, ranging from assessment of pesticide exposure among farmers in West Africa [6] and Peru [7] and flame retardant exposure among preschool children in the United States [4, 8, 9], to assessment of volatile organic chemicals emanating from the surface of human skin [10]. These studies have demonstrated that a commercial silicone wristband, worn by study participants, offers a non-invasive and simple way to quantify personal exposure to multiple chemicals from multiple microenvironments and within a multiday time period. Further, several studies have found significant correlations between the mass of chemicals accumulated on the wristband and biomarkers of internal exposure measured in blood or urine [11-13]. This sampling method opens a wide range of opportunities for larger scale exposure monitoring studies, especially in vulnerable populations such as children, due to its noninvasiveness (and thus low rejection rates of subject participation), simplicity, and cost-effectiveness.
To date, silicone wristbands have been employed in a small number of studies to measure polycyclic aromatic hydrocarbons (PAHs) [5], polybrominated diphenyl ethers (PBDEs) and novel flame retardants (NFRs) [4, 13], organophosphate esters (OPEs) [12], and pesticides [6, 9]. However, these previous studies on wristbands were limited to one group of compounds or to qualitative analysis. There is still the urgent need for an analytical method that can quantitatively analyze a broad range of SVOCs in this matrix.
The goal of this study was to further the development and application of silicone wristbands for exposure assessments for a wide range of SVOCs. Here we present an analytical method that combines the simultaneous extraction, as well as fractionation and quantitative analysis of four groups of SVOCs, including PBDEs, NFRs, OPEs, and PAHs, for a total of 77 compounds.
2. Materials and Methods
2.1. Standards
The BFR-PAR (Brominated Flame Retardant - native compounds stock solution) mixture [including BDE-7, 10, 15, 17, 28, 30, 47, 49, 66, 71, 77, 85, 99, 100, 119, 126, 138-140, 153, 154, 156, 169, 180, 183, 184, 191, 196, 197, 201, and 203-209, pentabromoethyl benzene (PBEB), hexabromobenzene (HBB), 1,2-bis(2,4,6-tribromophenoxy)ethane (TBE), and decabromodiphenyl ethane (DBDPE)], BDE-MXE (Brominated Diphenyl Ether – native solution mixture) solution mixture [including BDE-7, 15, 17, 28, 49, 47, 66, 71, 85, 99, 100, 119, 126, 138, 153, 154, 156, 169, 184, 183, 191, 196, 197, 206, 207, 209], and individual syn- and anti-DP, pentabromobenzene (PBBZ), tetrabromo-p-xylene (pTBX), 2-ethyl-hexyl tetrabromobenzoate (EHTBB), BDE-118, BDE-181, 13C12-BDE-209, di-(2-ethylhexyl)-tetrabromophthalate (BEHTBP) standards were purchased from Wellington Laboratories Inc. (Guelph, ON). Other individual compounds including BDE-77, -118, and -166 were purchased from AccuStandard, Inc. (New Haven, CT). The concentrations of PBDE/NFR calibration standard components ranged from 4 to 20 ng/mL.
Individual OPE standards [including tri-ethyl phosphate (TEP), tri-n-propyl phosphate (TPRP), tri-n-buytl phosphate (TNBP), tris(2-chloroethyl) phosphate (TCEP), tris[1-chloro-2-propyl] phosphate (TCIPP), tris(1,3-dichloro-2-propyl) phosphate (TDCIPP), tris(2-butoxyethyl) phosphate (TBOEP), 2-ethylhexyl diphenyl phosphate (EHDPHP), tris(2-ethylhexyl) phosphate (TEHP), triphenyl phosphate (TPHP), tri-o-tolyl phosphate (TOTP), tri-m-tolyl phosphate (TMTP), tri-p-tolyl phosphate (TPTP), tris(2-isopropylphenyl) phosphate (TIPPP), tris(3,5-dimethylphenyl) phosphate (TDMPP), d12-tris(2-chlorethyl) phosphate (d12-TCEP), and 13C18-triphenyl phosphate (13C12-TPHP )] were also purchased from Wellington Laboratories, Inc. (Guelph, ON). Other individual OPE standards [including tris(2,3-dibromopropyl) phosphate (TDBPP), triisopropyl phosphate (TIPRP), and tripentyl phosphate (TPEP)] were purchased from AccuStandard, Inc. (New Haven, CT) and tris(4-tert-butylphenyl) phospate (TBPP) was purchased from Sigma Aldrich (St. Louis, MO).
The PAH solution mixture [including acenaphthene, acenaphthylene, anthracene, benz[a]anthracene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[ghi]perylene, benzo[a]pyrene, chrysene, dibenz[a,h]anthracene, fluoranthene, fluorene, indeno[1,2,3-cd]pyrene, phenanthrene, pyrene] and individual PAH standards [including d10-phenanthrene, d10-pyrene, d12-perylene, d12-benz[a]anthracene, and d12-anthracene] were purchased from Ultra Scientific (now Agilent Technologies, Santa Clara, CA) and individual PAH standards [including coronene, benzo[e]pyrene, and retene) were purchased from ChemService, Inc. (West Chester, PA). The concentration of each PAH calibration standard component was 20 ng/mL.
2.2. Materials
Black silicone wristbands were purchased in bulk (www.24hourwristbands.com, Houston, TX); they measured approximately 1.2 cm × 20 cm × 0.2 cm and weighed 4.98 ± 0.02 g (average ± standard deviation, n = 13) after cleaning/drying. Hexane, acetone, ethyl acetate, dichloromethane, and isopropyl alcohol were all HPLC grade or higher (OmniSolv, VWR International, Chicago, IL). Deionized water was filtered to at least 16 MΩ-cm using a Barnstead D4641 purifier (ThermoScientific, Dubuque, IA). Alumina (MP Biomedicals, Santa Ana, CA), Florisil (Sigma-Aldrich, St. Louis, MO), and silica (Fisher Chemical, Fair Lawn, NJ) were all muffled at 300°C, cooled in a desiccator to ambient temperature, 3% (by weight) water deactivated, and stored in a desiccator for 12 hours to reach equilibrium before use. Additional muffled silica was acid deactivated (50% by weight) using 95-98% sulfuric acid (Sigma-Aldrich, St. Louis, MO) and stored in a desiccator for 12 hours before use. Anhydrous sodium sulfate (Fisher Chemical, Fair Lawn, NJ) was muffled at 500°C for 12 hours and cooled to ambient temperature before use. All glassware was washed with soap and water, rinsed with deionized water, air dried, covered in aluminum foil, and muffled at 500°C for 8 hours. Stainless steel tools, Teflon caps and stopcocks were washed with soap and water, rinsed with deionized water, placed in a drying oven at 80°C, and rinsed with dichloromethane before use.
2.3. Cleaning and deployment of wristbands
Wristbands were cleaned using two 18-hour Soxhlet extractions: first with ethyl acetate and hexane (1:1, v:v), and then with ethyl acetate and methanol (1:1, v:v), similarly to Hammel et al. (2016) and O’Connell et al. [5, 12]. Wristbands were allowed to dry for 12 hours, covered in the fume hood. Then, they were individually wrapped in foil, placed into a Ziploc bag, and stored in a freezer at −20°C until deployment.
Ten participants wore the wristbands on their dominant wrist continuously for seven days in Bloomington, Indiana. Seven participants wore one wristband and three participants wore duplicate wristbands. Participants were 18-50 years old, 5 males and 5 females, all non-smokers. Participants were instructed to wear the wristband through bathing and normal day to day activities, to keep it free from clothing cover as much as possible, and only to remove the wristband during swimming in a chlorinated pool. After seven days, the wristbands were collected and placed in a clean amber jar with a Teflon lid and stored at −20°C until extraction. The study was approved by Indiana University Institutional Review Board (Protocol #1703813059R001).
2.4. Sample extraction
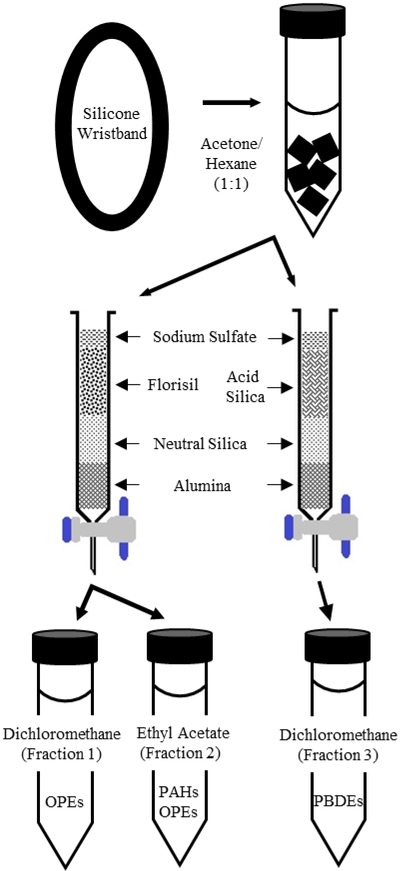
A simplified diagram of the extraction, cleanup, and fractionation procedures can be found in Figure 1. A more detailed flow chart of these procedures can be found in the Supporting Information (Figure S1).
Figure 1.
Simplified diagram of wristband extraction, column chromatography, and fractionation.
Prior to extraction, wristbands were placed into a deionized water bath and agitated for a few seconds to remove any surface debris on the wristband. The wristbands were briefly rinsed with isopropyl alcohol to remove water similarly to O’Connell et al. [5]. Once dry, the wristbands were cut into 0.5-1 cm pieces (about 20 pieces total) using pre-cleaned surgical scissors and placed into a 50 mL conical tube.
Cut wristbands in the tube were spiked with 100 μL PBDE/NFR (BDE-77, -166, and 13C12-209; 3, 5, and 4 ng/mL, respectively), 100 μL OPE (d12-TCEP and 13C12-TPHP; each 250 ng/mL), and PAH (d10-phenanthrene and d10-pyrene; each 400 ng/mL) surrogate standards and covered with 30 mL acetone and hexane mixture (1:1, v:v) to allow for expansion of the wristbands (3-4 times their original size). Wristbands were then ultrasonicated for 2 hours and left on the bench for 12 hours for complete extraction. The extract was transferred to a new tube, and wristbands were ultrasonicated once more with 20 mL of extraction solvent for another 2 hours. The two extracts were combined and concentrated to 1 mL using high purity nitrogen (99.998%; Praxair, Indianapolis, IN) with an N-Evap apparatus (Organomation Associates, Inc, Berlin, MA), twice exchanged to hexane, and diluted to 4 mL. Samples were vortexed for 30 seconds, split into two volumetrically equal subsamples, and loaded on two separate multi-layer chromatography columns.
2.5. Sample cleanup
The first column (glass, internal diameter [i.d.] 1 cm, length 25 cm) was dry packed with neutral alumina (3 cm; 3% water deactivated) and eluted with 25 mL of hexane until the alumina was saturated. Next, neutral silica (3 cm; 3% water deactivated) in a hexane slurry was added, followed by Florisil (4 cm; 3% water deactivated) and capped with a layer of anhydrous sodium sulfate (1 cm), and conditioned with hexane. The samples were eluted first with 40 mL of dichloromethane (Fraction 1, contained part of PAHs and OPEs, and BEHTBP) and then with 40 mL of ethyl acetate (Fraction 2, contained the rest of PAHs and OPEs). Fraction 1 was concentrated to 0.5 mL, twice solvent exchanged to hexane, and spiked with 100 μL OPE (d12-perlyne, d12-benz[a]anthracene, and d10-anthracene; each 400 ng/mL) and 50 μL PBDE/NFR (BDE-118 and -181; 5 and 10 ng/mL, respectively) internal standards. The deuterated PAH compounds were used as internal standards for OPE quantitation because the majority of the available deuterated OPE compounds elute early in the chromatogram. PBDE/NFR internal standards were spiked to this fraction to quantitate BEHTBP. Fraction 2 was evaporated to dryness, reconstituted with 1 mL hexane, and then spiked with 100 μL PAH internal standards (d12-perlyne, d12-benz[a]anthracene, and d10-anthracene; each 400 ng/mL).
The second column (glass, i.d. 1 cm, length 25 cm) was dry packed with neutral alumina (3 cm; 3% water deactivated) and eluted with 25 mL of hexane until the alumina was saturated. Next, neutral silica (3 cm; 3% water deactivated), followed by acidic silica (4 cm; 50% sulfuric acid deactivated), both in a hexane slurry, were added, and capped with a layer of anhydrous sodium sulfate (1 cm). The column was conditioned with hexane and the samples were eluted with 40 mL of dichloromethane (Fraction 3, contained PBDEs and NFRs). Samples were concentrated to 0.5 mL, exchanged to hexane twice, spiked with 50 μL PBDE/NFR internal standards (BDE-118 and -181), and further concentrated to ~100 μL.
2.6. Instrument analysis
Fractions 1 and 2 were analyzed for OPEs using an Agilent 6890 series gas chromatograph (GC) coupled to an Agilent 5973 mass spectrometer (MS) operated in the electron impact mode. A pulsed splitless injection of 1 μL at 285°C was used to introduce the sample to the GC column and high purity helium (99.999%; Indiana Oxygen, Indianapolis, IN) was used as the carrier gas at 1.5 mL/min. The column used to attain GC resolution was a 30 m × 0.25 mm × 0.25 pm Rtx-OPPesticides2 capillary column (Restek Corp., Bellefonte, PA). The GC oven temperature was held at 50°C for 3 min, increased to 100°C at 20°C/min, then to 170°C at 10°C/min and held for 3 min, then to 230°C at 12°C/min and held for 4 min, then to 260°C at 5°C/min, then to 300°C at 10°C/min and held for 14 min (total runtime 48.50 min). The GC/MS transfer line, ion source, and quadrupole temperatures were set at 285°C, 230°C, and 150°C, respectively. The list of monitored ions for OPEs can be found in Table S1. Examples of chromatograms of Fractions 1 and 2 for a blank, a matrix spike, and a worn wristband sample are shown in Figure S2 and S3, respectively.
Fraction 1 was also analyzed for PAHs using an Agilent 6890 series GC coupled to an Agilent 5973 MS operated in the electron impact mode. A pulsed splitless injection of 1 μL at 285°C was used to introduce the sample to the GC column and high purity helium (99.999%; Indiana Oxygen, Indianapolis, IN) was used as the carrier gas at 1.5 mL/min. The column used to attain GC resolution was a 30 m × 0.25 mm × 0.25 μm DB-5MS Ultra Inert capillary column (Agilent Technologies, Santa Clara, CA). The GC oven temperature was held at 70°C for 3 min, increased to 280°C at 30°C/min and held for 6 min, then to 300°C at 30°C/min and held for 12 min (total runtime 28.7 min). The GC/MS transfer line, ion source, and quadrupole temperatures were set at 285°C, 230°C, and 150°C, respectively. The list of monitored ions for PAHs is included in Table S1, and examples of chromatograms of Fraction 1 for a blank, a matrix spike, and a worn wristband sample can be found in Figure S4.
Fractions 1 and 3 were analyzed for PBDEs and NFRs on an Agilent 7890B series GC coupled to an Agilent 5977B MS operated in the electron capture negative ionization mode. A pulsed splitless injection of 1 μL at 285°C was used to introduce the sample to the GC column and high purity helium was used as the carrier gas with a flow rate of 54 mL/min. The column was a 15 m × 0.25 mm × 0.10 μm Rtx-1614 capillary column (Restek Corp., Bellefonte, PA). For better separation of the target analytes and to overcome interferences, the samples were injected twice using two different GC oven parameters. The first method held the oven temperature at 100°C for 2 min, then increased to 250°C at 25°C/min, then to 270°C at 3°C/min, then to 320°C at 25°C/min and held for 9 min (total runtime 25.7 min). The second method used slower temperature ramp and held the oven temperature at 100°C for 2 min, then increased to 250°C at 5°C/min, then to 270°C at 3°C/min, then to 320°C at 25°C/min and held for 9 min (total runtime 49.7 min). For both methods, the GC/MS transfer line was set at 310°C, and the ion source and quadrupole temperatures were 200°C and 150°C, respectively. High purity methane was used as the collision gas. Monitored ions for PBDEs and NFRs are included in Table S1, and examples of chromatograms of Fraction 3 for a blank, a matrix spike, and a worn wristband sample are shown in Figures S5 and S6.
2.7. Limits of quantification
PBDEs, NFRs, and PAHs were analyzed using a one-level calibration with internal standards for quantification. A 13-level calibration curve was used for PBDEs and NFRs linearity tests with a wide range of concentrations ranging from 0.10-500 ng; high correlation coefficients were obtained for all compounds (r2 > 0.993). A 9-level calibration curve was used for linearity tests for 13 PAHs, with concentrations ranging from 50-2,000 ng for all compounds; high correlation coefficients were obtained for all PAHs (r2 ≥ 0.999). The OPEs were analyzed using a 9-level quadratic calibration curve with concentrations ranging from 2.1-2,000 ng for all compounds (r2 > 0.997).
The limits of quantification (LOQ, ng) were calculated as the average concentrations of the analyte in the blanks plus two times its standard deviation. Seven pre-cleaned wristbands were extracted as procedural blanks. In cases of a non-detect, the instrument detection limit (IDL) was used for the LOQ calculation for PBDEs, NFRs, and PAHs, and the lowest amount from the calibration curve was used for OPEs (see Table S2 in the Supporting Information). The IDL was calculated using three times the standard deviation of the measurements of each analyte in ten low concentration injections. The LOQs ranged from 0.05 to 0.43 ng for PBDEs, from 0.08 to 0.81 ng for NFRs, from 1.00 to 100 ng for OPEs, and 0.6 to 5.2 ng for PAHs. The LOQs for each analyte are given in Table 1.
Table 1.
Limits of quantification (LOQ, ng) of target analytes, and median and mean percent matrix spike recoveries (± standard error, SE) in eight spiked wristband replicates.
| Compound | LOQ (ng) |
Median (%) |
Mean (%) ± SE |
Compound | LOQ (ng/g) |
Median (%) |
Mean (%) ± SE |
|
|---|---|---|---|---|---|---|---|---|
| Polybrominated diphenyl ethers (PBDEs) | Organophosphate esters (OPEs) | |||||||
| BDE-7 (4) | 0.07 | 95 | 94 ± 5 | TEP (400) | 26.7 | 38 | 58 ± 17 | |
| BDE-15 (4) | 0.07 | 134 | 141 ± 17 | TIPRP (200) | 6.31 | 41 | 60 ± 16 | |
| BDE-17 (4) | 0.06 | 102 | 104 ± 3 | TPRP (200) | 10.9 | 59 | 66 ± 11 | |
| BDE-28 (4) | 0.09 | 98 | 107 ± 6 | TNBP (1000) | 25.0 | 65 | 76 ± 11 | |
| BDE-47 (4) | 0.08 | 88 | 90 ± 2 | TCEP (500) | 21.0 | 75 | 87 ± 11 | |
| BDE-49 (4) | 0.10 | 109 | 112 ± 8 | TCIPP (1000) | 18.7 | 78 | 80 ± 9 | |
| BDE-66 (4) | 0.16 | 100 | 100 ± 2 | TPEP (200) | 13.3 | 64 | 87 ± 16 | |
| BDE-71 (4) | 0.10 | 79 | 80 ± 3 | TDCIPP (1000) | 18.6 | 77 | 81 ± 7 | |
| BDE-85 (4) | 0.05 | 86 | 88 ± 2 | TPHP (1000) | 12.5 | 72 | 74 ± 5 | |
| BDE-99 (4) | 0.12 | 86 | 87 ± 1 | TBOEP (1000) | 100 | 93 | 103 ± 20 | |
| BDE-100 (4) | 0.11 | 79 | 79 ± 1 | EHDP (500) | 56.0 | 72 | 74 ± 5 | |
| BDE-119 (4) | 0.12 | 80 | 83 ± 2 | TEHP (500) | 6.25 | 71 | 72 ± 9 | |
| BDE-126 (4) | 0.07 | 158 | 139 ± 18 | TOTP (200) | 3.68 | 88 | 87 ± 7 | |
| BDE-138 (8) | 0.09 | 90 | 98 ± 8 | TMTP (200) | 2.50 | 84 | 90 ± 7 | |
| BDE-153 (8) | 0.20 | 79 | 80 ± 2 | TPTP (200) | 5.00 | 92 | 90 ± 5 | |
| BDE-154 (8) | 0.24 | 64 | 64 ± 1 | TIPPP (200) | 1.00 | 92 | 86 ± 6 | |
| BDE-156+169 (8) | 0.23 | 81 | 82 ± 4 | TDMPP (200) | 8.62 | 85 | 89 ± 5 | |
| BDE-183 (8) | 0.20 | 72 | 68 ± 6 | TDBPP (1000) | 100 | 59 | 75 ± 16 | |
| BDE-184 (8) | 0.29 | 80 | 80 ±3 | TBPP (200) | 2.50 | 100 | 107 ± 14 | |
| BDE-191 (8) | 0.07 | 89 | 82 ± 12 | d12-TCEP (250; SS) | - | 100 | 95 ±8 | |
| BDE-196 (8) | 0.13 | 72 | 80 ±7 | 13C12-TPHP (250; SS) | - | 96 | 99 ±9 | |
| BDE-197 (8) | 0.09 | 63 | 71 ±7 | |||||
| BDE-206 (20) | 0.26 | 66 | 72 ±5 | Polycyclic aromatic hydrocarbons (PAHs) | ||||
| BDE-207 (20) | 0.39 | 65 | 74 ±7 | Acenaphthylene (400) | 4.93 | 60 | 57 ±8 | |
| BDE-209 (20) | 0.43 | 62 | 66 ±7 | Acenaphthene (400) | 5.21 | 64 | 60 ±7 | |
| BDE-77 (5; SS) | - | 101 | 100 ±3 | Fluorene (400) | 1.04 | 71 | 67 ± 6 | |
| BDE-166 (5; SS) | - | 86 | 87 ± 3 | Phenanthrene (400) | 2.27 | 83 | 79 ± 6 | |
| 13C12BDE-209 (4; SS) | - | 54 | 62 ± 9 | Anthracene (400) | 0.57 | 74 | 75 ± 4 | |
| Fluoranthene (400) | 1.17 | 93 | 96 ± 5 | |||||
| Pyrene (400) | 1.09 | 95 | 95 ± 3 | |||||
| Novel Flame Retardants (NFRs) | Retene (400) | 4.62 | 104 | 102 ± 6 | ||||
| pTBX (4) | 0.08 | 86 | 89 ± 3 | Benz[a]anthracene (400) | 1.28 | 89 | 89 ± 2 | |
| PBBZ (4) | 0.09 | 98 | 100 ± 4 | Chrysene (400) | 2.14 | 91 | 88 ± 3 | |
| EHTBB (5) | 0.81 | 81 | 80 ± 4 | Benzo [b]fluoranthene (400) | 1.36 | 81 | 78 ± 5 | |
| BEHTBP (5) | 0.26 | 84 | 70 ± 16 | Benzo[k]fluoranthene (400) | 1.43 | 68 | 68 ± 3 | |
| syn-DP (10) | 0.32 | 92 | 100 ± 6 | Benzo[e]pyrene (400) | 1.93 | 76 | 75 ± 4 | |
| anti-DP (10) | 0.65 | 92 | 99 ± 6 | d10-Phenanthrene (400; SS) | - | 76 | 71 ± 4 | |
| d10-Pyrene (400; SS) | - | 88 | 87 ± 3 | |||||
The spiked amount to the wristband matrix (ng) is listed in parenthesis after the compound name. SS: surrogate standard.
3. Results and Discussion
3.1. Chromatographic cleanup
Chromatographic cleanup procedures previously used for analysis of organic pollutants in wristbands include Florisil [12], C18 solid phase extraction cartridges [4], and 5% acid silica columns [13]. Some other methods (mostly focusing on the screening of analytes) did not include any cleanup or fractionation [5, 7, 14]. In the initial trials, we found several interferences in the extracts that were limiting the analysis of some target analytes: one was siloxane, which was released from the wristbands during extraction; the other were lipids, which were transferred from the skin to the wristbands during deployment due to the direct skin-wristband contact. The interferences affected quantification of some PBDE, NFR, and PAH peaks. To address these issues, we made a few adjustments. First, we extracted the worn wristbands using sonication and overnight resting on the benchtop at ambient temperature rather than using accelerated solvent, which allowed us to extract at a lower temperature than that of the pre-cleaning process. Then, we used a multi-layer acidic silica column to remove lipids. However, since acid degrades most OPE compounds, we split the sample into two parts and treated one half without the acid to elute OPEs and PAHs. Due to interferences while analyzing PBDEs and NFRs, we could not achieve chromatographic separation for peaks with retention times between 7 and 9.5 minutes, affecting 13 compounds, including surrogate standard BDE-77 and internal standard BDE-118, as well as BDE-47, -49, -66, -71, -85, -99, -100, -119, -126, -154, EHTBB, and HBB. To address these interferences, we analyzed PBDEs and NFRs using a method with a slower oven temperature program. Additionally, since BEHTBP was lost on the acidic silica column, we analyzed this compound in Fraction 1.
3.2. Quality Assurance /Quality Control
The accuracy and precision of the method were determined using cleaned wristbands spiked with known amounts of the target analytes (matrix spike samples). Table 1 shows the median and mean (with standard errors) percent recoveries of target compounds in eight matrix spike samples.
Generally, validation results demonstrated good recoveries and precision for most of the matrix analytes (59 out of 64). Most PBDE and NFR mean recoveries ranged from 64 to 112% with the standard error (SE) < 16 %. The exceptions were BDE-15 and -126 with mean recoveries of 141 ± 17% and 139 ± 18%, suggesting that signals from these two compounds were affected by the matrix effect. Our matrix spike experiment for PBDEs and NFRs did not include the following compounds (BDE-10, -30, -139, -140, -180, -201, 203-205, -208, PBEB, HBB, TBE, and DBDPE). However, these compounds have similar physio-chemical properties to those included in our matrix spike experiment; hence, we expect similar recoveries also for these compounds. For most OPEs, the mean recoveries ranged from 76 to 107%, with SE < 20%. The exceptions were TEP, TIPRP, and TPRP with somewhat lower recoveries of 58-66%. Similarly, PAH mean recoveries ranged from 57 to 102%, with SE below 8%. Mean recoveries of all surrogate standards ranged from 71 ± 4% to 100 ± 3%, with the exception of the recovery for 13C12BDE-209 at 62 ± 9%.
3.3. Application to deployed wristband samples
The method described here was used to process and analyze wristbands worn by ten participants for seven days. Table 2 shows the levels, detection frequencies, median, minimum, and maximum concentrations of the compounds detected in more than half of the wristbands. The data for all the compounds measured in wristbands can be found in the Supporting Information (Table S3). Figure 2 shows concentrations of the most frequently detected and most abundant compounds in each wristband.
Table 2.
Detection frequencies (D.F., %), median (Med), minimum (Min), and maximum (Max) levels (ng) for the most abundant compounds measured in deployed wristbands (WB). The results shown for WB 8, 9, and 10 are the averages of the compound levels in duplicate pairs.
| Compounds | D.F. (%) |
Med | Min | Max | WB 1 | WB 2 | WB 3 | WB 4 | WB 5 | WB 6 | WB 7 | WB 8 | WB 9 | WB 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polybrominated diphenyl ethers (PBDEs) | ||||||||||||||
| BDE-17 | 100 | 0.19 | 0.09 | 1.94 | 0.09 | 0.61 | 0.31 | 0.10 | 0.11 | 0.76 | 0.13 | 1.94 | 0.25 | 0.13 |
| BDE-28 | 100 | 0.80 | 0.26 | 7.16 | 0.26 | 2.11 | 1.45 | 0.44 | 0.58 | 3.97 | 0.69 | 7.16 | 0.90 | 0.43 |
| BDE-47 | 100 | 27.3 | 6.11 | 200 | 6.11 | 73.8 | 46.7 | 11.5 | 14.5 | 132 | 16.1 | 200 | 38.5 | 13.1 |
| BDE-49 | 100 | 2.03 | 0.39 | 7.80 | 0.39 | 5.24 | 2.05 | 1.00 | 0.98 | 5.46 | 2.00 | 7.80 | 5.08 | 0.70 |
| BDE-66 | 100 | 0.71 | 0.12 | 4.88 | 0.12 | 2.03 | 1.00 | 0.28 | 0.33 | 3.15 | 0.42 | 4.88 | 1.02 | 0.24 |
| BDE-71 | 62 | 0.46 | <0.10 | 2.36 | <0.10 | 1.55 | 0.40 | 0.43 | <0.10 | 0.26 | <0.10 | 0.48 | 2.36 | <0.10 |
| BDE-85 | 100 | 0.77 | 0.14 | 2.78 | 0.14 | 2.06 | 1.08 | 0.30 | 0.34 | 1.58 | 0.47 | 2.78 | 1.07 | 0.25 |
| BDE-99 | 100 | 18.4 | 3.52 | 87.9 | 3.52 | 49.1 | 28.6 | 7.84 | 9.52 | 52.4 | 11.2 | 87.9 | 25.6 | 8.13 |
| BDE-100 | 100 | 3.47 | 0.68 | 20.7 | 0.68 | 9.41 | 5.75 | 1.43 | 1.78 | 12.6 | 2.01 | 20.7 | 4.94 | 1.52 |
| BDE-119 | 100 | 0.28 | 0.15 | 0.56 | 0.28 | 0.42 | 0.53 | 0.15 | 0.44 | 0.24 | 0.27 | 0.56 | 0.25 | 0.15 |
| BDE-126 | 77 | 0.13 | <0.07 | 0.39 | <0.07 | 0.23 | 0.10 | <0.07 | <0.07 | 0.25 | 0.07 | 0.39 | 0.13 | 0.08 |
| BDE-153 | 100 | 1.36 | 0.22 | 4.06 | 0.22 | 3.54 | 1.79 | 0.59 | 0.62 | 4.06 | 1.12 | 3.99 | 1.61 | 0.54 |
| BDE-154 | 92 | 1.03 | <0.24 | 2.93 | <0.24 | 2.24 | 1.12 | 0.36 | 0.39 | 1.91 | 0.64 | 2.93 | 1.03 | 0.34 |
| BDE-180 | 69 | 0.34 | <0.06 | 0.92 | <0.06 | 0.73 | 0.07 | <0.06 | 0.34 | 0.92 | 0.10 | 0.51 | 0.07 | <0.06 |
| BDE-183 | 62 | 0.28 | <0.20 | 7.91 | <0.20 | 0.28 | 0.26 | <0.20 | <0.20 | 7.91 | 0.18 | 1.41 | 0.27 | <0.20 |
| BDE-196 | 54 | 1.24 | <0.13 | 3.52 | <0.13 | <0.13 | 0.43 | <0.13 | <0.13 | 3.52 | <0.13 | 1.08 | 1.40 | <0.13 |
| BDE-197 | 100 | 0.22 | <0.09 | 5.14 | <0.09 | 0.33 | 0.22 | 0.10 | <0.09 | 5.14 | 0.11 | 0.69 | 0.23 | 0.12 |
| BDE-206 | 100 | 1.08 | <0.26 | 10.5 | <0.26 | 2.67 | 0.80 | 0.88 | 0.96 | 10.5 | 1.08 | 3.94 | 0.86 | 1.98 |
| BDE-207 | 92 | 0.82 | <0.39 | 3.50 | <0.39 | 1.02 | 0.54 | <0.39 | 0.39 | 3.50 | 0.59 | 2.47 | 0.62 | 1.14 |
| BDE-209 | 100 | 15.6 | 5.90 | 61.0 | 16.6 | 15.7 | 5.90 | 15.4 | 15.2 | 47.2 | 12.9 | 61.0 | 13.3 | 24.7 |
| ∑BDEs | 76.3 | 28.4 | 412 | 28.4 | 173 | 99.1 | 40.8 | 46.5 | 297 | 50.2 | 412 | 99.4 | 53.4 | |
| New Flame Retardants (NFRs) | ||||||||||||||
| PBBZ | 92 | 0.24 | <0.09 | 2.39 | <0.09 | 0.55 | 0.10 | 0.37 | 0.21 | 0.11 | 0.20 | 0.37 | 2.39 | 0.24 |
| PBEB | 85 | 0.19 | <0.06 | 0.36 | 0.08 | 0.28 | 0.04 | <0.06 | 0.27 | 0.20 | 0.13 | 0.19 | 0.36 | 0.04 |
| HBB | 100 | 0.19 | 0.07 | 1.50 | 0.07 | 1.50 | 0.11 | 0.09 | 0.34 | 0.16 | 0.11 | 0.37 | 0.21 | 1.37 |
| EHTBB | 100 | 89.1 | 15.4 | 358 | 15.4 | 358 | 103 | 87.8 | 32.0 | 42.5 | 116 | 90.5 | 263 | 16.3 |
| TBE | 85 | 0.33 | <0.07 | 5.70 | 0.13 | 2.94 | 0.10 | 0.07 | 0.24 | 4.03 | 0.33 | 5.70 | 0.45 | <0.07 |
| BEHTBP | 85 | 193 | <0.26 | 354 | 25.0 | 193 | 303 | 64.4 | 48.5 | 354 | 199 | 265 | 114 | <0.26 |
| Syn-DP | 69 | 0.81 | <0.32 | 2.17 | <0.32 | 0.94 | <0.32 | <0.32 | <0.32 | 0.68 | 1.03 | 2.17 | 0.47 | 0.60 |
| Anti-DP | 69 | 2.08 | <0.65 | 11.5 | <0.65 | 2.27 | <0.65 | <0.65 | <0.65 | 5.71 | 0.68 | 11.5 | <0.65 | 1.88 |
| DBDPE | 92 | 10.3 | <0.26 | 66.0 | <0.26 | 66.0 | 1.06 | 5.45 | 10.3 | 8.36 | 10.5 | 31.1 | 4.53 | 62.3 |
| ∑NFRs | 356 | 40.7 | 625 | 40.7 | 625 | 407 | 158 | 91.9 | 415 | 327 | 407 | 385 | 82.7 | |
| Organophosphate esters (OPEs) | ||||||||||||||
| TEP | 54 | 96.7 | <26.7 | 1720 | <26.7 | 90.8 | <26.7 | 96.7 | <26.7 | 113 | <26.7 | 1720 | 35.2 | <26.7 |
| TPRP | 62 | 156 | <10.9 | 273 | 156 | <10.9 | <10.9 | 59.8 | <10.9 | <10.9 | <10.9 | 170 | 273 | 31.6 |
| TNBP | 77 | 93.1 | <25.0 | 881 | 37.8 | 87.4 | <25.0 | 56.4 | 53.4 | 166 | 881 | 98.9 | 129 | <25.0 |
| TCEP | 100 | 60.9 | 27.2 | 348 | 27.2 | 60.2 | 118 | 28.2 | 54.9 | 114 | 54.4 | 211 | 61.6 | 348 |
| TCIPP | 100 | 288 | 109 | 1050 | 109 | 420 | 286 | 205 | 1050 | 199 | 289 | 191 | 440 | 433 |
| TDCIPP | 100 | 759 | 168 | 2060 | 340 | 1620 | 2060 | 766 | 184 | 752 | 168 | 2030 | 1480 | 257 |
| TPHP | 100 | 290 | 72.1 | 1360 | 157 | 961 | 192 | 300 | 72.1 | 442 | 161 | 1360 | 878 | 279 |
| TBOEP | 100 | 2090 | 878 | 4000 | 1500 | 1240 | 878 | 4000 | 1550 | 3350 | 2090 | 2090 | 2220 | 2270 |
| EHDP | 85 | 245 | <56.0 | 979 | 78.4 | 273 | 542 | <56.0 | <56.0 | 979 | 165 | 414 | 217 | 199 |
| TEHP | 77 | 353 | <6.25 | 710 | <6.25 | 510 | 167 | 516 | 115 | 412 | 710 | 295 | 268 | <6.25 |
| TMTP | 85 | 41.1 | <2.50 | 433 | 37.8 | 54.0 | 139 | 23.4 | 12.0 | 41.1 | 23.5 | 433 | 285 | <2.50 |
| TIPPP | 54 | 31.9 | <1.00 | 3400 | <1.00 | 3400 | 24.7 | <1.00 | 33.9 | 29.8 | 13.2 | <1.00 | <1.00 | 58.5 |
| TDMPP | 54 | 44.9 | <8.62 | 144 | <8.62 | <8.62 | <8.62 | <8.62 | <8.62 | 39.6 | <8.62 | 29.9 | 50.2 | 144 |
| TDBPP | 54 | 3025 | <100 | 3520 | <100 | <100 | 3190 | 2990 | 2060 | 3060 | 3520 | <100 | <100 | 1040 |
| ∑OPEs | 7840 | 2440 | 9580 | 2440 | 8620 | 7600 | 8960 | 5220 | 9580 | 8080 | 9040 | 6340 | 5060 | |
| Polycyclic aromatic hydrocarbons (PAHs) | ||||||||||||||
| Acenaphthylene | 100 | 4.46 | 2.59 | 7.94 | 2.59 | 7.94 | 4.39 | 4.37 | 4.97 | 3.46 | 3.42 | 6.39 | 4.53 | 5.88 |
| Acenaphthene | 62 | 10.7 | <5.21 | 30.2 | <5.21 | 13.3 | 8.31 | <5.21 | <5.21 | <5.21 | <5.21 | 30.2 | 13.1 | 6.29 |
| Fluorene | 100 | 24.3 | 10.8 | 102 | 15.3 | 36.1 | 18.1 | 31.1 | 18.2 | 16.5 | 102 | 37.7 | 30.4 | 10.8 |
| Phenanthrene | 100 | 89.3 | 21.4 | 336 | 30.3 | 148 | 81.0 | 71.7 | 27.4 | 153 | 336 | 235 | 97.7 | 21.4 |
| Anthracene | 69 | 64.2 | <0.57 | 106 | 7.10 | 85.5 | <0.57 | <0.57 | <0.57 | <0.57 | 93.3 | 106 | 37.5 | 43.0 |
| Fluoranthene | 100 | 30.0 | 9.22 | 198 | 11.6 | 106 | 17.8 | 18.4 | 9.22 | 57.9 | 198 | 195 | 41.6 | 17.2 |
| Pyrene | 100 | 29.9 | 8.46 | 274 | 19.6 | 79.0 | 14.5 | 19.9 | 11.8 | 159 | 149 | 274 | 40.0 | 8.46 |
| Benz[a]anthracene | 100 | 16.0 | 0.36 | 212 | 4.52 | 39.2 | 21.6 | 36.9 | 2.95 | 81.8 | 24.6 | 212 | 131 | 0.36 |
| Chrysene | 77 | 19.4 | <2.14 | 143 | <2.14 | 29.3 | 2.76 | 2.45 | 1.67 | 66.6 | 27.4 | 143 | <2.14 | 11.4 |
| ∑PAHs | 273 | 76.2 | 1240 | 95.3 | 544 | 168 | 185 | 76.2 | 538 | 934 | 1240 | 395 | 125 | |
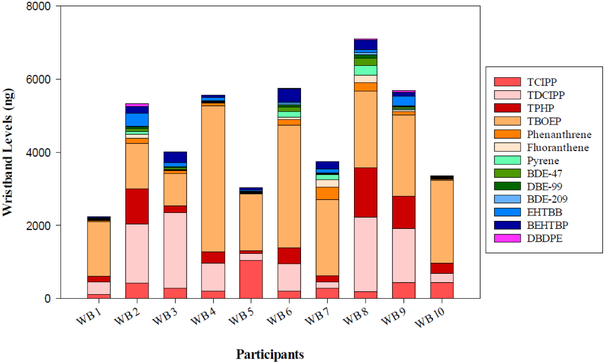
Figure 2.
Levels of the most abundant analytes (ng) measured in each of the deployed wristbands. Average levels analytes for duplicate wristband pairs are shown for WB 8, 9, and 10.
Average surrogate recoveries in the deployed wristband samples were 80 ± 6%, 85 ± 3%, and 44 ± 3%, for BDE-77, -166, and -209, respectively; 116 ± 5% and 121 ± 12% for d12-TCEP and 13C12-TPHP, respectively; and 91 ± 3% and 97 ± 3% for d10-phenanthrene and d10-pyrene, respectively. Seven procedural blanks were processed with the wristband samples. On average, the levels measured in blanks constituted less than 5% of the average sample levels for all analytes.
Two wristbands were worn by three participants and used as duplicates. The average levels of analytes in each of the duplicate pairs are included in Table 2, while the levels in individual duplicate wristbands are given in Table S3 in the Supporting Information. The relative percent differences (RPD) of the most abundant compounds in these duplicates are included in the Supporting Information (Table S4). For the majority of PBDEs, the RPDs were below 30% and for NFRs the RPD were all below 55%. For OPEs, the RPDs were within 20%, with the exceptions of TPRP (63% and 93% for two out of three duplicate pairs) and TPHP (56%) and TMTP (40%) for one duplicate pair. For the majority of PAHs, the RPD was within 20%. Overall, these results suggest good repeatability for the described method.
The levels of total PBDEs (∑PBDEs) and total NFRs (∑NFRs) in deployed wristbands ranged from 28.4 to 412 ng (median of 76.3 ng) and from 40.7 to 625 ng (median of 356 ng), respectively. The most abundant PBDEs, BDE-47, -99, and -209, were detected in 100% of the samples with levels ranging from 6.11 to 200 ng, 3.52 to 87.9 ng, and 5.90 to 61.0 ng, respectively. The most abundant NFRs were EHTBB, BEHTBP, and DBDPE and were detected in 85-100% of the samples with levels ranging from 15.4 to 358 ng, <0.26 to 354 ng, and <0.26 to 66.0 ng, respectively. Total OPE (∑OPE) levels in wristbands varied from 2,440 to 9,580 ng, with a median level of 7,840 ng. The most abundant OPEs, TPHP and TBOEP, were detected in all of the wristbands with the levels ranging from 72.1 to 1,360 ng (median of 290 ng) and 878 to 4,000 ng (median of 2,090 ng), respectively. Among the chlorinated OPEs, TCIPP and TDCIPP were the most abundant with the levels ranging from 109 to 1,050 ng (median of 288 ng) and 168 to 2,060 ng (median of 759 ng), respectively. PAHs were detected in 62-100% of the wristbands with total PAH (∑PAH) concentrations ranging from 76.2 to 1,240 ng (median of 273 ng). The most abundant PAHs included phenanthrene, fluoranthene, and pyrene with the levels ranging from 21.4 to 336 ng, 9.22 to 198 ng, and 8.46 to 274 ng, respectively.
In all wristbands, ∑OPE levels were 1-2 orders of magnitude higher than those of PBDEs and NFRs (see Figure 2). ∑PAHs generally were the second most abundant group of chemicals in half of the participants, and NFRs were the most abundant in the other half. PBDEs were the least abundant compounds in all wristbands, except for wristband #8 that had the highest levels of BDE-47, -99, and -209 compared to others. It is possible that these relatively high levels of PBDEs in this wristband are due to older foam furniture in participant’s home. Overall, the levels of the targeted chemical groups were comparable among the participants, with the exception of participant #1 who had the lowest levels for all four chemical groups.
Since silicone wristbands are relatively new passive samplers, there is only a limited number of studies published in the scientific literature, making a comparison with other studies difficult. In addition, some previously published studies report their results in concentrations (ng/h wristband). To allow for comparison, we converted the masses reported here into concentrations using an average wristband weight of 4.98 ± 0.02 g. Average BDE-47 and -99 levels measured in this study at 11.2 and 5.73 ng/g wristband, respectively, were lower than those previously reported for preschool children in Oregon at 213 and 154 ng/g wristband [4]. Our average EHTBB and BEHTBP concentrations of 22.7 and 35.1 ng/g wristband, respectively, were comparable with the previously reported EHTBB and BEHTBP concentrations among Oregon children (41.7 and 71.4 ng/g wristband, respectively) [4]. Further, the geometric mean of BDE-47, -99, and -209 measured in this study (6.21, 3.67, and 3.71 ng/g wristband, respectively), were lower than those measured in adults in North Carolina (55.9, 35.4, and 12.2 ng/g wristband, respectively) [13]. However, the geometric mean of BEHTBP in this study (27.3 ng/g wristband) was comparable to BEHTBP geometric mean (32.6 ng/g wristband) measured in adults in North Carolina [13].
The sum of TCEP, TCIPP, TDCIPP, and TPHP concentrations measured in this study (range of 128-766 ng/g wristband) was comparable with those reported in wristbands worn by preschool children (160-1,078 ng/g wristband) [4]. The geometric means of TDCIPP, TCIPP, and TPHP at 660, 300, and 329 ng/wristband, respectively, were also comparable to the geometric means in wristbands worn by adult participants from North Carolina at 1,251, 1,536, and 459 ng/wristband, respectively [12].
The PAH levels observed here (76.2 to 1,240 ng/wristband) were much lower than those reported in silicone wristbands worn by hot asphalt roofers (230 to 4,600 ng/wristband) [5], suggesting higher PAH exposure in occupational settings. Median phenanthrene and fluorene levels (89.3 and 24.3 ng/wristband, respectively) measured in this study were lower than those reported in wristbands worn by pregnant women in New York City (228 and 74 ng/wristband, respectively) [11], which could be related to higher traffic and combustion sources in New York City than in Bloomington, Indiana.
4. Conclusions
We developed an efficient method for the determination of a total of 77 analytes, including 35 PBDEs, 10 NFRs, 19 OPEs, and 13 PAHs, in silicone wristbands. This method offers a solution to chromatographic interferences associated with siloxanes from the wristband matrix, as well as lipids and other possible contributors from the skin that may come into contact with the wristband (e.g., lotions and fragrances). This method demonstrates that wristbands can be used to capture environmental exposure to a range of SVOCs. Wristbands worn by participants were sensitive and able to capture various levels of target analytes, ranging from less than 1 ng to close to 10,000 ng. These results were generally comparable to other studies, although the comparison is limited due to the paucity of published data. This is the first study that simultaneously extracted and quantitatively analyzed ~80 different SVOCs in wristbands. Further studies are needed to expand this method to other compounds, including pesticides, per- and poly-fluoroalkyl substances, and phthalates.
Supplementary Material
Highlights.
Silicone wristbands are a novel tool for assessing personal exposures
This is the first method for simultaneous quantitative analysis of 77 SVOCs
35 PBDEs, 9 NFRs, 19 OPEs, and 13 PAHs were simultaneously extracted and analyzed
Acknowledgements
This work was funded by National Institute of Environmental Health Sciences [NIEHS R15ES026394-01A1]. The authors would like to thank the study participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Peverly AA, Ma Y, Venier M, Rodenburg Z, Spak SN, Hornbuckle KC, Hites RA, Variations of flame retardant, polycyclic aromatic hydrocarbon, and pesticide concentrations in chicago's atmosphere measured using passive sampling. Environ Sci Technol. 49(2015), p.p. 5371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Venier M, Audy O, Vojta S, Becanova J, Romanak K, Melymuk L, Kratka M, Kukucka P, Okeme J, Saini A, Diamond ML, Klanova J, Brominated flame retardants in the indoor environment - comparative study of indoor contamination from three countries. Environ Int. 94(2016), p.p. 150–160. [DOI] [PubMed] [Google Scholar]
- [3].Stubbings WA, Schreder ED, Thomas MB, Romanak K, Venier M, Salamova A, Exposure to brominated and organophosphate ester flame retardants in U.S. childcare environments: Effect of removal of flame-retarded nap mats on indoor levels. Environ Pollut. 238(2018), p.p. 1056–1068. [DOI] [PubMed] [Google Scholar]
- [4].Kile ML, Scott RP, O'Connell SG, Lipscomb S, MacDonald M, McClelland M, Anderson KA, Using silicone wristbands to evaluate preschool children's exposure to flame retardants. Environ Res. 147(2016), p.p. 365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O'Connell SG, Kincl LD, Anderson KA, Silicone wristbands as personal passive samplers. Environ Sci Technol. 48(2014), p.p. 3327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Donald CE, Scott RP, Blaustein KL, Halbleib ML, Sarr M, Jepson PC, Anderson KA, Silicone wristbands detect individuals' pesticide exposures in west africa. R Soc Open Sci. 3(2016), p.p. 160433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bergmann AJ, North PE, Vasquez L, Bello H, DelCarmen Gastanaga Ruiz M, Anderson KA, Multi-class chemical exposure in rural Peru using silicone wristbands. J Expo Sci Environ Epidemiol. 27(2017), p.p. 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lipscomb ST, McClelland MM, MacDonald M, Cardenas A, Anderson KA, Kile ML, Cross-sectional study of social behaviors in preschool children and exposure to flame retardants. Environ Health. 16(2017), p.p. 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vidi PA, Anderson KA, Chen H, Anderson R, Salvador-Moreno N, Mora DC, Poutasse C, Laurienti PJ, Daniel SS, Arcury TA, Personal samplers of bioavailable pesticides integrated with a hair follicle assay of DNA damage to assess environmental exposures and their associated risks in children. Mutat Res. 822(2017), p.p. 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roodt AP, Naudé Y, Stoltz A, Rohwer E, Human skin volatiles: Passive sampling and GC x GC-TOFMS analysis as a tool to investigate the skin microbiome and interactions with anthropophilic mosquito disease vectors. J Chromat B. 1097-1098(2018), p.p. 83–93. [DOI] [PubMed] [Google Scholar]
- [11].Dixon HM, Scott RP, Holmes D, Calero L, Kind LD, Waters KM, Camann DE, Calafat AM, Herbstman JB, Anderson KA, Silicone wristbands compared with traditional polycyclic aromatic hydrocarbon exposure assessment methods. Anal Bioanal Chem. 410(2018), p.p. 3059–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hammel SC, Hoffman K, Webster TF, Anderson KA, Stapleton HM, Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ Sci Technol. 50(2016), p.p. 4483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hammel SC, Phillips AL, Hoffman K, Stapleton HM, Evaluating the use of silicone wristbands to measure personal exposure to brominated flame retardants. Environ Sci Technol, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Aerts R, Joly L, Sztemfeld P, Tsilikas K, De Cremer K, Castelain P, Aerts JM, Van Orshoven J, Somers B, Hendrickx M, Andjelkovic M, Van Nieuwenhuyse A, Silicone wristband passive samplers yield highly individualized pesticide residue exposure profiles. Environ Sci Technol. 52(2018), p.p. 298–307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.