Abstract
Background/Objective:
The premature mortality among patients with end-stage renal disease due to lupus nephritis (LN-ESRD) persisted in the U.S. between 1995 and 2006. We extended the analysis until 2014 for the latest trend and also examined key cause-specific mortality trends.
Methods:
Using the national registry of ESRD patients, we identified all patients with incident LN-ESRD between January 1, 1995 and December 31, 2014, divided into four five-year cohorts by calendar year of ESRD onset (1995–1999, 2000–2004, 2005–2009, 2010–2014). We assessed mortality rates within each cohort. We examined temporal trends in all-cause mortality and cause-specific mortality, adjusting for covariates.
Results:
We identified 20,974 individuals with incident LN-ESRD between 1995–2014. The mortality rate per 100 patient-years declined from 11.1 (95% CI 10.4–11.8) in 1995–1999 to 6.7 (95% CI 6.2–7.2) in 2010–2014 (p trend <0.01). Adjusted mortality hazard ratios in 2010–2014, compared with 1995–1999 were 0.68 (95% CI 0.58–0.78) for white patients, 0.67 (95% CI 0.57–0.78) for African Americans, and 0.51 (95% CI 0.38–0.69) for Hispanics. Deaths due to cardiovascular disease (CVD) and infection declined by 44% and 63%, respectively, from 1995–1999 to 2010–2014 (both p trend <0.01).
Conclusion:
Between 1995 and 2014, there was a considerable reduction in all-cause mortality among white, African American, and Hispanic patients in recent years, with reduced risk of death due to CVD and infections. Collectively, these trends provide an important benchmark of improving care in this high-risk population.
Systemic lupus erythematosus (SLE) is associated with multiple morbidities and premature mortality.(1–5) Lupus nephritis (LN) affects up to 50% of adults with SLE, and despite the introduction of improved, lower toxicity treatments in the past 15 years, including mycophenolate and low dose cyclophosphamide regimens,(6–8) up to 30% of patients with lupus nephritis progress to end-stage renal disease (ESRD).(9) Mortality among SLE patients is highest among this subgroup.(10) Compared to white patients, African Americans with LN-ESRD have increased mortality,(11) mediated in part by socioeconomic factors.(12)
The premature mortality among patients with LN-ESRD persisted in the US between 1995 and 2006,(13) but it is unknown whether there has since been a significant change in survival. To address this important gap in knowledge, we examined temporal mortality trends, including key cause-specific mortality trends, among patients with LN-ESRD in the U.S. extended from 1995 to 2014 using a national ESRD registry.
PATIENTS AND METHODS
Data Source and Study Population
We identified all patients with SLE (ICD-9: 710.0) indicated as the attributed cause of ESRD (i.e., LN-ESRD) who were registered in the United States Renal Data System (USRDS) between January 1, 1995 and December 31, 2014.(14) The USRDS is the national registry of ESRD patients in the U.S., representing > 94% of all patients who receive renal replacement therapy.(15) As a requirement for enrollment in Medicare, which pays for ESRD therapy for all U.S. patients eligible for Social Security, attending nephrologists are required to submit a Centers for Medicare and Medicaid Services (CMS) Medical Evidence Report (CMS 2728), which includes the cause of ESRD according to International Classification of Diseases, Ninth Revision (ICD-9) codes, within 45 days of a patient starting ESRD treatment. The accuracy of coding of SLE as the primary cause of ESRD in the USRDS has been previously studied and had 93% positive predictive value.(14)
Incident LN-ESRD patients were divided into four five-year sub-cohorts based on year of ESRD onset (1995–1999, 2000–2004, 2005–2009, and 2010–2014). We determined the date of ESRD onset as the earliest of the dialysis start date or date of kidney transplant. From the USRDS, we also obtained demographics (i.e., age, sex, and race/ethnicity), body mass index (BMI) at enrollment, U.S. Census region of residence (i.e., Northeast, Midwest, South, and West), relevant baseline comorbidities (i.e., diabetes, hypertension, coronary artery disease), initial ESRD therapy modality (i.e., renal transplant, hemodialysis, or peritoneal dialysis), death, and cause of death. Race (e.g., white, African American, Asian, other) and ethnicity (e.g., Hispanic and non-Hispanic) categories are not mutually exclusive. Death was determined by the CMS ESRD Death Notification Form (CMS-2746), also mandatory for attending nephrologists to complete.
Annual U.S. population estimates were obtained from the U.S. Census Bureau.(16)
Statistical Analysis
We compared baseline characteristics of individuals in the four 5-year sub-cohorts. The annual incidence rates (IR) of LN-ESRD, per million individuals in the U.S. population, and 95% confidence intervals were calculated for each 5-year period. We calculated mortality rates per 100 patient-years for each 5-year sub-cohort using Poisson regression. Patient-years of follow up for each subject were calculated as the amount of time from the index date (i.e., ESRD treatment initiation date) until either death or censoring at the end of their 5-year sub-cohort period (i.e., December 31, 1999 for the first sub-cohort) to ensure fair follow up time across sub-cohorts.(5, 17, 18) We compared all-cause mortality for each sub-cohort, with the 1995–1999 sub-cohort as the reference group, using Cox proportional hazards models. We adjusted for age, sex, and BMI in partially adjusted models. We additionally adjusted for smoking status, comorbidities at time of ESRD treatment onset (i.e., diabetes, hypertension, coronary artery disease, congestive heart failure, and cerebrovascular accident), geographic region, and first ESRD treatment modality in a fully-adjusted model. We performed subgroup analyses stratified by race (i.e., white, African American, Asian/Pacific Islander, and other), as well as by ethnicity (i.e., Hispanic and non-Hispanic). Patients with missing covariates were excluded from all models.
We also assessed the mortality trends due to cardiovascular disease, infection, and other/unknown causes as listed on form CMS-2746. Cardiovascular deaths included acute myocardial infarction, pericarditis/tamponade, atherosclerotic heart disease, cardiomyopathy, cardiac arrhythmia, cardiac arrest (cause unknown), valvular heart disease, congestive heart failure, cerebrovascular accident, and pulmonary embolism. Infectious deaths included septicemia due to internal vascular access/vascular access catheter, peritoneal access infectious complication (bacterial or fungal), peritonitis, central nervous system infection, septicemia due to peripheral vascular disease/gangrene, other septicemia, endocarditis, pulmonary infection, abdominal infection, and genitourinary infection. We adjusted for the competing risk of death from other causes in cause-specific death analyses using the Fine and Gray method.(19)
All p values were 2-sided with a significance threshold of alpha< 0.05. Statistical analyses were performed using SAS, version 9.4.
Data Use Agreement and Institutional Review
The data reported here have been supplied by the USRDS under an approved data use agreement. The interpretation and reporting of these data are the responsibility of the authors and should not be seen as official policy or interpretation of the U.S. government. This study was exempted from the Partner’s HealthCare Institutional Review Board.
RESULTS
Baseline Characteristics
Between 1995 and 2014, 20,974 individuals developed LN-ESRD in the U.S. (Table 1). The mean age at ESRD onset was 40 years, and 82% of subjects were female. African Americans comprised 48% of all subjects. Mean BMI rose from mean 24.8 kg/m2 (SD 6.7) between 1995–1999 to 27.3 kg/m2 (SD 7.8) between 2010–2014. The incidence of comorbid diabetes and hypertension increased (5.9 to 9.7 % and 69.9 to 85.7%, respectively) in more recent sub-cohorts, while congestive heart failure slightly declined (16.3 to 13.7%). Hemodialysis was the most frequent initial ESRD therapy throughout the study period (83–86%). The frequency of pre-emptive renal transplant as the initial ESRD modality rose from 2.2% between 1995–1999 to 4.4% between 2010–2014.
Table 1:
Characteristics of Patients with ESRD due to Lupus Nephritis in the United States between 1995–2014
| Year of Initial ESRD | 1995–1999 | 2000–2004 | 2005–2009 | 2010–2014 |
|---|---|---|---|---|
| Number of Cases | 4,861 | 5,413 | 5,540 | 5,160 |
| Annual Incidence (95% CI)* | 3.6 (3.4–3.8) | 3.8 (3.6–4.0) | 3.7 (3.5–3.9) | 3.3 (3.1–3.5) |
| Demographics | ||||
| Age (Mean, SD) | 40.1 (15.3) | 39.9 (15.5) | 39.4 (15.4) | 40.5 (15.7) |
| Sex (Female %) | 81.9 | 81.6 | 81.8 | 81.7 |
| Race (%) | ||||
| White | 42.4 | 41.8 | 41.8 | 43.3 |
| African American | 45.9 | 48.0 | 50.1 | 48.6 |
| Asian | 3.5 | 3.3 | 5.1 | 5.8 |
| Other | 8.4 | 6.9 | 3 | 2.3 |
| Hispanic | 15.9 | 17.8 | 19.6 | 19.4 |
| BMI (kg/m2, SD) | 24.8 (6.7) | 26.1 (7.0) | 26.8 (7.4) | 27.3 (7.8) |
| Region† (%) | ||||
| Northeast | 13.8 | 13.5 | 13.0 | 13.4 |
| Midwest | 19.2 | 18.8 | 17.8 | 17.1 |
| South | 41.6 | 43.0 | 45.6 | 44.6 |
| West | 20.1 | 19.9 | 19.1 | 20.7 |
| Comorbid Conditions (%) | ||||
| Diabetes | 5.9 | 3.9 | 9.4 | 9.7 |
| Hypertension | 69.9 | 75.9 | 82.7 | 85.7 |
| Coronary Artery Disease | 6.8 | 7.3 | 5.2 | 4.8 |
| Congestive Heart Failure | 16.3 | 15.2 | 13.8 | 13.7 |
| Peripheral Vascular Disease | 3.0 | 3.5 | 3.2 | 2.6 |
| Stroke or TIA | 5.0 | 5.3 | 5.2 | 5.2 |
| Current Smoking | 3.7 | 4.0 | 4.2 | 4.3 |
| History of Malignancy | 1.3 | 1.5 | 1.5 | 2.0 |
| Initial ESRD Treatment (%) | ||||
| Transplant | 2.2 | 3.0 | 3.8 | 4.4 |
| Hemodialysis | 83.3 | 86.4 | 86.3 | 82.6 |
| Peritoneal Dialysis | 14.5 | 10.6 | 9.9 | 12.9 |
BMI, body mass index; TIA, Transient Ischemic Attack
/1,000,000 US Population;
These four regions capture all of the United States. Patients from Puerto Rico, other US territories, and foreign countries were excluded from these analyses since US population census estimates do not include these individuals.
1,025 individuals (4.9%) were excluded from all models due to missing covariates.
Incidence of LN-ESRD
The overall IR of LN-ESRD per million U.S. population remained stable from 3.6 (95% CI 3.4–3.8) in the first sub-cohort (1995–1999) to 3.7 (95% CI 3.5–3.9) in the third sub-cohort (2005–2009), and then declined to 3.3 (95% CI 3.1–3.5) in the latest sub-cohort (2010–2014) (p trend 0.01).
Renal Transplantation
The frequency of undergoing renal transplantation, either as the initial modality or later receiving a renal transplant during each 5-year sub-cohort, remained stable over the study period (20.6% between 1995–1999, 20.4% between 2000–2004, 21.9% between 2005–2009, and 19.8% between 2010–2014, p trend=0.74). The mean duration of time between entering the USRDS and the time of renal transplantation among the recipients declined over the study period (1.29 years [SD 1.06] between 1995–1999, 1.25 [1.14] between 2000–2004, 1.27 [1.17] between 2005–2009, and 1.07 [1.12] between 2010–2014, p trend <0.01).
Mortality Trends
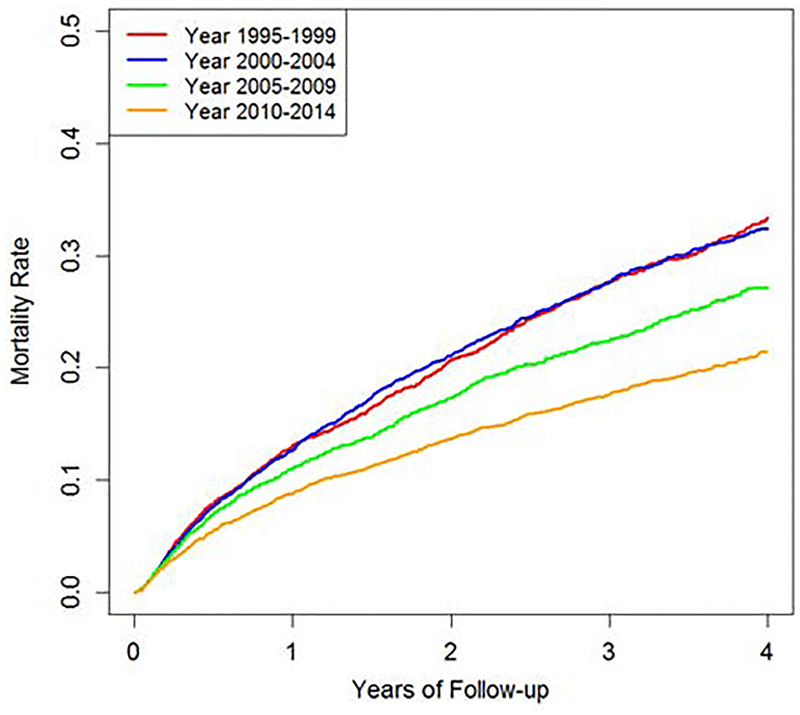
A total of 4,131 patients with LN-ESRD died during study follow-up (19.7%). The mortality rates per 100 patient-years were similar in the first two sub-cohorts, 1995–1999 and 2000–2004 (11.1 [95% CI 10.4–11.8] and 11.0 [95% CI 10.4–11.6], respectively), but declined significantly in the latest decade during the study, 2005–2009 and 2010–2014 (8.9 [95% CI 8.4–9.4] and 6.7 [95% CI 6.2–7.2], respectively, p trend <0.001) (Table 2 and Figure 1). The fully-adjusted hazard ratio (HR) for all-cause mortality in 2010–2014 was 0.68 (95% CI 0.61–0.75) compared to the reference sub-cohort, 1995–1999 (Table 2).
Table 2:
Temporal Trends in Risk of Death Among Persons with ESRD due to Lupus Nephritis (1995–2014)
| Year of ESRD Initiation | N | Follow-up Time (PY) | Number of Deaths* | Incidence of Death/ 100 PY (95% CI) |
Unadjusted HR (95% CI) |
Age-, Sex- Adjusted HR (95% CI) | Multivariate Adjusted† HR (95% CI) |
|---|---|---|---|---|---|---|---|
| 1995–1999 | 4,861 | 9,763 | 1,081 | 11.1 (10.4–11.8) |
1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| 2000–2004 | 5,413 | 11,001 | 1,209 | 11.0 (10.4–11.6) |
1.03 (0.94–1.13) |
1.04 (0.95–1.14) |
1.08 (0.99–1.18) |
| 2005–2009 | 5,540 | 11,958 | 1,062 | 8.9 (8.4–9.4) |
0.83 (0.76–0.91) |
0.85 (0.78–0.94) |
0.90 (0.82–0.99) |
| 2010–2014 | 5,160 | 11,640 | 779 | 6.7 (6.2–7.2) |
0.63 (0.57–0.70) |
0.63 (0.57–0.69) |
0.68 (0.61–0.75) |
| P-for-trend | <0.001 | <0.001 | <0.001 | <0.001 | |||
PY, patient years; HR, hazard ratio
Number of deaths and total follow up determined using the end of the sub-cohort (e.g., December 31st 1999 for the 1995–1999 sub-cohort) as the censoring date.
Adjusted for age, sex, BMI, diabetes, hypertension, current smoker, coronary artery disease, congestive heart failure, cerebrovascular accident, region, and first ESRD treatment modality
Figure 1: Cumulative Incidence of All-Cause Mortality in ESRD due to Lupus Nephritis (1995–2014).
Cumulative incidence function estimates for all-cause mortality by period of ESRD onset among patients with ESRD due to lupus nephritis in the U.S., 1995–2014
When patients were analyzed by race and ethnicity, the mortality rates had similar trends across the 5-year sub-cohort periods (Table 3). African American and white patients had similar reductions in mortality risk over time, with fully-adjusted HRs of 0.67 (95% CI 0.57–0.78) and 0.68 (0.58–0.78), respectively, in the final sub-cohort (2010–2014) compared with the first sub-cohort (1995–1999). The corresponding adjusted mortality HR was 0.51 (95% CI 0.38–0.69) in Hispanic patients. Asian LN-ESRD patients did not have significantly improved survival over time; however, the number of Asian patients were small, less than one third that of the Hispanic group (Table 3).
Table 3:
Temporal Trends in Risk of Death in ESRD due to Lupus Nephritis (1995–2014) Stratified by Race/Ethnicity
| Year of ESRD Initiation | N | Follow-up Time (PY) | Number of Deaths | Unadjusted HR (95% CI) |
Age-, Sex-, BMI-Adjusted HR (95% CI) | Multivariate Adjusted† HR (95% CI) |
|---|---|---|---|---|---|---|
| White | ||||||
| 1995–1999 | 2,063 | 4,090 | 490 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2000–2004 | 2,265 | 4,521 | 548 | 1.02 (0.90–1.17) | 1.04 (0.91–1.19) | 1.08 (0.95–1.23) |
| 2005–2009 | 2,317 | 5,008 | 452 | 0.77 (0.67–0.89) | 0.80 (0.70–0.92) | 0.93 (0.80–1.06) |
| 2010–2014 | 2,236 | 4,948 | 356 | 0.62 (0.54–0.72) | 0.62 (0.53–0.72) | 0.68 (0.58–0.78) |
| P-for-trend | <0.001 | <0.001 | <0.001 | |||
| African American | ||||||
| 1995–1999 | 2,231 | 4,402 | 497 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2000–2004 | 2,599 | 5,197 | 573 | 1.03 (0.90–1.17) | 1.03 (0.90–1.17) | 1.07 (0.94–1.23) |
| 2005–2009 | 2,777 | 5,879 | 555 | 0.88 (0.77–0.99) | 0.88 (0.77–0.99) | 0.87 (0.76–1.00) |
| 2010–2014 | 2,505 | 5,683 | 380 | 0.63 (0.55–0.73) | 0.62 (0.54–0.72) | 0.67 (0.57–0.78) |
| P-for-trend | <0.001 | <0.001 | <0.001 | |||
| Asian | ||||||
| 1995–1999 | 170 | 397 | 24 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2000–2004 | 177 | 408 | 22 | 0.88 (0.46–1.68) | 0.97 (0.51–1.86) | 1.22 (0.63–2.37) |
| 2005–2009 | 285 | 671 | 33 | 0.87 (0.49–1.57) | 0.94 (0.52–1.69) | 1.08 (0.59–1.99) |
| 2010–2014 | 301 | 717 | 29 | 0.72 (0.40–1.32) | 0.75 (0.41–1.37) | 0.90 (0.48–1.68) |
| P-for-trend | 0.20 | 0.31 | 0.56 | |||
| Hispanic | ||||||
| 1995–1999 | 773 | 1,632 | 126 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2000–2004 | 965 | 2,131 | 152 | 0.93 (0.72–1.20) | 0.96 (0.75–1.24) | 0.99 (0.76–1.28) |
| 2005–2009 | 1,085 | 2,493 | 128 | 0.70 (0.52–0.88) | 0.69 (0.53–0.89) | 0.69 (0.52–0.90) |
| 2010–2014 | 1,003 | 2,431 | 89 | 0.48 (0.36–0.64) | 0.47 (0.35–0.63) | 0.51 (0.38–0.69) |
| P-for-trend | <0.001 | <0.001 | <0.001 | |||
Adjusted for age, sex, BMI, diabetes, hypertension, current smoker, coronary artery disease, congestive heart failure, cerebrovascular accident, region, and first modality
Cause-Specific Mortality Trends
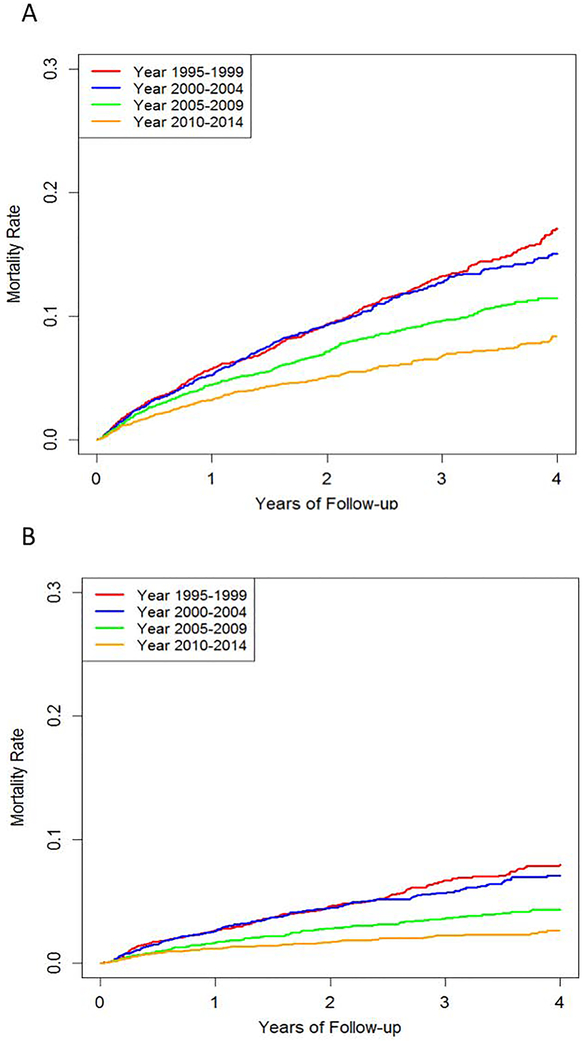
Cardiovascular disease (CVD) and infections were the first and second leading causes of death, respectively, among all patients with incident LN-ESRD. The rates of death due to CVD and infection both declined over the study period, similar to the all-cause mortality trend (Figure 2). The risk of death due to CVD declined by 44% in the latest sub-cohort (2010–2014) compared to the first sub-cohort (1995–1999) (adjusted HR 0.56 [95% CI 0.48–0.67]), after accounting for competing risks (Table 4). The risk of death due to infections also declined over the study period, with a 63% reduction in the most recent sub-cohort compared with the first sub-cohort (adjusted HR 0.37 [95% CI 0.29–0.47]), after accounting for competing risks. (Table 4). The cause of death was unknown in 826 subjects (20% overall).
Figure 2: Cumulative Incidence of Cause-Specific Mortality in ESRD due to Lupus Nephritis (1995–2014).
A. Mortality Due to Cardiovascular Disease B. Mortality Due to Infection
Cumulative incidence function estimates for mortality due to (A) cardiovascular disease and (B) infection, accounting for competing risk of death, by period of ESRD onset among patients with ESRD due to lupus nephritis in the U.S., 1995–2014.
Table 4:
Temporal Trends in Cause-Specific Risk of Death in ESRD due to Lupus Nephritis (1995–2014)
| Year of ESRD Initiation | N | Number of Deaths | Unadjusted HR (95% CI) |
Age-, Sex-, BMI-Adjusted HR (95% CI) | Multivariate† Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Cardiovascular Disease | |||||
| 1995–1999 | 4,861 | 475 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2000–2004 | 5,413 | 501 | 0.95 (0.83–1.08) | 0.96 (0.84–1.09) | 1.01 (0.88–1.17) |
| 2005–2009 | 5,540 | 414 | 0.76 (0.66–0.87) | 0.77 (0.68–0.89) | 0.82 (0.71–0.95) |
| 2010–2014 | 5,160 | 279 | 0.53 (0.45–0.61) | 0.53 (0.45–0.61) | 0.56 (0.48–0.67) |
| P-for-trend | <0.001 | <0.001 | <0.001 | ||
| Infection | |||||
| 1995–1999 | 4,861 | 218 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2000–2004 | 5,413 | 227 | 0.91 (0.75–1.10) | 0.91 (0.75–1.11) | 0.91 (0.74–1.12) |
| 2005–2009 | 5,540 | 152 | 0.57 (0.46–0.70) | 0.58 (0.47–0.72) | 0.58 (0.46–0.72) |
| 2010–2014 | 5,160 | 91 | 0.37 (0.29–0.47) | 0.37 (0.29–0.47) | 0.37 (0.28–0.48) |
| P-for-trend | 0.001 | <0.001 | <0.001 | ||
| Other | |||||
| 1995–1999 | 4,861 | 378 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2000–2004 | 5,413 | 458 | 1.05 (0.92–1.21) | 1.07 (0.93–1.23) | 1.15 (0.99–1.34) |
| 2005–2009 | 5,540 | 474 | 1.05 (0.91–1.20) | 1.09 (0.94–1.25) | 1.16 (1.00–1.35) |
| 2010–2014 | 5,160 | 390 | 0.91 (0.79–1.06) | 0.92 (0.80–1.07) | 1.01 (0.86–1.18) |
| P-for-trend | NS | NS | NS | ||
Adjusted for age, sex, BMI, diabetes, hypertension, current smoker, coronary artery disease, congestive heart failure, cerebrovascular accident, region, and first modality
DISCUSSION
In this study of nearly all patients with incident LN-ESRD in the U.S. over the past two decades, we observed a 32% reduction in mortality. Our findings expand on the previous studies that showed no change in mortality rates between 1995–2006(13) and non-significant improvement between 1995–2010 among incident LN-ESRD patients in the U.S.(13) We similarly found a stable trend among LN-ESRD patients during the first 10 years (1995–2004). However, by following the latest 5 more years, a clear improvement trend in mortality emerged across the latest decade (2005–2014). These trends persisted after adjusting for age, sex, BMI, smoking, comorbidities, and other potential confounders across the sub-cohorts. We observed similarly improved mortality among African Americans, Hispanics, and Whites. Finally, we observed a 44% lower risk of cardiovascular deaths and 63% lower risk of infection-related deaths during the study period, contributing to the declining overall mortality trend.
This improved survival among patients with LN-ESRD may be explained by a combination of improvements in the management of ESRD and of underlying SLE. To that end, the mortality trends observed here among LN-ESRD patients are consistent with a 28% reduction in all-cause mortality among all-cause ESRD patients between 2001–2015 in the USRDS.(20) Our findings may also be related to reduced use of intense immunosuppression following the transition to ESRD for patients with lupus nephritis compared with prior treatment patterns.(9) Reductions in cumulative corticosteroid exposure may also explain a portion of the reduction in deaths due to cardiovascular disease in this population, which also could have contributed to improved survival in recent years. Improved management of comorbidities including CVD may have also contributed to improved survival. Finally, the rate of pre-emptive renal transplantation increased slightly over this time-period, and transplantation has been shown to reduce mortality relative to treatment with hemodialysis in the general ESRD population,(9, 21) although this remained infrequent (<5%) even in the final period. The rate of undergoing eventual renal transplantation (as initial modality or later switching to renal transplantation) did not change across sub-cohorts. However, there was a modest reduction in duration of time prior to receiving a renal transplant in the most recent sub-cohort, which might have contributed to the observed survival trends. Further studies are needed to clarify the role of recent trends in access to renal transplantation and the impact of renal transplantation on survival among patients with LN-ESRD.
Multiple studies have previously shown a higher risk of premature death in African Americans with LN-ESRD than white patients.(11, 12) However, we found that African Americans with LN-ESRD had a similar level of improvement in all-cause mortality as white patients, suggesting that the mortality disparity did not change. There are multiple proposed factors contributing to worse outcomes among African Americans with lupus nephritis and ESRD that have been previously identified including differences in socioeconomic status(12) and genetic predisposition to renal disease progression such as the APOL1 mutation among some African American patients.(22, 23) African Americans in general also historically had lower rates of renal transplantation, in particular pre-emptive renal transplantation, than white patients with ESRD, although this may be improving in recent years.(24) While improved care for African Americans with lupus nephritis is likely still needed, our study indicates that the mortality gap has not worsened for African Americans with ESRD in recent years.
This study has several strengths but also limitations. As it was established by CMS for the enrollment of new-onset ESRD patients into Medicare, the USRDS contains data on nearly all new cases of ESRD in the U.S. population and thus these findings are highly generalizable. Furthermore, temporal mortality trend data according to race/ethnicity and cause of death in patients with LN-ESRD are additional strengths compared with previous studies.(13, 25) The accuracy of the LN-ESRD diagnosis has been previously verified in this database with a 93% positive predictive value. The USRDS does not include, however, those who are not eligible for Social Security and Medicare, thus excluding those who are not legal residents of the U.S., an underserved and vulnerable population at increased risk of poor outcomes from SLE. (14) Furthermore, we did not have data regarding clinical SLE disease activity measures, prior SLE treatment regimens, or cumulative corticosteroid exposure so we could not assess the impact of these features on survival. Furthermore, this study did not assess trends in the rate of progression to LN-ESRD among an identifiable number of SLE patients. Therefore, potential variation in the progression to ESRD may have affected the observed trend results. However, our adjustment for major baseline comorbidities would have likely helped control for potential variations in the level of sickness at baseline. Additionally, patient race and ethnicity are reported by the attending nephrologist and staff on the baseline enrollment form to CMS, and there may be biases and misclassification in this information. Cause of death was unknown for one fifth of patients, but it would be unlikely for there to be systematic differences in the reporting of those deaths across the time periods studied.
In summary, among nearly all patients with incident LN-ESRD in the U.S. from 1995–2014, we found substantial improvements in all-cause mortality in recent years. Although African Americans with LN-ESRD have been previously shown to have higher mortality than white patients, they had a similar level of temporal improvement in all-cause mortality as Whites, and Hispanics also had considerable mortality reductions. Overall rates of death due to CVD and infection considerably improved, suggesting improved ESRD care as well as improved management of immunosuppression and underlying SLE might have minimized these complications. Collectively, these trends provide an important benchmark of improving care in this high-risk population.
Acknowledgments
Funding:
Dr. Jorge is supported in part by the T32 Ruth L. Kirschstein Institutional National Research Service Award from NIAMS (T32-AR-007258).
Dr. Wallace is supported in part by the Scientist Development Award from the Rheumatology Research Foundation and the T32 Ruth L. Kirschstein Institutional National Research Service Award from NIAMS (T32-AR-007258).
Dr. Choi is supported by the National Institutes of Arthritis and Musculoskeletal and Skin Diseases (P50-AR-060772).
Footnotes
Commercial Interests:
The authors have no relevant commercial interests or other conflicts of interest to disclose.
REFERENCES
- 1.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, et al. Mortality in systemic lupus erythematosus. Arthritis Rheumatol 2006;54:2550–7. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y, Choi S, Ji J, Song G. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus 2016;25:727–34. [DOI] [PubMed] [Google Scholar]
- 3.Aviña-Zubieta JA, To F, Vostretsova K, De Vera MA, Sayre EC, Esdaile JM. Risk of Myocardial Infarction and Stroke in Newly Diagnosed Systemic Lupus Erythematosus: A General Population-Based Study. Arthritis Care Res (Hoboken) 2017. [DOI] [PubMed] [Google Scholar]
- 4.Navarra SV, Leynes MS. Infections in systemic lupus erythematosus. Lupus 2010;19:1419–24. [DOI] [PubMed] [Google Scholar]
- 5.Jorge AM, Lu N, Zhang Y, Rai SK, Choi HK. Unchanging premature mortality trends in systemic lupus erythematosus: a general population-based study (1999–2014). Rheumatology (Oxford) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan TM, Tse KC, Tang CS, Mok MY, Li FK. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol 2005;16:1076–84. [DOI] [PubMed] [Google Scholar]
- 7.Walsh M, Solomons N, Lisk L, Jayne DR. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis with poor kidney function: a subgroup analysis of the Aspreva Lupus Management Study. Am J Kidney Dis 2013;61:710–5. [DOI] [PubMed] [Google Scholar]
- 8.Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, Garrido Ed Ede R, Danieli MG, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002;46:2121–31. [DOI] [PubMed] [Google Scholar]
- 9.Sabucedo AJ, Contreras G. ESKD, Transplantation, and Dialysis in Lupus Nephritis. Semin Nephrol 2015;35:500–8. [DOI] [PubMed] [Google Scholar]
- 10.Almaani S, Meara A, Rovin BH. Update on Lupus Nephritis. Clin J Am Soc Nephrol 2017;12:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sule S, Fivush B, Neu A, Furth S. Increased risk of death in African American patients with end-stage renal disease secondary to lupus. Clin Kidney J 2014;7:40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nee R, Martinez-Osorio J, Yuan CM, Little DJ, Watson MA, Agodoa L, et al. Survival Disparity of African American Versus Non-African American Patients With ESRD Due to SLE. Am J Kidney Dis 2015;66:630–7. [DOI] [PubMed] [Google Scholar]
- 13.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, et al. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum 2011;63:1681–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broder A, Mowrey WB, Izmirly P, Costenbader KH. Validation of Systemic Lupus Erythematosus Diagnosis as the Primary Cause of Renal Failure in the US Renal Data System. Arthritis Care Res (Hoboken) 2017;69:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2016. researcher’s guide to the USRDS database Bethesda (MD): NIH, National Institute of Diabetes and Digestive and Kidney Diseases [Available from: https://www.usrds.org/2016/rg/2016_USRDS_Researchers_Guide_16.pdf.
- 16.Population and Housing Unit Estimates United States Census Bureau [Available from: https://www.census.gov/programs-surveys/popest/data/data-sets.html.
- 17.Zhang Y, Lu N, Peloquin C, Dubreuil M, Neogi T, Aviña-Zubieta J, et al. Improved survival in rheumatoid arthritis: a general population-based cohort study. Ann Rheum Dis 2017;76:408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallace ZS, Zhang Y, Lu N, Stone JH, Choi HK. Improving Mortality in End-Stage Renal Disease due to Granulomatosis with Polyangiitis from 1995 to 2014. Arthritis Care Res (Hoboken) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999;94:496–509. [Google Scholar]
- 20.US Renal Data System 2017 Annual Report 2017 [Available from: https://www.usrds.org/adr.aspx.
- 21.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999;341:1725–30. [DOI] [PubMed] [Google Scholar]
- 22.Kruzel-Davila E, Wasser WG, Skorecki K. APOL1 Nephropathy: A Population Genetics and Evolutionary Medicine Detective Story. Semin Nephrol 2017;37:490–507. [DOI] [PubMed] [Google Scholar]
- 23.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol 2014;66:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Odds for receiving a kidney transplant now equal for black, white and Hispanic candidates [press release]. July 12, 2017. 2017.
- 25.Sexton DJ, Reule S, Solid C, Chen SC, Collins AJ, Foley RN. ESRD from lupus nephritis in the United States, 1995–2010. Clin J Am Soc Nephrol 2015;10:251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]