Abstract
Background:
Recent studies have demonstrated that Th2 responses have the ability to antagonize Th17 responses. In mouse models of allergic asthma, blockade of Th2-effector cytokines results in elaboration of Th17 responses and associated increases in pulmonary neutrophilia. While these can be controlled by simultaneous blockade of Th17-associated effector cytokines, clinical trials of anti-IL-17/IL-17RA blocking therapies have demonstrated increased of risk of bacterial and fungal infections. Identification of minimally effective doses of cytokine-blocking therapies with the goal of reducing the potential emergence of infection-related complications is a translationally relevant goal.
Objective:
In the current report, we examine whether combined blockade of IL-13 and IL-17A, at individually sub-therapeutic levels, can limit the development of allergic asthma while sparing expression of IL-17A-associated anti-microbial effectors.
Methods:
House dust mite was given intratracheally to A/J mice. Anti-IL-13 and anti-IL-17A antibodies were administered individually, or concomitantly at sub-therapeutic doses. Airway hyper-reactivity, lung inflammation, magnitude of Th2- and Th17-associated cytokine production and expression of IL-13- and IL-17A-induced genes in the lungs was assessed.
Results:
Initial dosing studies identified sub-therapeutic levels of IL-13 and IL-17A blocking mAbs that have a limited effect on asthma parameters and do not impair responses to microbial products or infection. Subsequent studies demonstrated that combined sub-therapeutic dosing with IL-13 and IL-17A blocking mAbs resulted in significant improvement in airway hyperresponsiveness (AHR) and expression of IL-13-induced gene expression. Importantly, these doses neither exacerbated nor inhibited production of Th17-associated cytokines, or IL-17A-associated gene expression.
Conclusion:
This study suggests that combining blockade of individual Th2 and Th17 effector cytokines, even at individually sub-therapeutic levels, may be sufficient to limit disease development while preserving important anti-microbial pathways. Such a strategy may therefore have reduced potential for adverse events associated with blockade of these pathways.
Introduction
Mechanistic studies of allergic asthma have demonstrated that excessive production of Th2-associated cytokines contributes to the pathogenesis of allergic asthma1,2. Indeed, much of the pathology observed in allergic asthma can be ascribed to specific effects of the Th2 cytokines IL-4, IL-5 and IL-13. For example, IL-4 drives increased production of IgE that defines an atopic response2–4. In contrast, IL-5 drives the eosinophil-dominant inflammatory response that defines most allergic diseases by contributing to eosinophil development/maturation, enhancing eosinophil survival and inducing the production of eosinophil-recruiting chemokines5,6. Finally, IL-13 is considered a key effector molecule that can induce many of the pathological changes observed in the asthmatic airway, including smooth muscle hypertrophy, goblet cell hyperplasia, airway remodeling, and increased airway hyperresponsiveness7,8. Based on the expected primacy of these Th2 cytokines in the pathogenesis of allergic asthma, blockade of IL-4, IL-5, and IL-13 (or their receptors) is actively being investigated for their therapeutic efficacy in the treatment of allergic asthma9–17.
However, it is increasingly clear that the Th2 paradigm is insufficient to explain the full spectrum of asthma severity. For example, induction of Th1-associated immune responses, with or without concomitant Th2 responses, are associated with more severe airway pathology and increased resistance to common therapeutic approaches in mouse models of asthma and in humans18–20. Similarly, multiple lines of evidence suggest that induction of a mixed Th2/Th17 cytokine profile is associated with the development of more severe disease pathogenesis. Severe asthmatics display increased numbers of lung-infiltrating IL-17A+ or IL-13+/IL-17+ CD4 T cells21–23, increased serum or sputum Th17 cytokine levels23–26, and increased frequency of single nucleotide polymorphisms in the genes encoding IL17A and IL17F24,27–31. Moreover, Th17 responses may be associated with a “frequent exacerbator” phenotype that is associated with increased steroid usage, reduced quality of life, and more rapid decline in FEV1/FVC ratios compared to severe asthmatics with a lower frequency of exacerbations32. Thus, there is great interest in better understanding how interactions between distinct immune responses can contribute to the development of severe disease and influence the effectiveness of therapeutic regimens.
While the mechanisms through which IL-17A and IL-17A-secreting cells contribute to the development of asthma remain unclear, a number of possibilities have been proposed, including induction of negative regulators of glucocorticoid signaling33,34, a direct pathogenic role for neutrophils in the development of allergic disease35, or molecular synergy between IL-13- and IL-17A-induced intracellular signaling pathways36. Regardless, the combined actions of multiple immune pathways likely define unique asthma phenotypes that may respond differently to therapeutic interventions. For example, while blockade of IL-13 in mouse models has been ascribed a powerful inhibitory role on the development of allergic asthma37–39, recent evidence suggests an antagonistic relationship between Th2 cytokines and Th17 cells40–43. As such, inhibition of IL-13 in mixed Th2/Th17 mouse models of asthma drove increased recruitment of CD4+IL-17A+ T cells and neutrophils to the lung and increased expression of IL-17A-driven gene expression40. Combined blockade of both IL-13 and IL-17A abrogated the amplified Th17 response and protected the lung from increased neutrophil accumulation and increased expression of IL-17A-associated genes40. Given the importance of IL-17A in clearance of bacterial and fungal infections44, and the role of Th2 responses in controlling parasitic infections45, chronic blockade of these pathways may result in increased susceptibility to these sorts of infections. Indeed, bacterial and fungal infections are a commonly reported adverse event in clinical trials testing efficacy of IL-17A/IL-17RA-blocking therapies46–48. Thus, the goal of the current study was to determine if combining blockade of IL-13 and IL-17A, at individually sub-therapeutic levels, is sufficient to limit asthma severity with reduced potential for adverse infectious events associated with blockade of these pathways in a unique mouse model of mixed Th2/Th17-associated severe allergic asthma.
Materials and Methods
Mice:
All animals were randomly assigned to treatment groups upon arrival in our animal facility. Male A/J mice were purchased from Jackson Laboratories, and housed in an SPF facility at Cincinnati Children’s Hospital Medical Center. Animals were housed with a 12 hours light/dark cycle and free access to food and water. All experiments were approved by the CCHMC IACUC.
Assessment of asthmatic phenotype.
Mice were treated with 40 μl PBS, or 200 μg house dust mite extract (HDM) (Greer Laboratories, Lenoir, NC) i.t. on days 0, 14 and 21. To block the effect of endogenous cytokines throughout the experimental protocol, animals were treated with the indicated concentrations of monocloncal antibodies (mAbs) to IL-13 (CNTO 8666; rat IgG2a), IL-17A (CNTO 8096; rat IgG2a) or an isotype control rat IgG2a (CNTO 6601) on days −2, 2, 12, 16, 19 and 23. Bronchoalveolar lavage fluid (BALF) was collected in HBSS and cells were placed on slides by cytocentrifugation, and stained with Diff-Quik (Dade Behring). Differential cell counts were determined using morphologic criteria under a light microscope by evaluation of ≥ 500 cells/slide. Whole lung cells were restimulated with HDM for 72 hours and cytokine production was assessed by ELISA using mAbs (eBioscience). For IL-4, IL-13 and IL-10, O.D.s were compared to a standard curve to obtain absolute concentrations. For IL-17A, O.D. values, minus the plate blank were generated for each sample, O.D. values from all replicates were averaged, and this value was displayed. To measure AHR, mice were anesthetized with sodium pentobarbital and xylazine (100 and 20 mg/mL respectively). Invasive measurements of airway responsiveness were made with the Flexivent apparatus (SCIREQ, Montreal, Quebec, Canada). Mouse tracheas were cannulated with a 20-gauge blunt needle, and the mice were ventilated at 150 breaths/min and 3.0 cm H2O positive end-expiratory pressure. Two total lung capacity perturbations were then performed for airway recruitment before baseline measurement and subsequent methacholine challenges were performed. Dynamic resistance was assessed after exposure to increasing concentrations of aerosolized methacholine (0, 12.5, 25, and 50 mg/mL). The highest dynamic resistance value with a coefficient of determination of 0.9 or greater (as determined by using Flexivent software) was used to determine the dose-response curve. Individuals collecting data were blinded as to the experimental group of the animals in question. The asthma phenotype of animals from different experimental groups was assessed in a random order.
Assessment of anti-microbial responses.
To determine whether low dose anti-IL-17A inhibits anti-microbial responses, mice were given anti-IL-17A (75 or 200 μg), or control IgG2a (200 μg) i.p. and 18 hours later challenged LPS (1 μg) i.t. Inflammatory cells in BALF were determined as above. Alternatively, animals were treated with anti-IL-17A (75 or 200 μg) or control IgG2a (200 μg) one hour prior to, and 24 hours after intratracheal infection with 1 × 106 – 1 × 108 S. pneumoniae. 48 hours after infection, animals were sacrificed. Upon sacrifice lungs were harvested to assess pulmonary gene expression by RT-PCR.
Quantitative real-time PCR:
PCR primer pairs for S14, Alox15, Arg1, Tff2, Cebpb, Cebpd, Bd2, Cxcl1, Cxcl2, Cxcl3 and Csf3 were designed to span an intronic region to avoid co-amplification of genomic DNA. Primer sequences are indicated in Table 1. Gene expression was analyzed by real-time PCR using the LightCycler system (Roche) and SYBR green.
TABLE 1.
List of mouse primers
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| S14 | GAGGAGTCTGGAGACGACGA | TGGCAGACACCAAACACATT |
| Alox15 | CGGTCTACTTGTCTCCCTGC | CTTGATCCCATCCAGAAGGA |
| Arg1 | CATGAGCTCCAAGCCAAAGT | TTTTTCCAGCAGACCAGCTT |
| Tff2 | AGGACTGTGCCAGTCGAAAC | CTCGGCAGTAGCAACTCTCA |
| Cebpb | GTTTCGGGACTTGATGCAAT | CCCCGCAGGAACATCTTTA |
| Cebpd | TAAGGAGATGGACGCGTTTC | GTTAGGCCAACTGTTCTCCG |
| Bd2 | AAGTATTGGATACGAAGCAG | TGGCAGAAGGAGGACAAATG |
| Cxcl1 | ACCCAAACCGAAGTCATAGC | TCTCCGTTACTTGGGGACAC |
| Cxcl2 | CCAACCACCAGGCTACAGG | GCGTCACACTCAAGCTCTG |
| Cxcl3 | CAGCCACACTCCAGCCTA | CACAACAGCCCCTGTAGC |
| Csf3 | ATGGCTCAACTTTCTGCCCAG | CTGACAGTGACCAGGGGAAC |
Statistical Analysis:
To determine differences between multiple groups, analysis of variance (ANOVA) was used with post hoc comparisons using Tukey’s method. Significance was assumed at p < 0.05.
Results:
Blockade of IL-13 completely abrogates HDM-induced AHR with the potential for exacerbation of Th17-associated responses
While Th2 responses are central to the pathogenesis of allergic asthma, it is increasingly clear that more severe forms of asthma are associated with concomitant development of Th2 responses and other types of immune responses (e.g. Th1, Th17). Recent work examining augmentation of potentially pathogenic Th17 responses in the face of anti-Th2 therapy40 has demonstrated the utility of combined approaches to simultaneously block Th2 and Th17 responses. However, blockade of effector cytokines is not without risk itself – particularly a risk of increased infection. As such, we wished to determine whether simultaneous blockade of Th2 and Th17 associated cytokines, at doses that fail to individually demonstrate any therapeutic (or pathological) effects, would be a useful strategy for treatment of allergic asthma. To determine if combined application of sub-therapeutic doses of IL-13 and IL-17A blocking antibodies could ameliorate the pathogenesis of Th2/Th17 mediated-asthma we made use of HDM-exposed A/J mice. Application of the common human aeroallergen house dust mite (HDM) to the lungs on days 0, 14 and 21 in A/J mice drives a mixed Th2/Th17 response and results in severe lung pathology and airway dysfunction that was recently found to demonstrate increased steroid refractory characteristics compared to other common laboratory strains49–52.
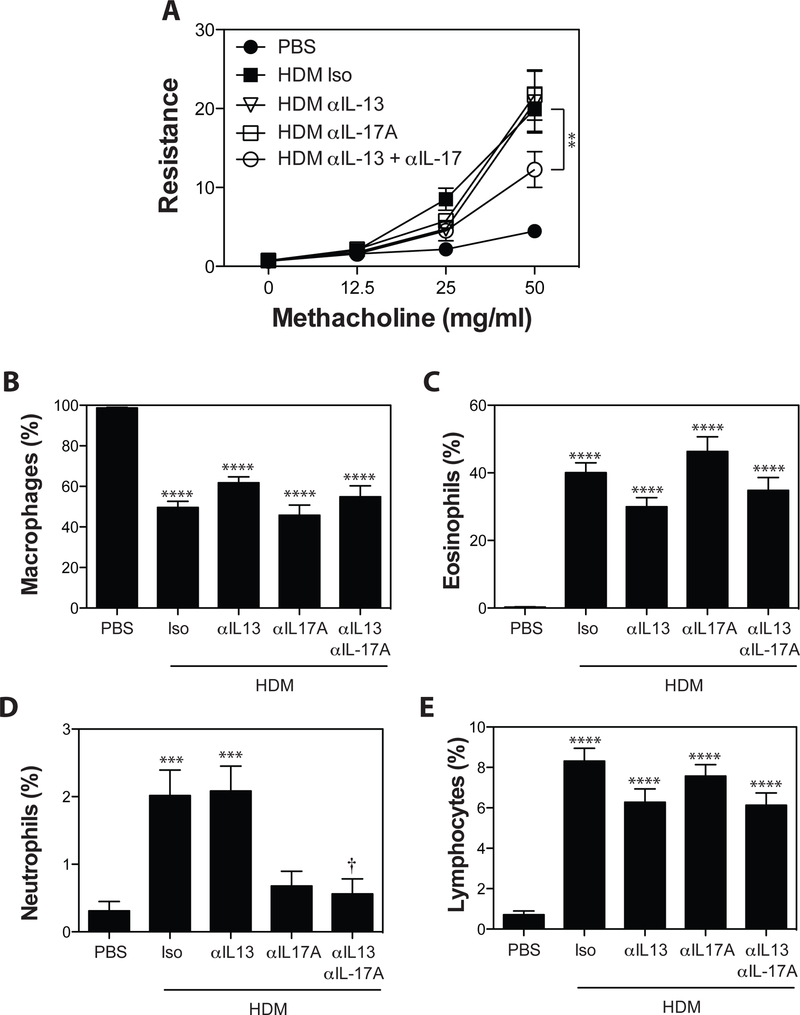
To identify sub-therapeutic doses of anti-IL-13, mAbs were administered at doses of 200 μg, 75 μg, 25 μg, 8 μg, 2 μg and 0.5 μg/injection 48 hours prior to, and 48 hours following each HDM exposure. As expected, HDM exposure in A/J mice induced robust AHR associated with marked eosinophil accumulation in the BAL fluid, and a smaller, but significant accumulation of neutrophils and lymphocytes (Fig 1). Administration of anti-IL-13 at high doses (200 μg/injection) completely abrogated HDM-induced AHR (Fig 1A). Similar effects were observed at 75 μg and 25 μg of anti-IL-13 (data not shown). Lower concentrations of anti-IL-13 (8 μg/injection) only partially reduced AHR (Fig 1A), while doses of 2 and 0.5 μg did not influence AHR. In examining the inflammatory response in the BAL (Fig 1B-E), we found that IL-13 blockade, at any concentration, did not significantly impact macrophage (Fig 1B) or lymphocyte (Fig 1E) recruitment, and eosinophil recruitment was significant reduced only at the 200 μg dose (Fig 1C). While there was a trend towards increased neutrophil recruitment following blockade of IL-13, this did not reach statistical significance (Fig 1D). Thus, IL-13-blockade can abrogate key features of the asthma phenotype in our model, but lower doses have limited effect.
Figure 1. Blockade of IL-13 abrogates HDM-induced AHR and reduces HDM-induced eosinophil accumulation.
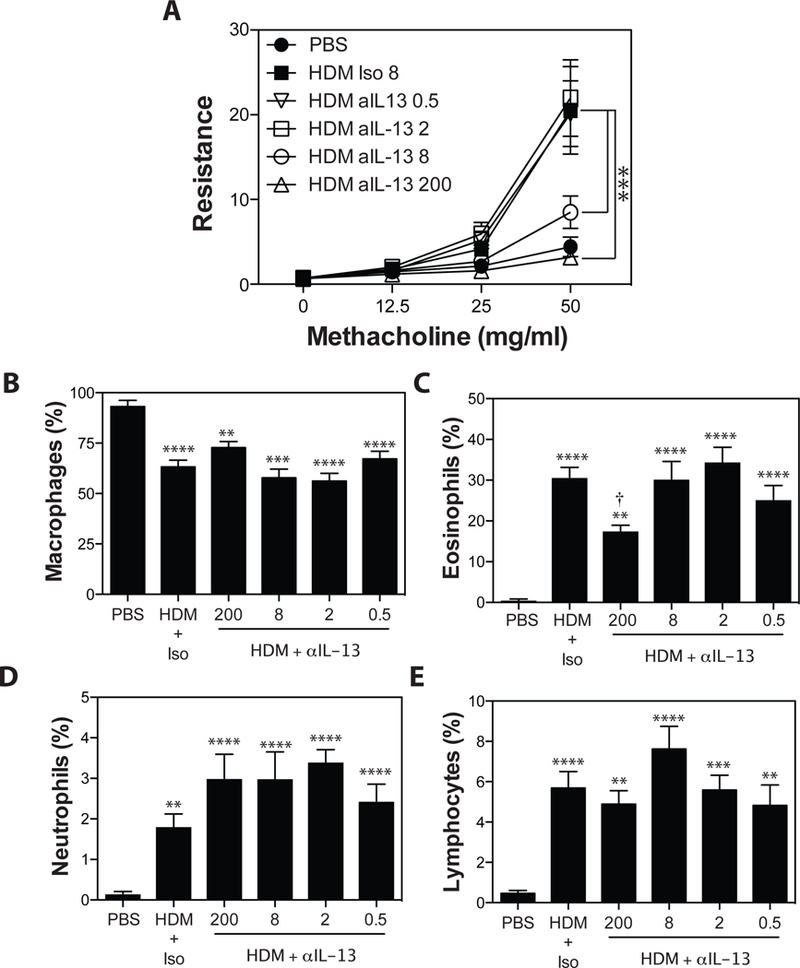
Asthma was induced as in Materials and Methods. Anti-IL-13 (0.5, 2, 8, or 200 μg/injection), or Rat IgG2a (200μg/injection) was administered i.p. 48 hours before, and 48 hours after each HDM exposure. (A) methacholine-induced AHR was measured by Flexivent. BALF was collected, and the number of macrophages (B), eosinophils (C), neutrophils (D), and lymphocytes (E) were assessed. Data are mean ± SEM of n = 6–12 mice from 2 independent experiments. **p < 0.01, ***p < 0.001, and **** p < 0.0001 versus PBS. † < 0.05 versus isotype.
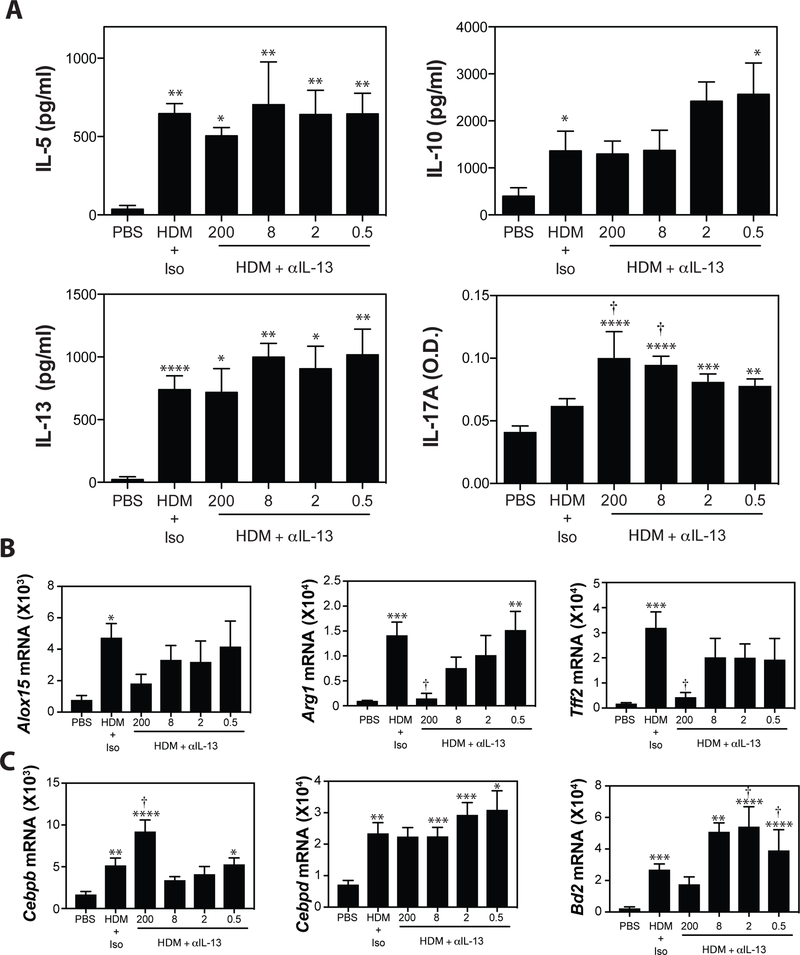
To further assess the effects of IL-13 blockade on the magnitude of the T cell immune response, total lung cells were restimulated with HDM in vitro for 72 hours and levels of Th1- (IFNγ), Th2- (IL-5, IL-10, IL-13) and Th17- (IL-17A) associated cytokines were assessed by ELISA. As expected, HDM restimulation of lung cell cultures from in vivo HDM-exposed animals induced readily detectable levels of IL-5, IL-10, IL-13 and IL-17A, but not IFNγ. Anti-IL-13, at any dose tested, did not significantly impact the production of Th1- (data not shown) or Th-2 associated cytokines (Fig 2A). Consistent with previous reports of an inhibitory effect of IL-13 on Th17 cells41–43 high doses (200 μg, 8 μg) of anti-IL-13 blocking significantly elevated production of IL-17A in HDM-restimulated lung cells cultures (Fig 2A). Doses lower than 8 μg had no effect on IL-17A cytokine production.
Figure 2. IL-13 blockade reduces IL-13-induced gene expression, while increasing Th17 responses.
(A) Lung cells were restimulated in vitro with HDM and IL-5, IL-10, IL-13, and IL-17A production was evaluated by ELISA. (B) Pulmonary expression of IL-13-driven genes (Alox15, Arg1, Tff2) and (C) IL-17A-induced genes (Cebpb, Cebpd, Bd2) was assessed by RT-PCR analysis. Data are mean ± SEM of n = 6–12 mice from 2 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, and **** p < 0.0001 versus PBS. † < 0.05 versus isotype.
To directly assess the magnitude of IL-13 driven gene expression in the HDM-exposed lung, we assessed the production of a panel of IL-13-induced genes (Alox15, Arg1, Tff2) (Fig 2B) and IL-17A-induced genes (Cebpb, Cebpd, Bd2) (Fig 2C) in the lung by RT-PCR. As expected, blockade of IL-13 with 200μg of anti-IL-13 had a profound effect on IL-13-induced gene expression, reducing expression of all genes examined close to baseline. Similar effects were observed with blockade at 75 μg and 25 μg (data not shown). While lower concentrations of anti-IL-13 blocking mAbs tended to decrease expression of these genes in a dose-dependent manner, this did not reach statistical significance (Fig 2B). Consistent with our observation of increased production of IL-17A in cultures of HDM-stimulated lung cell cultures, we also observed increased expression of IL-17A-assocated genes following administration of anti-IL-13 (Fig 2C). The observation that 8 μg/injection of anti-IL-13 partially abrogated AHR, while 2 μg/injection had no impact on allergen-induced AHR suggested that the maximal dose of anti-IL-13 that did not alter AHR was somewhere between these values. As such we chose 4 μg/injection of anti-IL-13 for subsequent studies.
Blockade of IL-17A reduces HDM-induced AHR.
To identify sub-therapeutic doses of anti-IL-17A, mAbs were administered at doses of 200 μg, 75 μg and 25 μg/injection 48 hours prior to, and 48 hours following each HDM exposure. Consistent with our previous studies demonstrating a pathogenic role for IL-17A in HDM-exposed A/J mice49, the highest dose (200 μg/injection) of anti-IL-17A resulted in a significant, but incomplete, reduction in allergen-induced AHR (Fig 3A). Lower doses of anti-IL-17A (75 μg, 25 μg/injection) had no effect on AHR. While anti-IL-17A (at any dose), had no significant impact on HDM-induced recruitment of macrophages, eosinophils or lymphocytes, the highest dose of anti-IL-17A almost completely abrogated neutrophil accumulation in the BALF (Fig 3B-E).
Figure 3. Blockade of IL-17A reduces HDM-induced AHR and abrogates pulmonary neutrophil recruitment.
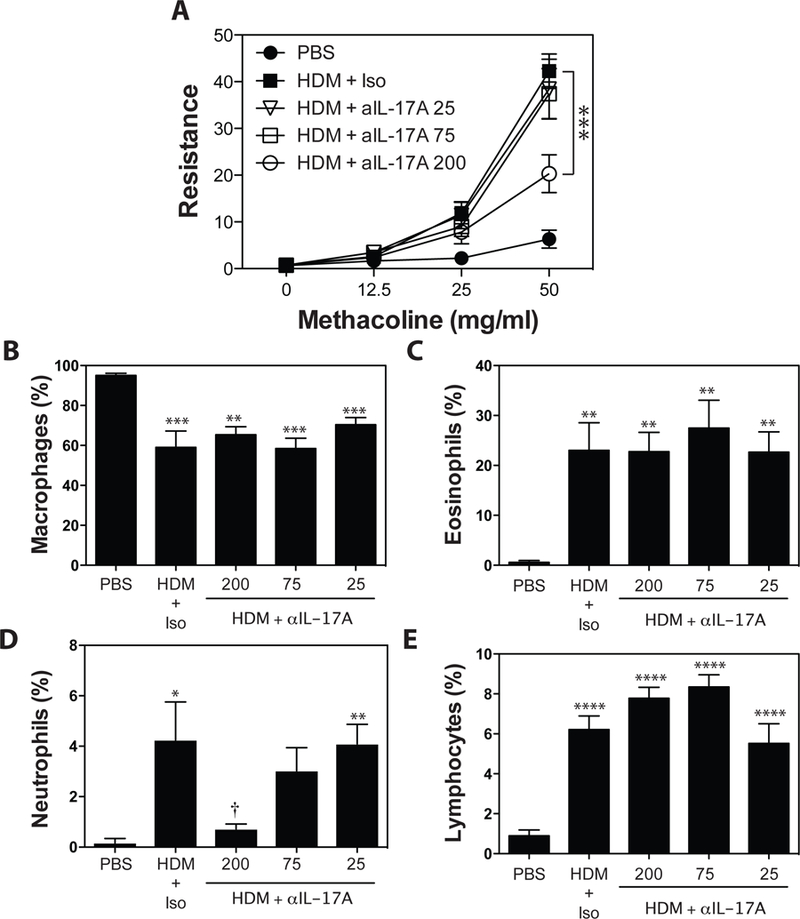
Asthma was induced as in Materials and Methods.. Anti-IL-17A (25, 75, or 200 μg/injection), or Rat IgG2a (200μg/injection) was administered i.p. 48 hours before, and 48 hours after each HDM exposure. (A) methacholine-induced AHR was measured by Flexivent. BALF was collected, and the number of macrophages (B), eosinophils (C), neutrophils (D), and lymphocytes (E) were assessed. Data are mean ± SEM of n = 7 – 9 mice in 2 independent experiments. **p < 0.01, ***p < 0.001, and **** p < 0.0001 versus PBS. † < 0.05 versus isotype.
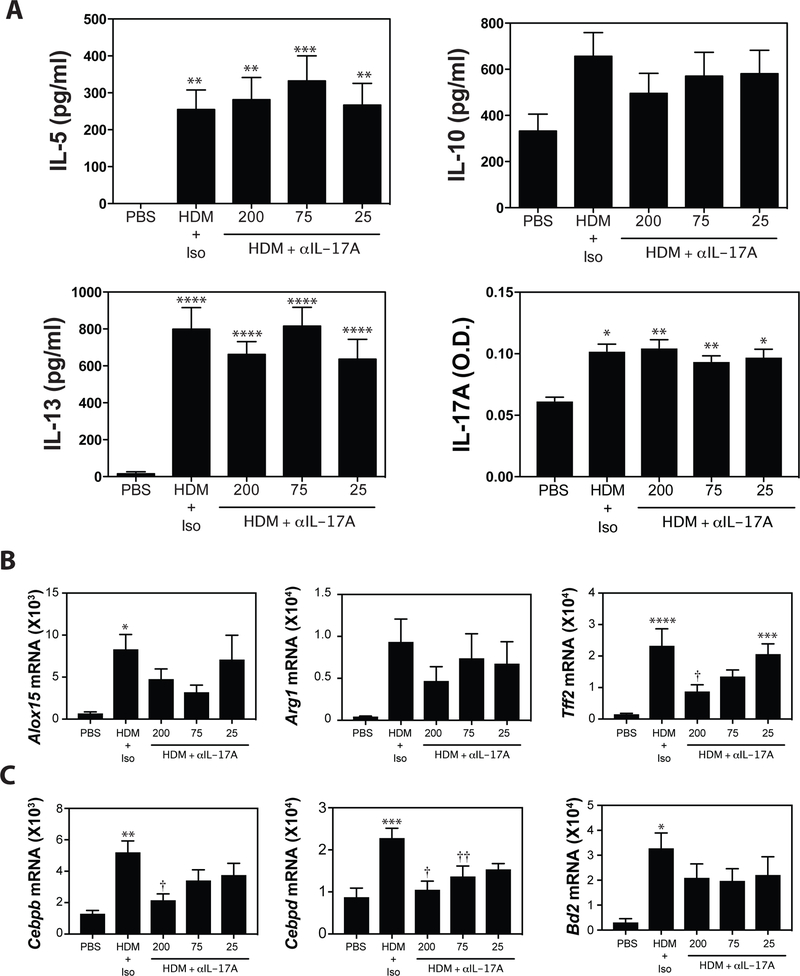
Assessment of cytokine production in HDM-restimulated lung cell cultures revealed that in vivo blockade of IL-17A had no significant impact on T cell cytokine production (Fig 4A). To assess the impact of in vivo IL-17A blockade on pulmonary responses to IL-13 and IL-17A, we assessed the expression of IL-13 and IL-17A-induced genes in whole lung tissue by RT-PCR. Consistent with a role for IL-17A in augmenting IL-13 responses2,36,49, anti-IL-17A reduced expression of Alox15, Arg1, and significantly decreased Tff2 mRNA levels (Fig 4B) at the highest dose. Not surprisingly, blockade of IL-17A at the highest dose (200 μg/injection) resulted in significantly reduced expression of IL-17A-associated genes (Fig 4C). Lower doses of anti-IL-17A did not have a significant effect on expression of IL-17A-induced genes. As 75μg of anti-IL-17A failed to alter AHR, infiltration of inflammatory cells in the BAL, T cell derived cytokine production, or the production of IL-13- or IL-17A-associated gene expression, this dose of anti-IL-17A was chosen for subsequent experiments.
Figure 4. Blockade of IL-17A reduces expression of both IL-13 and IL-17-induced genes.
(A) Lung cells were restimulated in vitro with HDM and IL-5, IL-10, IL-13, and IL-17A production was evaluated by ELISA. (B) Pulmonary expression of IL-13-driven genes (Alox15, Arg1, Tff2) and (C) IL-17A-induced genes (Cebpb, Cebpd, Bd2) was assessed by RT-PCR analysis. Data are mean ± SEM of n = 7 – 9 mice in 2 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, and **** p < 0.0001 versus PBS. † < 0.05 and †† < 0.01 versus isotype.
Low dose IL-17A blockade does not impact anti-bacterial responses.
To determine whether low dose anti-IL-17A inhibits anti-microbial responses, mice were given anti-IL-17A (75 or 200 μg), or control IgG2a (200 μg) i.p. and 18 hours later challenged LPS (1 μg) i.t. Animals were sacrificed at 4, 24 and 48 hours to assess airway inflammatory infiltrates and IL-17A-associated gene expression. LPS exposure induced a rapid pulmonary inflammation consisting of mostly neutrophils, which peaked at 24 hours and started to diminish by 48 hours (Fig 5A-B). High-dose anti-IL-17A significantly reduced total inflammatory cells and neutrophils at 24 and 48 hours after LPS challenge (Fig 5A - B), while 75 μg of anti-IL-17A had no significant impact. 200, μg of anti-IL-17A significantly reduced expression of Cebpd (compared to anti-IL-17A (75 μg) animals) and Csf3 (compared to control animals) (Fig 5C).
Figure 5. Administration of low dose anti-IL-17A does not alter LPS-induced neutrophil recruitment, or pro-inflammatory gene expression.
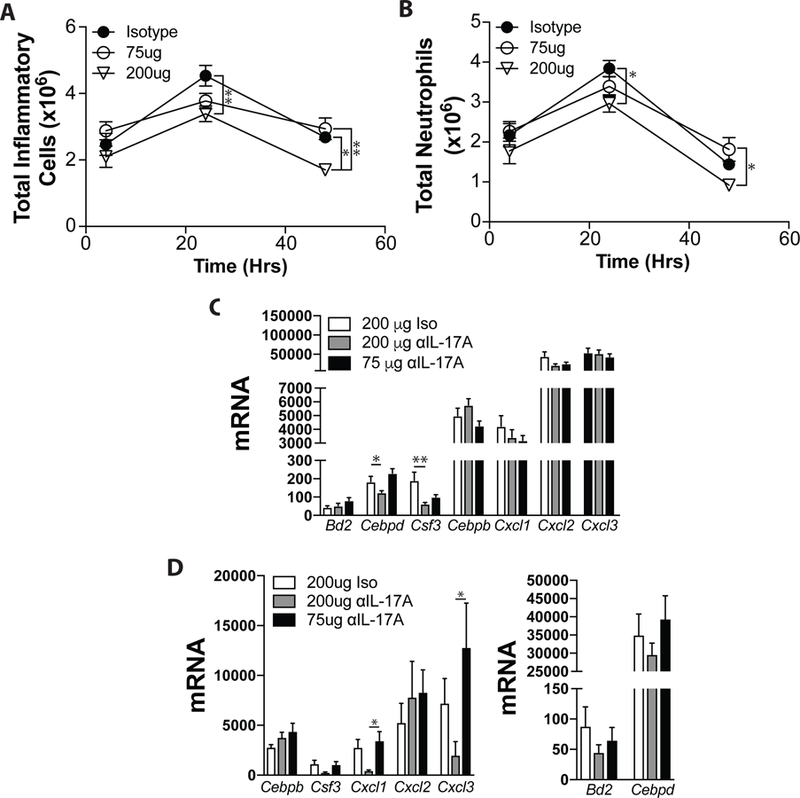
(A-C) Responses to LPS were assessed as in Materials and Methods. The absolute number of inflammatory cells (A), and neutrophils (B) were assessed 4, 24 and 48 hours later. (C) Expression of IL-17A-induced gene expression was assessed at 24 hrs by RT-PCR. Data are shown as mean + SEM of n = 10 – 12 mice. (D-E) Responses to infection with S. pneumoniae were assessed as in Materials and Methods. Animals were sacrificed 48 hours after infection to assess expression of IL-17A-induced genes. Data are shown as mean ± SEM of n = 10 – 12 mice from 3 independent experiments. *p < 0.05 and **p < 0.01 versus isotype.
As an alternative approach, animals were treated with anti-IL-17A (75 or 200 μg) or control IgG2a (200 μg) one hour prior to, and 24 hours after intratracheal infection with 1 × 106 – 1 × 108 S. pneumoniae. 48 hours after infection, animals were sacrificed. 200 μg of anti-IL-17A was associated with significantly decreased pulmonary expression of the anti-microbial genes Cxcl1 and Cxcl3, and strong trends towards decreased expression of Bd2, Csf3, Cebpd, and Cxcl3 (Fig 5E). No differences in mortality were observed (not shown). Collectively, these data suggest that the lower doses of anti-IL-17A selected for further study are not associated with diminished anti-bacterial responses.
Administration of sub-therapeutic levels of anti-IL-13 and IL-17A significantly improve lung function in HDM-treated A/J mice.
To determine if simultaneous administration of sub-therapeutic doses of anti-IL-13 and anti-IL-17A had any impact on the asthma phenotype, HDM-sensitized A/J mice were treated with 4 μg of anti-IL-13, 75 μg of anti-IL-17A or both antibodies in combination. As expected, administration of anti-IL-17A at 75 μg/injection had no impact on AHR (Fig 6A). Importantly, 4 μg/injection of ant-iL-13 did not alter AHR (Fig 6A), validating the choice of this concentration for these studies. In contrast, compared to animals treated with isotype control mAb, co-administration of anti-IL-13 and IL-17A reduced HDM-induced AHR by ~40% (Fig 6A). Examination of BAL cellularity (Fig 6B-D) demonstrated that combined IL-13/IL-17A blockade had limited effect on BAL cellularity (Fig 6B-E). Eosinophils and lymphocytes tended to be reduced in all anti-IL-13 treated groups, but this did not reach statistical significance (Fig 6C, 6E). Similarly BAL neutrophil levels were reduced in the BAL of animals receiving anti-IL-17A, although this only reached statistical significance in anti-IL-13 + anti-IL-17A treated animals.
Figure 6. Administration of sub-therapeutic levels of anti-IL-13 and IL-17A significantly improve lung function and limit neutrophil recruitment in HDM-treated A/J mice.
Asthma was induced as in Materials and Methods. Anti-IL-17A (75 μg/injection), anti-IL-13 (4 μg/injection), anti-IL-17A + anti IL-13, or Rat IgG2a (79 μg/injection) was administered i.p. 48 hours before, and 48 hours after each HDM exposure. (A) methacholine-induced AHR was measured by Flexivent. BALF was collected, and the number of macrophages (B), eosinophils (C), neutrophils (D), and lymphocytes (E) were assessed. Data are mean ± SEM of n = 10 – 12 mice from 2 independent experiments. ***p < 0.001 and ****p < 0.0001 versus PBS. † < 0.05 versus isotype.
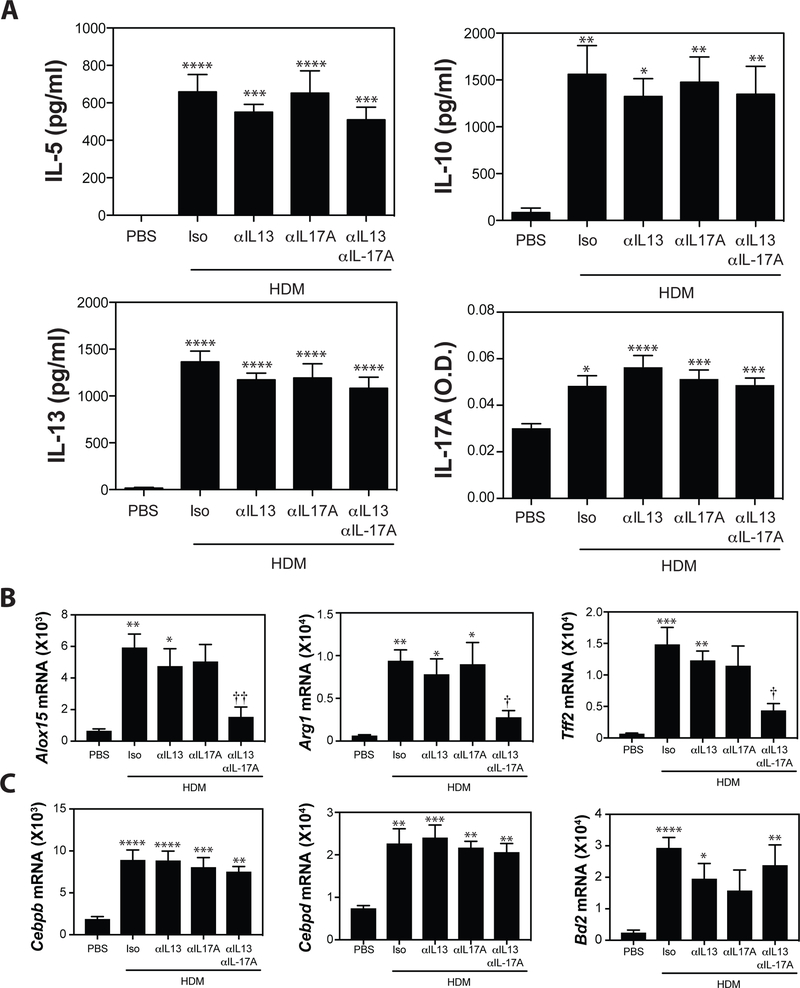
Blockade of IL-13 or IL-17A had limited impact on the production of Th2, or Th17-derived cytokines following HDM-restimulation of whole lung cell cultures (Fig 7A), or on the frequency of IL-17A+CD4+, IL-13+CD4+, or IL-13+IL-17A+CD4+ T cells in the lungs of low dose anti-IL-13, low dose anti-IL-17A, or combined low dose anti-IL-13 + IL-17A treated animals (Supplementary Figure 1). In contrast, expression of IL-13-induced genes Alox15, Arg1, and Tff2 was significantly reduced in animals treated with anti-IL-13 and IL-17A (Fig 7B) consistent with our previously reported synergism between IL-13 and IL-17A36,51. Importantly, there was no significant impact of either anti-IL-13 or anti-IL-17A, alone or in combination, on the expression of Th17-associated genes like Cebpb, Cebpd, and Bd2 (Fig 7C). Additional Th17-associated targets that are representative of the “Th17 signature” observed elsewhere40 including Cxcl1, Cxcl2, Cxcl3 and Csf3 show similar patterns of expression (Supplementary Figure 2). Collectively, these data indicate that simultaneous administration of individually sub-therapeutic doses of anti-IL-13 and anti-IL-17A has the capacity to limit the development of allergen induced AHR and IL-13-induced gene expression, with limited impact on the capacity to mount Th17-associated responses.
Figure 7. Simultaneous blockade of IL-13 and IL-17A at sub-therapeutic individual doses significantly reduces IL-13-induced gene expression, without impacting expression of IL-17A-induced genes.
(A) Lung cells were restimulated in vitro with HDM and IL-5, IL-10, IL-13, and IL-17A production was evaluated by ELISA. (B) Pulmonary expression of IL-13-driven genes (Alox15, Arg1, Tff2) and (C) IL-17A-induced genes (Cebpb, Cebpd, Bd2) was assessed by RT-PCR analysis. Data are mean ± SEM of n = 10 – 12 mice from 2 independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, and **** p < 0.0001 versus PBS. † < 0.05 and †† < 0.01 versus isotype.
Discussion
While asthma is driven by aberrant Th2 cell responses, it is increasingly evident that severe forms of disease are frequently associated with mixed Th2/Th1 or Th2/Th17 responses18–32,49. Interestingly, recent evidence also suggests that Th2 effector cytokines can actively inhibit Th17-associated responses, either at the level of Th17-cell cytokine production, or downstream interaction between IL-13 and IL-17A-driven signaling pathways36,41–43. Consistent with this observation, blockade of Th2 effector cytokines in allergic asthma elevates Th17-associated responses and pulmonary neutrophilia40. Not surprisingly, concomitant blockade of Th2- and Th17-associated cytokines limits the augmentation of Th17-associated responses that results from blockade of Th2 responses, and reverses airway neutrophilia40, suggesting that combining therapeutic approaches may provide additional benefit. However, herein we make the novel observation that concomitant blockade of IL-13 and IL-17A, at doses that are sub-therapeutic when administered alone, reduce the magnitude of pathological features of asthma (AHR, IL-13-induced gene expression) while sparing the IL-17A-dependent responses associated with protection from bacterial infections. Importantly, one of the more common adverse events associated with blockade of IL-17A/IL-17RA in early clinical trials is the increased susceptibility to bacterial or fungal infections46–48. Thus, the demonstration that low dose anti-IL-17A has no impact on responses to bacterial products or the response to infection, while maintaining the ability to influence asthma severity, is a highly relevant observation emerging from the current study – particularly as bacterial or fungal infections are frequent triggers for asthma exacerbations53,54. Thus, we argue that exploration of combined, sub-therapeutic dosing regimens is an important avenue for future experimental studies.
Similarly, Th2 responses are thought to be critical mediators of anti-parasitic responses45. While exacerbation of parasitic responses has not been observed in clinical trials, parasitic infections expected to be most impacted are not typically endemic in the US or Europe55, where most clinical trials evaluating the efficacy of Th2 targeting therapies for the treatment of allergic diseases are currently underway. Regardless, it is encouraging that while expression of anti-parasitic mediators (Alox15, Tff2, Arg1)56–58 is reduced in IL-13 + IL-17A treated animals, they are not returned to baseline. As IL-17A has not been described as a critical mediator of anti-parasitic immunity, it seems unlikely that anti-parasitic immunity would be significantly impacted in animals treated with anti-IL-13 and IL-17.
Each gene product tested contributes either to AHR, or anti-microbial responses. Arginase 1 generates L-ornithine which contributes to airway remodeling and hyperresponsiveness59, while decreasing NO, a smooth muscle relaxant60. Elevated arginase activity is associated with reduced lung function61,62. Trefoil factor 2 (Tff2) is implicated in organ regeneration and healing63, its expression is elevated in asthma, and is required for IL-13 and allergen-induced AHR56. 15-lipoxygenase (Alox15) catabolizes arachidonic acid, generating lipid mediators such as lipoxin and eoxin64. Alox15−/− mice demonstrate reduced airway inflammation, Th2 cytokine production and airway remodeling65. Cebpb and Cebpd encode C/EBPβ and C/EBPδ which promote synergy between IL-17A and other pro-inflammatory cytokines by enhancing mRNA stability66. Bd2 encodes defensin β−2, a bactericidal protein stimulated by LPS67 which contributes to clearance of select bacteria68,69. Finally, Cxcl1, Cxcl2, and Cxcl3 encode neutrophil-recruiting, while Csf3 encodes G-CSF, a cytokine that promotes neutrophil differentiation70. Thus, altered expression of any IL-13 or IL-17A induced gene studied may impact allergic or anti-bacterial responses.
While airway inflammation contributes to AHR, inflammation-independent factors such as smooth muscle contractility, airway remodeling, mucus hypersecretion, and airway remodeling also contribute71–73. Our observation that concomitant IL-13/IL-17A blockade significantly reduces AHR, with no impact on airway inflammation suggests that IL-13/IL-17A blockade targets inflammation-independent components of AHR. Indeed, IL-13 signaling in epithelial cells is sufficient to induce AHR38, and IL-17A exacerbates AHR through augmenting IL-13 responses36. It is possible that IL-13/IL-17A pathways targeted are epithelium specific.
It is also important to note that inhibition of IL-13 with high doses of IL-13-blocking mAb did result in enhanced IL-17A production in HDM-restimulated lung cell cultures and elevated expression of IL-17A-dependent gene expression in the context of IL-13 blockade. The mechanisms responsible for these changes are not entirely clear, however, it has been observed that Th17 cells express the IL-13Rα1 and that signaling through this receptor inhibits the capacity of Th17 cells to make IL-17A41–43. As such, it is conceivable that blocking IL-13 relieves this brake on IL-17A production, allowing for increased capacity for IL-17A production. However, we have also observed that concomitant activation of IL-13 and IL-17A-induced signaling pathways in the same cell results in significant downregulation of IL-17A-induced gene expression in cells exposed to both cytokines36. Thus the effect on increased expression of IL-17A-induced genes is due to a combined effect of both increased IL-17A production, and increased responsiveness of pulmonary cells to IL-17A-induced signaling.
One shortcoming of the current study is that the anti-cytokine therapies are administered prophylactically, prior to the initiation of disease, rather than in a more therapeutic model. While typically viewed as effector cytokines, a number of studies suggest that IL-13 or IL-17A can influence the development an adaptive immune response through a number of different mechanisms. Blockade of IL-17A during sensitization, has been shown to be sufficient to inhibit the development of HDM induced asthma74. Interestingly, IL-17A has been demonstrated to enhance dendritic cell differentiation and release from the bone marrow75, co-stimulatory molecule expression49 and proinflammatory cytokine production74. Thus blocking IL-17A during sensitization may limit the capacity of pulmonary dendritic cells (DCs) to drive asthma development. Similarly, ILC2-derived IL-13 has been proposed as an important regulator of asthma development through the regulation of DC migration from the lung to LN and subsequent induction of Th2 immunity76,77. However, we did not see significant alteration in the frequency of IL-17A+CD4+, IL-13+CD4+, or IL-13+IL-17A+CD4+ T cells in the lungs of low dose anti-IL-13, low dose anti-IL-17A, or combined low dose anti-IL-13 + IL-17A treated animals (data not shown). As such, we feel it is unlikely that the inhibitory effect of combined low dose anti-IL-13 and anti-IL-17A administration is due to inhibition of T cell immunity. Rather, we expect that blockade of IL-17A and/or IL-13 was operant primarily during the effector/challenge phase of the immune response.
In conclusion, the current study supports published reports demonstrating that combined blockade of Th2 and Th17 effector molecules may have improved benefit over blockade of Th2 effector cytokines alone, particularly in models of mixed Th2/Th17 asthma. However, this study also suggests that the beneficial impact of combined Th2/Th17-targeting therapeutic approaches in asthma might still be evident even when the blocking antibodies are administered in quantities that were individually unable to impact disease outcomes. In addition to the benefits inherent in reducing the dose of administered therapeutic agents while retaining efficacy, we also observe that induction of Th17-driven anti-microbial responses are impacted to a lesser degree with such sub-therapeutic dosing strategies. As pulmonary bacterial and fungal infections are common triggers of asthma exacerbations, preserving the capacity to effectively combat these infections may have additional, unappreciated clinical benefit in the treatment of severe asthma.
Supplementary Material
Acknowledgements
This work was funded through a Research Funding Agreement provided by Janssen Research & Development, LLC (IPL) and NHLBI R01 HL122300 (IPL). We would like to thanks Phoebe Franz for expert technical assistance.
References:
- 1.Finkelman FD, Hogan SP, Hershey GK, Rothenberg ME, Wills-Karp M. Importance of cytokines in murine allergic airway disease and human asthma. J Immunol 2010;184(4):1663–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson MJ, Prout M, Mearns H, et al. IL-4 Haploinsufficiency Specifically Impairs IgE Responses against Allergens in Mice. The Journal of Immunology 2017;198:1815–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pène J, Rousset F, Brière F, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proceedings of the National Academy of Sciences 1988;85:6880–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkelman FD, Katona IM, Urban JF, et al. IL-4 is required to generate and sustain in vivo IgE responses. The Journal of Immunology 1988;141:2335–2341. [PubMed] [Google Scholar]
- 5.Hogan SP, Mould AW, Young JM, et al. Cellular and molecular regulation of eosinophil trafficking to the lung. Immunol Cell Biol 1998;76(5):454–460. [DOI] [PubMed] [Google Scholar]
- 6.Pope SM, Brandt EB, Mishra A, et al. IL-13 induces eosinophil recruitment into the lung by an IL-5- and eotaxin-dependent mechanism. J Allergy Clin Immunol 2001;108(4):594–601. [DOI] [PubMed] [Google Scholar]
- 7.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13: central mediator of allergic asthma. Science 1998;282(5397):2258–2261. [DOI] [PubMed] [Google Scholar]
- 8.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine 2015;75(1):68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev 2017;9:CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntontsi P, Papathanassiou E, Loukides S, Bakakos P, Hillas G. Targeted anti-IL-13 therapies in asthma: current data and future perspectives. Expert Opin Investig Drugs 2018;27(2):179–186. [DOI] [PubMed] [Google Scholar]
- 11.Barranco P, Phillips-Angles E, Dominguez-Ortega J, Quirce S. Dupilumab in the management of moderate-to-severe asthma: the data so far. Ther Clin Risk Manag 2017;13:1139–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med 2016;4(10):781–796. [DOI] [PubMed] [Google Scholar]
- 13.Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011;365(12):1088–1098. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel S, Castro M, Corren J, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016;388(10039):31–44. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel SE, Wang L, Pirozzi G. Dupilumab in persistent asthma. N Engl J Med 2013;369(13):1276. [DOI] [PubMed] [Google Scholar]
- 16.Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014;371(13):1198–1207. [DOI] [PubMed] [Google Scholar]
- 17.Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med 2017;5(5):390–400. [DOI] [PubMed] [Google Scholar]
- 18.Gauthier M, Chakraborty K, Oriss TB, et al. Severe asthma in humans and mouse model suggests a CXCL10 signature underlies corticosteroid-resistant Th1 bias. JCI Insight 2017;2(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chambers ES, Nanzer AM, Pfeffer PE, et al. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-gamma(high) immunophenotypes: Potential benefits of calcitriol. J Allergy Clin Immunol 2015;136(3):628–637 e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oriss TB, Raundhal M, Morse C, et al. IRF5 distinguishes severe asthma in humans and drives Th1 phenotype and airway hyperreactivity in mice. JCI Insight 2017;2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YH, Voo KS, Liu B, et al. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med 2010;207(11):2479–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irvin C, Zafar I, Good J, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol 2014;134(5):1175–1186 e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Ramli W, Prefontaine D, Chouiali F, et al. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J Allergy Clin Immunol 2009;123(5):1185–1187. [DOI] [PubMed] [Google Scholar]
- 24.Bazzi MD, Sultan MA, Al Tassan N, et al. Interleukin 17A and F and asthma in Saudi Arabia: gene polymorphisms and protein levels. J Investig Allergol Clin Immunol 2011;21(7):551–555. [PubMed] [Google Scholar]
- 25.Bullens DM, Truyen E, Coteur L, et al. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir Res 2006;7:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasegawa T, Uga H, Mori A, Kurata H. Increased serum IL-17A and Th2 cytokine levels in patients with severe uncontrolled asthma. Eur Cytokine Netw 2017;28(1):8–18. [DOI] [PubMed] [Google Scholar]
- 27.Agache I, Ciobanu C, Agache C, Anghel M. Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med 2010;104(8):1131–1137. [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi M, Takahashi D, Hizawa N, et al. IL-17F sequence variant (His161Arg) is associated with protection against asthma and antagonizes wild-type IL-17F activity. J Allergy Clin Immunol 2006;117(4):795–801. [DOI] [PubMed] [Google Scholar]
- 29.Wang JY, Shyur SD, Wang WH, et al. The polymorphisms of interleukin 17A (IL17A) gene and its association with pediatric asthma in Taiwanese population. Allergy 2009;64(7):1056–1060. [DOI] [PubMed] [Google Scholar]
- 30.Jin EH, Choi EY, Yang JY, Chung HT, Yang YS. Significant Association between IL-17F Promoter Region Polymorphism and Susceptibility to Asthma in a Korean Population. Int Arch Allergy Immunol 2010;155(2):106–110. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Zhang Y, Han D, Zhang L. Association between polymorphisms in cytokine genes IL-17A and IL-17F and development of allergic rhinitis and comorbid asthma in Chinese subjects. Hum Immunol 2012;73(6):647–653. [DOI] [PubMed] [Google Scholar]
- 32.Ricciardolo FLM, Sorbello V, Folino A, et al. Identification of IL-17F/frequent exacerbator endotype in asthma. J Allergy Clin Immunol 2017;140(2):395–406. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez-Tello A, Halwani R, Hamid Q, Al-Muhsen S. Glucocorticoid receptor-beta up-regulation and steroid resistance induction by IL-17 and IL-23 cytokine stimulation in peripheral mononuclear cells. J Clin Immunol 2013;33(2):466–478. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez-Tello A, Semlali A, Chakir J, et al. Induction of glucocorticoid receptor-beta expression in epithelial cells of asthmatic airways by T-helper type 17 cytokines. Clin Exp Allergy 2010;40(9):1312–1322. [DOI] [PubMed] [Google Scholar]
- 35.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med 2009;180(8):720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall SL, Baker T, Lajoie S, et al. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J Allergy Clin Immunol 2017;139(2):462–471 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomlinson KL, Davies GC, Sutton DJ, Palframan RT. Neutralisation of interleukin-13 in mice prevents airway pathology caused by chronic exposure to house dust mite. PLoS One 2010;5(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuperman DA, Huang X, Koth LL, et al. Direct effects of interleukin-13 on epithelial cells cause airway hyperreactivity and mucus overproduction in asthma. Nat Med 2002;8(8):885–889. [DOI] [PubMed] [Google Scholar]
- 39.Yang G, Volk A, Petley T, et al. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine 2004;28(6):224–232. [DOI] [PubMed] [Google Scholar]
- 40.Choy DF, Hart KM, Borthwick LA, et al. TH2 and TH17 inflammatory pathways are reciprocally regulated in asthma. Sci Transl Med 2015;7(301):301ra129. [DOI] [PubMed] [Google Scholar]
- 41.Newcomb DC, Boswell MG, Huckabee MM, et al. IL-13 regulates Th17 secretion of IL-17A in an IL-10-dependent manner. J Immunol 2012;188(3):1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newcomb DC, Boswell MG, Zhou W, et al. Human TH17 cells express a functional IL-13 receptor and IL-13 attenuates IL-17A production. J Allergy Clin Immunol 2011;127(4):1006–1013 e1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Newcomb DC, Zhou W, Moore ML, et al. A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol 2009;182(9):5317–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev 2008;226:160–171. [DOI] [PubMed] [Google Scholar]
- 45.Allen JE, Sutherland TE. Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin. Semin Immunol 2014;26(4):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab Inhibition of Interleukin-17A in Patients with Psoriatic Arthritis. N Engl J Med 2015;373(14):1329–1339. [DOI] [PubMed] [Google Scholar]
- 47.McInnes IB, Mease PJ, Ritchlin CT, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford) 2017;56(11):1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imafuku S, Torisu-Itakura H, Nishikawa A, Zhao F, Cameron GS, Japanese U-SG. Efficacy and safety of ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis: Subgroup analysis of a placebo-controlled, phase 3 study (UNCOVER-1). J Dermatol 2017;44(11):1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lajoie S, Lewkowich IP, Suzuki Y, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol 2010;11(10):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewkowich IP, Lajoie S, Clark JR, Herman NS, Sproles AA, Wills-Karp M. Allergen uptake, activation, and IL-23 production by pulmonary myeloid DCs drives airway hyperresponsiveness in asthma-susceptible mice. PLoS One 2008;3(12):e3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewkowich IP, Herman NS, Schleifer KW, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function. J Exp Med 2005;202(11):1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serra MF, Cotias AC, Pao CRR, et al. Repeated Allergen Exposure in A/J Mice Causes Steroid-Insensitive Asthma via a Defect in Glucocorticoid Receptor Bioavailability. J Immunol 2018;201(3):851–860. [DOI] [PubMed] [Google Scholar]
- 53.Darveaux JI, Lemanske RF Jr., Infection-related asthma. J Allergy Clin Immunol Pract 2014;2(6):658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kanchongkittiphon W, Mendell MJ, Gaffin JM, Wang G, Phipatanakul W. Indoor environmental exposures and exacerbation of asthma: an update to the 2000 review by the Institute of Medicine. Environ Health Perspect 2015;123(1):6–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest 2008;118(4):1311–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wills-Karp M, Rani R, Dienger K, et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med 2012;209(3):607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brys L, Beschin A, Raes G, et al. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J Immunol 2005;174(10):6095–6104. [DOI] [PubMed] [Google Scholar]
- 58.Pesce JT, Ramalingam TR, Mentink-Kane MM, et al. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 2009;5(4):e1000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.North ML, Grasemann H, Khanna N, Inman MD, Gauvreau GM, Scott JA. Increased ornithine-derived polyamines cause airway hyperresponsiveness in a mouse model of asthma. Am J Respir Cell Mol Biol 2013;48(6):694–702. [DOI] [PubMed] [Google Scholar]
- 60.Perez-Zoghbi JF, Bai Y, Sanderson MJ. Nitric oxide induces airway smooth muscle cell relaxation by decreasing the frequency of agonist-induced Ca2+ oscillations. J Gen Physiol 2010;135(3):247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lara A, Khatri SB, Wang Z, et al. Alterations of the arginine metabolome in asthma. Am J Respir Crit Care Med 2008;178(7):673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maarsingh H, Dekkers BG, Zuidhof AB, et al. Increased arginase activity contributes to airway remodelling in chronic allergic asthma. Eur Respir J 2011;38(2):318–328. [DOI] [PubMed] [Google Scholar]
- 63.Hoffmann W Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 2005;62(24):2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feltenmark S, Gautam N, Brunnstrom A, et al. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc Natl Acad Sci U S A 2008;105(2):680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andersson CK, Claesson HE, Rydell-Tormanen K, Swedmark S, Hallgren A, Erjefalt JS. Mice lacking 12/15-lipoxygenase have attenuated airway allergic inflammation and remodeling. Am J Respir Cell Mol Biol 2008;39(6):648–656. [DOI] [PubMed] [Google Scholar]
- 66.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 2009;9(8):556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrison GM, Davidson DJ, Dorin JR. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett 1999;442(1):112–116. [DOI] [PubMed] [Google Scholar]
- 68.Wu M, McClellan SA, Barrett RP, Hazlett LD. Beta-defensin-2 promotes resistance against infection with P. aeruginosa. J Immunol 2009;182(3):1609–1616. [DOI] [PubMed] [Google Scholar]
- 69.Wu M, McClellan SA, Barrett RP, Zhang Y, Hazlett LD. Beta-defensins 2 and 3 together promote resistance to Pseudomonas aeruginosa keratitis. J Immunol 2009;183(12):8054–8060. [DOI] [PubMed] [Google Scholar]
- 70.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony stimulating factors. Dev Comp Immunol 2004;28(5):509–554. [DOI] [PubMed] [Google Scholar]
- 71.Meurs H, Gosens R, Zaagsma J. Airway hyperresponsiveness in asthma: lessons from in vitro model systems and animal models. Eur Respir J 2008;32(2):487–502. [DOI] [PubMed] [Google Scholar]
- 72.Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol 2006;118(3):551–559; quiz 560–551. [DOI] [PubMed] [Google Scholar]
- 73.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: Airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 2010;138(2 Suppl):4S–10S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chenuet P, Fauconnier L, Madouri F, et al. Neutralization of either IL-17A or IL-17F is sufficient to inhibit house dust mite induced allergic asthma in mice. Clin Sci (Lond) 2017;131(20):2533–2548. [DOI] [PubMed] [Google Scholar]
- 75.Antonysamy MA, Fanslow WC, Fu F, et al. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol 1999;162(1):577–584. [PubMed] [Google Scholar]
- 76.Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 2014;40(3):425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gold MJ, Antignano F, Halim TY, et al. Group 2 innate lymphoid cells facilitate sensitization to local, but not systemic, TH2-inducing allergen exposures. J Allergy Clin Immunol 2014;133(4):1142–1148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.