Abstract
Objective:
Inflammation occurs during the progression of abdominal aortic aneurysm (AAA). Interleukin-33 (IL-33) is a pleiotropic cytokine with multiple immunomodulatory effects, yet its role in AAA remains unknown.
Approach and Results:
Immunoblot, immunohistochemistry, and immunofluorescent staining revealed increased IL-33 expression in adventitia fibroblasts from mouse AAA lesions. Daily intraperitoneal administration of recombinant IL-33 or transgenic IL-33 expression ameliorated peri-aorta CaPO4 injury- and aortic elastase exposure-induced AAA in mice, as demonstrated by blunted aortic expansion, reduced aortic wall elastica fragmentation, enhanced AAA lesion collagen deposition, attenuated T-cell and macrophage infiltration, reduced inflammatory cytokine production, skewed M2 macrophage polarization, and reduced lesion matrix metalloproteinase (MMP) expression and cell apoptosis. Flow cytometry analysis, immunostaining, and immunoblot analysis showed that exogenous IL-33 increased CD4+Foxp3+ regulatory T cells (Tregs) in spleens, blood, and aortas in peri-aorta CaPO4-treated mice. Yet, ST2-deficiency muted these IL-33 activities. Tregs from IL-33-treated mice also showed significantly stronger activities in suppressing smooth muscle cell inflammatory cytokine and chemokine expression, macrophage MMP expression, and in increasing M2 macrophage polarization than those from vehicle-treated mice. In contrast, IL-33 failed to prevent AAA and lost its beneficial activities in CaPO4-treated mice after selective depletion of Tregs.
Conclusion:
Together, this study established a role of IL-33 in protecting mice from AAA formation by enhancing ST2-dependent aortic and systemic Treg expansion and their immunosuppressive activities.
Keywords: Interleukin-33, abdominal aortic aneurysm, regulatory T cells, ST2
INTRODUCTION
Abdominal aortic aneurysm (AAA) is a chronic vascular inflammatory disease with permanent dilations and destructive remodelling of the abdominal aorta, which leads to aortic rupture in elderly persons.1 AAA pathophysiology is complicated and contains massive aortic tissue inflammatory response and extracellular matrix (ECM) degeneration.2,3 Aortic tissue inflammatory response is characterized by the infiltration of inflammatory cells and local expression of inflammatory cytokines and proteases.4 Additionally, endothelial cells, smooth muscle cells (SMCs), and fibroblasts in the vasculature also produce proteases to destroy the medial elastica and aggravate the progression of AAA.2,5 Current therapeutic measures rely merely on invasive surgical repairs.1,6 Therefore, it is urgent to explore non-invasive possibilities for these patients.
IL-33, a member of the IL-1 family, is constitutively expressed in endothelial and epithelial cells at barrier sites, and its expression is induced in infiltrated inflammatory cells at the site of inflammation.7–9 As an alarmin, IL-33 is rapidly released from damaged or necrotic cells.9 ST2, the receptor for IL-33, has two primary isoforms: the transmembrane isoform (ST2L) and the soluble isoform (sST2) that serves as a decoy receptor for IL-33.10 ST2 is expressed on immune cells, including Th2 cell, activated Th1 cells, regulatory T cells (Tregs), and type 2 innate lymphoid cells (ILC2).10−13 Through binding to ST2 on these cells, IL-33 serves pleiotropic functions in both adaptive and innate immunities. Accumulated evidence suggests an important role of IL-33 in regulating inflammatory responses.9 It is involved in the pathogenesis of lung inflammation,14,15 intestinal inflammation,12,16 infectious diseases,11,17 cancer immunity,18 autoimmune disorders,19 and cardiovascular diseases.20–22 In transverse aortic constriction (TAC)-induced mouse heart failure, mechanical stress-induced cardiac hypertrophy, cardiac dysfunction, myocardium fibrosis and inflammation, and post-injury survival were all impaired in IL-33-deficient mice.23 Similarly, IL-33 reduced angiotensin II- and phenylephrine-induced cardiomyocyte hypertrophy in mice by inhibiting I-κBα activity and NF-κB nuclear binding activity. Soluble ST2 blocked the anti-hypertrophic activity of IL-33.20 These studies tested a direct role of IL-33 in regulating cardiac hypertrophy. Recently, IL-33 was found in mouse aorta, where it played a protective role in atherosclerosis.24 Yet, the role of IL-33 in AAA remains unknown.
In this study, we demonstrated that IL-33 was elevated in AAA lesions, and adventitial fibroblasts were the major source. Repletion or over-expression of IL-33 protected against AAA injury. We also showed that IL-33 induced ST2-dependent Treg expansion. IL-33 lost its AAA-protective activity in the absence of Tregs.
METHODS
Data available on request from the authors.
Mice
Male C57BL/6J mice aged 10–12 weeks were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). IL-33 transgenic mice on a C57BL/6J background were kindly provided by Dr. Rong Mu from the Department of Rheumatology and Immunology, Peking University Peoples Hospital (Beijing, China). ST2–/– mice on a C57BL/6J background were obtained from Dr. Andrew McKenzie (Medical Research Council Laboratory of Molecular Biology, University of Cambridge, Cambridge, U.K.). DEREG (depletion of regulatory T-cell) transgenic mice on a C57BL/6J background were purchased from the Jackson Laboratory (Bar Harbour, ME). AAA incidence in female mice is much lower than that in male mice in all tested experimental AAA models.25 Therefore, we used only male mice throughout this study. All animal studies were approved by the Animal Care and Utilization Committee of Huazhong University of Science and Technology. Experiments were conducted in accordance with the NIH guidelines.
AAA models
Calcium phosphate (CaPO4) model: AAA was induced by CaPO4 in mice at the age of 10 to 12 weeks as described previously.26 Briefly, mice were anaesthetized with ketamine (50 mg/kg−1) and pentobarbital sodium (50 mg/kg−1), and the infrarenal region of the abdominal aorta was isolated and a small piece of gauze soaked in 0.5 M CaCl2 was applied around the aorta. After 10 minutes, the gauze was replaced by another piece of PBS-soaked gauze for 5 minutes. Control mice were similarly treated with 0.5 M NaCl for 15 minutes. Sham-operated mice were used as experimental controls.
Elastase model: AAA was induced by elastase exposure in mice at the age of 10 to 12 weeks as described previously.27 After anaesthesia, the aorta was isolated from the renal vein to the iliac bifurcation and was bathed in either 10 μl of 100% porcine pancreatic elastase (#E1250, Sigma–Aldrich, St. Louis, MO) or heat-inactivated elastase (control) for 10 min. After elastase exposure, the incision was stitched with a 4–0 suture.
Mice were sacrificed 7 days (CaPO4 model) or 14 days (elastase model) after the surgery, and the abdominal aorta was harvested and photographed (Nikon D7200) to determine its external diameter by using Image-Pro Plus computer-assisted image analysis software. The adventitial circumferences at the maximal expanded portion of the infrarenal aortas were quantified as the maximal abdominal aortic diameter. At least 3 measurements of the maximal expanded portion of the infrarenal aorta of each mouse were averaged before calculating the mean of each experimental group. AAA was defined as an aortic diameter increase by 50% or greater (≥0.75 mm) compared to the sham-operated group (n=17, 0.50±0.02 mm).28
Treatment and groups
For the measurement of IL-33 and ST2 expression from AAA lesions, C57BL/6J mice were randomly divided into two groups that were treated with CaPO4 or NaCl (n=5 per group). To test the effect of IL-33 in CaPO4 induced AAA, mice were randomly divided into 5 groups: 1) C57BL/6J mice treated with the recombinant IL-33 (#34-8332-85, eBioscience, San Diego, CA, n=15); 2) C57BL/6J mice treated with vehicle (PBS, n=15), in which the mice were injected intraperitoneally with 1 μg of IL33 diluted in PBS or PBS containing 0.5% bovine serum albumin daily after the operation for 7 days; 3) IL-33 transgenic group (IL-33TG, n=13); 4) non-transgenic (NTG) group (n=13); 5) Sham operated group (n=17). To test the effect of IL-33 in elastase-induced AAA, mice were randomly divided into 3 groups: 1) C57BL/6J mice treated with the recombinant IL-33 (n=10); 2) C57BL/6J mice treated with vehicle (PBS, n=10), in which the mice were injected intraperitoneally with 2 μg of IL33 diluted in PBS or PBS containing 0.5% bovine serum albumin every two days after the operation for 14 days; and 3). C57BL/6J mice underwent the same procedure but with heat-inactivated elastase (n=5). To investigate the involvement of Tregs in IL-33-mediated protection against AAA, DEREG mice were randomly divided into 5 groups and subjected to CaPO4-induced AAA: 1) DEREG mice subjected to a sham operation (n=10); 2) DEREG mice subjected to the AAA model injected intraperitoneally (i.p.) with 200 μl PBS (n=8); 3) DEREG mice subjected to the AAA model injected i.p. with 1 μg diphtheria toxin (Merck, Germany) (n=10); 4) DEREG mice subjected to the AAA model injected i.p. with 1 μg IL-33 (n=8); 5) DEREG mice subjected to the AAA model injected i.p. with 1 μg IL-33 and 1 μg diphtheria toxin (n=9). Diphtheria toxin or PBS was injected i.p. for 2 consecutive days before the operation and every two days after the operation, and IL-33 was injected i.p. daily after the operation for 7 days.
Histology and immunohistochemical (IHC) analyses
After the mice were sacrificed, aortas from the ascending aorta to the bifurcation of the iliac artery were isolated. Whole aortas were harvested, fixed for 24 h in 4% paraformaldehyde, cut at the site of maximal diameter, and embedded in paraffin for cross section preparation. Aortic sections (4 μm each) were collected serially from the proximal to the distal aorta. At least 10 sections were prepared and analysed per mouse. Serial sections were analysed using the elastica van Gieson staining kit according to the manufacturer’s protocol (#115974, EMD Millipore, Burlington, MA) for elastin assessment. Elastin degradation was graded as 1, < 25% degradation; 2, 25% to 50% degradation; 3, 50% to 75% degradation; or 4, > 75% degradation.3 Sirius Red staining were analysed according to the manufacturer’s protocol (#09400–10, 0.1% Sirius Red; Polysciences Inc, Warrington, PA) and presented as percentage of positive area. Apoptotic cells in lesions were determined with the in situ apoptosis detection kit according to the manufacturer’s instructions (#ab206386, Abcam, Cambridge, MA) and presented as positive numbers per mm2.
Serial paraffin sections were also used for immunostaining using the following primary antibodies: CD3, CD68, MMP-2, MMP-9, and IL-33 with detailed information listed in Supplementary Table I. Apoptotic cells in lesions were determined with the TUNEL apoptosis detection kit according to the manufacturer’s instructions (#ab206386, Abcam). We counted the number of IL-33-positive cells, CD68-positive macrophages, CD3-positive T cells, Foxp3-positive Tregs, and TUNEL-positive apoptotic cells. MMP-2 and MMP-9 were quantified and presented as percentage of positive area. All staining were imaged under the OLYMPUS BX51 microscope. We randomly selected five mice from each experimental group for immunohistological analysis based on the availability. All examinations were conducted by two trained, independent observers blinded to the genotype and treatment. A mean value was determined from at least four sections from each animal.
For immunofluorescent staining, paraffin sections were permeabilized with 0.3% TritonX-100 in PBS for 5 minutes and blocked with 5% foetal bovine serum and 10% donkey serum in PBS at room temperature for 1 hour. Tissues were stained for 2 hours at room temperature in the presence of goat anti-IL-33, rabbit anti-vimentin, or rabbit anti-α-SMA, followed by staining for 1 hour at room temperature with PE-donkey anti-goat secondary antibody, FITC-donkey anti-rabbit secondary antibody, or PE-Foxp3 (Supplementary Table I). Tissue sections were further stained with DAPI for 5 minutes before imaging (Nikon A1Si).
Cell cultures
To examine the suppressive activity of IL-33-expanded Tregs in vitro, we isolated spleen CD4+CD25+ Tregs from IL-33- or PBS-treated mice with a mouse CD4+CD25+ Regulatory T cell Isolation Kit (#130-091-041, MiltenyiBiotec, Bergisch-Gladbach, Germany). Flow cytometry analysis confirmed that the purity of the Tregs was greater than 95% (Supplementary Figure I). Next, 1×105 Tregs from IL-33- or PBS-treated mice were co-cultured with 10×105 mouse aortic smooth muscle cells (MOVAS, #CRL-2797, American Type Culture Collection [ATCC], Manassas, VA) or macrophage cells (RAW264.7, #SC-6003, ATCC) in 12-well plates for 48 hours with anti-CD3 antibody (2 μg/ml; #16-0032-85, eBioscience) and anti-CD28 antibody (1 μg/ml; #16-0281-85, eBioscience). RAW264.7 cells were harvested for protein and mRNA analyses. Additionally, before collecting, the MOVAS were treated with 0.05 M CaPO4 (0.05 M CaCl2 with phosphate buffered saline) for another 30 min to induce inflammatory cytokine production.
Flow cytometry analysis
To detect T-cell subsets in mice, mononuclear cells from the randomly selected spleen and blood were isolated by Ficoll density gradient (#GE17-1440-02, Sigma-Aldrich). The cells were first stained with PE/Cy7- or APC-anti-CD4 and APC-anti-ST2 for 30 min and washed with PBS. Then, the cells were fixed, permeabilized, and stained with Alexa Fluor®-anti-Ki67 and PE-anti-Foxp3 (Supplementary Table I). Other reagents used for flow cytomety are all listed in Supplementary Table I
To determine IL-33-producing cells in AAA lesions, we sorted the CD45+ and CD45– fractions from the digested aortas. Briefly, aortas were minced into small pieces and then digested in 1× Aorta Dissociation Enzyme stock Solution (125 U/ml collagenase type XI, 60 U/ml hyaluronidase type I-s, 60 U/ml DNase1, and 450 U/ml collagenase type I, all enzymes were obtained from Sigma-Aldrich) at 37°C for 1 h. Cell suspensions were prepared by filtering through a cell strainer (#322350, 70 μm size, BD Bioscience, Billerica, MA).29 The harvested cells were labelled with FITC-anti-CD45 (Supplementary Table I). Flow cytometry was performed using a FACS Calibur (BD Immunocytometry Systems, San Jose, CA). The results were analysed using FlowJo 7.6.1 (TreeStar Inc, Ashland, OR).
Western blot
Equal amounts of total protein from tissues or cells were electrophoresed on 10% SDS polyacrylamide gels under a reducing condition and transferred to PVDF membranes. After incubation in 5% non-fat milk for 2 hours at room temperature, membranes were incubated overnight at 4 °C with the primary antibodies, including mouse anti-β-actin monoclonal antibody, rat anti-Foxp3 monoclonal antibody, goat anti-IL-33 polyclonal antibody, and rabbit anti-ST2 polyclonal antibody (Supplementary Table I). Membranes were washed in tween-PBS and incubated with HRP-conjugated species-appropriate secondary antibodies (1:3000) for 2 hours at room temperature. An enhanced chemiluminescence (ECL) kit (Pierce, Rockford, IL) was used to detect all protein bands, and β-actin was used as a house-keeping protein for analysis between groups.
RT-PCR
Total RNA was isolated from randomly selected aortas or cells using Trizol Isolation Reagent (#15596018, Invitrogen, Carlsbad, CA). Reverse transcription was performed using the PrimeScript RT reagent kit (#RR064B, Takara Biotechnology, Dalian, China), and RT-PCR was completed using the SYBR Green Master Mix (#RR066A, Takara, Japan) on an ABI PRISM 7900 Sequence Detector system (Applied Biosystems, Foster City, CA). mRNA levels were standardized with GAPDH, and all experiments were completed in triplicates. The primers used for RT-PCR were listed in Supplementary Table II.
ELISA
The concentration of IL-33 in mouse plasma was analysed using ELISA kits (#88-7333-22, eBioscience, San Diego, CA) according to the manufacturer’s instructions. All samples were detected in duplicates.
Statistical analysis
Data were presented as mean ± SEM. To assess the statistical significance, Kolmogorov–Smirnov test with Lilliefors’ correction was first employed to determine data normality. Equal variance between groups was tested by the F test. If evaluation of similar variances was passed, then student’s t-test was used for comparison between two groups. Nonparametric Mann–Whitney test was used where data were not normally distributed. For comparisons among three or more groups, all data passed both normality and equal variance tests. A one-way or two-way ANOVA test plus a post hoc Bonferroni test was used. In all cases, statistical significance was concluded where the two-tailed probability was less than 0.05. All analyses were performed using GraphPad Prism 6.0 and SPSS 18.0 software.
RESULTS
IL-33 is upregulated in experimental AAA
IL-33 is found in mouse aortas,24 yet its expression in AAA lesions has not been tested. To determine the expression of IL-33 in AAAs, we produced CaPO4-induced AAA in mice. As previously reported,26,30 after perivascular application of PBS following CaCl2 to the infrarenal abdominal aorta, the external aortic diameter increased by almost 2-fold at day 8 compared with NaCl-treated mice (data not shown). Proteolytic activation produced both the 20-kDa and 18-kDa active forms of IL-33.31 Immunoblot analysis demonstrated significantly increased production of both the 20-kDa and 18-kDa active forms of IL-33 in CaPO4-induced AAA lesions relative to those in the aortic extracts from NaCl-treated mice (Figure 1A/1B), although AAA production did not significantly increase plasma IL-33 levels compared with those from NaCl-treated mice as determined by ELISA (data not shown). RT-PCR also demonstrated much higher IL-33 mRNA levels in AAA lesions from CaPO4-treated mice than that in aortas from NaCl-treated mice (Figure 1C). Immunoblot analysis also detected increased expression of IL-33 receptor ST2 in AAA lesions from CaPO4-treated mice than that from the control mice (Figure 1D/1E). Immunostaining showed more IL-33-positive cells within the aortic wall in CaPO4-induced AAA mice than those in NaCl control mice (Figure 1F/1G). Endothelial cells, fibroblasts, SMCs, and infiltrated inflammatory cells are known to produce IL-33.8 To determine the cell type(s) that produce IL-33 in AAA lesions, we sorted the CD45+ and CD45– fractions from the digested aortas and quantified the mRNA levels by RT-PCR. In AAA lesions, CD45– cells were the major cell population that expressed IL-33 (Figure 1H). This finding was confirmed by immunofluorescent double staining, by which the majority of the IL-33-positive cells were vimentin+ and α-SMA− fibroblasts located at the adventitia (Figure 1I).
Figure 1.
Expression of IL-33 and its receptor ST2 in mouse AAA lesions. Immunoblot (A), relative protein level (B), and RT-PCR analysis (C) of IL-33 expression in aortic tissues of mice from both the NaCl and CaPO4 groups (n=5 per group). Immunoblot (D) and relative protein level (E) of ST2 in aortic tissues from NaCl- and CaPO4-treated mice. Representative immunostaining of IL-33 in the aorta (F) and quantitative analysis of IL-33-positive cells (G) (n=5 per group). The arrows indicate the IL-33-positive cells (brown). Goat IgG was used as antibody isotype negative control. Scale bar: 50 μm. H. RT-PCR analysis of IL-33 mRNA levels in CD45+ and CD45– fractions from digested aortas (n=8 per group). I. Immunofluorescent co-staining of IL-33 with fibroblast vimentin and SMC α-smooth muscle actin (α-SMA) in AAA lesions. All sections were from CaPO4-induced AAA mice. Rabbit IgG was used as corresponding antibody isotype controls. Scale bar: 100 μm. Data are Mean±SEM. *P<0.05 and **P<0.01, unpaired t test was used in B, and E, Mann–Whitney test was used in C, G, and H.
IL-33 protects mice from CaPO4-induced AAA
High levels of IL-33 in AAA lesions suggest a role of IL-33 in this aortic disease. We tested this hypothesis by giving CaPO4-induced AAA mice a recombinant IL-33 or producing CaPO4-induced AAA in IL-33-transgenic mice (IL-33TG). Relative to PBS-treated mice, exogenous IL-33 administration reduced aortic dilatation (1.29±0.05 mm vs. 0.84±0.07 mm, P<0.05, Figure 2A/2B), protected the aortic wall elastica from fragmentation, and increased aortic wall collagen deposition (Figure 2C/2D). IL-33-transgenic mice, in which the plasma IL-33 level determined by ELISA and IL-33 expression in the aortic tissues tested by immunoblot and RT-PCR were increased compared with those from non-transgenic (NTG) control mice (Supplementary Figure IIA–D), also displayed much smaller aortic diameters (1.21±0.05 mm vs. 0.72±0.04 mm, P<0.05, Figure 2A/2B) and had fewer elastica fragmentations and more collagen deposition than those from NTG mice (Figure 2C/2D). Further, infiltration of CD3+ T cells and CD68+ macrophages and the expression of inflammatory cytokines and chemokines such as IL-6 and MCP-1 (monocyte chemotactic protein-1) were also decreased in IL-33-treated or IL-33-transgenic mice (Figure 3A–3C). Meanwhile, administration of IL-33 markedly elevated the expression of M2 macrophage markers Arg-1, CD206, and Fizz1 and reduced the expression of M1 macrophage markers iNOS, CD86, and TNF-α in aortic lesions (Figure 3D).17,32 Flow cytometry analysis confirmed the increase of CD206+CD11c– M2 macrophages and decrease of CD11c+CD206– M1 macrophages33 after IL-33 treatment (Figure 3E). MMP-2 and MMP-9 are two important matrix metalloproteinases responsible for the degradation of medial elastica due to their elastinolytic activities.5 IL-33 treatment (Supplementary Figure IIIA–E) or transgenic expression (Supplementary Figure IVA–E) reduced AAA lesion MMP-2- and MMP-9-positive areas and their mRNA levels. AAA lesions from these mice also contained many fewer TUNEL-positive cells than PBS-treated control mice (Supplementary Figure IIIF/IVF).
Figure 2.
AAA lesion size, elastica fragmentation, and collagen deposition. Mice treated with IL-33 or PBS or IL-33-transgenic (IL-33TG) or non-transgenic (NTG) mice were used to induce AAA by CaPO4 aortic injury. NaCl-treated mice served as baseline control. A. Representative photographs of abdominal aortic fragments. B. Maximal external diameters of infrarenal aortas were detected 7 days after surgery (n=13~15 per group). C. Representative images of elastica Verhoeff–Van Gieson staining and Sirius red collagen staining. Scale bar: 50 μm. D. Assessment of medial elastica fragmentation and collagen Sirius red-positive area (n=5 per group). Elastica fragmentation grading keys are shown to the left. Data are Mean±SEM. *P<0.05 vs. PBS group, #P<0.05 vs. NTG group, one-way ANOVA was used in B, Mann–Whitney test was used in D.
Figure 3.
Inflammatory responses in AAA lesions from PBS- or IL-33-treated mice and mice with (IL-33TG) and without (NTG) IL-33 transgene. A. Representative images of CD3+ T-cell and CD68+ macrophage staining. Rabbit IgG and mouse IgG were used as isotype controls for CD3 and CD68 antibodies, respectively. Scale bar: 50 μm. B. Quantification of CD3+ T-cell and CD68+ macrophage numbers in aortic tissues (n=5 per group). C-E. RT-PCR analysis of inflammatory chemokine and cytokine and M1/M2 macrophage gene expression and gate strategy and flow cytometry analysis of CD11c+CD206– M1 and CD11c–CD206+ M2 macrophages of AAA lesions from different groups of mice as indicated (n=5 per group). Data are Mean±SEM. *P<0.05 and **P<0.01 vs. PBS group, #P<0.05 and ##P<0.01 vs. NTG group, unpaired 2-tailed t test.
As a potent Th2 cytokine,34,35 IL-33 administration may affect T-cell subset polarization, which also affects AAA development.36,37 To test this possibility, we performed RT-PCR analysis of AAA lesion tissue extract and found that IL-33 administration reduced lesion Th1 cytokine IFN-γ and Th17 cytokine IL17, but increased lesion Th2 cytokine IL-4 (Supplementary Figure VA). Yet, flow cytometry analysis of splenocytes from the same mice found no significant difference in CD4+Ifng+, CD4+IL4+, CD4+IL17a+ T-cell subsets between AAA mice treated with or without IL-33 (Supplementary Figure VB). In mice without CaPO4-induced aortic injury, administration of IL-33 or PBS also did not cause dilation at the abdominal aortas (data not shown). Histological analysis demonstrated that, in addition to the protection of CaPO4-indcued AAA, IL-33 administration or transgene expression showed no effect to the lung, liver, kidney, or heart (Supplementary Figure VI). Collectively, these findings suggest a protective role of IL-33 in experimental AAA.
IL-33 induces ST2-dependent Treg expansion in AAA mice
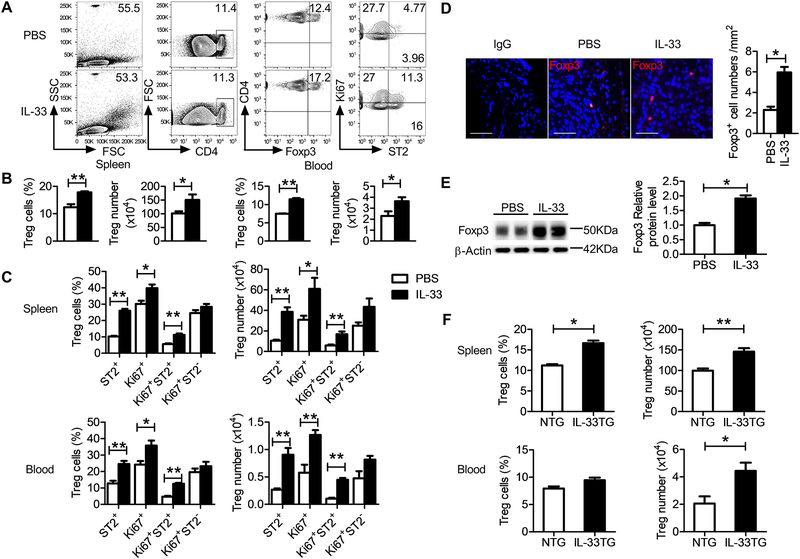
Prior studies demonstrated a protective role of Tregs in the formation and expansion of experimental AAAs.38 From CaPO4-induced AAA mice, IL-33 treatment increased CD4+Foxp3+ Treg cell percentage or absolute number in the blood and spleens (Figure 4A/4B). Flow cytometry analysis also showed that IL-33 increased splenic and blood proliferating Tregs (Ki67+CD4+Foxp3+), ST2-positive Tregs (ST2+CD4+Foxp3+), and proliferating ST2-positive Tregs (Ki67+ST2+CD4+Foxp3+), although IL-33-induced increase of proliferating ST2-negative Ki67+ST2–CD4+Foxp3+ Tregs did not reach statistical significance (Figure 4C). Immunofluorescent staining also revealed a marked elevation of Foxp3+ Tregs in AAA lesions from IL-33-treated mice (Figure 4D). Consistently, immunoblot analysis revealed an elevated level of Foxp3 protein in AAA lesion preparation from IL-33-treated mice, compared with that from PBS-treated control mice (Figure 4E). We obtained similar observations from mice with transgenic overexpression of IL-33. Spleen and blood CD4+Foxp3+ Treg percentage or absolute number from the IL-33TG mice was also elevated compared with those from the NTG mice after CaPO4-induced AAA (Figure 4F). IL-33-induced Treg expansion required ST2 expression. From wild-type (WT) and ST2-deficient ST2–/– mice with CaPO4-induced AAA, IL-33-induced CD4+Foxp3+ Treg elevation was detected only in spleens and blood from WT mice but not in those from ST2–/– mice (Supplementary Figure VIIA–B). These observations suggest that IL-33 protects AAA development in mice by enhancing lesional and systemic Treg expansion in an ST2-dependent manner.
Figure 4.
Increased Treg expansion in mice after IL-33 administration or transgenic expression. A. Gate strategy and flow cytometry analysis of splenic Foxp3+ Tregs and ST2 or Ki67 expression. B-C. Percentages and numbers of Foxp3+ Tregs in the spleen and blood from IL-33- and PBS-treated mice (n=5 per group). D. Representative immunofluorescent staining of Foxp3-positive cells and quantification of Foxp3-positive cells (n=5 per group) in AAA lesions from PBS- and IL-33-treated mice. Rat IgG was used as Foxp3 antibody isotype control. Scale bar: 50 μm. E. Western blot and relative Foxp3 protein level in AAA lesions from PBS- and IL-33-treated mice (n=5 per group). F. Percentages and numbers of CD4+Foxp3+ Tregs in the spleen and blood from NTG and IL-33TG mice (n=5 per group). Data are Mean±SEM. *P<0.05 and **P<0.01, unpaired 2-tailed t test.
IL-33 enhances Treg immunosuppressive activity in AAA
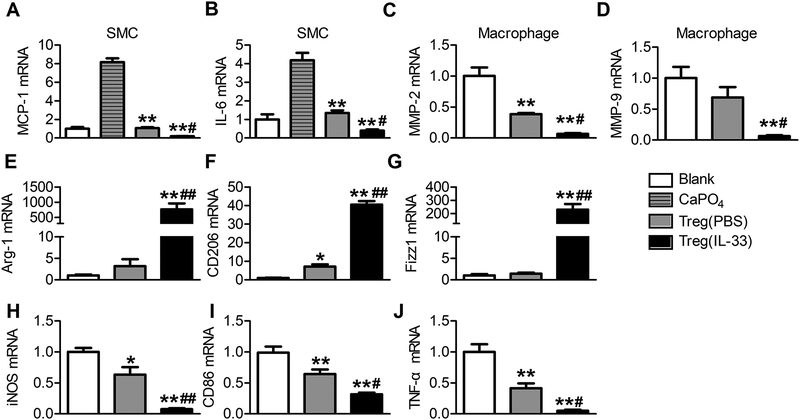
Tregs protect mice from AAA development with their immunosuppressive activities to inhibit immune cell activation and vascular cell inflammation.38–41 IL-33 treatment may not only increase ST2-dependent Treg proliferation, but also affect Treg immunosuppressive activities. We tested this possibility using splenic Tregs from PBS- and IL-33-treated AAA mice. Isolated Tregs were co-cultured with mouse aortic SMCs or RAW264.7 macrophages. In SMCs, CaPO4 induced the expression of chemokine MCP-1 and cytokine IL-6. Tregs from PBS-treated mice suppressed the expression of these inflammatory molecules. Yet, Tregs from IL-33-treated mice showed much stronger activity in inhibiting SMC expression of MCP-1 and IL-6 than those from PBS-treated mice (Figure 5A/5B). In RAW264.7 macrophages, Tregs from PBS-treated mice reduced the expression of MMP-2, yet Tregs from IL-33-treated mice displayed significantly stronger activity in blunting the expressions of both MMP-2 and MMP-9 (Figure 5C/5D). In macrophages, we also found that Tregs from IL-33-treated mice were more potent than those from PBS-treated mice in enhancing the expression of M2-related genes (Arg-1, CD206, Fizz1) (Figure 5E–5G) and suppressing the expression of M1 markers (iNOS, CD86, TNF-α) (Figure 5H–5J).
Figure 5.
IL-33 enhances Treg immunosuppressive activity on SMCs and macrophages. Mouse aortic SMCs or RAW264.7 cells were incubated with Tregs from PBS or IL-33-treated mice at ratios of 10:1 in the presence of anti-CD3 (2 μg/ml) and anti-CD28 (1 μg/ml). After 48 hours of co-culture, CaCl2 (0.05 M) and PBS (0.05 M) were added to the supernatant of the SMCs for 30 min. A-B. IL-6 and MCP-1 mRNA levels in SMCs. **P<0.01 vs. CaPO4, #P<0.05 vs. Tregs (PBS), one-way ANOVA. C-J. RT-PCR detected the mRNA levels of MMP-2, MMP-9, TNF-α, Arg-1, CD206, Fizz1, iNOS and CD86 in RAW264.7 cells. Data are representative of five independent experiments. Data are Mean±SEM. *P<0.05 and **P<0.01 vs. blank, #P<0.05 and ##P<0.01 vs. Tregs (PBS), one-way ANOVA.
Selective depletion of Foxp3+ Tregs mutes IL-33 activity in AAA
Next we asked whether IL-33 suppressed AAA development via expansion of Treg cells and enhanced Treg function. To test this possibility, we depleted Tregs using the DEREG mice that express a DT (diphtheria toxin) receptor and enhanced green fluorescent protein (eGFP) fusion protein under the control of Foxp3 locus, allowing both detection and inducible depletion of Foxp3+ Treg cells.42 Prior studies showed that DT-mediated Treg depletion in DEREG mice did not cause autoimmune diseases, tissue damages, or organ inflammatory cell infiltrations, but enhanced T-cell activation with increased number of splenic CD4+CD44+CD62Llow and CD8+CD44+CD62Llow T cells. Treg depletion reduced splenic CD4+ and CD8+ T cells in DEREG mice, although such reductions did not reach statistic significance.42,43
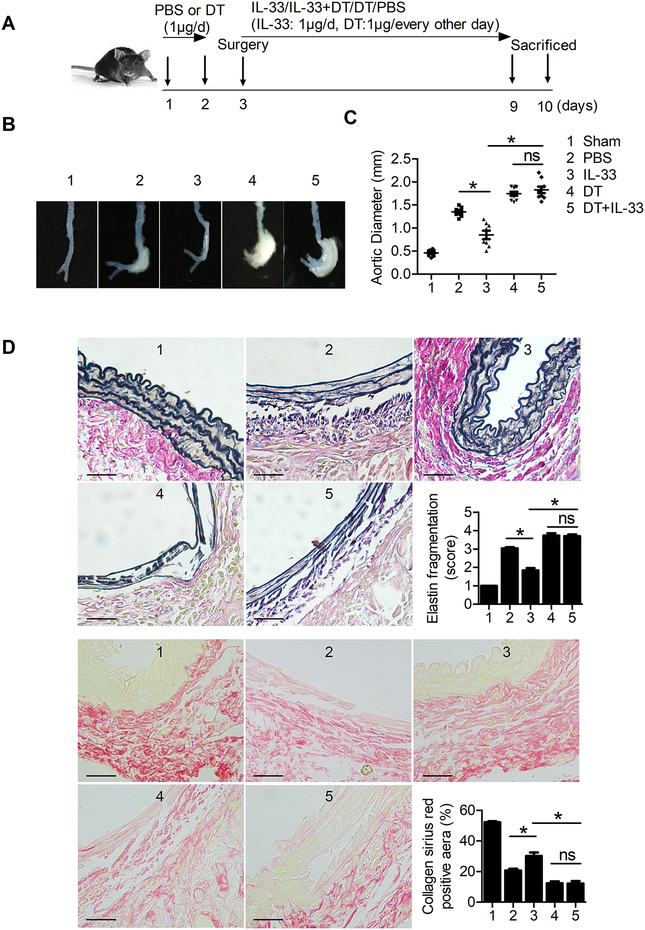
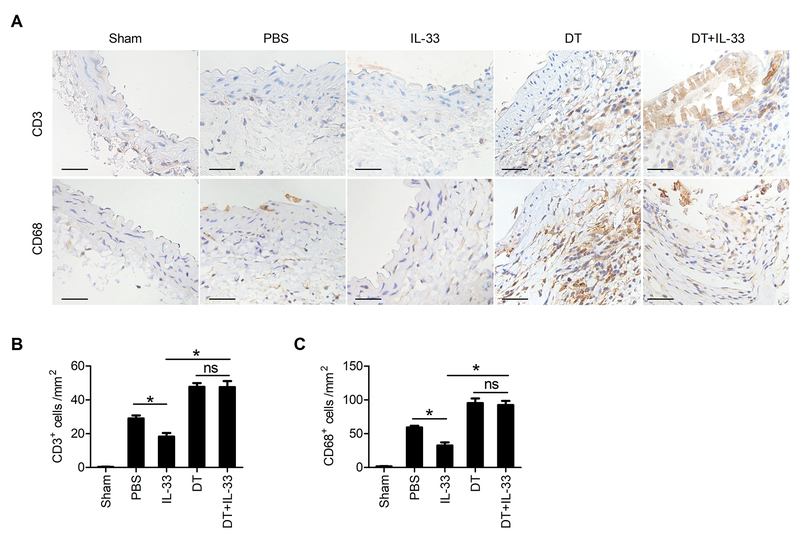
We produced CaPO4-induced AAA in DEREG mice while mice were treated with and without DT and IL-33 (Figure 6A). Flow cytometry analysis showed that DT treatment depleted splenic and blood CD4+Foxp3+ Tregs irrespective of IL-33 administration (Supplementary Figure VIIIA–B). Although the difference in survival did not differ between AAA mice with and without DT treatment (data not shown), the aortic diameters in DT-treated AAA mice were significantly bigger than those without DT treatment. IL-33 treatment reduced CaPO4-induced AAA growth only in mice without DT treatment but not in DT-treated mice (Figure 6B/6C). Upon depletion of Treg cells, the protective roles of IL-33 in suppressing aortic wall elastica fragmentation and in increased AAA lesion collagen deposition were both muted (Figure 6D). AAA lesion T-lymphocyte and macrophage infiltration (Figure 7A/7B), MMP-2 and MMP-9 expression, and TUNEL-positive apoptotic cell counts (Supplementary Figure IXA–B) were also increased in DT-treated mice. IL-33 treatment reduced these AAA lesion variables only in mice without DT treatment but not in Treg-depleted mice. To test whether increased AAA lesion size and aortic tissue remodelling (Figure 6) and lesion CD3+ T cells and CD68+ macrophages in DT-treated mice (Figure 7) were due to altered systemic inflammatory responses because of Treg depletion, we measured splenic total CD4+ T cells, lymphocytes, and splenocytes. Besides reduced splenic CD4+ T cells as expected,43 DT treatment did not affect total spenocytes, total lymphocytes (Supplementary Figure VIIIC), or bodyweight gains (not shown). Although a direct role of IL-33 on Treg in AAA development requires Treg-specific silencing of IL-33 signalling, IL-33-induced AAA reduction in DEREG control mice but not in DT-treated DEREG mice suggests an association between IL-33-Treg interaction and AAA development.
Figure 6.
CaPO4-induced AAA size, lesion elastica fragmentation, and collagen deposition in DEREG mice with different treatments, including PBS (n=8), IL-33 (n=8), diphtheria toxin (DT, n=10), or IL-33 with DT (n=9). Sham-operated DEREG mice (n=10) served as the controls. A. Experimental procedure and timeline of surgery and treatment. B. Representative photographs of abdominal aortic fragments from the 5 groups of mice. C. Maximal external diameter of the infrarenal aortas at 7 days after the operation. D. Representative images of Verhoeff–Van Gieson elastin staining and Sirius red collagen staining in 5 groups of mice and the assessment of medial elastica fragmentation and collagen deposition (n=5 per group). Scale bar: 50 μm. Data are Mean±SEM. *P<0.01, one-way ANOVA.
Figure 7.
CD3+ T cells and CD68+ macrophages in CaPO4-induced AAA lesions from DEREG mice with different treatments, including PBS (n=8), IL-33 (n=8), DT (n=10), or IL-33 with DT (n=9). Sham-operated DEREG mice (n=10) served as the controls. A. Representative images of CD3+ T cells and CD68+ macrophages in 5 groups of mice. B-C. Quantification of CD3+ T cells and CD68+ macrophages in AAA lesions. Scale bar: 50 μm. Data are Mean±SEM. *P<0.01, one-way ANOVA.
IL-33 suppresses elastase exposure-induced AAA in mice
To support our conclusion and to test further a role of IL-33 in suppressing AAA formation, we produced porcine pancreatic elastase exposure-induced AAA in mice. In this second model, IL-33 treatment also reduced the AAA lesion sizes, reduced aortic wall elastica fragmentation, and increased lesion collagen deposition (Supplementary Figure XA–C). Heat-inactivated elastase was used as experimental control. Again, IL-33 treatment markedly decreased AAA lesion T-lymphocyte and macrophage infiltration, reduced lesion cytokine and chemokine expression, promoted lesion macrophage polarization towards a M2 phenotype (Supplementary Figure XIA–C), reduced lesion MMP-2 and −9 protein and mRNA levels, and reduced lesion cell apoptosis (Supplementary Figure XIIA–F).
DISCUSSION
Emerging evidence suggests that IL-33 plays a role in regulating immune responses and associated inflammatory diseases.9 Recent studies demonstrated an increase of IL-33 in the vasculature of atherosclerosis-prone apolipoprotein E-deficient Apoe–/– mice, and IL-33 treatment reduced atherosclerosis in these mice by inducing a Th1-to-Th2 switch and ox-LDL antibody production.24 IL-33 has also been considered as a biomarker associated with the development of chronic heart failure and acute myocardial infarction. Administration of IL-33 reduced cardiac hypertrophy and myocardial fibrosis and improved cardiac function in TAC mouse models.20,22–24,44,45 Here we demonstrated a protective role of IL-33 in mouse experimental AAAs. Administration of exogenous IL-33 or over-expression of IL-33 using IL-33-transgenic mice attenuated AAA development in two independent mouse AAA models: CaPO4-ignited peri-aortic injury-induced and aortic elastase exposure-induced AAAs. Therefore, like in other cardiovascular diseases, IL-33 acted as an anti-inflammatory cytokine in AAA pathogenesis. Both CaPO4 and elastase caused damages to the aortas and induced IL-33 production. Such post-injury induction of IL-33 may act as a protective signal to the aorta. In mouse CaPO4-induced AAA lesions, we detected elevated IL-33 expression in the adventitia fibroblasts. This observation may explain why peri-aortic fibroblasts slowed the development of mouse experimental AAA. In addition to release matrix elastase inhibitors as previously reported,46 fibroblasts may also release IL-33 to slow AAA development, although our study did not provide direct evidence to support this hypothesis.
Tregs, a subpopulation of CD4+ T lymphocytes with immunosuppressive activity, are involved in the regulation of multiple diseases such as autoimmunity, inflammation, and cardiovascular diseases. Recently, a number of studies confirmed that Tregs regulate the pathogenesis of AAA.38–41,47 Analyses of the peripheral blood of AAA patients showed a reduced frequency of CD4+CD25+ T cells accompanied by decreased expression of Foxp3 and impaired immunosuppressive activity of Tregs in AAA patients.47 Treg depletion by anti-CD25 antibodies or genetic disruption of CD80/CD86 aggravated the development of angiotensin-II-induced AAA.39 Adoptive transfer or endogenous expansion of Tregs prevented AAA formation.40 All these studies identified a protective role for Tregs on AAA and suggested that targeting Tregs may become a potential treatment of AAA. In this study, we demonstrated that Tregs from IL-33-treated mice showed much greater activity in suppressing SMC production of IL-6 and MCP-1 and macrophage expression of MMP-2/9 than control Tregs. Although not tested in this study, Tregs are known to suppress T-cell activation and proliferation.48 IL-33-expanded Tregs exhibited elevated immunosuppressive capacity against the proliferation of CD4+ and CD8+ T cells.49 The activities of IL-33-treated Tregs in reducing vascular cell expression of MCP-1 and possibly other untested chemokines and in regulating T-cell activation and proliferation may all contribute to the reduced AAA lesion CD3+ T cells and CD68+ macrophages that we detected from IL-33-treated mice. Macrophages exist in different phenotypes in the innate immune system, including the pro-inflammatory M1 phenotype and the anti-inflammatory M2 phenotype.50 M1 macrophages were increased in human and mouse models of AAA. Adoptive transfer of M2 macrophages preserved arterial wall elastica.51 Here we found that IL-33 increased M2-type macrophages and associated gene expression but reduced M1-type macrophages and their gene expression in AAA lesions. We also demonstrated that IL-33-expanded Tregs had much greater activity to induce the M1-to-M2 switch than control Tregs. Using ST2-deficient mice and Treg-depleted DEREG mice, we report that IL-33 protected mice from AAA development by inducing ST2-dependent Treg proliferation. IL-33 failed to induce Treg expansion in ST2-deficient mice and lost its AAA-protective activity in Treg-depleted DEREG mice. Although we proposed the hypothesis that IL-33 may act directly on the ST2 on Tregs and affect Treg biology, it is possible that IL-33 may affect Treg biology indirectly. Recent study by Molofsky et al. indicated that IL-33-activated ILC2s also induced Treg accumulation through the interactions between Treg ICOS and ILC2 ICOSL.13 Morita et al. reported that IL-33 promoted Treg expansion by stimulating mast cells to produce IL-2.52 These prior studies suggest that IL-33 may also use ILC2, mast cells, and other unidentified cells to affect Treg proliferation and immunosuppressive activity to block AAA development, a possibility that merits further investigation.
Together, our study provided evidence from two experimental AAA models that IL-33 plays a protective role in attenuating AAA growth by controlling Treg proliferation and its immunosuppressive activities. Recombinant IL-33 or regimens to increase endogenous IL-33 expression may restrict the progression of mature AAA or prevent human AAA development.
Supplementary Material
HIGHLIGHTS.
Mouse AAA lesions contained much greater levels of IL-33 than normal aortas. Exogenous IL-33 or transgenic expression of IL-33 reduced AAA progression, lesion inflammation, and aortic wall remodeling.
IL-33 treatment increased regulatory T-cell (Treg) expansion in the spleens, blood, and aortas. Tregs from IL-33-treated mice demonstrated significantly higher activities in suppressing smooth muscle cell chemokine and cytokine expression, macrophage matrix metalloproteinase expression, and in increasing M2 macrophage polarization than those from PBS-treated mice.
In mice with selectively depleted Tregs, IL-33 no longer protected mice from AAA formation, suggesting that Tregs mediated the IL-33 activities in protecting mice from AAA formation.
ACKNOWLEDGEMENTS
We thank Prof. Rong Mu (Department of Rheumatology and Immunology, Peking University Peoples Hospital) for kindly providing the IL-33 transgenic mice and Prof. Andrew McKenzie (Medical Research Council Laboratory of Molecular Biology, University of Cambridge, Cambridge, U.K.) for providing the ST2–/– mice.
SOURCES OF FUNDING
This work was supported by grants from the National Natural Science Foundation of China [No. 81525003, 81720108005, 91639301 to X.C.; No. 81400364, 81770503 to N.X.; No. 81670361 to T.T.T.; No. 81500186 to S.F.N.; No. 81600390 to M.Y.L.], the Fundamental Research Funds for the Central Universities [2017KFYXJJ227 to X.C.; 2016YXMS246 to N.X.], and the National Institute of Health [HL123568 and HL60942 to GPS].
Nonstandard Abbreviations and Acronyms:
- AAA
abdominal aortic aneurysm
- MMP
matrix metalloproteinase
- ECM
extracellular matrix
- SMCs
smooth muscle cells
- ILC2
type 2 innate lymphoid cell
- IL-33TG
IL-33-transgenic mice
- NTG
non-transgenic control mice
- WT
wild-type
- RT-PCR
reverse transcription polymerase chain reaction
- Tregs
regulatory T-cell
- PBS
phosphate buffer saline
- DEREG
depletion of regulatory T-cell
- DT
diphtheria toxin
- APOE
apolipoprotein E
- ox-LDL
oxidatively modified low-density lipoprotein
- TAC
transverse aortic constriction
Footnotes
DISCLOSURES
None.
REFERENCES:
- 1.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8:92–102. [DOI] [PubMed] [Google Scholar]
- 2.Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of AAA progression. Part 1: extracellular matrix degeneration. Nat Rev Cardiol. 2009;6:464–474. [DOI] [PubMed] [Google Scholar]
- 3.Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of abdominal aortic aneurysm progression. Part 2: inflammation. Nat Rev Cardiol. 2009;6:543–552. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006; 26(5): 987–994. [DOI] [PubMed] [Google Scholar]
- 5.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michel JB, Martin-Ventura JL, Egido J, Sakalihasan N, Treska V, Lindholt J, Allaire E, Thorsteinsdottir U, Cockerill G, Swedenborg J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–110. [DOI] [PubMed] [Google Scholar]
- 8.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011: 103–110. [DOI] [PubMed] [Google Scholar]
- 9.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in Tissue Homeostasis, Injury and Inflammation. Immunity. 2015;42:1005–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. [DOI] [PubMed] [Google Scholar]
- 11.Baumann C, Bonilla WV, Fröhlich A, Helmstetter C, Peine M, Hegazy AN, Pinschewer DD, Löhning M. T-bet- and STAT4-dependent IL-33 receptor expression directly promotes antiviral Th1 cell responses. Proc Natl Acad Sci U S A. 2015;112:4056–4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiering C, Krausgruber T, Chomka A, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Löhning M, Belkaid Y, Fallon PG, Powrie F. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513;564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molofsky AB, Gool van F, Liang HE, Dyken van SJ, Nussbaum JC, Lee J, Bluestone JA, Locksley RM. Interleukin-33 and Interferon-γ Counter-Regulate Group 2 Innate Lymphoid Cell Activation during Immune Perturbation. Immunity. 2015;43:161–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–5750. [DOI] [PubMed] [Google Scholar]
- 15.Zhiguang X, Wei C, Steven R, Wei D, Wei Z, Rong M, Zhanguo L, Lianfeng Z. Over-expression of IL-33 leads to spontaneous pulmonary inflammation in mIL-33 transgenic mice. Immunol Lett. 2010;131:159–165. [DOI] [PubMed] [Google Scholar]
- 16.Duan L, Chen J, Zhang H, Yang H, Zhu P, Xiong A, Xia Q, Zheng F, Tan Z, Gong F, Fang M. Interleukin-33 ameliorates experimental colitis through promoting Th2/Foxp3⁺ regulatory T-cell responses in mice. Mol Med. 2012;18:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Besnard AG, Guabiraba R, Niedbala W, Palomo J, Reverchon F, Shaw TN, Couper KN, Ryffel B, Liew FY. IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells; M2 macrophages regulatory T cells. PLoS Pathog. 2015;11:e1004607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao K, Li X, Zhang L, Bai L, Dong W, Gao K, Shi G, Xia X, Wu L, Zhang L. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells inhibits tumor growth and metastasis in mice. Cancer Lett. 2013;335:463–471. [DOI] [PubMed] [Google Scholar]
- 19.Barbour M, Allan D, Xu H, Pei C, Chen M, Niedbala W, Fukada SY, Besnard AG, Alves-Filho JC, Tong X, Forrester JV, Liew FY, Jiang HR. IL-33 attenuates the development of experimental autoimmune uveitis. Eur J Immunol. 2014;44:3320–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demyanets S, Kaun C, Pentz R, Krychtiuk KA, Rauscher S, Pfaffenberger S, Zuckermann A, Aliabadi A, Gröger M, Maurer G, Huber K, Wojta J. Components of the interleukin-33/ST2 system are differentially expressed and regulated in human cardiac cells in cells of the cardiac vasculature. J Mol Cell Cardiol. 2013;60:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seki K, Sanada S, Kudinova AY, Steinhauser ML, Ha V, Gannon J, Lee RT. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009;2:684–691. [DOI] [PubMed] [Google Scholar]
- 23.Veeraveedu PT, Sanada S, Okuda K, Fu HY, Matsuzaki T, Araki R, Yamato M, Yasuda K, Sakata Y, Yoshimoto T, Minamino T. Ablation of il-33 gene exacerbate myocardial remodeling in mice with heart failure induced by mechanical stress. Biochem Pharmacol. 2017;138:73–80. [DOI] [PubMed] [Google Scholar]
- 24.Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, Baker AH, McInnes IB, Liew FY. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robinet P, Milewicz DM, Cassis LA, Leeper NJ, Lu HS, Smith JD. Consideration of sex differences in design and reporting of experimental arterial pathology studies-statement from atvb council. Arterioscler Thromb Vasc Biol. 2018;38:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamanouchi D, Morgan S, Stair C, Seedial S, Lengfeld J, Kent KC, Liu B. Accelerated aneurysmal dilation associated with apoptosis and inflammation in a newly developed calcium phosphate rodent abdominal aortic aneurysm model. J Vasc Surg. 2012;56:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao F, Chambon P, Offermanns S, et al. Disruption of TGF-b signaling in smooth muscle cell prevents elastase-induced abdominal aortic aneurysm. Biochemical and Biophysical Research Communications. 2014;454:137–143. [DOI] [PubMed] [Google Scholar]
- 28.Wei Z, Wang Y, Zhang K, et al. Inhibiting the Th17/IL-17A-related inflammatory responses with digoxin confers protection against experimental abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2014;34:2429–2438. [DOI] [PubMed] [Google Scholar]
- 29.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun W, Pang Y, Liu Z, Sun L, Liu B, Xu M, Dong Y, Feng J, Jiang C, Kong W, Wang X. Macrophage inflammasome mediates hyperhomocysteinemia-aggravated abdominal aortic aneurysm. J Mol Cell Cardiol. 2015;81:96–106. [DOI] [PubMed] [Google Scholar]
- 31.Lefrancais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. Il-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin g. Proc Natl Acad Sci U S A. 2012;109:1673–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai L, Li Z, Li Q, Guan H, Zhao S, Liu R, Wang R, Zhang J, Jia Y, Fan J, Wang N, Reddy JK, Shyy JY, Liu E. Mediator 1 is atherosclerosis protective by regulating macrophage polarization. Arterioscler Thromb Vasc Biol. 2017;37:1470–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takamura M, Kurokawa K, Ootsuji H, Inoue O, Okada H, Nomura A, Kaneko S, Usui S. Long-term administration of eicosapentaenoic acid improves post-myocardial infarction cardiac remodeling in mice by regulating macrophage polarization. J Am Heart Assoc. 2017;6:e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murakami-Satsutani N, Ito T, Nakanishi T, Inagaki N, Tanaka A, Vien PTX, Kibata K, Inaba M, Nomura S. Il-33 promotes the induction and maintenance of th2 immune responses by enhancing the function of ox40 ligand. Allergol Int. 2014;63:443–455. [DOI] [PubMed] [Google Scholar]
- 35.Krueger WA, Lenhart FP, Neeser G, Ruckdeschel G, Schreckhase H, Eissner HJ, Forst H, Eckart J, Peter K, Unertl KE. Influence of combined intravenous and topical antibiotic prophylaxis on the incidence of infections, organ dysfunctions, and mortality in critically ill surgical patients: A prospective, stratified, randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med. 2002;166:1029–1037. [DOI] [PubMed] [Google Scholar]
- 36.Schonbeck U, Sukhova GK, Gerdes N, Libby P. T(h)2 predominant immune responses prevail in human abdominal aortic aneurysm. Am J Pathol. 2002;161:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curci JA, Thompson RW. Adaptive cellular immunity in aortic aneurysms: Cause, consequence, or context? J Clin Invest. 2004;114:168–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Wu W, Lindholt JS, Sukhova GK, Libby P, Yu X, Shi GP. Regulatory T cells in human angiotensin II-induced mouse abdominal aortic aneurysms. Cardiovasc Res. 2015;107:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ait-Oufella H, Wang Y, Herbin O, Bourcier S, Potteaux S, Joffre J, Loyer X, Ponnuswamy P, Esposito B, Dalloz M, Laurans L, Tedgui A, Mallat Z. Natural regulatory T cells limit angiotensin II-induced aneurysm formation and rupture in mice. Arterioscler Thromb Vasc Biol. 2013;33:2374–2379. [DOI] [PubMed] [Google Scholar]
- 40.Meng X, Yang J, Zhang K, An G, Kong J, Jiang F, Zhang Y, Zhang C. Regulatory T cells prevent angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E knockout mice. Hypertension. 2014;64:875–882. [DOI] [PubMed] [Google Scholar]
- 41.Yodoi K, Yamashita T, Sasaki N, Kasahara K, Emoto T, Matsumoto T, Kita T, Sasaki Y, Mizoguchi T, Sparwasser T, Hirata K. Foxp3+ regulatory T cells play a protective role in angiotensin II-induced aortic aneurysm formation in mice. Hypertension. 2015;65:889–895. [DOI] [PubMed] [Google Scholar]
- 42.Lahl K, Loddenkemper C, Drouin C, Freyer J, Arnason J, Eberl G, Hamann A, Wagner H, Huehn J, Sparwasser T. Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med. 2007;204:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayer CT, Ghorbani P, Kuhl AA, Stuve P, Hegemann M, Berod L, Gershwin ME, Sparwasser T. Few foxp3(+) regulatory t cells are sufficient to protect adult mice from lethal autoimmunity. Eur J Immunol. 2014;44:2990–3002. [DOI] [PubMed] [Google Scholar]
- 44.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-Más J, Lax A, Asensio-López Mdel C, Fernandez-Del Palacio MJ, Caballero L, Santarelli G, Januzzi JL, Pascual-Figal DA. Modulation of IL-33/ST2 system in postinfarction heart failure: correlation with cardiac remodelling markers. European Journal Of Clinical Investigation. 2014;44:643–651. [DOI] [PubMed] [Google Scholar]
- 46.Giraud A, Zeboudj L, Vandestienne M, Joffre J, Esposito B, Potteaux S, Vilar J, Cabuzu D, Kluwe J, Seguier S, Tedgui A, Mallat Z, Lafont A, Ait-Oufella H. Gingival fibroblasts protect against experimental abdominal aortic aneurysm development and rupture through tissue inhibitor of metalloproteinase-1 production. Cardiovasc Res. 2017;113:1364–1375. [DOI] [PubMed] [Google Scholar]
- 47.Yin M, Zhang J, Wang Y, Wang S, Böckler D, Duan Z, Xin S. Deficient CD4+CD25+ T regulatory cell function in patients with abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2010;30:1825–1831. [DOI] [PubMed] [Google Scholar]
- 48.Joly AL, Seitz C, Liu S, Kuznetsov NV, Gertow K, Westerberg LS, Paulsson-Berne G, Hansson GK, Andersson J. Alternative splicing of foxp3 controls regulatory t cell effector functions and is associated with human atherosclerotic plaque stability. Circ Res. 2018;122:1385–1394. [DOI] [PubMed] [Google Scholar]
- 49.Biton J, Khaleghparast Athari S, Thiolat A, Santinon F, Lemeiter D, Herve R, Delavallee L, Levescot A, Roga S, Decker P, Girard JP, Herbelin A, Boissier MC, Bessis N. In vivo expansion of activated foxp3+ regulatory t cells and establishment of a type 2 immune response upon il-33 treatment protect against experimental arthritis. J Immunol. 2016;197:1708–1719. [DOI] [PubMed] [Google Scholar]
- 50.Wang N, Liang H, Zen K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014;5:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dale MA, Xiong W, Carson JS, Suh MK, Karpisek AD, Meisinger TM, Casale GP, Baxter BT. Elastin-Derived Peptides Promote Abdominal Aortic Aneurysm Formation by Modulating M1/M2 Macrophage Polarization. J Immunol. 2016;106:4536–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morita H, Arae K, Unno H, et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity. 2015;43:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.