Abstract
Objective
Mutations in Krüppel like factor-11 (KLF11), a gene also known as maturity-onset diabetes of the young type 7, contribute to the development of diabetes. KLF11 has anti-inflammatory effects in endothelial cells and beneficial effects on stroke. However, the function of KLF11 in the cardiovascular system is not fully unraveled. In this study, we investigated the role of KLF11 in vascular smooth muscle cell (VSMC) biology and arterial thrombosis.
Approach and Results
Using a ferric chloride-induced thrombosis model, we found that the occlusion time was significantly reduced in conventional Klf11 knockout (Klf11 KO) mice while bone marrow transplantation could not rescue this phenotype, suggesting that vascular KLF11 is critical for inhibition of arterial thrombosis. We further demonstrated that VSMC specific Klf11 knockout mice also exhibited significantly reduced occlusion time. The expression of tissue factor (TF, encoded by the F3 gene), a main initiator of the coagulation cascade, was increased in the artery of Klf11 KO mice, as determined by real-time quantitative PCR and immunofluorescence. Furthermore, VSMCs isolated from Klf11 KO mouse aortas showed increased TF expression, which was rescued by KLF11 overexpression. In human aortic smooth muscle cells, siRNA-mediated knockdown of KLF11 increased TF expression. Consistent results were observed upon adenovirus-mediated overexpression of KLF11. Mechanistically, KLF11 downregulates F3 at the transcriptional level as determined by reporter and chromatin immunoprecipitation assays.
Conclusions
Our data demonstrate that KLF11 is a novel transcriptional suppressor of F3 in VSMCs, constituting a potential molecular target for inhibition of arterial thrombosis.
Keywords: vascular biology, vascular disease, vascular smooth muscle, thrombosis, tissue factor
Introduction
Thrombosis is a common pathology underlying many cardiovascular diseases: myocardial infarction, stroke and venous thromboembolism, which collectively cause more than one fourth of deaths worldwide.1 The primary pathogenic process in arterial thrombosis is the rupture of the atherosclerotic plaque, which promotes platelet recruitment, adhesion, aggregation and activation and results in thrombus growth.2 Given the importance of the vascular smooth muscle cells (VSMCs) in vessel homeostasis and pathogenesis of vascular diseases,3 it is of great interest to identify molecular signaling pathways that mediate the effects of both physiological and pathophysiological stimuli in VSMCs.
The Krüppel-like factor (KLF) family is a subclass of Cys2/His2 zinc-finger DNA-binding proteins.4 The KLF family plays critical roles in the maintenance of vascular homeostasis and further affects multiple vascular diseases.5, 6 In fact, emerging data from population genetics studies suggest that KLF11 gene polymorphisms are significantly associated with type 2 diabetes.7 Maturity-onset diabetes of the young type 7 (MODY7), an early-onset type 2 diabetes mellitus, is caused by mutations in the KLF11 gene.7 In pancreatic β-cells, KLF11 regulates insulin transcription by directly binding or via increasing the expression of another MODY gene, pancreatic-duodenal homeobox-1 (PDX-1).8, 9 Moreover, KLF11 reduces hepatic triglyceride levels by increasing fatty acid oxidation.10 Apart from the metabolic disorder, diabetes accelerates vascular pathology and enhances thrombotic risk,11, 12 which account for the major causes of morbidity and mortality in diabetic patients.13
As a transcription factor, KLF11 is expressed ubiquitously and is abundant in adipose tissue, testis, breast, artery and lung (GTEx database). RNA sequencing data show that the mRNA level of KLF11 is modest in human endothelium and moderate in human aortic smooth muscle cells (HASMCs).6 Prior studies from others and our group demonstrated that KLF11 plays an important role in maintaining vascular homeostasis.14, 15 Specifically, KLF11 inhibits endothelial activation in the presence of inflammatory stimuli and functions as a peroxisome proliferator-activated receptor-γ co-regulator to attenuate middle cerebral artery occlusion-induced stroke in mice.16 These in vitro and in vivo observations form the basis of the current view that KLF11 is a vessel protective factor. However, the role of KLF11 in VSMC biology and thrombosis has not been explored.
In the present study we focused on VSMC KLF11 and, using in vivo VSMC-specific loss-of-function approaches, demonstrated that this factor is an inhibitor of experimental arterial thrombosis through transcriptional repression of tissue factor (TF, encoded by F3 gene) in VSMCs.
Materials and Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. An extended version of this section is available online as Extended Materials and Methods.
Animals
Conventional KLF11 knockout mice (Klf11 KO) and wild type (WT) mice in the C57BL/6J background were previously described14 and the KO was confirmed (Supplemental Figure I). The floxed-KLF11 (Klf11fl/fl) mice were generated by injecting blastocysts developed from Klf11 targeted ES cells (Klf11tm2a(KOMP)Wtsi, UCDavis, KOMP) into C57BL/6J mice. Exon 3 of the Klf11 gene was flanked by LoxP elements. The genotype was determined from mouse tail clippings and a pair of PCR primers flanking the downstream LoxP region (Supplemental Figure IIIA). The inducible smooth muscle cell specific Klf11 knockout (Sm-Klf11 KO) mice were generated by cross-breeding Klf11fl/fl mice with Myh11-CreERT2 mice (019079, Jackson Laboratory).17 The primer sequences for mouse genotyping are shown (Supplemental Table I). The Myh11-CreERT2-(+)/Klf11fl/fl mice (Sm-Cre/Klf11fl/fl +TAM) and Myh11-CreERT2-(−)/Klf11fl/fl mice (Klf11fl/fl +TAM) were treated with tamoxifen (intraperitoneal injections, 80mg/kg per day for 5 consecutive days, indicated as +TAM). Myh11-CreERT2-(+)/Klf11fl/fl mice treated with the same volume of vehicle corn oil (Sm-Cre/Klf11fl/fl + Oil) were used as the control group. Fourteen days after tamoxifen or corn oil treatment, the reduction of KLF11 expression in the aorta was confirmed by real-time quantitative PCR and western blot (Supplemental Figure IIIB-C). Since the Myh11-Cre/ERT2 transgene is inserted on the Y chromosome,18 only male Sm-Cre/Klf11fl/fl mice can be generated using this approach. All animal care and experimental procedures were approved by the University of Michigan Animal Care and Use Committee.
Ferric Chloride-Induced Carotid Artery Thrombosis Model
Ferric chloride (FeCl3)-induced carotid artery thrombosis was conducted as previously described 19 and detailed in the “Extended Materials and Methods”.
Bone Marrow Transplantation (BMT)
The protocol for syngeneic BMT was previously described20, 21 and detailed in the “Extended Materials and Methods”.
Prothrombin Time (PT) and Activated Partial Thromboplastin Time (aPTT) Measurements
Platelet-poor-plasma (PPP) was collected as previously described 22 and detailed in the “Extended Materials and Methods”.
Tail-Bleeding Assay
Conventional Klf11 KO mice and WT mice (8–10-week-old male) were used in this experiment. As previously described,23 a transverse incision at the 5 mm distal end of the tail was performed and the tail was immersed in saline at 37°C. Bleeding time was recorded as the time to cessation of bleeding.
Reagents and Antibodies
Reagents and Antibodies are listed in the “Extended Materials and Methods”.
Cell Culture
Human aortic smooth muscle cells (HASMCs, CC-2571, Lonza) were cultured in SmGM™−2 medium containing 5% FBS (CC-3182, Lonza) and used within 10 passages. Before thrombin stimulation, the HASMCs were made quiescent with DMEM/F12 with 0.5% fetal bovine serum (FBS) for 48 hours. A7r5 cells (CRL-1444™, ATCC) were cultured in DMEM/F12 supplemented with 10% FBS) and 50mg/ml of a penicillin/streptomycin mix. Mouse aortic smooth muscle cells (MASMCs) were isolated from the conventional Klf11 KO mice and WT mice (3–4-week-old male) as previously described.17, 24 Details are described in the “Extended Materials and Methods”. The purity of MASMCs was validated by immunostaining for α-smooth muscle actin (α-SMA). All cells were cultured in a 5% CO2 humidified incubator at 37°C.
Preparation of Washed Murine Platelets and Platelet Aggregation Assay
The collection of washed murine platelets and platelet aggregation assay were performed as previously described23 and detailed in the “Extended Materials and Methods”.
Protein Extracts and Western Blot
All protein extracts and western blot were performed as previously described.25
Adenoviral Constructs
Adenoviral vectors overexpressing human KLF11 or LacZ control were generated as previously described.26 The procedures are detailed in the “Extended Materials and Methods”.
Statistical Analysis
All quantitative data are presented as mean ± SEM. Statistical analysis were performed using the GraphPad Prism 7. All data were first subjected to Shapiro-Wilk normality test and F test to evaluate homogeneity of variances. For normally distributed data with similar variances among groups, unpaired Student t test with Welch’s correction was used for two-group comparisons and one-way analysis of variance (ANOVA) followed by Tukey’s test was used for more than two groups’ comparisons. Two-way ANOVA followed by Bonferroni test was applied for comparisons of grouped data under different conditions. Nonparametric Mann-Whitney test was used for data not normally distributed. All results were representative from at least four independent experiments.
Results
KLF11 Deficiency Aggravates Arterial Thrombosis In Vivo
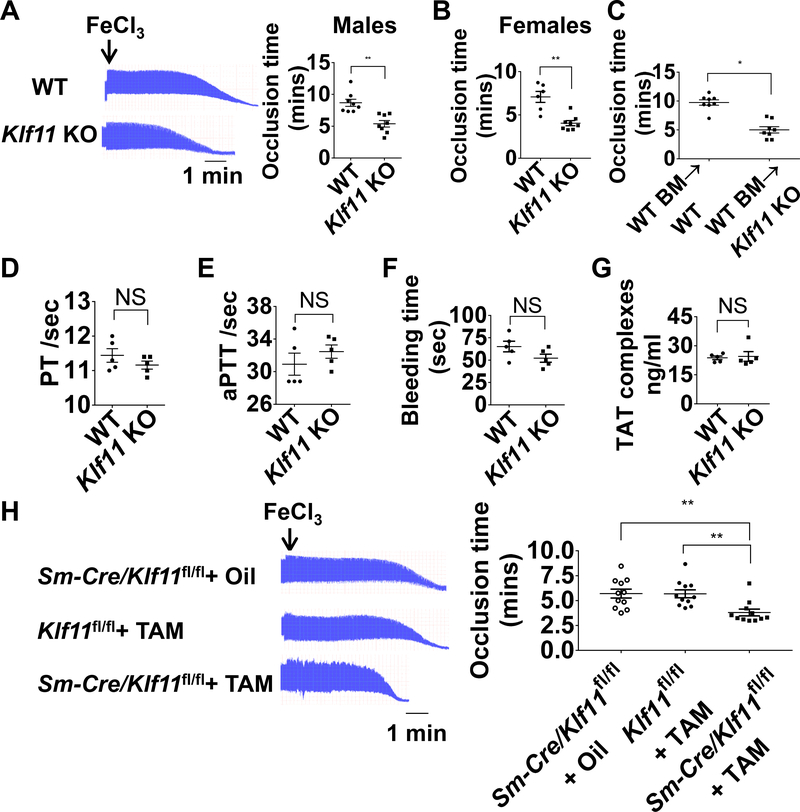
To assess the role of KLF11 in arterial thrombosis, we used the conventional Klf11 KO mice previously reported14 and applied a FeCl3-induced thrombosis model for studying arterial thrombosis.19 The occlusion time in the Klf11 KO male mice was significantly reduced to an average of 62% of that in WT C57BL/6J male mice (Figure 1A). A similar pro-thrombotic phenotype was also observed in Klf11 KO female mice, with the occlusion time reducing to 56% of that in the WT C57BL/6J female mice (Figure 1B). To exclude the effects of Klf11 KO in blood cells (platelets, neutrophils, macrophages, etc.) in this pro-thrombotic phenotype, we performed bone marrow transplantation (BMT) in Klf11 KO and WT mice. The reduced occlusion time in the Klf11 KO group transplanted with WT bone marrow was not rescued when compared with WT mice transplanted with WT bone marrow (Figure 1C).
Figure 1. KLF11 (Krüppel-like Factor 11) deficiency aggravates arterial thrombosis.
A-C, The left carotid arteries of WT (wild type) and conventional Klf11 KO (knockout) mice were subjected to 10% FeCl3 to induce arterial thrombosis. A, Representative images of blood flow detected by ultrasound are shown with each division representing 8 seconds (left) and the corresponding occlusion time (right) determined in WT and Klf11 KO male mice (n=8/group). B, Occlusion time in WT and Klf11 KO female mice (n=6–8/group). C, WT male mice transplanted with WT bone marrow were designated as WT BM→WT, Klf11 KO male mice transplanted with WT bone marrow were designated as WT BM→Klf11 KO mice. The carotid artery occlusion time after bone marrow transplantation was recorded as in A (n=8/group). **P<0.01 or *P<0.05 using unpaired Student t-test. D-G, PT (prothrombin time), aPTT (activated partial thromboplastin time), bleeding time and TAT (thrombin-antithrombin) complexes were measured from WT and Klf11 KO male mice (n=5/group). NS, no significance using unpaired Student t-test (D, F, G) or nonparametric Mann-Whitney test (E). H, The left carotid arteries of Sm-Cre/Klf11fl/fl+TAM (Myh11-CreERT2/Klf11fl/fl+tamoxifen) mice and controls: Sm-Cre/Klf11fl/fl+ Oil (Myh11-CreERT2/Klf11fl/fl+ corn oi) and Klf11fl/fl+TAM (Klf11fl/fl+ tamoxifen) mice, were subjected to 10% FeCl3 to induce thrombosis. Representative images of blood flow detected by ultrasound are shown and the occlusion time in control and Sm-Klf11 KO mice was recorded (n=11/group). **P<0.01 using one-way ANOVA followed by Tukey’s test.
The hemostatic status in the Klf11 KO male mice was evaluated by measuring the function of coagulation factors and platelets. Our results showed that KLF11 did not alter the prothrombin time (PT) and activated partial thromboplastin time (aPTT), which reflect the function of extrinsic/common or intrinsic coagulation pathways, respectively (Figure 1D-E). Bleeding time and thrombin-antithrombin (TAT) complexes were also unchanged in the Klf11 KO mice, indicating that the general hemostatic status was normal in the conventional Klf11-deficient mice (Figure 1F-G). To evaluate whether the deficiency of KLF11 in megakaryocytes can affect the platelet function, we isolated washed platelets and performed thrombin induced platelet aggregation assay. The maximum platelet aggregation induced by thrombin did not show significant differences between WT and conventional Klf11 KO mice (Supplemental Figure II).
Smooth Muscle Cell Specific KLF11 Deficiency Aggravates FeCl3-Induced Arterial Thrombosis
Taking into account that the hemostatic status in the circulation was not affected and the bone marrow transplantation cannot rescue the occlusion time in the conventional Klf11 KO mice, the phenotype in Klf11 KO mice might result from molecular changes specific to the vascular wall. To further identify the role of vascular smooth muscle cell (VSMC) KLF11 in thrombosis, we generated tamoxifen-inducible Sm-Klf11 KO (Myh11-CreERT2/Klf11fl/fl + TAM) mice (Supplemental Figure IIIA). Klf11fl/fl mice treated with tamoxifen (Klf11fl/fl + TAM) and Myh11-CreERT2/Klf11fl/fl mice treated with corn oil (Sm-Cre/Klf11fl/fl + Oil) were used as controls. KLF11 deficiency in the aortic media of Sm-Klf11 KO mice was confirmed at the mRNA and protein levels (Supplemental Figure IIIB-C). The specificity of the Myh11-CreERT2 in the aorta had been confirmed previously, as no expression was detected in cells from the blood and bone marrow.27 In the FeCl3 thrombosis model, Sm-Klf11 KO mice exhibited a similar pro-thrombotic phenotype as that observed in the conventional Klf11 KO mice. The occlusion time in the Sm-Klf11 KO mice was significantly reduced to an average of 67% of that in the two control mouse groups (Figure 1H). Our data suggest that VSMC KLF11 protects against arterial thrombosis.
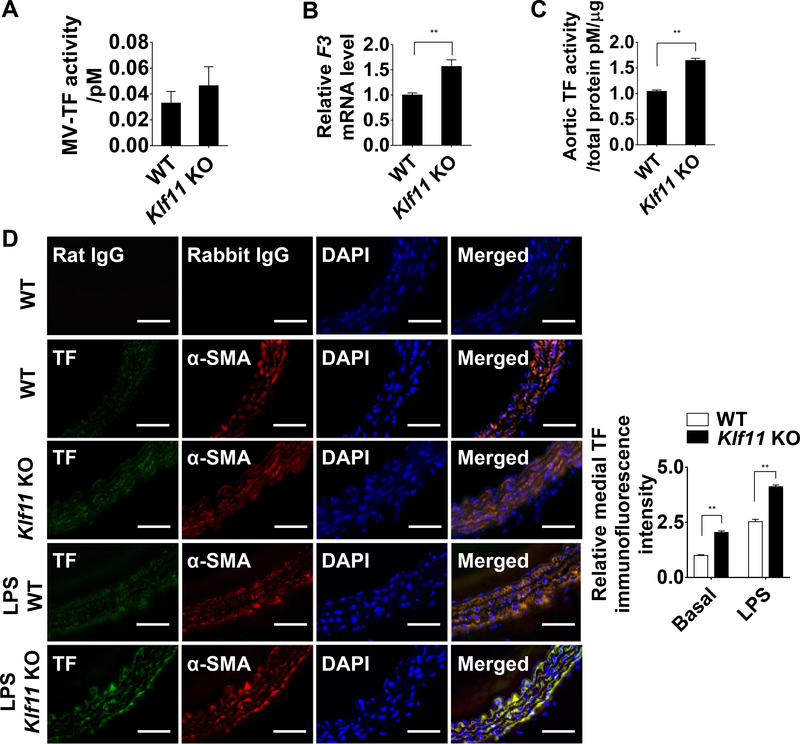
TF Expression is Increased in the Vascular Wall of Conventional Klf11 KO Mice
TF is an important initiator of the coagulation cascade, which can generate insoluble fibrins and form a thrombus. The vascular wall TF contributes to arterial thrombosis in cardiovascular diseases such as atherosclerotic plaque rupture and myocardial infarction.28 VSMC TF is critical for arterial thrombus formation in the mouse FeCl3 thrombosis model.19 To determine whether TF is an effector mediating the enhanced thrombosis resulting from KLF11 deficiency, we measured the expression of TF in aorta of conventional Klf11 KO mice and WT mice in basal conditions. First, the activity of circulating microvesicle-associated TF (MV-TF) showed no significant differences between Klf11 KO and WT mice (Figure 2A). We applied anti-mouse TF 1H1 antibody29 or rat IgG to validate the specificity of this assay. Next, we observed increased F3 mRNA level and TF activity in the isolated carotid artery of conventional Klf11 KO mice (Figure 2B-C). The elevated aortic TF activity can also be blocked by TF 1H1 antibody. Furthermore, the immunofluorescence staining data showed that TF protein was consistently upregulated in the vascular wall, and co-localized with α-smooth muscle actin (α-SMA), a specific marker of VSMCs. Moreover, the Klf11 KO mice also showed increased TF expression under lipopolysaccharide (LPS)-induced inflammatory conditions (Figure 2D). The relative TF intensity in the vascular wall was quantified and statistically analyzed. Collectively, our data suggest that KLF11 negatively regulates TF levels in VSMCs in vivo. Similar to the data in Figure 1G, no significant difference in TAT complexes was observed between WT and Klf11 KO mice at basal conditions. However, there was a higher TAT complexes in Klf11 KO mice after LPS treatment (Supplemental Figure IV).
Figure 2. KLF11 (Krüppel-like Factor 11) deficiency induces TF (tissue factor) expression in arterial wall.
A, Activity of microvesicles-associated tissue factor (MV-TF) in the plasma after preincubation with IgG or TF 1H1 antibody (n=6/group). Data are presented by subtracting the amount of FXa generated in the presence of TF 1H1 antibody from the amount of total FXa generated in the presence of IgG. B, F3 mRNA level of carotid arteries from WT and Klf11 KO mice. The mRNA level was normalized by 18S and is presented relative to the WT group set as 1 (n=4/group). C, The aortic TF activity was measured and presented as in A, after preincubation with IgG or TF 1H1 antibody and normalized to the total protein quantity (n=6/group). **P<0.01 using unpaired Student t-test (A-C). D, Expression of TF (Alexa 647, displayed in green) and α-SMA (α-smooth muscle actin, Alexa 568, displayed in red) in mouse aorta at basal level or 4 hours after LPS (30 µg/kg) tail vein injection was visualized by immunofluorescence staining. Respective IgG staining was used as negative control. Scale bars=50 µm. Quantification was performed from 4 mice, randomly selecting 3 different medial regions from each specimen and dividing the TF immunofluorescence intensity by medial area (indicated by α-SMA positive cells). Data are presented relative to the basal level of WT group set as 1. **P<0.01 using two-way ANOVA followed by Bonferroni test.
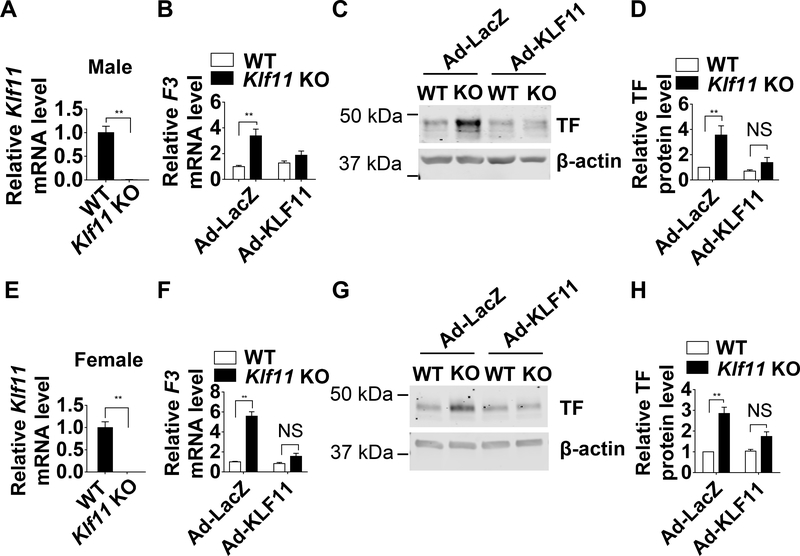
KLF11 Overexpression Rescues the TF Upregulation in KLF11-Deficient MASMCs
As a complementary and necessary approach, mouse aortic smooth muscle cells (MASMCs) were isolated from male and female conventional Klf11 KO mice and WT mice. The isolated MASMCs were characterized by immunostaining for α-SMA (Supplemental Figure V). The Klf11 deficiency was confirmed by real-time quantitative PCR (Figure 3A, E). A higher TF expression was observed in the MASMCs from both male and female Klf11 KO mice compared with WT mice (Figure 3B-D, F-H, Ad-LacZ). In both genders, restoration of KLF11 can rescue the phenotype in Klf11 KO MASMCs. As expected, the upregulated TF expression was significantly alleviated after KLF11 overexpression, at both mRNA and protein levels (Figure 3B-D, F-H, Ad-KLF11). These results indicate that endogenous KLF11 is required to prevent excessive TF upregulation, a hallmark of VSMC involvement in thrombosis.19
Figure 3. KLF11 (Krüppel-like Factor 11) overexpression rescues the TF (tissue factor) upregulation in KLF11-deficient MASMCs (mouse aortic smooth muscle cells).
MASMCs were isolated from male (A-D) and female (E-H) WT or Klf11 KO mice. A and E, Klf11 mRNA level of MASMCs from WT and Klf11 KO mice. The mRNA level was normalized by 18S and is presented relative to the WT group set as 1 (n=4/group). **P<0.01 using unpaired Student t-test. B-H, MASMCs isolated from WT or Klf11 KO mice were infected with Ad-LacZ or Ad-KLF11 (50 MOI). B and F, F3 mRNA level of MASMCs was normalized by 18S and is presented relative to the WT infected with Ad-LacZ group set as 1 (n=4/group). C and G, Representative western blot of TF protein level. D and H, Band density from 4 independent western blots was quantitatively analyzed and normalized against β-actin. The WT infected with Ad-LacZ group was set as 1. **P<0.01 or NS, no significance using two-way ANOVA followed by Bonferroni test (B, D, F, H).
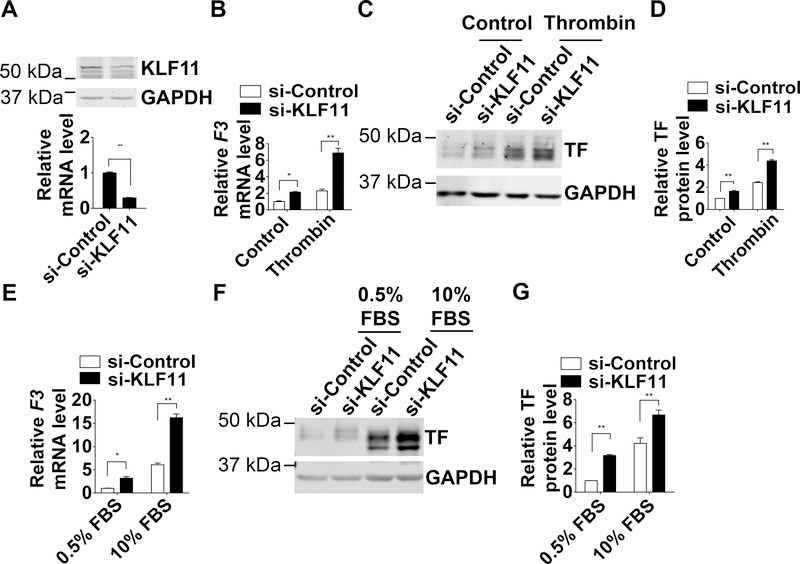
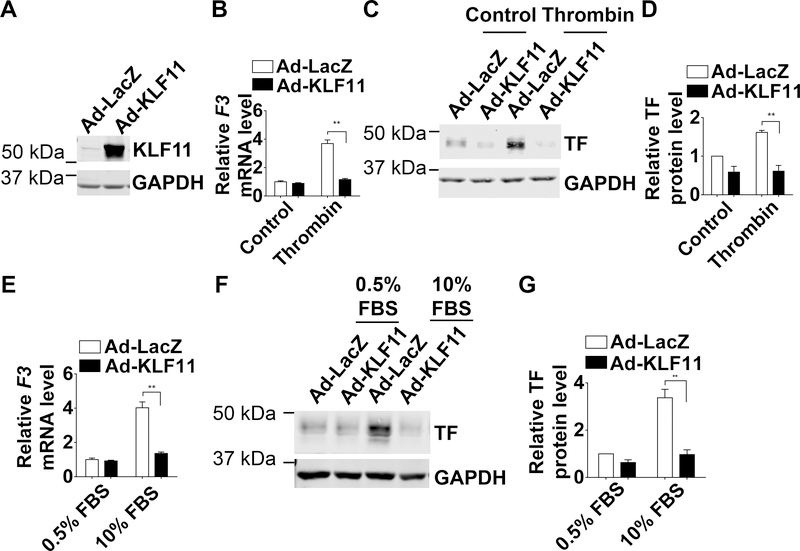
KLF11 Inhibits TF Expression in HASMCs
To determine whether KLF11 is essential to regulate TF in VSMCs, we measured TF expression upon KLF11 knockdown in HASMCs. The efficiency of siRNA-mediated KLF11 knockdown was confirmed at the mRNA and protein levels (Figure 4A). The KLF11 knockdown in HASMCs increased both the basal and thrombin-induced expression of TF at mRNA and protein levels (Figure 4B-D). Similar effects were observed in HASMCs stimulated with 10% FBS-containing culture medium (Figure 4E-G).
Figure 4. TF (Tissue factor) expression is increased upon KLF11 (Krüppel-like Factor 11) knockdown in HASMCs (human aortic smooth muscle cells).
HASMCs were transfected with si-Control or si-KLF11 (40 nM) and 24 hours later serum starved with 0.5% FBS (fetal bovine serum) for 48 hours. Three days after transfection, HASMCs were exposed to thrombin (3.24 µg/ml) (B-D) or 10% FBS (E-G) for 4 hours. A, The knockdown efficiency of KLF11 was determined by real-time quantitative PCR and western blot. The mRNA level was normalized by GAPDH and is presented relative to HASMCs transfected with si-Control group set as 1 (n=4/group). **P<0.01 using unpaired Student’s t-test. B and E, F3 mRNA level of HASMCs was normalized by GAPDH and is presented relative to HASMCs transfected with si-Control group set as 1 (n=4/group). C and F, Representative western blot showing the protein level of TF. D and G, Band density from 4 independent western blots was quantitatively analyzed and normalized against GAPDH. HASMCs transfected with si-Control group was set as 1. *P<0.05, **P<0.01 using two-way ANOVA followed by Bonferroni test (B, D, E, G).
Apart from TF, other factors can also affect the formation of arterial thrombus. In HASMCs, the expression of other thrombosis related factors such as tissue factor pathway inhibitor (TFPI) and protease activated receptor-1 (PAR-1) and inflammatory genes such as monocyte chemoattractant protein-1 (MCP-1) and interleukin 1 beta (IL-1β) was not significantly changed in the KLF11-deficient HASMCs (Supplemental Figure VI).
Further, we upregulated KLF11 in primary HASMCs to determine whether KLF11 regulates TF in vitro. In HASMCs, adenovirus-mediated overexpression of KLF11 (Figure 5A) significantly inhibited the thrombin-induced TF expression at both mRNA and protein levels (Figure 5B-D). Similarly, KLF11 also suppressed TF expression in HASMCs stimulated with 10% FBS-containing culture medium (Figure 5E-G). Thus, our data suggest that KLF11 potently inhibits TF in human VSMCs under either thrombin stimulation or normal serum conditions.
Figure 5. TF (Tissue factor) expression is decreased upon KLF11 (Krüppel-like Factor 11) overexpression in HASMCs (human aortic smooth muscle cells).
HASMCs were infected with Ad-LacZ or Ad-KLF11 (50 MOI). Twelve hours after infection, HASMCs were serum starved with 0.5% FBS (fetal bovine serum) for 48 hours and then stimulated to thrombin (3.24 µg/ml) (B-D) or 10% FBS (E-G) for 4 hours. A, The overexpression of KLF11 was determined by western blot. B and E, F3 mRNA level of HASMCs from each group was normalized by GAPDH and is presented relative to HASMCs infected with Ad-LacZ group set as 1 (n=4/group). C and F, Representative western blot showed the protein level of TF. D and G, Band density from 4 independent western blots was quantitatively analyzed and normalized against GAPDH. HASMCs infected with Ad-LacZ group was set as 1. **P<0.01 using two-way ANOVA followed by Bonferroni test (B, D, E, G).
Interestingly, KLF11 overexpression also decreased tumor necrosis factor alpha (TNF-α)-induced TF expression in human umbilical vein endothelial cells (HUVECs) at both mRNA and protein levels (Supplemental Figure VII), implicating that endothelial KLF11 also may have an important role in thrombosis.
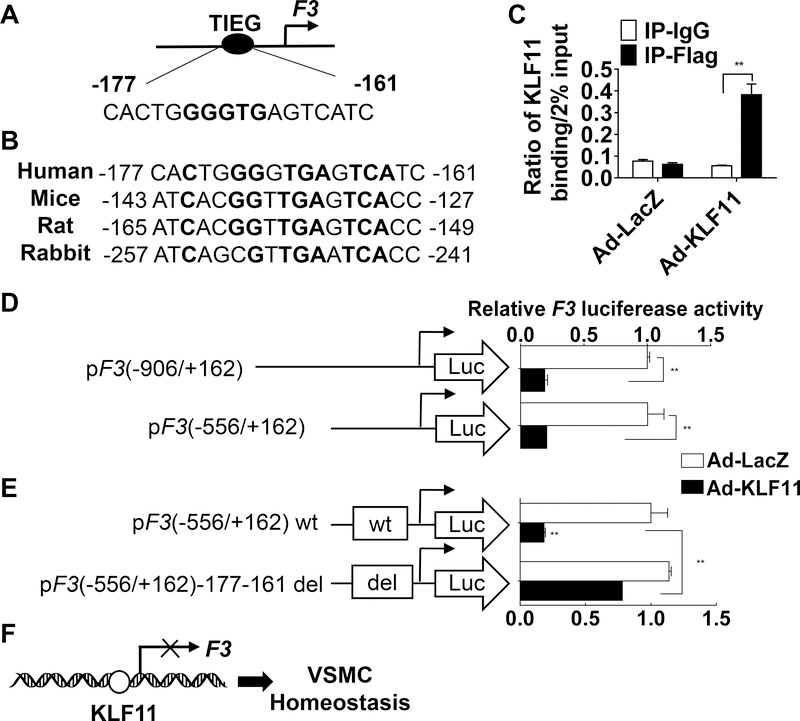
KLF11 Inhibits F3 Transcription
Next, we determined the mechanism that mediates the regulation of F3by KLF11. KLF11 was originally identified as the transforming growth factor-β-inducible early gene 2 (TIEG2), with a preference to bind at GC-rich sequences (GGGTG).30 Transcription factor binding site analysis of the human F3 gene (Genomatix) revealed a relatively conserved TIEG binding site (−177 to −161 base pairs) upstream of the F3 transcription start site (Figure 6A). The TIEG binding site is conserved among human, mouse, rat and rabbit (Figure 6B). To determine whether this TIEG binding site is a functional KLF11 binding region, we performed chromatin immunoprecipitation (ChIP) assay in the HASMCs infected with Ad-flag-KLF11 or Ad-LacZ. Our data suggest that KLF11 can bind to the region containing this TIEG binding site (Figure 6C). To determine whether KLF11 regulates F3 at the transcriptional level, we generated luciferase reporter constructs, which were under the control of different lengths (−906/+162 and −556/+162) of the human F3 promoter. In A7R5 cells, a rat aortic smooth muscle cell line, transfected with different reporter constructs, KLF11 overexpression significantly reduced the luciferase activity (Figure 6D). Next, we deleted the TIEG bind site (−177 to −161) in the F3 promoter-driven luciferase construct. As expected, the deletion of the TIEG binding site significantly attenuated the KLF11 inhibition of F3 luciferase activity (Figure 6E) from the reporter plasmid. In conclusion, we identified that KLF11 inhibits F3 expression at transcription level through direct binding to the F3 promoter (Figure 6F).
Figure 6. KLF11 (Krüppel-like Factor 11) inhibits F3 (coagulation factor III) transcription.
A, A diagram showing the simplified structure of the human F3 promoter region with an illustration of the TIEG (transforming growth factor-beta-inducible early gene) binding site. KLF binding region is shown in bold. B, The bold bases indicate the conservation of the TIEG binding site among species. C, HASMCs were infected with Ad-LacZ or Ad-Flag KLF11. Forty-eight hours after infection, the binding of KLF11 to the F3 promoter was determined by ChIP assays using an antibody against Flag (n=4/group). D-E, A7r5 cells were transfected with two different length (D), or wt (wild type) or del (region deleted) (E) luciferase reporter driven by the F3 promoter and then infected with Ad-LacZ or Ad-KLF11 (50 MOI). Two days later, the luciferase activity was measured and normalized by Renilla activity. The results are presented relative to A7r5 transfected with pF3 (−906/+162) (D) or wt (E) and infected with Ad-LacZ group set as 1 (n=4/group). **P<0.01 using two-way ANOVA followed by Bonferroni test (C, D, E). F, Schematic summary: KLF11 inhibits F3 transcription by directly binding to the F3 promoter region.
Discussion
In the current study, we observed an increase of arterial thrombosis in both genetically engineered conventional Klf11 KO mice and VSMC-specific Klf11 KO mice. In cultured human aortic smooth muscle cells, we demonstrated that KLF11 inhibits TF expression. Mechanistically, KLF11 directly binds to F3 promoter region and thereby suppresses the transcription of F3. This study demonstrated a potential role for VSMC KLF11 in arterial thrombosis.
The KLF family modulates cardiovascular activity through regulation of metabolism and inflammation in the cardiovascular system.4–6 Endothelial KLF2 and KLF4 have been reported to inhibit thrombus formation by inhibiting the transcription of pro-thrombotic factors (e.g. plasminogen activator inhibitor 1 and TF) and increasing the expression of anti-thrombotic factors (e.g. thrombomodulin) under inflammatory conditions.6, 31–33
We previously identified that KLF11 inhibits endothelial activation14 and attenuates endothelial dysfunction in the mouse middle brain artery occlusion-induced stroke model.16 Our current finding that VSMC KLF11 inhibits arterial thrombosis advances the understanding of the protective role of KLF11 in vascular diseases. Population genetics studies identified that mutations in KLF11 gene are positively associated with type 2 diabetes.7 Cardiovascular events are the major causes of death in diabetes.34 Our study points to a potentially beneficial effect of KLF11 on cardiovascular complications in the diabetic patients. Follow-up studies are warranted to determine the role of VSMC KLF11 in diabetes associated cardiovascular diseases such as atherosclerosis, thrombosis, and angiogenesis.
In this study, we used the FeCl3 thrombosis model, a widely used mouse arterial thrombosis model,35 to study the role of KLF11 in the vascular wall in vivo. The penetration of FeCl3 from adventitia triggers thrombosis.36 In this study, we found that conventional Klf11 KO mice were more pro-thrombotic. The bone marrow transplantation study and the measurement of MV-TF activity excluded a potential involvement of KLF11 from blood cells and circulation-derived TF. Similar pro-thrombotic phenotype was observed in the Sm-Klf11 KO mice, which further demonstrated the anti-thrombotic effects of KLF11 in the vascular wall under basal conditions.
TF is critical in maintaining the balance between hemostasis and thrombosis.37 VSMCs in human atherosclerotic plaques express high levels of TF.38–40 Interestingly, a previous study using a low-TF mice demonstrated that the vascular wall derived TF, rather than leukocytes derived TF, is responsible for the macrovascular thrombosis.41 Therefore, the lower expression of TF in the vascular wall, especially in the VSMCs, can limit the initiation of the TF-dependent coagulation cascade and thus be a potentially protective mechanism for the prolonged occlusion time in vivo. Compared with endothelial cells (ECs), VSMCs have a higher constitutive expression of TF and are considered as the primary source of TF in the arterial wall.42 The expression of TF can be rapidly induced in VSMCs after artery injury43 and contributes to thrombosis events after plaque rupture.38, 44 The SM22-driven VSMC-specific TF-deficient mice showed an increase in occlusion time in FeCl3-induced arterial thrombosis, indicating a key role of VSMC-derived TF in arterial thrombosis.19 Although TF is not the only key factor in thrombosis formation, inhibition of TF activity by a monoclonal antibody45 or administration of its counterpart recombinant TFPI46 showed beneficial effects, which make inhibition of TF a potential pharmaceutical target for thrombosis. In addition, it has been reported that the deficiency of TFPI in smooth muscle cells can reduce the occlusion time in FeCl3 model.47 However, in this study, we found that TFPI expression was not significantly changed in the KLF11-deficient VSMCs. Therefore, the increased TF can at least partially account for the pro-thrombotic phenotype in the KLF11-deficient VSMCs.
It is well known that thrombin causes positive feedback effects on the coagulation cascade, including promoting the contact activation pathway.48 However, numerous studies indicated that thrombin can also induce TF expression in VSMCs.49–52 Thrombin can bind to protease-activated receptors (PARs) on human aortic SMCs and activate protein kinase B (PKB), protein kinase C (PKC) and mitogen-activated protein kinase (MAPK) pathways, which could induce TF expression.53–55 Our data demonstrated that KLF11 directly binds to the F3 promoter and inhibits its activity in VSMCs, which account for the KLF11-dependent decreased F3 transcription under thrombin stimulation. The effect of KLF11 on thrombin-activated signaling pathways (e.g. PARs, PKB, PKC and MAPK) warrants future investigation.
In the present study, we demonstrated that KLF11 binds to F3 promoter to inhibit its transcription (Figure 6). In basal conditions (serum starvation), TF is expressed at very low level in VSMCs, and endogenous KLF11 is abundant enough to maintain the low level of TF by direct binding to the F3 promoter. Thus, overexpression of KLF11 did not further inhibit F3 transcription (Figure 5). The ChIP (Figure 6C) and luciferase (Figure 6D-E) experiments were performed in normal growth conditions (10% FBS). Under these conditions, we observed the inhibitory effect of KLF11 on F3 transcription (Figure 5). Upon thrombin or serum stimulation, overexpressed KLF11 may compete with other transcription factors such as activator protein-1 (AP-1),56 whose binding region (−172–160bp) at the F3 promoter overlaps with the KLF11 binding region (−177–161bp). On the other hand, KLF11 deficiency may vacate the KLF11 binding site and abolish the repressive effect of KLF11 on F3 promoter activity. Therefore, KLF11 deficiency can increase F3 transcription even under basal conditions (Figure 4).
The FeCl3 model is a commonly used experimental model to study arterial thrombosis.57 However, whether the endothelium is denudated and internal elastic lamina is intact in this model are still controversial.58, 59 The mechanisms mediating the VSMC derived effect on arterial thrombosis in the FeCl3 model remain to be investigated. Noteworthy, despite an intact internal elastic lamina upon FeCl3 infiltration,58, 59 VSMC-specific TF KO inhibited the thrombus formation in the FeCl3 model.19 In the present study, we found that smooth muscle cell specific Klf11 KO mice had a pro-thrombotic phenotype in association with increased TF, indicating that VSMC KLF11 can maintain anti-thrombotic state through transcriptional control of F3. We also found that KLF11 overexpression potently inhibited tumor necrosis factor alpha (TNF-α)-induced TF expression in HUVECs at both the mRNA and protein levels (Supplemental Figure VII), although it should be highlighted that endogenous levels of KLF11 in ECs are low.6 Since the contribution of endothelium is unclear in the FeCl3 model,58, 59 the function of EC KLF11 in thrombosis warrants further investigation independently in other appropriate thrombosis models.
Interestingly, although there was no significant difference in TAT complexes between WT and Klf11 KO mice at basal conditions, there was a higher TAT complexes in Klf11 KO mice after LPS treatment (Figure 1G and Supplemental Figure IV). At basal conditions, TF is constitutively expressed in vascular wall and expressed much less in circulation. 60, 61 After LPS stimulation, TF expression is significantly induced not only in vascular wall, but also monocytes. 62, 63 We have demonstrated that KLF11 inhibits TF expression in VSMCs and ECs (Figure 3–5 and Supplemental Figure VII). Whether KLF11 also has inhibitory effects on TF expression in monocytes and thereby inhibits the pro-thrombotic status under LPS conditions warrants future investigations. Moreover, the conversion of prothrombin to thrombin is the common pathway in coagulation cascade. 61 Under LPS-treated conditions, apart from the increased TF expression, the abnormality of other coagulation factors may also contribute to the elevated TAT complexes in Klf11 KO mice.
In summary, utilizing gain- and loss-of-function strategies, we demonstrated an important homeostatic role of KLF11 as an anti-thrombotic factor in the vascular wall. We uncovered KLF11-dependent transcriptional inhibition of F3 in VSMCs as the potential mechanism underlying this anti-thrombotic effect. Our findings extend the current understanding of the roles of KLF11 in the vascular system. Manipulation of this novel molecular target could contribute to therapeutic strategies aimed at controlling thrombosis under pathological conditions and diabetic vascular pathologies at large.
Supplementary Material
Highlights.
VSMC-specific KLF11 protects against arterial thrombosis.
Tissue factor is increased in the KLF11-deficient VSMCs.
KLF11 inhibits F3 at the transcriptional level.
Acknowledgements
W. Liang, Y. Fan, H. Lu and J. Sun performed experiments and analyzed results; W. Liang and Y. Fan wrote the paper; W. Hu, Z. Chang, H. Wang, T. Zhu, J. Wang, M. T. Garcia-Barrio, R. Adili, M. Holinstat, D. Eitzman, and J. Zhang contributed to discussion of the project and manuscript. M. T. Garcia-Barrio did critical editing and data analysis; Y. Fan and Y.E. Chen. designed research and discussed results.
The authors would like to thank Dr. Audrey Cleuren at University of Michigan for the help on PT and aPTT experiments; Dr. Wenjie Li and Ms. Megan Hawley at University of Michigan for the assistance on the platelet aggregation experiments; Dr. Daniel Kirchhofer at Genentech Inc for providing the rat anti-mouse TF 1H1 antibody; Dr. Nigel Mackman and Dr. Yohei Hisada at University of North Carolina at Chapel Hill for the kind help on the TF activity assays; Dr. Wei Zhou at Harvard Medical School for the assistance on database analysis.
Sources of funding
This work was partially supported by NIH grants HL068878, HL137214, and HL134569 (Y.E.C.), HL138094 (Y.F.), HL138139 (J.Z.), and American Heart Association grants 18PRE34000005 (W.L), 14SDG19880014 (Y.F.) and 17PRE33400179 (H.L.).
Nonstandard Abbreviations and Acronyms
- KLF
krüppel-like factor
- VSMC
vascular smooth muscle cell
- HASMC
human aortic smooth muscle cell
- MASMC
mouse aortic smooth muscle cell
- Myh11
smooth muscle cell myosin heavy chain 11
- WT
wild type
- KO
knockout
- TF
tissue factor
- TFPI
tissue factor pathway inhibitor
- TIEG
transforming growth factor-β-inducible gene
- aPTT
activated partial thromboplastin time
- PT
prothrombin time
- BMT
bone marrow transplantation
- siRNA
small interfering RNA
- Ad
adenovirus
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- FeCl3
ferric chloride
Footnotes
Disclosures
None
References
- 1.Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, Hylek EM, Kakkar A, Konstantinides SV, McCumber M, Ozaki Y, Wendelboe A, Weitz JI, Day ISCfWT. Thrombosis: A major contributor to global disease burden. Arterioscler Thromb Vasc Biol 2014;34:2363–2371. [DOI] [PubMed] [Google Scholar]
- 2.Wolberg AS, Aleman MM, Leiderman K, Machlus KR. Procoagulant activity in hemostasis and thrombosis: Virchow’s triad revisited. Anesth Analg 2012;114:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michel JB, Li Z, Lacolley P. Smooth muscle cells and vascular diseases. Cardiovasc Res 2012;95:135–137. [DOI] [PubMed] [Google Scholar]
- 4.McConnell BB, Yang VW. Mammalian kruppel-like factors in health and diseases. Physiol Rev 2010;90:1337–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alaiti MA, Orasanu G, Tugal D, Lu Y, Jain MK. Kruppel-like factors and vascular inflammation: Implications for atherosclerosis. Curr Atheroscler Rep 2012;14:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan Y, Lu H, Liang W, Hu W, Zhang J, Chen YE. Kruppel-like factors and vascular wall homeostasis. J Mol Cell Biol 2017;9:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V et al. Role of transcription factor klf11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A 2005;102:4807–4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnefond A, Lomberk G, Buttar N et al. Disruption of a novel kruppel-like transcription factor p300-regulated pathway for insulin biosynthesis revealed by studies of the c.−331 ins mutation found in neonatal diabetes mellitus. J Biol Chem 2011;286:28414–28424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Zapico ME, van Velkinburgh JC, Gutierrez-Aguilar R, Neve B, Froguel P, Urrutia R, Stein R. Mody7 gene, klf11, is a novel p300-dependent regulator of pdx-1 (mody4) transcription in pancreatic islet beta cells. J Biol Chem 2009;284:36482–36490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Chen Q, Yang M, Zhu B, Cui Y, Xue Y, Gong N, Cui A, Wang M, Shen L, Zhang S, Fang F, Chang Y. Mouse klf11 regulates hepatic lipid metabolism. J Hepatol 2013;58:763–770. [DOI] [PubMed] [Google Scholar]
- 11.Hess K, Grant PJ. Inflammation and thrombosis in diabetes. Thromb Haemost 2011;105 Suppl 1:S43–54. [DOI] [PubMed] [Google Scholar]
- 12.Pechlivani N, Ajjan RA. Thrombosis and vascular inflammation in diabetes: Mechanisms and potential therapeutic targets. Front Cardiovasc Med 2018;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vazzana N, Ranalli P, Cuccurullo C, Davi G. Diabetes mellitus and thrombosis. Thromb Res 2012;129:371–377. [DOI] [PubMed] [Google Scholar]
- 14.Fan Y, Guo Y, Zhang J, Subramaniam M, Song CZ, Urrutia R, Chen YE. Kruppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-kappab signaling pathway. Arterioscler Thromb Vasc Biol 2012;32:2981–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glineur C, Gross B, Neve B, Rommens C, Chew GT, Martin-Nizard F, Rodriguez-Pascual F, Lamas S, Watts GF, Staels B. Fenofibrate inhibits endothelin-1 expression by peroxisome proliferator-activated receptor alpha-dependent and independent mechanisms in human endothelial cells. Arterioscler Thromb Vasc Biol 2013;33:621–628. [DOI] [PubMed] [Google Scholar]
- 16.Yin KJ, Fan Y, Hamblin M, Zhang J, Zhu T, Li S, Hawse JR, Subramaniam M, Song CZ, Urrutia R, Lin JD, Chen YE. Klf11 mediates ppargamma cerebrovascular protection in ischaemic stroke. Brain 2013;136:1274–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherepanova OA, Gomez D, Shankman LS et al. Activation of the pluripotency factor oct4 in smooth muscle cells is atheroprotective. Nat Med 2016;22:657–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Shao F, Hinds A, Yao S, Ram-Mohan S, Norman TA, Krishnan R, Fine A. Retinoic acid signaling is essential for airway smooth muscle homeostasis. JCI Insight 2018;3:e120398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood 2009;113:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilley RE, Pedersen B, Pawlinski R, Sato Y, Erlich JH, Shen Y, Day S, Huang Y, Eitzman DT, Boisvert WA, Curtiss LK, Fay WP, Mackman N. Atherosclerosis in mice is not affected by a reduction in tissue factor expression. Arterioscler Thromb Vasc Biol 2006;26:555–562. [DOI] [PubMed] [Google Scholar]
- 21.Luo W, Wang H, Ohman MK, Guo C, Shi K, Wang J, Eitzman DT. P-selectin glycoprotein ligand-1 deficiency leads to cytokine resistance and protection against atherosclerosis in apolipoprotein e deficient mice. Atherosclerosis 2012;220:110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Wang X, Degen JL, Ginsburg D. Reduced thrombin generation increases host susceptibility to group a streptococcal infection. Blood 2009;113:1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeung J, Tourdot BE, Adili R, Green AR, Freedman CJ, Fernandez-Perez P, Yu J, Holman TR, Holinstat M. 12(s)-hetre, a 12-lipoxygenase oxylipin of dihomo-gamma-linolenic acid, inhibits thrombosis via galphas signaling in platelets. Arterioscler Thromb Vasc Biol 2016;36:2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamblin M, Chang L, Chen YE. Isolation and culture of vascular smooth muscle cells. Manual of research techniques in cardiovascular medicine 2013:125–130. [Google Scholar]
- 25.Lu H, Fan Y, Qiao C, Liang W, Hu W, Zhu T, Zhang J, Chen YE. Tfeb inhibits endothelial cell inflammation and reduces atherosclerosis. Sci Signal 2017;10:eaah4214. [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, Lu H, Liang W, Garcia-Barrio MT, Guo Y, Zhang J, Zhu T, Hao Y, Zhang J, Chen YE. Endothelial tfeb (transcription factor eb) positively regulates postischemic angiogenesis. Circ Res 2018;122:945–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK. Klf4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taubman MB, Wang L, Miller C. The role of smooth muscle derived tissue factor in mediating thrombosis and arterial injury. Thromb Res 2008;122 Suppl 1:S78–81. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhofer D, Moran P, Bullens S, Peale F, Bunting S. A monoclonal antibody that inhibits mouse tissue factor function. J Thromb Haemost 2005;3:1098–1099. [DOI] [PubMed] [Google Scholar]
- 30.Pollak NM, Hoffman M, Goldberg IJ, Drosatos K. Kruppel-like factors: Crippling and un-crippling metabolic pathways. JACC Basic Transl Sci 2018;3:132–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Z, Kumar A, SenBanerjee S, Staniszewski K, Parmar K, Vaughan DE, Gimbrone MA Jr., Balasubramanian V, Garcia-Cardena G, Jain MK. Kruppel-like factor 2 (klf2) regulates endothelial thrombotic function. Circ Res 2005;96:e48–57. [DOI] [PubMed] [Google Scholar]
- 32.Boon RA, Fledderus JO, Volger OL, van Wanrooij EJ, Pardali E, Weesie F, Kuiper J, Pannekoek H, ten Dijke P, Horrevoets AJ. Klf2 suppresses tgf-beta signaling in endothelium through induction of smad7 and inhibition of ap-1. Arterioscler Thromb Vasc Biol 2007;27:532–539. [DOI] [PubMed] [Google Scholar]
- 33.Zhou G, Hamik A, Nayak L et al. Endothelial kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest 2012;122:4727–4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjornsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess mortality among persons with type 2 diabetes. N Engl J Med 2015;373:1720–1732. [DOI] [PubMed] [Google Scholar]
- 35.Sachs UJ, Nieswandt B. In vivo thrombus formation in murine models. Circ Res 2007;100:979–991. [DOI] [PubMed] [Google Scholar]
- 36.Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (eitzman series). Arterioscler Thromb Vasc Biol 2007;27:2079–2093. [DOI] [PubMed] [Google Scholar]
- 37.Grover SP, Mackman N. Tissue factor: An essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 2018;38:709–725. [DOI] [PubMed] [Google Scholar]
- 38.Marmur JD, Thiruvikraman SV, Fyfe BS, Guha A, Sharma SK, Ambrose JA, Fallon JT, Nemerson Y, Taubman MB. Identification of active tissue factor in human coronary atheroma. Circulation 1996;94:1226–1232. [DOI] [PubMed] [Google Scholar]
- 39.Annex BH, Denning SM, Channon KM, Sketch MH Jr., Stack RS, Morrissey JH, Peters KG. Differential expression of tissue factor protein in directional atherectomy specimens from patients with stable and unstable coronary syndromes. Circulation 1995;91:619–622. [DOI] [PubMed] [Google Scholar]
- 40.Tremoli E, Camera M, Toschi V, Colli S. Tissue factor in atherosclerosis. Atherosclerosis 1999;144:273–283. [DOI] [PubMed] [Google Scholar]
- 41.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, Wakefield TW, Mackman N, Fay WP. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood 2005;105:192–198. [DOI] [PubMed] [Google Scholar]
- 42.Mackman N, Taubman MB. Does tissue factor expression by vascular smooth muscle cells provide a link between c-reactive protein and cardiovascular disease? Arterioscler Thromb Vasc Biol 2008;28:601–603. [DOI] [PubMed] [Google Scholar]
- 43.Marmur JD, Rossikhina M, Guha A, Fyfe B, Friedrich V, Mendlowitz M, Nemerson Y, Taubman MB. Tissue factor is rapidly induced in arterial smooth muscle after balloon injury. J Clin Invest 1993;91:2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A 1989;86:2839–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrow DA, Murphy SA, McCabe CH, Mackman N, Wong HC, Antman EM. Potent inhibition of thrombin with a monoclonal antibody against tissue factor (sunol-ch36): Results of the proximate-timi 27 trial. Eur Heart J 2005;26:682–688. [DOI] [PubMed] [Google Scholar]
- 46.Abraham E, Reinhart K, Opal S et al. Efficacy and safety of tifacogin (recombinant tissue factor pathway inhibitor) in severe sepsis: A randomized controlled trial. JAMA 2003;290:238–247. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Jin K, Xiao J, Ma J, Ma D. Endogenous tissue factor pathway inhibitor in vascular smooth muscle cells inhibits arterial thrombosis. Front Med 2017;11:403–409. [DOI] [PubMed] [Google Scholar]
- 48.Spronk H, Borissoff J, ten Cate H. New insights into modulation of thrombin formation. Curr Atheroscler Rep 2013;15:363. [DOI] [PubMed] [Google Scholar]
- 49.Taubman MB, Marmur JD, Rosenfield CL, Guha A, Nichtberger S, Nemerson Y. Agonist-mediated tissue factor expression in cultured vascular smooth muscle cells. Role of ca2+ mobilization and protein kinase c activation. J Clin Invest 1993;91:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herkert O, Diebold I, Brandes RP, Hess J, Busse R, Gorlach A. Nadph oxidase mediates tissue factor-dependent surface procoagulant activity by thrombin in human vascular smooth muscle cells. Circulation 2002;105:2030–2036. [DOI] [PubMed] [Google Scholar]
- 51.BelAiba RS, Djordjevic T, Bonello S, Artunc F, Lang F, Hess J, Gorlach A. The serum- and glucocorticoid-inducible kinase sgk-1 is involved in pulmonary vascular remodeling: Role in redox-sensitive regulation of tissue factor by thrombin. Circ Res 2006;98:828–836. [DOI] [PubMed] [Google Scholar]
- 52.Gorlach A, BelAiba RS, Hess J, Kietzmann T. Thrombin activates the p21-activated kinase in pulmonary artery smooth muscle cells. Role in tissue factor expression. Thromb Haemost 2005;93:1168–1175.15968404 [Google Scholar]
- 53.Chung SW, Park JW, Lee SA, Eo SK, Kim K. Thrombin promotes proinflammatory phenotype in human vascular smooth muscle cell. Biochem Biophys Res Commun 2010;396:748–754. [DOI] [PubMed] [Google Scholar]
- 54.Jeong JY, Son Y, Kim BY, Eo SK, Rhim BY, Kim K. Multiple signaling pathways contribute to the thrombin-induced secretory phenotype in vascular smooth muscle cells. Korean J Physiol Pharmacol 2015;19:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djordjevic T, Hess J, Herkert O, Gorlach A, BelAiba RS. Rac regulates thrombin-induced tissue factor expression in pulmonary artery smooth muscle cells involving the nuclear factor-kappab pathway. Antioxid Redox Signal 2004;6:713–720. [DOI] [PubMed] [Google Scholar]
- 56.Yasumoto H, Kim S, Zhan Y, Miyazaki H, Hoshiga M, Kaneda Y, Morishita R, Iwao H. Dominant negative c-jun gene transfer inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia in rats. Gene Ther 2001;8:1682–1689. [DOI] [PubMed] [Google Scholar]
- 57.Schoenwaelder SM, Jackson SP. Ferric chloride thrombosis model: Unraveling the vascular effects of a highly corrosive oxidant. Blood 2015;126:2652–2653. [DOI] [PubMed] [Google Scholar]
- 58.Barr JD, Chauhan AK, Schaeffer GV, Hansen JK, Motto DG. Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood 2013;121:3733–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eckly A, Hechler B, Freund M, Zerr M, Cazenave JP, Lanza F, Mangin PH, Gachet C. Mechanisms underlying fecl3-induced arterial thrombosis. J Thromb Haemost 2011;9:779–789. [DOI] [PubMed] [Google Scholar]
- 60.Drake TA, Morrissey JH, Edgington TS. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 61.Mackman N The role of tissue factor and factor viia in hemostasis. Anesthesia and analgesia 2009;108:1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood 2005;105:2764–2770. [DOI] [PubMed] [Google Scholar]
- 63.Bode M, Mackman N. Regulation of tissue factor gene expression in monocytes and endothelial cells: Thromboxane a2 as a new player. Vascular pharmacology 2014;62:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.