Abstract
OBJECTIVE
We assessed the excess medical expenditures for adults newly diagnosed with diabetes, for up to 10 years before and after diabetes diagnosis.
RESEARCH DESIGN AND METHODS
Using the 2001–2013 MarketScan data, we identified people with newly diagnosed diabetes among adults aged 25–64 years (diabetes cohort) and matched them with people who did not have diagnosed diabetes (control cohort) using 1:1 propensity score matching. We followed these two cohorts up to 610 years from the index date, with annual matched cohort sizes ranging from 3,922 to 39,726 individuals. We estimated the yearly and cumulative excess medical expenditures of the diabetes cohorts before and after the diagnosis of diabetes.
RESULTS
The per capita annual total excess medical expenditure for the diabetes cohort was higher for the entire 10 years prior to their index date, ranging between $1,043 in year 210 and $4,492 in year 21. Excess expenditure spiked in year 1 ($8,109), declined in year 2, and then increased steadily, ranging from $4,261 to $6,162 in years 2–10. The cumulative excess expenditure for the diabetes cohort during the entire 20 years of follow-up was $69,177 ($18,732 before and $50,445 after diagnosis).
CONCLUSIONS
People diagnosed with diabetes had higher medical expenditures compared with their counterparts, not only after diagnosis but also up to 10 years prior to diagnosis. Managing risk factors for type 2 diabetes and cardiovascular disease before diagnosis, and for diabetes-related complications after diagnosis, could alleviate medical expenditure in people with diabetes.
Diabetes, a common chronic condition, imposes a large economic burden on the American health care system. The direct medical cost of diabetes was estimated at $176 billion in 2012 (1), which increased to $237 billion in 2017 (2). People who have diagnosed diabetes spend more on health care for treatment, diabetes-related complications, and comorbidities compared with those without the disease (1–6). They also spend more during the years before the diagnosis of diabetes, possibly because they may already have undiagnosed diabetes, prediabetes, or other chronic diseases such as cardiovascular diseases (7–9). The age- and sex-adjusted average medical expenditures for people with diagnosed diabetes in the U.S. was 2.3 times as large as expenditures in the absence of diabetes in 2012 and 2017 (1,2).
Examining the changes in medical expenditures over time among people diagnosed with diabetes compared with those who were not diagnosed with diabetes is critical to understanding the trajectory of financial burdens imposed by diabetes and to provide cost parameters needed by many diabetes cost-effectiveness simulation models. Information on the excess expenditure before diabetes diagnosis can also guide efforts to identify strategies for preventing diabetes and cardiovascular diseases.
Two previous studies on the trajectory of medical expenditures for people with diabetes focused on their spending patterns either before (10) or after (11) the diagnosis. However, these studies were conducted decades ago using a study sample from a single managed care organization. Another study recently estimated medical expenditures among people with diabetes, both before and after the diagnosis; however, the study was limited to military veterans from three southern states and with a short follow-up of 64 years (12). Treatment of diabetes and cardiovascular disease has changed during the last two decades, which might have resulted in a change in the medical expenditure pattern. Furthermore, studies using data from a particular organization or special population may not represent the medical expenditure patterns of the broader population diagnosed with diabetes in the U.S.
Using a contemporary, wide regional coverage and longer follow-up data, we estimated the trajectory of excess medical expenditures for people newly diagnosed with diabetes, compared with those who were not diagnosed with diabetes, for up to a 20-year period surrounding their diagnosis of diabetes.
RESEARCH DESIGN AND METHODS
Data Source
The data for this study were from the 2001–2013 Truven Health MarketScan Commercial Claims and Encounters (CCE) database. This database contains data from active employees, early retirees, and dependents insured by employer-sponsored plans contributed by ;100 payers (13). CCE data contain detailed records on patients’ enrollment, outpatient services, inpatient admissions, prescription drugs, and costs of services. Outpatient claims include services that occurred outside of an inpatient admission, such as in a physician’s office, patient’s home, or hospital out-patient facility, as well as laboratory testing. Inpatient claims include those associated with hospital admission, such as physician, surgeon, independent lab-oratories, and medication charges. Pharmaceutical claims were payments for prescription drugs in outpatient settings, including diabetes supplies such as insulin pumps, pens, syringes, glucose monitors, and test strips. The health plans and services data, along with dates and times, provide a full continuum of care that can be linked with an encrypted unique identification number at the patient level. A major advantage of MarketScan claims data is their large comprehensive and high-quality coding that includes fully paid and adjudicated claims and patients’ out-of-pocket expenses. Additionally, the MarketScan database can be used to create longitudinal data, providing an opportunity to construct a study cohort and follow them over multiple years using their unique identification numbers.
Study Population
We included adults aged 25–64 years who were fully and continuously enrolled in fee-for-service plans from 2001 to 2013 and had prescription drug coverage. We excluded those with gestational diabetes and those who were not fully enrolled during 2001–2013. We also excluded those on partially or fully capitated plans because the encounter data often did not have full accounts for costs, thus limiting our ability to calculate the total expenditure (13).
Identification of Study Cohorts and Follow-up
We identified two study cohorts. The diabetes cohort consisted of those first diagnosed with diabetes during the study period, whereas the control cohort were those not diagnosed with diabetes during the study period and matched with case subjects. To ensure that the diabetes case was a newly diagnosed case, a person was required to have no medical claims associated with diabetes for at least the two previous years (14). In addition, the person was required to have at least two outpatient claims with diabetes codes (ICD-9 code 250.xx) at least 30 days apart, or have at least one inpatient diabetes claim (ICD-9 = 250.xx) during the 2-year case identification period (15). For each identified case subject, we required a minimum of 2 years of enrollment after the diagnosis date. Therefore, there were no case subjects identified in 2012 and 2013. Previous studies using administrative claims data with a 2-year case identification period had very high sensitivity and specificity (16–19). We assigned an index date (diagnosis date) for the new case subject as the first claim date with a diabetes code in an outpatient or inpatient setting (15).
For each identified new case subject, we used a propensity score matching method to identify a control subject. Propensity scores were estimated using a probit model, controlling for age, sex, health plan, and geographical locations (U.S. census region and metropolitan statistical area). We used 1:1 propensity score matching to select a matched control subject for each case subject in the year of diagnosis using the nearest neighbor algorithm. We chose this algorithm to retain all the case subjects from the diabetes cohort (20). For the matched control subject, we assigned the same index date as their corresponding case subject. After case-control matching, we assessed the quality of matching by examining the standardized difference between the means of the diabetes cohort and the control cohort for each covariate (21). The excess expenditure for each age/sex group cannot be estimated when age/sex was used as a covariate (22), thus we used the same propensity score matching method for individual age (25–44, 45–54, and 55–64 years) and sex (male and female) groups, separately.
The matched diabetes and control cohorts were followed up to 10 years before and up to 10 years after the index date. New cases of diabetes were identified yearly from 2003 to 2011, each contributing a minimum of 2 years to up to 10 years of follow-up, backward and forward, within the 2001–2013 time frame. For example, newly diagnosed diabetes case subjects in 2003 and their matched control subjects contributed up to 2 years backward (2001–2002) and up to 10 years forward (2004–2013) from the index date. Because everyone in the cohorts had the same length of enrollment, the censuring was based on the end of the study (2013), reaching age 65 years, or the matched control subject being diagnosed with diabetes. Applying our inclusion and exclusion criteria, a total of 254,049 people were qualified in 2001 for our study. The total sample size for the matched cohort for each follow-up year ranged between 3,922 and 39,726 individuals (Table 1). The sample sizes for matched cohort by age-group and sex are presented in Supplementary Table 1.
Table 1.
Matched cohort size by follow-up years before and after the index date
| Years, before and after index date |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | −10 | −9 | −8 | −7 | −6 | −5 | −4 | −3 | −2 | −1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| 2003 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3,922 | 3,922 | 3,922 | 3,922 | 3,922 | 3,922 | 3,922 | 3,922 | 3,922 | 3,922 | 3,922 | 3,922 |
| 2004 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4,080 | 4,080 | 4,080 | 4,080 | 4,080 | 4,080 | 4,080 | 4,080 | 4,080 | 4,080 | 4,080 | 4,080 | 0 |
| 2005 | 0 | 0 | 0 | 0 | 0 | 0 | 4,218 | 4,218 | 4,218 | 4,218 | 4,218 | 4,218 | 4,218 | 4,218 | 4,218 | 4,218 | 4,218 | 4,218 | 0 | 0 |
| 2006 | 0 | 0 | 0 | 0 | 0 | 4,704 | 4,704 | 4,704 | 4,704 | 4,704 | 4,704 | 4,704 | 4,704 | 4,704 | 4,704 | 4,704 | 4,704 | 0 | 0 | 0 |
| 2007 | 0 | 0 | 0 | 0 | 4,782 | 4,782 | 4,782 | 4,782 | 4,782 | 4,782 | 4,782 | 4,782 | 4,782 | 4,782 | 4,782 | 4,782 | 0 | 0 | 0 | 0 |
| 2008 | 0 | 0 | 0 | 4,494 | 4,494 | 4,494 | 4,494 | 4,494 | 4,494 | 4,494 | 4,494 | 4,494 | 4,494 | 4,494 | 4,494 | 0 | 0 | 0 | 0 | 0 |
| 2009 | 0 | 0 | 4,844 | 4,844 | 4,844 | 4,844 | 4,844 | 4,844 | 4,844 | 4,844 | 4,844 | 4,844 | 4,844 | 4,844 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2010 | 0 | 4,428 | 4,428 | 4,428 | 4,428 | 4,428 | 4,428 | 4,428 | 4,428 | 4,428 | 4,428 | 4,428 | 4,428 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2011 | 4,254 | 4,254 | 4,254 | 4,254 | 4,254 | 4,254 | 4,254 | 4,254 | 4,254 | 4,254 | 4,254 | 4,254 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 4,254 | 8,682 | 13,526 | 18,020 | 22,802 | 27,506 | 31,724 | 35,804 | 39,726 | 39,726 | 39,726 | 39,726 | 35,472 | 31,044 | 26,200 | 21,706 | 16,924 | 12,220 | 8,002 | 3,922 |
In 2001, a total of 245,049 adults met the inclusion criteria.
Estimation of Medical Expenditures
For each follow-up year, we estimated per capita annual medical expenditures in total and by component (outpatient care, inpatient care, and prescription drugs), age-group at diagnosis (25–44, 45–54, and 55–64 years), and sex. The medical expenditures included all the paid claims during the period of follow-up. The expenditures in the follow-up year 1 also included the expenses that incurred on the day of diabetes diagnosis if in outpatient setting or expenses of hospitalizations during which diabetes was diagnosed. We estimated excess expenditures as the difference in mean expenditures between the diabetes and matched control cohorts (10,11), and differences in expenditures between cohorts were tested using paired Student t test (23). We also calculated total cumulative predicted medical expenditures during the follow-up periods.
We conducted two sets of sensitivity analyses. The first analysis was to assess the extent to which the excess medical expenditures in year 1 would change when excluding the expenses on the day of diabetes diagnosis if in outpatient setting or expenses of hospitalizations during which diabetes was diagnosed (10,11). The second analysis was to assess the extent to which the excess medical expenditure would change when case subjects were allowed to be control subjects before they became case subjects (10,11).
All medical expenditures were adjusted to 2013 U.S. dollars using the medical care part of the consumer price index (available at http://www.bls.gov/cpi/tables/home.htm). Data were managed in SAS version 9.3, and the propensity score matching (using psmatch2 algorithm) and data analysis were conducted using Stata version 14.
RESULTS
Descriptive Results
A total of 19,863 new case subjects with diabetes along with the same number of matched control subjects were identified. The average number of follow-up years was 6 years, before or after the index date. The number of person-years evaluated was 477,712.
Both cohorts had a slightly higher proportion of females than males, predominantly living in a metropolitan statistical area in the South or Midwest and enrolled in preferred provider organizations (Supplementary Table 2). There were no statistically significant differences in descriptive characteristics between the two cohorts (Supplementary Table 2). The estimated standardized difference for each covariate in each matching year was <1%, suggesting no significant imbalances in covariates used in propensity score matching.
Estimated Total Medical Expenditures
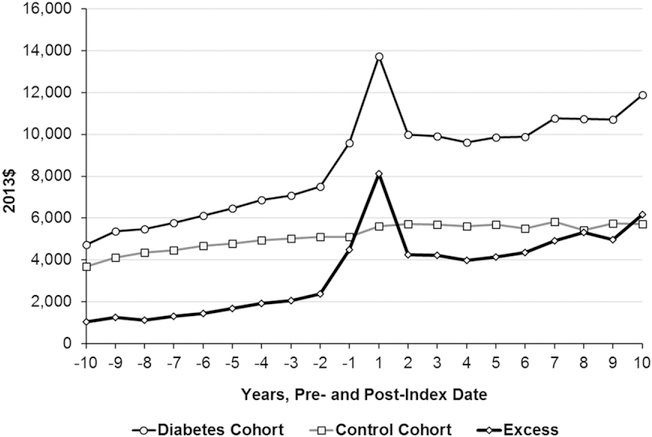
The per capita annual total medical expenditures for the diabetes cohort increased from $4,722 in year 10 before the index date to $9,586 the year before the index date (Fig. 1 and Supplementary Table 3). After the index date, the peak expenditure was $13,727 in year 1, declining in year 2 ($9,985), and then increasing gradually to year 10 ($11,886). The expenditures in the peak year were 1.4 times as large as in the previous year or year 2. The annual average medical expenditure after the index date was 1.6 times as large as the expenditure before the index date ($10,703 vs. $6,498).
Figure 1—
Mean total and excess medical expenditures during the 10 years before and after the index date (diabetes diagnosis) by cohort. The expenditures in follow-up year 1 also include the cost incurred on the day of diagnosis if diagnosed in outpatient setting or the cost incurred for whole admission if diagnosed in inpatient setting. The control cohort includes people not diagnosed with diabetes during the study period. Excess = average expenditures for diabetes cohort – average expenditures for control cohort. All the excess medical expenditures are statistically significant (P < .05, paired Student t test).
For the control cohort, the per-capita annual total medical expenditure increased gradually over time, starting from $3,679 in year 10 before the index date to $5,724 in year 10 after the index date (Fig. 1 and Supplementary Table 3). There were no spikes in expenditures as were seen in the diabetes cohort. The annual average medical expenditure after the index date was 1.2 times as large as the expenditure before the index date ($5,658 vs. $4,625).
Annual total excess medical expenditures were consistently higher for the diabetes cohort, with excesses increasing from $1,043 in year 10 before the index date to $4,492 the year before the index date (Fig. 1 and Supplementary Table 3). Excess expenditure peaked in year 1 after the index date ($8,109), declined in year 2 ($4,261), remained stable until year 6, and then increased steadily to year 10 ($6,162). The excess expenditure in the peak year was 1.8 times as large as that in the year prior to diagnosis and 1.9 times as large as in year 2 after diagnosis. The annual average excess medical expenditure after the index date was 2.7 times as large as that before the index date ($5,044 vs. $1,873).
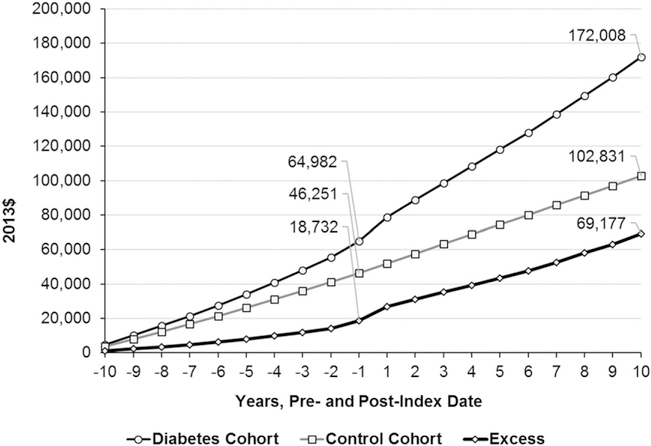
During the entire 20 years before and after the index date, the total per capita cumulative medical expenditure for the diabetes cohort was $172,008, an excess of $69,177 (before the index date $18,732 and after the index date $50,445) over the expenditure of the control cohort ($102,831) (Fig. 2 and Supplementary Table 3). Proportionally, the excess cumulative medical expenditures were higher after the diabetes diagnosis than before the diagnosis (1.9 vs. 1.4) (Supplementary Table 3).
Figure 2—
Mean cumulative total and excess medical expenditures during the 10 years before and after the index date by cohort. The expenditures in follow-up year 1 also include the cost incurred on the day of diagnosis if diagnosed in outpatient setting or the cost incurred for whole admission if diagnosed in inpatient setting. The control cohort includes people not diagnosed with diabetes during the study period. Excess = average expenditures for diabetes cohort – average expenditures for control cohort.
Estimated Medical Expenditures by Component
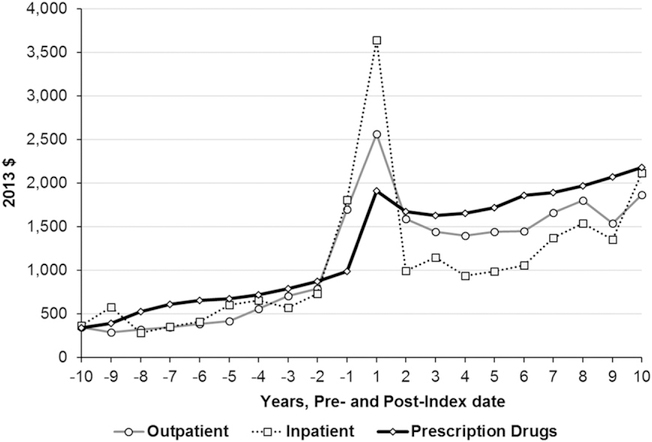
For outpatient care, excess medical expenditures before the index date increased as the index date approached, spiked in year 1 after diagnosis, decreased in year 2, remained relatively stable between years 3 and 6, and increased afterward (Fig. 3). The annual average excess expenditures were significantly higher during the periods both before and after the index date. The annual average excess expenditure was 2.9 times as large as that before the index date (Supplementary Table 4).
Figure 3—
Mean excess medical expenditures during the 10 years before and after the index date by medical expenditure component. The expenditures in follow-up year 1 also include the cost incurred on the day of diagnosis if diagnosed in outpatient setting or the cost incurred for whole admission if diagnosed in inpatient setting. The control cohort includes people not diagnosed with diabetes during the study period. Excess = average expenditures for diabetes cohort – average expenditures for control cohort. All the excess medical expenditures are statistically significant (P < 0.05, paired Student t test), except for the outpatient and inpatient expenditures in follow-up year −10.
For inpatient care, the pattern was similar to that of outpatient care, except for a much larger spike in year 1 after the index date and a sharp increase after year 6 through year 10 (Fig. 3). Again, the annual average excess expenditures of the diabetes cohort were significantly higher, during both periods before and after the index date. The annual average excess expenditure after the index date was 2.4 times as large as that before the index date (Supplementary Table 4).
For prescription drugs, the excess expenditures increased as the index date approached. However, the spike in year 1 after the index date was much smaller than for outpatient and inpatient care, and excess expenditures continued to increase after year 3 after the index date (Fig. 3). The annual average excess expenditure after the index date was 2.8 times as large as that before the index date (Supplementary Table 4).
Estimated Total Medical Expenditures by Subgroup
For all age-groups at diagnosis, the per capita annual total excess medical expenditure increased as they approached diagnosis (Supplementary Table 5). Average annual excess expenditures before the index date increased with age: aged 25–44 ($1,546), 45–54 ($1,999), and 55– 64 years ($2,085). In year 1 after the index date, the excess expenditure spiked for all the age-groups, being larger for the older age-groups, and then fell in year 2. Thereafter, excess expenditures generally increased for the younger age-groups and decreased for older age-groups. Average annual excess expenditures after the index date generally decreased with age: aged 25–44 ($5,223), 45–54 ($5,112), and 55–64 years ($4,134). The ratio of average annual excess expenditure after versus before the index date decreased with age: 3.4 for people aged 25–44, 2.6 for those 45–54, and 2.0 for those 55–64 years (Supplementary Table 5).
The per capita annual total excess medical expenditures by sex followed a pattern similar to that observed in the overall study population. Before the index date, annual average excess expenditures for men and women were similar ($1,885 vs. $1,863), but after the index date, they were lower for women than for men ($4,836 vs. $4,950) (Supplementary Table 6).
Sensitivity Analysis
When excluding the expenses that occurred on the day of diabetes diagnosis, the excess expenditure in year 1 decreased by 16% overall (outpatient 4% and inpatient 33%) (Supplementary Tables 3A and 4A), by 16–18% across age-groups (Supplementary Table 5A), and by 16–17% among males and females (Supplementary Table 6A). However, there were little changes in the ratio of total annual average expenditure before versus after the index date (overall 2.6).
When the case-control matching allowed a case subject to be a control subject before they became a case subject (Supplementary Tables 1A and 2A), the annual average excess expenditures both before and after the index date and the 20-year cumulative excess expenditures were slightly lower than that in which the control cohort included people not diagnosed with diabetes during the study period (Supplementary Tables 3 and 3B). For example, for total expenditures, thesewere$1,813 vs.$1,873 before the index date, $5,022 vs. $5,044 after the index date, and $68,349 vs. $69,177 cumulative excess expenditures. However, the ratio of annual average excess expenditures after versus before the index date was slightly larger (2.8 vs. 2.7) (Supplementary Tables 3 and 3B). The results of the sensitivity analysis by age-group and sex are presented in Supplementary Tables 4B–6B.
CONCLUSIONS
Using the contemporary data with a wide geographical coverage and a long followup, this study estimated the trajectories of medical expenditures for adults newly diagnosed with diabetes compared with similar people not diagnosed with diabetes. We found significant excess total medical expenditures among a diabetes cohort up to 10 years before diagnosis and increasing excesses as the cohort approached diagnosis. This pattern was consistent for all components of total expenditures, both sexes, and all agegroups. We also found a substantial spike in the excess total expenditures in the 1st year of diagnosis, which fell in year 2, remained relatively stable, and then elevated after year 6.
Our results show that those diagnosed with diabetes incurred higher medical expenditures years before diagnosis than those who were not diagnosed with diabetes, which is consistent with findings from previous studies (7,10,12,24,25). Nichols et al. (10) found significant excess costs among people diagnosed with diabetes compared with a matched control subject up to 8 years before diagnosis, and the cost increased at a faster rate as the diagnosis approached. Our estimated average annual excess expenditures were higher than that estimated by Nichols et al. (10) ($2,055 vs. $1,722 in 2013 dollars). Of the three cost components, our results showed that prescription drugs had the highest excess expenditures before the index date. In contrast, Nichols et al. (10) found that inpatient costs were the highest. This discrepancy may be in part due to differences in insurance plans and that our study population was younger than theirs (average age 51 vs. 60 years). Further, over the past 20 years, the way in which diabetes is diagnosed, the severity of diabetes, or the lag between the time of onset and diagnosis may have changed. Using Veterans Affairs data, Olson et al. (12) also found that for 4 years before diagnosis, the average excess expenditure was highest for inpatient care, followed by outpatient care and prescription drugs. However, the average annual expenditures were approximately one-third lower than what we observed. This difference could be due to the composition of the population and the health care system, as the sample was made up of veterans who were predominantly male, older (average age 63 years), sicker, and poorer than the general population, but who had better quality of care.
The substantial increases in expenditures and excess expenditures prior to diabetes diagnosis may be due to higher health care utilization associated with the natural progression of diabetes, including prediabetes or undiagnosed diabetes, before approaching diagnosis. The higher prevalence of comorbidities, such as cardiovascular conditions (12,26), obesity, hyperlipidemia, hypertension, and others, may have also led to a greater utilization of health services (8,24). This suggests that managing the risk factors of diabetes, such as obesity, hypertension, and cardiovascular conditions, could not only lower the risk of developing type 2 diabetes and related complications but also reduce subsequent costs.
Excess medical expenditures were much higher in the 1st year after diabetes diagnosis than in either the previous year or the following year. This is true in total expenditure, by expenditure component, and by subgroup. This observed pattern is consistent with results from other diabetes incidence-based studies using Canadian data (15,27), U.S. data (11,12), and Swedish data (28). In the 1st year after diagnosis, the diabetes cohort incurred expenditures 2.7 times as large as the expenditures of matched control subjects. This ratio is higher than that found by Brown et al. (11) (2.1 times as large) and Rosella et al. (15) (1.9 times as large for males and 2.0 times as large for females) but is much lower than the results found by Jonsson et al. (29) (5 times as large for males and 6.1 times as large for females). The higher excess cost in Jonsson et al. (29) could be due to a younger study population (aged 15– 34 years) who were predominantly on insulin (90%). Our estimated expenditure ratios in other years were also larger than the corresponding estimates from other studies (10–12). In addition to differences in health plans and health care systems, the excess could be associated with the age structure of the population, with younger age-groups having higher ratios. Our estimated average expenditure ratio between people with and without diabetes is smaller than that estimated by the American Diabetes Association (ADA) (1.9 vs. 2.3) (1,2). This could be due to the difference in data and methods used between our study and the ADA study.
Our finding that hospital inpatient care accounted for a higher proportion of excess expenditure compared with other components in the 1st year after the diagnosis is consistent with results from other studies (11,27,29). This could be associated with the diagnosis of diabetes as a biproduct of other conditions and the higher propensity of admission if diagnosed with diabetes (11). The large spike in outpatient expenditures and small spike in prescription medication could be attributed to increased medical attention of both diabetes and nondiabetes conditions. The stable excess medical expenditures generally after years 2–6 of diagnosis across the components could be a reflection of similar treatment patterns. The gradual increase in excess expenditure after year 6 of diagnosis could be due to the effect of increasing inpatient care and prescription medications for treating diabetes and cardiovascular diseases. The relatively steeper increase after year 9 of diagnosis across the component could be due to an increasing prevalence of complications.
Although those diagnosed with diabetes had substantial excess medical expenditures before diagnosis, the excess expenditures exacerbated after diabetes diagnosis. Excess expenditures during the 10 years after diagnosis were 2.6 times as large as prior to diagnosis. This finding implies that delaying diabetes may lower excess medical expenditures and provides justification for interventions starting years before diabetes onset. Previous studies have demonstrated that lifestyle interventions or metformin treatment among people with impaired glucose tolerance or among those at high risk of diabetes can prevent or delay the progression to type 2 diabetes (30–32). One study reported that over a 10-year period, lifestyle interventions for high risk adults were cost-effective and that metformin treatments were cost saving (33). Another study reported that among high-risk populations, screening for diabetes and prediabetes is cost saving (34).
The strengths of our study are founded on its use of a large recent data set with wide geographical coverage, the long periods of follow-up, and use of the propensity score matching method, which addresses the selection bias in the study population by having balances in matching covariates between case and control subjects. However, our study had several limitations. First, the MarketScan database is a convenience sample, and our study sample only represents those privately insured, aged 25–64 years. Therefore, our results may not reflect all U.S. adults. Second, due to data limitations, we could not distinguish between type 1 and type 2 diabetes, and therefore our results represent a combination of the two. However, it is likely that a large majority in the diabetes cohort had type 2 diabetes because type 1 diabetes is nearly always diagnosed before our diabetes cohort start age, i.e., 27 years, and in the general adult population (over 18 years), over 95% of adults with diabetes have type 2 (35). Third, also due to data limitations, we could not control for important demographic factors, such as race/ethnicity, HbA1c level, and obesity status in case-control matching. Further, there could be some lags in time between true onset and diagnosis of diabetes, hence matching of individuals could not be ascertained based on true risk of diabetes. Thus, part of excess medical expenditures before the index date could be attributed to factors such as obesity. Finally, our study sample was limited to those who were fully insured during the entire study period. This could have led to underestimated excess medical expenditures due to survival bias, as those who were excluded because they died during the study period likely had higher expenditures than those who remained in the panel (15). Further, those who had gaps in insurance coverage may have had a different pattern of medical expenditures than those with continuous coverage.
Privately insured adults diagnosed with diabetes have larger medical expenditures as compared with those not diagnosed with diabetes. These excess expenditures are substantial after the diabetes diagnosis, but also up to 10 years prior to a diabetes diagnosis. Annual average excess medical expenditures after the diabetes diagnosis are over twice as high as before the diagnosis. Our results suggest that by identifying those at high risk of diabetes, delaying or preventing diabetes by managing their risks and managing diabetes effectively could alleviate substantial medical expenditures and burdens to health care systems.
Supplementary Material
Acknowledgments.
The authors thank Clarice Conley, the web content writer-editor at CDC, for her editorial contributions.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Prior Presentation. Preliminary findings of this study were presented at the 76th Scientific Sessions of the American Diabetes Association, New Orleans, LA, 10–14 June 2016.
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alva ML, Gray A, Mihaylova B, Leal J, Holman RR. The impact of diabetes-related complications on healthcare costs: new results from the UKPDS (UKPDS 84). Diabet Med 2015;32:459–466 [DOI] [PubMed] [Google Scholar]
- 4.Li R, Bilik D, Brown MB, et al. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care 2013;19: 421–430 [PMC free article] [PubMed] [Google Scholar]
- 5.Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med 2013; 45:253–261 [DOI] [PubMed] [Google Scholar]
- 6.Zhuo X, Zhang P, Kahn HS, Bardenheier BH, Li R, Gregg EW. Change in medical spending attributable to diabetes: national data from 1987 to 2011. Diabetes Care 2015;38:581–587 [DOI] [PubMed] [Google Scholar]
- 7.Nichols GA, Brown JB. Higher medical care costs accompany impaired fasting glucose. Diabetes Care 2005;28:2223–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulliford MC, Charlton J, Latinovic R. Increased utilization of primary care 5 years before diagnosis of type 2 diabetes: a matched cohort study. Diabetes Care 2005;28:47–52 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Dall TM, Chen Y, et al. Medical cost associated with prediabetes. Popul Health Manag 2009;12:157–163 [DOI] [PubMed] [Google Scholar]
- 10.Nichols GA, Glauber HS, Brown JB. Type 2 diabetes: incremental medical care costs during the 8 years preceding diagnosis. Diabetes Care 2000;23:1654–1659 [DOI] [PubMed] [Google Scholar]
- 11.Brown JB, Nichols GA, Glauber HS, Bakst AW. Type 2 diabetes: incremental medical care costs during the first 8 years after diagnosis. Diabetes Care 1999;22:1116–1124 [DOI] [PubMed] [Google Scholar]
- 12.Olson DE, Zhu M, Long Q, et al. Increased cardiovascular disease, resource use, and costs before the clinical diagnosis of diabetes in veterans in the southeastern U.S. J Gen Intern Med 2015;30:749–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truven Health Analytics. MarketScan research databases: commercial claims and encounters Medicare supplemental data, data year 2012 edition Ann Arbor, MI, Truven Health Analytics, 2013 [Google Scholar]
- 14.Sloan FA, Belsky D, Ruiz D Jr., Lee P Changes in incidence of diabetes mellitus-related eye disease among US elderly persons, 1994–2005. Arch Ophthalmol 2008;126:1548–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosella LC, Lebenbaum M, Fitzpatrick T, et al. Impact of diabetes on healthcare costs in a population-based cohort: a cost analysis. Diabet Med 2016;33:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–516 [DOI] [PubMed] [Google Scholar]
- 17.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual 1999;14:270–277 [DOI] [PubMed] [Google Scholar]
- 18.Chen G, Khan N, Walker R, Quan H. Validating ICD coding algorithms for diabetes mellitus from administrative data. Diabetes Res Clin Pract 2010;89:189–195 [DOI] [PubMed] [Google Scholar]
- 19.Amed S, Vanderloo SE, Metzger D, et al. Validation of diabetes case definitions using administrative claims data. Diabet Med 2011; 28:424–427 [DOI] [PubMed] [Google Scholar]
- 20.Durden ED, Alemayehu B, Bouchard JR, Chu BC, Aagren M. Direct health care costs of patients with type 2 diabetes within a privately insured employed population, 2000 and 2005. J Occup Environ Med 2009;51: 1460–1465 [DOI] [PubMed] [Google Scholar]
- 21.Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS One 2010;5:e11501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rose S, Laan MJ. Why match? Investigating matched case-control study designs with causal effect estimation. Int J Biostat 2009;5: Article 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008;27: 2037–2049 [DOI] [PubMed] [Google Scholar]
- 24.Icks A, Haastert B, Giani G, Rathmann W. Incremental prescription and drug costs during the years preceding diabetes diagnosis in primary care practices in Germany. Exp Clin Endocrinol Diabetes 2006;114:348–355 [DOI] [PubMed] [Google Scholar]
- 25.Nichols GA, Arondekar B, Herman WH. Medical care costs one year after identification of hyperglycemia below the threshold for diabetes. Med Care 2008;46:287–292 [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care 2002;25:1129–12. 1134 [DOI] [PubMed] [Google Scholar]
- 27.Johnson JA, Pohar SL, Majumdar SR. Health care use and costs in the decade after identification of type 1 and type 2 diabetes: a population-based study. Diabetes Care 2006; 29:2403–2408 [DOI] [PubMed] [Google Scholar]
- 28.Sabale U, Bodega˚rd J, Sundstro¨m J, et al. Healthcare utilization and costs following newly diagnosed type-2 diabetes in Sweden: a follow-up of 38,956 patients in a clinical practice setting. Prim Care Diabetes 2015;9: 330–337 [DOI] [PubMed] [Google Scholar]
- 29.Jonsson PM, Marke LA, Nystro¨m L, Wall S, Ostman J. Excess costs of medical care 1 and 8 years after diagnosis of diabetes: estimates from young and middle-aged incidence cohorts in Sweden. Diabetes Res Clin Pract 2000;50:35–47 [DOI] [PubMed] [Google Scholar]
- 30.Knowler WC, Barrett-Connor E, Fowler SE, et al. ; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li G, Zhang P, Wang J, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol 2014;2:474–480 [DOI] [PubMed] [Google Scholar]
- 32.Li G, Zhang P, Wang J, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008; 371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 33.Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012;35:723–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatterjee R, Narayan KM, Lipscomb J, et al. Screening for diabetes and prediabetes should be cost-saving in patients at high risk. Diabetes Care 2013;36:1981–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. National diabetes statistics report, 2017 [Internet]. U.S. Department of Health and Human Services, 2017. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 22 November 2017 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.