Abstract
Background:
The transcatheter aortic valve replacement (TAVR) procedure was developed to provide patients with severe aortic stenosis an alternative to the surgical aortic valve replacement. Since the approval of the original SAPIEN the technology has rapidly evolved. While several approaches can be used for valve deployment, as delivery systems have become smaller and more flexible, the transfemoral approach has become the dominant technique for valve deployment.
Methods and Results:
145 patients undergoing TAVR receiving one of four valve types (Sapien, Sapien XT, Sapien3 or CoreValve) via the femoral artery were included in this study. Platelet count, white blood cells count (WBC), Interleukin-6 (IL-6), and Serum Amyloid A (SAA) were determined before and after TAVR. Platelet counts declined after the procedure regardless of the valve type and were dependent upon the baseline platelet count. Use of conscious sedation blunted the decline in platelet count. With the newer generation valves, the rise in WBC post-TAVR was lower than observed with the Sapien, in keeping with less systemic inflammation. Consistent with WBC, IL-6 levels were lower following deployment of the newer generation valves. Elevations in plasma SAA, which occur following myocardial injury, were not reduced with the newer valves.
Conclusions:
Evolution of the TAVR technology has occurred rapidly over the last five years. The newer devices and smaller delivery systems are associated with less systemic inflammation, as reflected in WBC and plasma IL-6 levels. However, the acute phase reactant SAA remains unchanged, possibly reflecting different triggers for SAA following TAVR.
Keywords: Platelet, transcatheter aortic valve replacement, inflammation
Introduction
The transcatheter aortic valve replacement (TAVR) procedure was developed over two decades ago to provide patients with severe aortic stenosis at high risk for surgery an alternative to the surgical aortic valve replacement (SAVR). The PARTNER clinical trial established the clinical efficacy of TAVR, either for inoperable or high risk patients, and resulted in FDA approval for the Edwards SAPIEN device, although the procedure came with higher rates of bleeding and stroke than conventional surgery. Shortly thereafter, the Medtronic CoreValve gained approval for use in the United States. The Sapien valve family has rapidly evolved since first approval in 2011. The Sapien XT, which featured changes in the stent material and design that allowed for use with a smaller delivery device, was approved in 2014. Both the Sapien and Sapien XT devices were associated with major vascular events; moreover, the development of significant post-procedural aortic insufficiency that occurred in approximately 5% of patients, was associated with worse long-term outcomes. The Sapien 3 features a longer frame length design to minimize aortic insufficiency and can be delivered by a 14-French catheter (or 16 French for the larges valve size). Several approaches can be used for TAVR deployment, including transfemoral, transapical, and transaortic. As the delivery systems have become smaller and more flexible, the transfemoral approach has become the dominant method for valve deployment. Concomitantly, a reduction in bleeding and stroke has been observed with the newest generation of valves. While initially approved for inoperable or high risk patients, approval has been extended to patients at intermediate risk of death from aortic valve surgery in 2016, and trials in low risk patients are underway.
Optimal management of patients after TAVR is incompletely understood. Transcatheter heart valve (THV) thrombosis can occur after the procedure. One study of 460 patients receiving either the Edwards Sapien XT or Sapien 3 valve indicated that 7% of patients experienced THV following TAVR. Clinical thrombosis is associated with serious clinical manifestations that included stroke, cardiogenic shock, and death (1). Based on data of the benefit of dual anti-platelet therapy (DAPT) after percutaneous coronary stent implantation, the TAVR trials were originally conducted with patients on DAPT, which is the current standard of care. However, retrospective analysis suggests that patients on oral anticoagulant therapy may experience less THV thrombosis (2). Several clinical studies are examining anti-platelet only versus anti-coagulation regimens. Intriguingly, the GALILEO trial of rivaroxaban versus antiplatelet therapy after TAVR was stopped early for harm with higher mortality, thromboembolic events and bleeding in patients randomized to rivaroxaban, suggesting a beneficial effect of anti-platelet therapy after TAVR. To better understand the impact of TAVR, we report the effect on platelet count and biomarkers of inflammation of different generations of valves.
Methods and Materials
Patients and Study Design
The Institutional Review Board at the University of Kentucky approved the study. One hundred a forty-five patients undergoing TAVR with either the Edward’s Sapien valve (n = 22), Sapien XT valve (n = 20), Medtronic CoreValve (n = 17) or the Sapien 3 valve (n = 86) were enrolled prospectively between October 2012 and December 2017. All patients gave written informed consent before the procedure. At the time of enrollment an option was given to the patient to provide baseline venous blood sampling for biomarker analysis. Blood samples on this subset of patients were also collected at 24 hours after the procedure. All patients received 600 mg clopidogrel at the time of the procedure irrespective of home medications. Platelet counts and white blood cell counts (WBC) were obtained from the electronic medical record.
Biomarker Analysis
Biomarker analysis was conducted on plasma obtained from a CTAD (citrate – adenosine-theophylline – dextrose) tube emented with EDTA to a final concentration of 10 μM. Plasma was separated by centrifugation for 10 minutes at 3000g and supernatant was immediately harvested and stored at −80°C. IL-6 was assayed using a multiplex system (Millipore) and read using a MAGPIX multiplex reader. SAA was measured using an Abnova human SAA ELISA kit (EL10015L).
Statistics
All study data were entered into a dedicated electronic database (REDCap). Two way repeated measures ANOVA was used to determine significant differences over time among the valve types for platelet count and white blood cell counts. Two way repeated measures ANOVA was used to determine significance between platelet count and sedation method. Pearson correlation and linear regression were used to determine significance and R2 for correlation of platelet count loss with baseline platelet count. Mann-Whitney analysis was used to determine significance between baseline and 24 hour post-op IL-6 for each valve type and One Way ANOVA on ranks was used to compare significant differences in IL-6 among the valve types. Paired t-test was used to determine significance between baseline and 24 hour post-op SAA for each valve type and One Way ANOVA on ranks was used to compare significant differences in SAA among the valve types. Statistical analysis was executed on SigmaPlot (version 13.0).
Results
Thrombo-inflammatory changes following TAVR
Following TAVR, a transient decrease in platelet count has been reported, and the extent of the decline reportedly correlates with baseline platelet count, general anesthesia, and left ventricular ejection fraction predicted severity of post-TAVR thrombocytopenia (3). Platelet count typically recovers approximately 72 hours after the procedure. We and others have demonstrated that persistent acquired thrombocytopenia after TAVR is associated with worse outcomes at 8 weeks (4) and 1 year mortality (5).
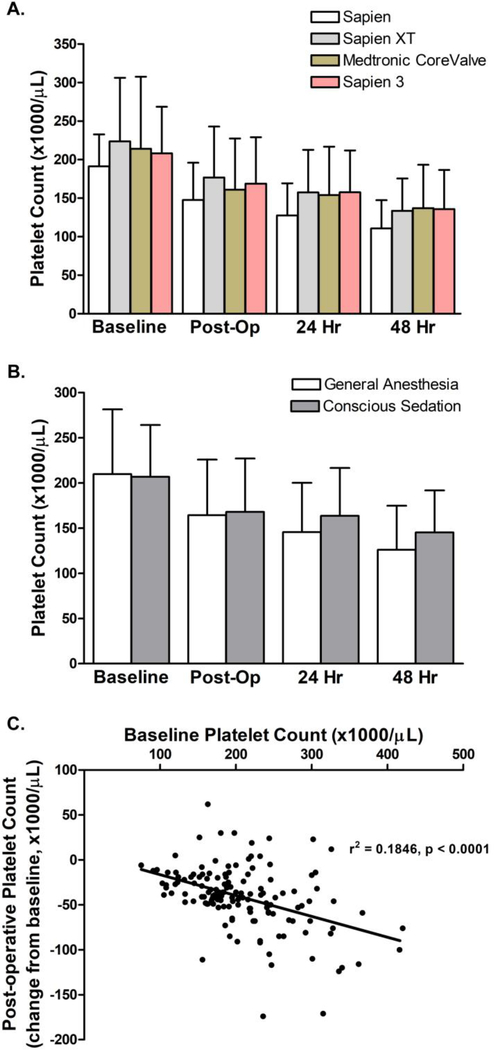
We sought to determine whether valve type influenced platelet count after transfemoral TAVR (Figure 1A) and observed a decline in platelet count within 48 hours in patients who received Sapien (4), Sapien XT, Medtronic CoreValve, and the Sapien 3 valve. As has been the case nationwide, our institutional practice has advanced to use of conscious sedation rather than general anesthesia for the procedures. Given the reports that general anesthesia predicted the extent of platelet decline, we examined whether there was a difference in platelet count in cohorts receiving conscious sedation versus general anesthesia. While platelet count declined in both cohorts, patients receiving general anesthesia had a significantly greater decline in platelet number over the first 48 hours (Figure 1B). As previously reported, baseline platelet count correlates with the extent of absolute decline in platelet number (Figure 1C). For all valve types, platelet counts declines by approximately 10% per day.
Figure 1. Platelet count declines following TAVR in every generation of valve and is mildly blunted when conscious sedation is used.
(A) Mean and standard deviation of platelet counts in cohorts undergoing transfemoral access TAVR (n = 22 for Sapien, n= 20 for Sapien XT, n = 17 for CoreValve, and n = 86 for Sapien 3). No significant difference was detected in platelet decline between any of the valve types (p = 0.622) as determined using a two way repeated measures ANOVA. (B) Mean and standard deviation of platelet counts in cohorts undergoing transfemoral access TAVR with general anesthesia (n = 90) or conscious sedation (n= 65). There was a significant difference in platelet decline between the sedation methods (p = 0.026) as determined using a two way repeated measures ANOVA. (C) Pearson correlation analysis was conducted between baseline platelet count and the change of platelet count observed post-operatively.
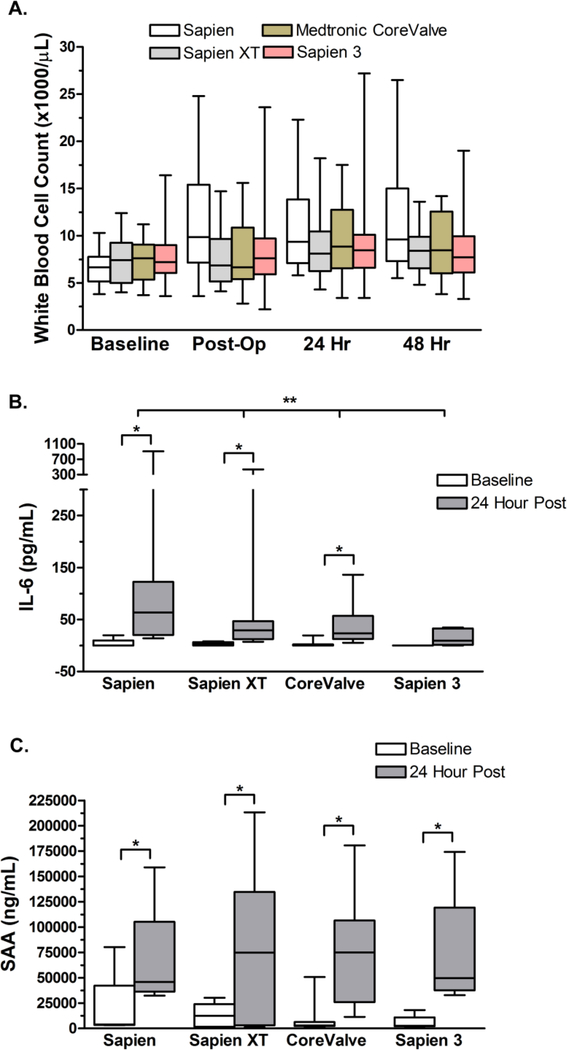
WBC and inflammatory biomarkers increase following the TAVR procedure (4, 6–9). We previously noted that the transapical approach elicited a larger systemic inflammatory response than the transfemoral approach. The post-procedural increase in WBC is lower in the newer generation of valves, especially the Sapien 3 (Figure 2A). The newer valves are also associated with attenuated increases in plasma levels of IL-6 after the procedure (Figure 2B). Interestingly, the acute phase reactant serum amyloid A (SAA) increased to similar levels after deployment of all of valve types (Figure 2C).
Figure 2. White blood cells count increases and inflammation are blunted with newer generation valves following the TAVR procedure.
(A) Inter quartile range (box), median (horizontal line) and extent of data set (whiskers) for WBC in the cohort of patients undergoing transfemoral TAVR none of whom underwent valve-in-valve procedures. A significant increase WBC count was observed after implantation of the Sapien valve (n = 22: P = 0.012) but not the newer Sapien XT (n = 20), Medtronic CoreValve (n = 17), or the Sapien 3 (n = 86) valve. Significance was determined using a two way repeated measures ANOVA. (B) Inter quartile range (box), median (horizontal line) and extent of data set (whiskers) for the indicated inflammatory biomarker in the cohort of patients undergoing transfemoral TAVR at baseline and 24 hours post-procedure in patients who received Sapien (n = 9), Sapien XT (n = 13), CoreValve (n = 13), and Sapien 3 (n = 6) valves. Significant differences between baseline and 24 hour post-op samples were determined by Mann-Whitney analysis and indicated by * where p < 0.05. No difference was observed in baseline inflammatory values among the cohorts. A significant difference was seen between 24 hour post-op samples between the valve types for IL-6 (p = 0.026) as calculated using One Way ANOVA on Ranks with significance considered as p < 0.05 and indicated by **. (C) Serum Amyloid A (SAA) was measured in baseline and 24 hour post-op samples in a subset of samples from patients receiving Sapien (n = 4), Sapien XT (n = 7), CoreValve (n = 8), and Sapien 3 (n = 6). Significant differences between baseline and 24 hour post-op samples were determined by paired t-test and indicated by * where p < 0.05. No differences were observed in baseline SAA levels between cohorts (p = 0.509) nor in 24 hour post-op samples (p = 0.985) using One Way ANOVA on Ranks.
Future Directions
The reasons for the decline in platelet count following TAVR remain unknown. It is possible that the platelets are removed from circulation by adherence to the prosthetic valve, damage, or cleared from circulation e.g. via attachment to inflamed or activated vascular endothelium perhaps elicited by the procedure. Platelets may be trapped in circulating microemboli or in the formation of platelet-leukocyte aggregates and thereby appear to be lower in number in circulation. Finally, it is possible that platelet biogenesis is disrupted by the procedure. The observation that the decline appears to be a fixed percentage of platelets per day may hint at an acute defect in production or a subset of platelets affected by the procedure. Whether these declines in platelet count contribute to THV thrombosis is not clear. Autopsy results of THV thrombus have revealed mild macrophage inflammatory cell infiltrate underneath the thrombus but not leaflet injury or lymphocyte infiltration suggestive of an autoimmune condition (10). These observations suggest that the interplay with inflammation may contribute to THV thrombosis. With use of the 4th generation of Sapien 3 valves, composed of a cobalt-chromium frame, bovine pericardial tissue leaflets, and a polyethylene terephthalate skirts to reduce paravalvular leak and designed for use with a small (14F) delivery system, the overall inflammatory response appears to be blunted in that both WBC and IL-6 levels are lower. The valve design or smaller delivery system or both could account for less systemic inflammation. Additionally, the newer generation valves have been associated with shorter procedure times, less contrast use, and lower rates of predilatation (11), which might also reduce inflammation. We have a single implanting team at our institution. To determine if operator experience contributed to our findings, we compared early and later implants for each valve type and observed no differences in biomarker patterns, suggesting that it was changes in the valve and/or deployment systems that accounted for the lower systemic inflammation. Despite less inflammation, the acute phase reactant SAA, which is particularly sensitive to myocardial injury, was elevated to a similar extent with all of the valve types, suggesting that it may be detecting a different mechanism of injury perhaps due to valve deployment itself. Additional work is needed to understand how these early signatures may influence later events that could be of clinical significance.
Key Points:
-
-
The TAVR procedure has evolved rapidly over the past decade with the development of new generation valves which have allowed less invasive approaches and the use of conscious sedation over general anesthesia.
-
-
Despite the improvements in valves, approach, and sedation the decline in platelet count following TAVR has persisted.
-
-
Increases in systemic inflammation following TAVR, as reported by plasma IL-6, has declined with the evolution of newer valve models.
-
-
Elevations in plasma SAA, which occur following myocardial injury, were not reduced with the newer valves.
-
-
Additional work is needed to understand how these early signatures may influence later events that could be of clinical significance.
Acknowledgments
Funding Sources: TRS was supported in part by the T32HL091812 from the Heart Lung and Blood Institute, National Institute of Health. The content is solely the responsibility of the authors and does no necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest: None
Disclosures: None
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Hafiz AKA, Ramadan R, Poulin M, Andalib A, Phillips C, Bhatt D, Reardon M, Kleiman N, Popma J Clinical or Symptomatic Leaflet Thrombosis Following Transcatheter Aortic Valve Replacement: Insights from the U.S. FDA MAUDE Database. Structural Heart. 2017;1(5–6):256–64. [Google Scholar]
- 2.Hansson NC, Grove EL, Andersen HR, Leipsic J, Mathiassen ON, Jensen JM, et al. Transcatheter Aortic Valve Thrombosis: Incidence, Predisposing Factors, and Clinical Implications. J Am Coll Cardiol. 2016;68(19):2059–69. [DOI] [PubMed] [Google Scholar]
- 3.Zhu Q, Liu X, He W, He Y, Tang M, Sun Y, et al. Predictors of Thrombocytopenia after Self-Expandable Transcatheter Aortic Valve Replacement: A Single-Center Experience from China. Cardiology. 2018;139(3):151–8. [DOI] [PubMed] [Google Scholar]
- 4.Sexton TR, Wallace EL, Chen A, Charnigo RJ, Reda HK, Ziada KM, et al. Thromboinflammatory response and predictors of outcomes in patients undergoing transcatheter aortic valve replacement. J Thromb Thrombolysis. 2016;41(3):384–93. [DOI] [PubMed] [Google Scholar]
- 5.Dvir D, Genereux P, Barbash IM, Kodali S, Ben-Dor I, Williams M, et al. Acquired thrombocytopenia after transcatheter aortic valve replacement: clinical correlates and association with outcomes. Eur Heart J. 2014;35(38):2663–71. [DOI] [PubMed] [Google Scholar]
- 6.Jansen F, Rohwer K, Vasa-Nicotera M, Mellert F, Grube E, Nickenig G, et al. CD-144 positive endothelial microparticles are increased in patients with systemic inflammatory response syndrome after TAVI. Int J Cardiol. 2016;204:172–4. [DOI] [PubMed] [Google Scholar]
- 7.Lindman BR, Goldstein JS, Nassif ME, Zajarias A, Novak E, Tibrewala A, et al. Systemic inflammatory response syndrome after transcatheter or surgical aortic valve replacement. Heart. 2015;101(7):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinning JM, Wollert KC, Sedaghat A, Widera C, Radermacher MC, Descoups C, et al. Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am Heart J. 2015;170(4):821–9. [DOI] [PubMed] [Google Scholar]
- 9.Uhle F, Castrup C, Necaev AM, Grieshaber P, Lichtenstern C, Weigand MA, et al. Inflammation and Its Consequences After Surgical Versus Transcatheter Aortic Valve Replacement. Artif Organs. 2018;42(2):E1–E12. [DOI] [PubMed] [Google Scholar]
- 10.De Marchena E, Mesa J, Pomenti S, Marin YKC, Marincic X, Yahagi K, et al. Thrombus formation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2015;8(5):728–39. [DOI] [PubMed] [Google Scholar]
- 11.Pilgrim T, Lee JKT, O’Sullivan CJ, Stortecky S, Ariotti S, Franzone A, et al. Early versus newer generation devices for transcatheter aortic valve implantation in routine clinical practice: a propensity score matched analysis. Open Heart. 2018;5(1):e000695. [DOI] [PMC free article] [PubMed] [Google Scholar]