Abstract
Ray Guillery had broad research interests that spanned cellular neuroanatomy, but was perhaps best known for his investigation of the connectivity and function of the thalamus, especially the visual pathways. His work on the genetics of abnormal vision in albino mammals served as an early paradigm for genetic approaches for studying brain connectivity of complex species in general, and remains of major relevance today. This work, especially on the Siamese cat, illustrates the complex relationship between genotype and physiology of cerebral cortical circuits, and anticipated many of the issues underlying the imperfect relationship between genes, circuits, and behavior in mammalian species including human. This review also briefly summarizes studies from our own lab inspired by Ray Guillery’s legacy that continue to explore the relationship between genes, structure and behavior in human cerebral cortex.
Keywords: Siamese Cat, genetics, brain development, cerebral cortex
When Ray Guillery died last April, neuroscience lost a great mind and a great person, a giant intellectual leader who was so understated that his remarkable contributions may not be as widely known as they should be. Those of us fortunate enough to have spent an extended period of time with him during our training believe that our careers bear his indelible imprint, if not being largely defined by his influence. In this piece I will try to capture Ray’s approach to science and its effects on me, with apologies that there is no clear way to describe this other than being autobiographical. His thoughts and imprint live on, and readers unfamiliar with the man will undoubtedly find his way of thinking about the brain to be interesting and informative (Guillery, 2017).
I will never forget the first lab meeting when I heard Ray present. I was a first-year graduate student, and this meeting captures in my mind the essence of his genius, recognizable by those who knew him. He was teaching us about the phenomenon of transsynaptic degeneration, discovered by Bernhard von Gudden (Muller, 2001), whose work Ray was studying at the time: removing the eyes of newborn animals to understand how this manipulation affected the patterns of connectivity of the remaining eye. He told us not only about von Gudden’s work, but the entire story of von Gudden, who was best known as the personal psychiatrist for “mad” Prince Ludwig of Bavaria, the benefactor of Richard Wagner, and the designer and builder of Neuschwanstein (the castle in Bavaria that is the model for the Disneyland castle). And to cap it off, he told us the enduring mystery story of how von Gudden and Ludwig died together under the most mysterious of circumstances, drowned in 3 feet of water in the midst of controversy about Ludwig’s fitness to lead the country (Guillery, 2011).
This was Ray. He was always putting science into a larger cultural-philosophical context, bringing in art and history and humor. We would talk science and philosophy over lunch in a subbasement conference room at the University of Chicago, eating our sandwiches and drinking black coffee. At some point I would make some poorly-informed philosophical generalization, and he would pounce on me and tell me I was shooting from the hip. We talked about Kuhn’s “Structure of Scientific Revolutions” (Kuhn, 1962), and what really counted as a revolution in neuroscience and what did not. He told me about how 19th century neuroanatomists were influenced by their culture and intellectual traditions—those west of the Rhein river (such as Campbell, and Cajal) by the empiricists such as Berkeley and Hume, while those east of the Rhein (such as Kolliker, Brodmann, and the Vogts) by the idealists such as Kant. We can see this in the classification of cerebral cortical areas: Cajal describes 9 layers in some areas, and 5 layers in other areas, describing them one at a time as they appear under the microscope. In contrast, Brodmann postulates an over-arching developmental-functional-evolutionary “Uhrstruktur” of a conserved 6-layer structure—but nonetheless one that is subject to absence of layers in some places and duplication of layers in other places (Brodmann & Garey, 2006). Which system is better? How do we define which system is better? Though Ray loved to poke fun at the Germanic culture into which he was born, such decisions like this are based on scientific utility, and here he concluded that Brodmann’s concept of a shared 6-layered structure has certainly been biological insightful and useful.
Of course, one of Ray’s greatest scientific heroes was Ramon y Cajal, and he transmitted that love to me by talking about the man, his work, and his unique character as he had understood it (he himself never having met Cajal). When he indicated that anyone serious about neuroanatomy had to read Cajal’s “Histologie du Systeme Nerveux” (at that time not available in English), I promptly embarked on teaching myself enough French so that I could stumble through it. Ray also encouraged me to take extra coursework in genetics, unusual for a budding neuroscientist, but which had a permanent influence on my future course.
Ray taught us Peter Medawar’s credo that Science is the “Art of the Soluble” (Medawar, 1967). By that he meant to take an impossibly difficult problem—understanding the function of the brain, for instance—and find within that a problem that was interesting, but nonetheless “soluble” on some level—for which definitive data could be found, or a specific hypothesis disproven. The disproof of hypotheses was how Medawar writes that science moved forward, leading to his famous critique of psychoanalysis as being useless because it was essentially non-disprovable. It was not just this approach of finding soluble problems that influenced me, but the discipline of reflecting on how we we find soluble problems that has had a lasting effect. Medawar’s books are still as timely now as then, and I recommend them to students and postdocs.
One of the many projects ongoing in Ray’s laboratory at that time was the study of the Siamese cat (Guillery, 1969; Guillery et al., 1974; LaMantia, 2018; Mason & Guillery, 2018; Taylor, 2018). Siamese cats are a temperature-sensitive albino mutation, hence their dark points, and they manifest abnormalities of the visual system that Ray and others described in albinos of many, apparently all, mammalian species, including humans (Guillery, 1971; Guillery et al., 1971; Guillery & Kaas, 1973; Guillery et al., 1975). In each albino, a majority of the retinal ganglion cell fibers from the temporal retina that would normally project to the ipsilateral (same side) lateral geniculate nucleus instead send their axons contralaterally—to the same spot but the wrong side of the brain. This creates a disorderly map of the retina in the LGN and, if transmitted unaltered to the cortex, a highly abnormal visual map on cortex in which a single cortical column would receive input from two different points of the same retina--in essence, data from two noncorresponding points in visual space—hence a fundamentally ambiguous visual map that would seem to prevent normal motor behavior (Figure 1). Yet, Ray and Jon Kaas showed that relatively normal visual behavior on the part of Siamese cats in their Midwestern colony reflects the suppression at the level of the cerebral cortex of much of the abnormal input, and Viven Casagrande showed that the visually guided behavior of Siamese cats is generally excellent (Guillery & Casagrande, 1977; Casagrande et al., 1978). Even more amazingly, Hubel and Wiesel showed in their own “Boston” Siamese colony an alternative solution—a true developmental miracle--in which the globally abnormal visual map is recognized as abnormal prior to the eyes opening, and is reorganized to reconstruct at the level of the cortex an orderly map of the world (Hubel & Wiesel, 1971; Kaas & Guillery, 1973; Guillery & Casagrande, 1977; Cooper & Blasdel, 1980).
Figure 1.
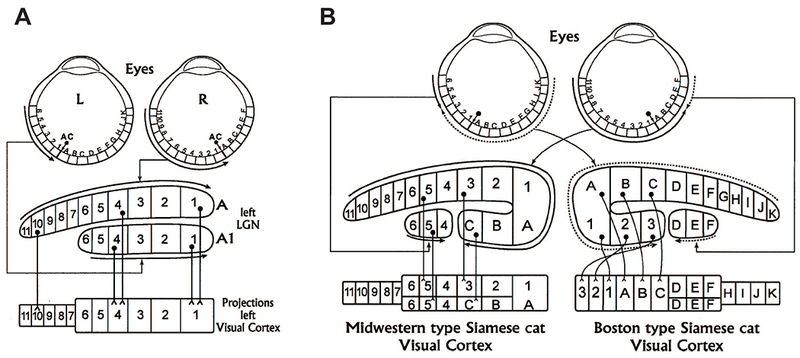
The Siamese cat abnormality, as an example of albino abnormalities in mammals. Taken from Kaas (Kaas, 2005) (with permission), the left panel illustrates the normal pattern of partial decussation of visual fibers from the retina to the geniculate, and their normal pattern of projection to visual cortex. The right panel summarizes the abnormal patterns of decussation seen in the Siamese cat, in which fibers from temporal retina that normally project ipsilaterally undergo abnormal crossing at the optic chiasm. This panel also illustrates the two ways in which this abnormal visual input is corrected at the level of the visual cortex, either by a relatively normal pattern of geniculocortical projection (along with relative suppression of the abnormal input) in Midwestern Siamese cats, or reorganization and re-mapping of the abnormal input in the Boston cats.
Thus, the Siamese cat presents two fundamental and fascinating problems in developmental biology. One relates to how retinal ganglion cells are instructed to project to one side or another of the brain, and what is the role of pigment—which is only detectably present in the pigmented retina and not in the neural retina—in this process? This issue is discussed elsewhere in this issue (Mason, 2018). And the second developmental problem, much more profound, is about how the ‘non-genetic’, or perhaps epigenetic, map reorganization (prior to visual experience) takes place. This latter question raised fascinating issues of the roles and limits of genetic explanations, taken up in a review article by Gunter Stent at the time.
“For the viewpoint that the structure and function of the nervous system of an animal is specified by its genes provides too narrow a context for actually understanding developmental processs and thus sets a goal for the genetic approach that is unlikely to be reached. Here ‘too narrow’ is not to mean that a belief in genetic specification of the nervous system necessarily implies a lack of awareness that in development there occurs an interaction between genes and environment, a fact of which all practitioners of the genetic approach are certainly aware. Rather, ‘too narrow’ means that the role of the genes, which, thanks to the achievements of molecular biology, we now know to be the specification of the primary structure of protein molecules, is at too many removes from the processes that actually ‘build nerve cells and specify neural circuits which underlie behavior’ to provide an appropriate conceptual framework for posing the developmental questions that need to be answered.”(Stent, 1981)
To a naïve, second-year graduate student the non-genetic, compensatory alteration of thalamo-cortical pathways that occurs in Siamese cats, in which disordered mapping is either selectively suppressed or globally reorganized into a continuous map of visual space, harkened philosophically to Emanuel Kant’s Critique of Pure Reason (Kant, 1998), in which he proposed that space, time and the categories are forms imposed by the mind (or brain) on the stuff of sensory experience. Hence, this forced visual reorganization represents a demand that the brain, or the “unity of consciousness” of Kant, can only function according to certain rules that in this case demand an orderly representation of visual space at all costs. Thus, neural mechanisms at the level of cortex demand orderly maps of the world, and when they are disrupted at the retinogeniculate level by albinism, they are reconstructed at the level of cortex by nongenetic mechanisms.
Back then, as I was casting about for a PhD thesis project, defining the biological instantiation of Kant’s “a priori” knowledge in the thalamo-cortical reorganization of Siamese cats, did not seem to satisfy Medawar’s dictum of the “Art of the Soluble” by a good bit. Even the seemingly simpler problem raised by the albino--defining how pigment might regulate axon crossing--seemed difficult and not well modeled by mice, where the genetics is good but the animals have only a puny uncrossed visual projection so that the magnitude of the effect of albino mutations is small. So my thesis with Ray settled for soluble problems. For example, working with Ray and Ed Polley, we described the timing and pattern of neurogenesis of the cat’s retinal ganglion cells, finding that distinct subtypes (medium, large, and small) are generated sequentially within a given spot of the retina, forming multiple waves of production (Walsh et al., 1983; Walsh & Polley, 1985). And Ray and I examined the pattern of outgrowth of retinal axons through the optic nerve, chiasm and optic tract in relation to these patterns of neurogenesis (Walsh & Guillery, 1985; Walsh, 1986; Guillery & Walsh, 1987a; b), finding that axons organized themselves in the optic system according to the sequence of production of the parent neurons (Torrealba et al., 1981). Our thinking was that, even if these studies would not solve the albino problem (and they did not), they might be useful background for getting at it. Notably three papers from my PhD thesis with Ray (Polley & Walsh, 1984; Walsh & Polley, 1985; Walsh, 1986) were published without him as a co-author, which was as unheard of then as it is today, demonstrating Ray’s tremendous generosity as a scientist and mentor.
Many studies from Ray’s lab at that time were done on ferrets, an animal that Ray introduced into neuroscience as an ideal model to study development. While at Wisconsin, with its large veterinary school, he explored many different mammalian species in terms of their visual system organization, and in terms of genetically induced retinogeniculate abnormalities (Guillery, 1971; Guillery et al., 1979; Linden et al., 1981; Huang & Guillery, 1985). While their general brain and visual organization and timing of development resembles the cat and other Carnivora, ferrets are born in very large litters (6–12 kits), and only 40–42 days after conception, right in the middle of cerebral cortical neurogenesis (Jackson et al., 1989), so that half of cortical neurogenesis occurs after birth, and much of retinogeniculate axon maturation occurs postnatallay as well (Cucchiaro & Guillery, 1984). He not only used them for postnatal manipulations, but also pioneered and described how remarkably amenable they are to fetal surgery.
Ray left Chicago for Oxford just as I finished my PhD, and at Oxford, he continued work on the organization and development of retinal projections to the thalamus, albino abnormalities, and thalamic structure (LaMantia, 2018; Mason & Guillery, 2018; Taylor, 2018). He also showed how sensory inputs wire into circuits that have already formed with their own internal logic, and studied how corticothalamic and thalamocortical projections organize and reorganize on their respective routes (Mitrofanis & Guillery, 1993; Guillery, 1995; Sherman & Guillery, 1996; Adams et al., 1997; Molnár, 2018; Onat et al., 2018). This work also resulted in his first book, with Murray Sherman, Exploring the Thalamus (Sherman & Guillery, 2001), and eventually to his second and final book (Guillery, 2017), where he increasingly thinks about relationships between thalamic and cortical and other connections, and how they relate functionally to our experience of the world.
I left Chicago soon after finishing my PhD with Ray, and continued to residency training in neurology at Massachusetts General Hospital, which at that time was a hotbed of pioneering human neurogenetics research, with the initiation of work to map and clone genes for Huntington’s disease, familial Alzheimer’s disease, and others (Martin, 1989). My subsequent postdoctoral fellowship with Connie Cepko involved learning molecular biology and more genetics, but was similarly descriptive from an anatomical point of view, with a focus on describing patterns of neurogenesis of cerebral cortical neurons using retroviral gene transfer. We developed libraries of retroviruses with DNA barcodes that could track clones of sibling cells regardless of where they migrated in the brain (Walsh & Cepko, 1988; Walsh & Cepko, 1992; Walsh & Cepko, 1993), so that we could extend methods she developed in the retina to studying the cerebral cortex. I continued to correspond with Ray about our findings, got his comments and support on some of our early papers, and adopted the ferret as an experimental animal for cerebral cortical cell lineage mapping as well after I started my own lab (Reid et al., 1997; Ware et al., 1999). In fact, our lab recently generated one of the first engineered neurological knockouts in ferrets, showing how much better they model defects of human cortical development than mice, and dedicated that paper to him posthumously (Johnson et al., 2018).
My return to studying genes and cerebral cortical development—and now specifically human cerebral cortical development—started unexpectedly a few months after setting up my own lab in 1993. At a meeting in Venice I heard a short talk by Peter Huttenlocher, child neurologist and a former colleague of Ray’s from the University of Chicago, and one of my teachers when I was in medical school there. Ray and Peter were good friends and both had suffered from the Nazi Regime as young children—Ray by escaping Germany in the middle of the night before the war (Sherman et al., 2017), and Peter suffering through the war and its aftermath in Germany as a young child before leaving for America in the late 40s (Lin et al., 2013). Peter presented a family with an inherited malformation of the cerebral cortex, called periventricular nodular heterotopia (Huttenlocher et al., 1994)(Figure 2) which he already suggested was X-linked but lethal to males. When I heard him speak it seemed like an epiphany, one of those rare moments in science where I literally felt my heart race and my palms sweat, because here was a “soluble” problem in human developmental neurogenetics, to map and hopefully clone the gene responsible for the Huttenlocher syndrome. Peter had already shown that the gene was on the X chromosome, so how hard could it be to identify it, and then we could understand how that gene relates to the abnormal cerebral cortical neuronal migration. Of course, this problem seemed soluble for the very reason that it lacked many of the subtleties and the Kantian scope inherent in understanding the wiring changes of the albino abnormality.
Figure 2.
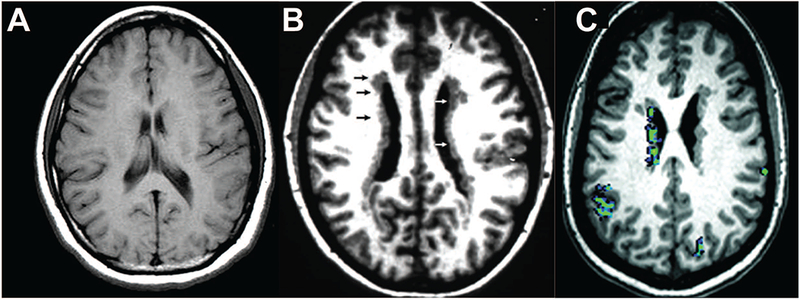
Periventricular nodular heterotopia. The first image (A), shows an axial MRI scan from a normal individual, showing the normal configuration of the cerebral cortex, and ventricular lining. The ventricles show white matter signal right down to the ventricular surface, except for a small part of the ventricle shown on the left side where the body of the caudate nucleus appears near the ventricular surface. The middle image (B) shows an MRI scan of a woman with periventricular nodular heterotopia due to a mutation in the FLNA gene, in this case a de novo mutation not shared by her parents. The small arrows highlight the continuous lining of the ventricular surface on both sides with irregular nodules that show identical signal characteristics to normal cerebral cortex. Figure C is adapted from Christodoulou et al (2012) (with permission) and shows resting-state functional connectivity MRI with bold oxygenation level-dependent (BOLD) imaging. The periventricular nodules in this patient are highly active, and their activity is synchronized with overlying cortex, suggesting that these abnormally placed nodules are structurally and functionally integrated into cerebral cortical circuits.
Our lab dove into the mapping (Eksioglu et al., 1996) and cloning of the FLNA gene responsible for Huttenlocher’s periventricular nodular heterotopia (Fox et al., 1998; Sheen et al., 2001). And this led to similar studies of “double cortex” syndrome (Figure 3), another X-linked cortical malformation, and the identification of the DCX gene responsible for that disorder, and the finding that DCX is a uniquely specific marker of newborn neurons (Allen et al., 1998; Gleeson et al., 1998; Gleeson et al., 1999). That led to studies of dozens of other genetic malformations of the cerebral cortex (Figure 3), and from there we ventured more broadly into study of genetic intellectual disabilities and autism spectrum disorders. So, in many ways my subsequent career—analyzing genes that are essential for normal formation and function of the human cerebral cortex—was a direct followup, and an ongoing tribute to, the ideas that Ray first brought out, to define how the cerebral cortex is defined developmentally by a set of genes. Yet what Ray’s work had already shown was how these genes nonetheless do not account for many of the most interesting and mysterious aspects of human brain function.
Figure 3.
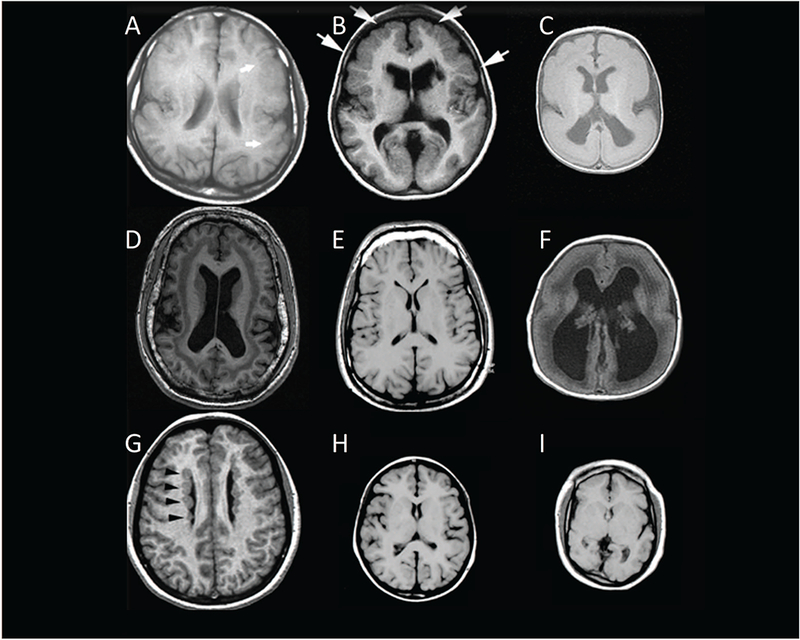
Diverse human brain malformations. The panel shows axial MRI scans from a normal individual (E) surrounded by MRI scans of brains from 8 individuals with Mendelian disorders of cerebral cortical development. A, perisylvian polymicrogyria, presents with normal patterns of cortical folding frontally and posteriorly, with disrupted gyral folding in the perisylvian region (arrows). These patients have a wide range of intellectual and epilepsy phenotypes from almost normal to severely epileptic and intellectually disabled. B shows bilateral frontoparietal polymicrogyria, reflecting biallelic mutation in GPR56, associated with severe intellectual and motor disability. C shows classical lissencephaly, with a smooth, thick cortex, reflecting abnormal neuronal migration, and associated with intractable neonatal epilepsy, severe motor disability, and usually early death. D shows “double cortex” syndrome, in this case due to a female with heterozygous mutations in the X-linked DCX gene, and again showing a very wide range of phenotypes, generally proportional to the thickness of the abnormal subcortical band of neurons, and including intellectual disability and seizures. F shows Walker-Warbug lissencephaly, also associated with severe disability, intractable epilepsy, and early death. G shows periventricular nodular heterotopia, with the abnormally located neurons highlighted by arrows, and associated with FLNA mutation. This condition is generally associated with normal intelligence and variable seizures, and with some patients being clinically asymptomatic altogether. H shows primary microcephaly, in this case due to biallelic mutation in ASPM, and associated with a cortex that is 50–60% reduced in volume, but relatively normally patterned, with normal cortical thickness, and associated with good motor function, intellectual disability, but usually some language development. I shows a patient with complex microcephaly with simplified and abnormal gyral patterning, in this case reflecting biallelic mutation in WDR62, and associated with more severe intellectual disability and motor delay.
Though the developmental basis for the albino misrouting is still not completely understood, the description of the cortical mapping abnormality of human albinos has progressed considerably with noninvasive imaging methods (Morland et al., 2001; Hoffmann et al., 2003; von dem Hagen et al., 2005; Bridge et al., 2014). Remarkably, humans (Guillery et al., 1975; Guillery, 1990; Hedera et al., 1994; Hoffmann et al., 2003; Kaule et al., 2014) as well as other primates (Guillery et al., 1984), often show the sort of conflicting cortical maps described by Ray and Jon Kaas in the Midwestern Siamese cats, in which there are two noncorresponding and mirror-reversed maps of visual space overlapping in the same hemisphere of primary visual cortex (Guillery, 1990). Stated in other words, area 17 of the right hemisphere has a map of the left visual hemifield as the textbook would say, with the midline represented at the 17–18 border and eccentricities of the visual field moving from center to left periphery mapping away from the border. However, that same area 17 also has a second, overlapping map of the right visual hemifield, with the midline again at the 17–18 border, but now eccentricities from center to right periphery also mapping away from the border, overlapping the other, mirror reversed visual map. How do two mirror-reversed maps of noncorresponding points in visual space on the same suite of neurons make sense? And furthermore how do albino humans, despite decreased visual acuity and depth perception, develop normal reading ability (Cole et al., 1987; MacDonald et al., 2012; Bridge et al., 2014; Huurneman et al., 2016b; a; c; 2017), remarkably normal motor behavior, and largely neurotypical cognition?
These questions raise broader questions that remain unanswered despite studies of thousands of patients with abnormally formed brains, caused by specific genetic defects, which has only deepened the mystery about the tremendous range of shape and form of the human brain that is often compatible with remarkably normal intelligence, and outward appearance and behavior. The variety of patterns of human cerebral cortical development that are consistent with neurotypical cognitive and behavioral development is enormous, suggesting that the “normal” pattern of human brain development can be amazingly broad and permissive. For example, in Huttenlocher’s periventricular heterotopia, typical patients have normal IQ and are behaviorally and cognitively indistinguishable from normal, although there is an increased rate of dyslexia, and patients are often first diagnosed when they have a brain MRI scan for other reasons (Chang et al., 2005; Chang et al., 2007). Yet, these patients have large numbers of cerebral cortical neurons arranged in irregular blobs centimeters from their normal locations (Figure 2), usually structurally and functionally connected to the overlying cerebral cortex in bizarre patterns that belie the neat 6-layered structure of textbook cerebral cortex (Christodoulou et al., 2012; Christodoulou et al., 2013; Shafi et al., 2015). Are patients using these abnormally located cortical neurons in conscious thought, or are they capable of normal conscious thought despite the apparent interference of these abnormally positioned neurons? The genetic aspect of this is the tremendously wide variety of shapes and sizes that the human brain can be transformed into by the action of highly penetrant, Mendelian genes, that can arrest neurons in the wrong place, damage a hemisphere, or more. An extreme example is presented by hemimegalencephaly, the abnormal overgrowth of one cerebral hemisphere, reflecting somatic, mosaic mutations (present in some neurons but not all neurons), in genes such as MTOR, AKT3, PIK3CA, and PIK3R that all encode members of the MTOR pathway regulating cell proliferation and growth (Poduri et al., 2012; D’Gama et al., 2015; D’Gama et al., 2017). The pathologically enlarged hemisphere never functions properly because of the mutation, and is the source of intractable epilepsy beginning at birth. Now the common treatment is surgical removal, or surgical disconnection, of the entire hemisphere, since the opposite hemisphere usually is normal and does not contain the mutation. Yet following hemisphere removal, living on just one cerebral hemisphere, many of these children can develop cognitively and behaviorally remarkably well (Figure 4).
Figure 4.
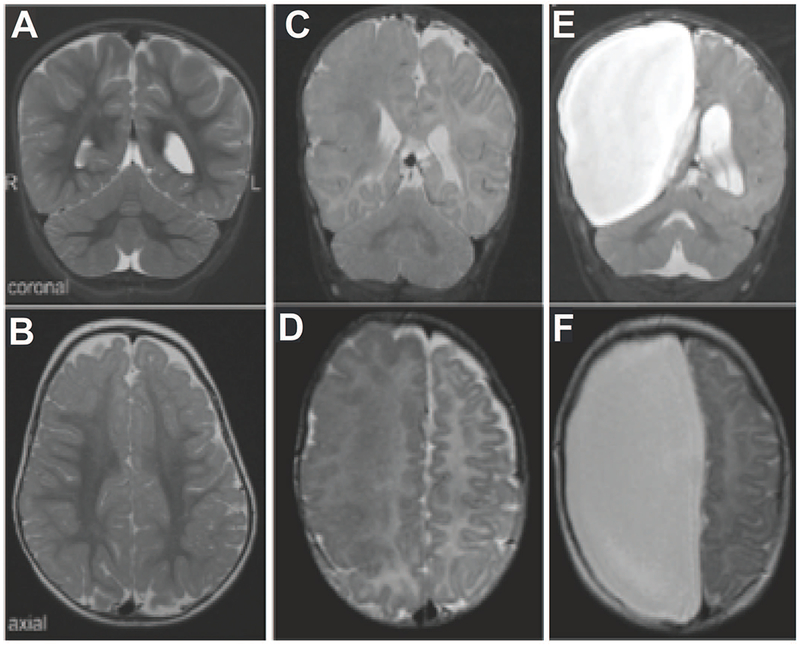
Hemimegalencephaly before and after hemispherectomy. The entire right hemisphere was removed because of intractable epilepsy, replaced by mere cerebrospinal fluid (bright white). The child, who had suffered dozen of seizures a day, and was weak on the left because of the abnormal hemisphere, did very well after the surgery, going on to learn to walk, speak fluently, and read at grade level. Adapted from Poduri et al (Poduri et al., 2012).
Just as mysterious as the diversity of genetic abnormalities consistent with a relatively “typical” apparent experience of the world is the diversity of abnormal responses that can occur when the same highly penetrant genetic mutation occurs in different individuals. Many genetic mutations, including deletion or duplication of chromosome segment 22q11.2 (Fine et al., 2005), or deletion or duplication of chromosome 16p11.2 (Weiss et al., 2008; Hanson et al., 2010) and certain highly penetrant point mutations--such as point mutations in TSC2 (Numis et al.), SHANK3 (Durand et al., 2007; Guilmatre et al., 2014), CHD8 (Bernier et al., 2014), and others--can cause a range of phenotypes in different individuals carrying essentially the same mutation. In most cases, individuals carrying such highly penetrant mutations are abnormal neurologically somehow, but the exact manifestations—whether intellectual disability, autistic symptoms, epilepsy, motor weakness, psychotic symptoms, or virtually no symptoms at all—are often remarkably variable between individuals with the same mutation, even from the same family (Manzini et al., 2014). This variability of neurological manifestations of a shared genetic mutation again harkens back to Stent and to the Boston and Midwestern Siamese cats, where the neurophysiological and behavioral output of a given mutation reflects additional causes that we have yet to identify. Those many years ago, the Siamese cat already provided a way to conceptualize the neurobiology of such differences—that the same genetic mutation might have diverse effects on higher order cortical wiring in different individuals. But we know less about what these other underlying causes of variability—or variable penetrance, in genetic parlance—might be. There are reasons to suspect that “common variation” at multiple sites in the genome might play a role, but I have always felt that the development of each complex brain is a singular history, in which the genes only establish initial conditions of a complex algorithm, and that stochastic, nongenetic, aspects of development might also play a role.
In his last year of life, Ray finished an amazing book that I recommend to all (Guillery, 2017), in which he proposed a plausible, unifying, neurobiological model to explain our private sense of self—i.e., a continuous internal sense of being and continuity. He presents a compelling argument that this psychological sensation reflects patterns of parallel recurrent projections through the brain, with central cortical centers essentially receiving a parallel report of outgoing motor signals via branched collaterals. His approach is radical in proposing specific connectomic pathways underlying some of our experiences that we think of as most uniquely human, while also suggesting that these same pathways—and perhaps analogous experiences—appear to be shared by other mammals. I find this model particularly appealing because it provides a simple explanation for how the wide variety of genetic forms of the human brain share certain common anatomical projection patterns that in turn might underlie commons patterns of thought. Ray’s book is completely neurobiological, while being admittedly speculative; but he has left us with a remarkable new vision that promises to move some one of the most “un-soluble” problems in neuroscience—those involving our experience of consciousness itself--into the realm of the testable.
Acknowledgements:
Research in the author’s lab is supported by grants from the NIMH (U01MH106883 and R01 MH083565) and the NINDS (R01NS032457 and R01 NS035129), and by the Frontiers Program of the Paul G. Allen Family Foundation. C.A.W. is an Investigator of the Howard Hughes Medical Institute. The author has no conflicts of interest.
References:
- Adams NC, Lozsadi DA & Guillery RW (1997) Complexities in the thalamocortical and corticothalamic pathways. Eur J Neurosci, 9, 204–209. [DOI] [PubMed] [Google Scholar]
- Allen KM, Gleeson JG, Shoup SM & Walsh CA (1998) A YAC contig in Xq22.3-q23, from DXS287 to DXS8088, spanning the brain-specific genes doublecortin (DCX) and PAK3. Genomics, 52, 214–218. [DOI] [PubMed] [Google Scholar]
- Bernier R, Golzio C, Xiong B, Stessman HA, Coe BP, Penn O, Witherspoon K, Gerdts J, Baker C, Vulto-van Silfhout AT, Schuurs-Hoeijmakers JH, Fichera M, Bosco P, Buono S, Alberti A, Failla P, Peeters H, Steyaert J, Vissers L, Francescatto L, Mefford HC, Rosenfeld JA, Bakken T, O’Roak BJ, Pawlus M, Moon R, Shendure J, Amaral DG, Lein E, Rankin J, Romano C, de Vries BBA, Katsanis N & Eichler EE (2014) Disruptive CHD8 mutations define a subtype of autism early in development. Cell, 158, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, von dem Hagen EA, Davies G, Chambers C, Gouws A, Hoffmann M & Morland AB (2014) Changes in brain morphology in albinism reflect reduced visual acuity. Cortex, 56, 64–72. [DOI] [PubMed] [Google Scholar]
- Brodmann K & Garey LJ (2006) Brodmann’s Localisation in the Cerebral Cortex. Springer, New York. [Google Scholar]
- Casagrande VA, Guillery RW & Harting JK (1978) Differential effects of monocular deprivation seen in different layers of the lateral geniculate nucleus. J Comp Neurol, 179, 469–485. [DOI] [PubMed] [Google Scholar]
- Chang BS, Katzir T, Liu T, Corriveau K, Barzillai M, Apse KA, Bodell A, Hackney D, Alsop D, Wong ST & Walsh CA (2007) A structural basis for reading fluency: white matter defects in a genetic brain malformation. Neurology, 69, 2146–2154. [DOI] [PubMed] [Google Scholar]
- Chang BS, Ly J, Appignani B, Bodell A, Apse KA, Ravenscroft RS, Sheen VL, Doherty MJ, Hackney DB, O’Connor M, Galaburda AM & Walsh CA (2005) Reading impairment in the neuronal migration disorder of periventricular nodular heterotopia. Neurology, 64, 799–803. [DOI] [PubMed] [Google Scholar]
- Christodoulou JA, Barnard ME, Del Tufo SN, Katzir T, Whitfield-Gabrieli S, Gabrieli JD & Chang BS (2013) Integration of gray matter nodules into functional cortical circuits in periventricular heterotopia. Epilepsy Behav, 29, 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christodoulou JA, Walker LM, Del Tufo SN, Katzir T, Gabrieli JD, Whitfield-Gabrieli S & Chang BS (2012) Abnormal structural and functional brain connectivity in gray matter heterotopia. Epilepsia, 53, 1024–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GF, Conn P, Jones RB, Wallace J & Moore VR (1987) Cognitive functioning in albino children. Dev Med Child Neurol, 29, 659–665. [DOI] [PubMed] [Google Scholar]
- Cooper ML & Blasdel GG (1980) Regional variation in the representation of the visual field in the visual cortex of the Siamese cat. J Comp Neurol, 193, 237–253. [DOI] [PubMed] [Google Scholar]
- Cucchiaro J & Guillery RW (1984) The development of the retinogeniculate pathways in normal and albino ferrets. Proc R Soc Lond B Biol Sci, 223, 141–164. [DOI] [PubMed] [Google Scholar]
- D’Gama AM, Geng Y, Couto JA, Martin B, Boyle EA, LaCoursiere CM, Hossain A, Hatem NE, Barry BJ, Kwiatkowski DJ, Vinters HV, Barkovich AJ, Shendure J, Mathern GW, Walsh CA & Poduri A (2015) Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol, 77, 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Gama AM, Woodworth MB, Hossain AA, Bizzotto S, Hatem NE, LaCoursiere CM, Najm I, Ying Z, Yang E, Barkovich AJ, Kwiatkowski DJ, Vinters HV, Madsen JR, Mathern GW, Blumcke I, Poduri A & Walsh CA (2017) Somatic Mutations Activating the mTOR Pathway in Dorsal Telencephalic Progenitors Cause a Continuum of Cortical Dysplasias. Cell Rep, 21, 3754–3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M & Bourgeron T (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nature genetics, 39, 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksioglu YZ, Scheffer IE, Cardenas P, Knoll J, DiMario F, Ramsby G, Berg M, Kamuro K, Berkovic SF, Duyk GM, Parisi J, Huttenlocher PR & Walsh CA (1996) Periventricular heterotopia: an X-linked dominant epilepsy locus causing aberrant cerebral cortical development. Neuron, 16, 77–87. [DOI] [PubMed] [Google Scholar]
- Fine SE, Weissman A, Gerdes M, Pinto-Martin J, Zackai EH, McDonald-McGinn DM & Emanuel BS (2005) Autism spectrum disorders and symptoms in children with molecularly confirmed 22q11.2 deletion syndrome. J Autism Dev Disord, 35, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JW, Lamperti ED, Eksioglu YZ, Hong SE, Feng Y, Graham DA, Scheffer IE, Dobyns WB, Hirsch BA, Radtke RA, Berkovic SF, Huttenlocher PR & Walsh CA (1998) Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron, 21, 1315–1325. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Allen KM, Fox JW, Lamperti ED, Berkovic S, Scheffer I, Cooper EC, Dobyns WB, Minnerath SR, Ross ME & Walsh CA (1998) Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell, 92, 63–72. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Minnerath SR, Fox JW, Allen KM, Luo RF, Hong SE, Berg MJ, Kuzniecky R, Reitnauer PJ, Borgatti R, Mira AP, Guerrini R, Holmes GL, Rooney CM, Berkovic S, Scheffer I, Cooper EC, Ricci S, Cusmai R, Crawford TO, Leroy R, Andermann E, Wheless JW, Dobyns WB, Walsh CA & et al. (1999) Characterization of mutations in the gene doublecortin in patients with double cortex syndrome. Ann Neurol, 45, 146–153. [DOI] [PubMed] [Google Scholar]
- Guillery RW (1969) An abnormal retinogeniculate projection in Siamese cats. Brain Res, 14, 739–741. [DOI] [PubMed] [Google Scholar]
- Guillery RW (1971) An abnormal retinogeniculate projection in the albino ferret (Mustela furo). Brain Res, 33, 482–485. [DOI] [PubMed] [Google Scholar]
- Guillery RW (1990) Normal and abnormal visual field maps in albinos. Central effects of non-matching maps. Ophthalmic Paediatr Genet, 11, 177–183. [DOI] [PubMed] [Google Scholar]
- Guillery RW (1995) Anatomical evidence concerning the role of the thalamus in corticocortical communication: a brief review. J Anat, 187 (Pt 3), 583–592. [PMC free article] [PubMed] [Google Scholar]
- Guillery RW (2011) The visual pathways in history https://urldefense.proofpoint.com/v2/url?u=https-3A__history.medsci.ox.ac.uk_seminars_history-2Dof-2Dmedical-2Dsciences-2Dseminar-2Dseries_prof-2Dray-2Dguillery-2Dthe-2Dvisual-2Dpathways-2Din-2Dhistory-2Dmaps-2Dof-2Dthe-2Dworld-2Din-2Dthe-2Dbrain_&d=DwIFaQ&c=qS4goWBT7poplM69zy_3xhKwEW14JZMSdioCoppxeFU&r=MwD1kalBGJIi_iASGjfxLuPAZcuzSbCv0iJKqBxNKcqcz75n0lFuTlJUWzKC3S4p&m=b5d-b6oxoO9_eHk4fyXRVC3elpmbmnaEpWYX42gzMg0&s=sfxyFG_E6k-5jBkSw68yU1eF4Feybmag1E8sdP28ApQ&e=.
- Guillery RW (2017) The Brain as a Tool: A Neuroscientist’s Account. Oxford University Press, Oxford. [Google Scholar]
- Guillery RW, Amorn CS & Eighmy BB (1971) Mutants with abnormal visual pathways: an explanation of anomalous geniculate laminae. Science (New York, N.Y, 174, 831–832. [DOI] [PubMed] [Google Scholar]
- Guillery RW & Casagrande VA (1977) Studies of the modifiability of the visual pathways in Midwestern Siamese cats. J Comp Neurol, 174, 15–46. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Casagrande VA & Oberdorfer MD (1974) Congenitally abnormal vision in Siamese cats. Nature, 252, 195–199. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Hickey TL, Kaas JH, Felleman DJ, Debruyn EJ & Sparks DL (1984) Abnormal central visual pathways in the brain of an albino green monkey (Cercopithecus aethiops). J Comp Neurol, 226, 165–183. [DOI] [PubMed] [Google Scholar]
- Guillery RW & Kaas JH (1973) Genetic abnormality of the visual pathways in a “white” tiger. Science (New York, N.Y, 180, 1287–1289. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Oberdorfer MD & Murphy EH (1979) Abnormal retino-geniculate and geniculo-cortical pathways in several genetically distinct color phases of the mink (Mustela vison). J Comp Neurol, 185, 623–655. [DOI] [PubMed] [Google Scholar]
- Guillery RW, Okoro AN & Witkop CJ Jr. (1975) Abnormal visual pathways in the brain of a human albino. Brain Res, 96, 373–377. [DOI] [PubMed] [Google Scholar]
- Guillery RW & Walsh C (1987a) Changing glial organization relates to changing fiber order in the developing optic nerve of ferrets. Journal of Comparative Neurology, 265, 203–217. [DOI] [PubMed] [Google Scholar]
- Guillery RW & Walsh C (1987b) Early uncrossed component of the developing optic nerve with a short extracerebral course: A light and electron microscopic study of fetal ferrets. J. Comp. Neurol, 265, 218–223. [DOI] [PubMed] [Google Scholar]
- Guilmatre A, Huguet G, Delorme R & Bourgeron T (2014) The emerging role of SHANK genes in neuropsychiatric disorders. Dev Neurobiol, 74, 113–122. [DOI] [PubMed] [Google Scholar]
- Hanson E, Nasir RH, Fong A, Lian A, Hundley R, Shen Y, Wu BL, Holm IA & Miller DT (2010) Cognitive and behavioral characterization of 16p11.2 deletion syndrome. J Dev Behav Pediatr, 31, 649–657. [DOI] [PubMed] [Google Scholar]
- Hedera P, Lai S, Haacke EM, Lerner AJ, Hopkins AL, Lewin JS & Friedland RP (1994) Abnormal connectivity of the visual pathways in human albinos demonstrated by susceptibility-sensitized MRI. Neurology, 44, 1921–1926. [DOI] [PubMed] [Google Scholar]
- Hoffmann MB, Tolhurst DJ, Moore AT & Morland AB (2003) Organization of the visual cortex in human albinism. J Neurosci, 23, 8921–8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K & Guillery RW (1985) A demonstration of two distinct geniculocortical projection patterns in albino ferrets. Brain Res, 352, 213–220. [DOI] [PubMed] [Google Scholar]
- Hubel DH & Wiesel TN (1971) Aberrant visual projections in the Siamese cat. J Physiol, 218, 33–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR, Taravath S & Mojtahedi S (1994) Periventricular heterotopia and epilepsy. Neurology, 44, 51–55. [DOI] [PubMed] [Google Scholar]
- Huurneman B, Boonstra FN & Goossens J (2016a) Perceptual Learning in Children With Infantile Nystagmus: Effects on 2D Oculomotor Behavior. Invest Ophthalmol Vis Sci, 57, 4229–4238. [DOI] [PubMed] [Google Scholar]
- Huurneman B, Boonstra FN & Goossens J (2016b) Perceptual Learning in Children With Infantile Nystagmus: Effects on Reading Performance. Invest Ophthalmol Vis Sci, 57, 4239–4246. [DOI] [PubMed] [Google Scholar]
- Huurneman B, Boonstra FN & Goossens J (2016c) Perceptual Learning in Children With Infantile Nystagmus: Effects on Visual Performance. Invest Ophthalmol Vis Sci, 57, 4216–4228. [DOI] [PubMed] [Google Scholar]
- Huurneman B, Boonstra FN & Goossens J (2017) Predictors of Sensitivity to Perceptual Learning in Children With Infantile Nystagmus. Invest Ophthalmol Vis Sci, 58, 4162–4172. [DOI] [PubMed] [Google Scholar]
- Jackson CA, Peduzzi JD & Hickey TL (1989) Visual cortex development in the ferret. I. Genesis and migration of visual cortical neurons. J. Neurosci, 9, 1242–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Sun X, Kiodani A, Borges-Monroy R, Girskis KM, Ryu SC, Wang PP, Komal P, Gonzalez DM, Woo YM, Yan Z, Liang B, Smith RS, Chatterjee M, Coman D, Papademetris X, Staib LH, Hyder F, Mandeville JB, Grant E, Im K, Kwak H, Englehardt JF, Walsh CA & Bae B-I (2018) Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH (2005) Serendipity and the Siamese cat: the discovery that genes for coat and eye pigment affect the brain. ILAR J, 46, 357–363. [DOI] [PubMed] [Google Scholar]
- Kaas JH & Guillery RW (1973) The transfer of abnormal visual field representations from the dorsal lateral geniculate nucleus to the visual cortex in Siamese cats. Brain Res, 59, 61–95. [DOI] [PubMed] [Google Scholar]
- Kant I (1998) Critique of Pure Reason. Cambridge University Press, Cambridge. [Google Scholar]
- Kaule FR, Wolynski B, Gottlob I, Stadler J, Speck O, Kanowski M, Meltendorf S, Behrens-Baumann W & Hoffmann MB (2014) Impact of chiasma opticum malformations on the organization of the human ventral visual cortex. Hum Brain Mapp, 35, 5093–5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn TS (1962) The Structure of Scientific Revolutions. University of Chicago Press, Chicago. [Google Scholar]
- LaMantia AL (2018) The strengths of the genetic approach to understanding neural systems development and function: Ray Guillery’s synthesis. The European Journal of Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DC, Xu L, Ding LW, Sharma A, Liu LZ, Yang H, Tan P, Vadgama J, Karlan BY, Lester J, Urban N, Schummer M, Doan N, Said JW, Sun H, Walsh M, Thomas CJ, Patel P, Yin D, Chan D & Koeffler HP (2013) Genomic and functional characterizations of phosphodiesterase subtype 4D in human cancers. Proc Natl Acad Sci U S A, 110, 6109–6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DC, Guillery RW & Cucchiaro J (1981) The dorsal lateral geniculate nucleus of the normal ferret and its postnatal development. J Comp Neurol, 203, 189–211. [DOI] [PubMed] [Google Scholar]
- MacDonald JT, Kutzbach BR, Holleschau AM, Wyckoff S & Summers CG (2012) Reading skills in children and adults with albinism: the role of visual impairment. J Pediatr Ophthalmol Strabismus, 49, 184–188. [DOI] [PubMed] [Google Scholar]
- Manzini MC, Xiong L, Shaheen R, Tambunan DE, Di Costanzo S, Mitisalis V, Tischfield DJ, Cinquino A, Ghaziuddin M, Christian M, Jiang Q, Laurent S, Nanjiani ZA, Rasheed S, Hill RS, Lizarraga SB, Gleason D, Sabbagh D, Salih MA, Alkuraya FS & Walsh CA (2014) CC2D1A regulates human intellectual and social function as well as NF-kappaB signaling homeostasis. Cell Rep, 8, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JB (1989) Molecular genetic studies in the neuropsychiatric disorders. TINS, 12, 130–137. [DOI] [PubMed] [Google Scholar]
- Mason CA & Guillery RW (2018) Tracking the actions of a single gene from the eye to the brain. The European Journal of Neuroscience. [Google Scholar]
- Medawar PB (1967) The Art of the Soluble Oxford Univ. Press., Oxford. [Google Scholar]
- Mitrofanis J & Guillery RW (1993) New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci, 16, 240–245. [DOI] [PubMed] [Google Scholar]
- Molnár Z (2018) Cortical layer with no known function. The European Journal of Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morland AB, Baseler HA, Hoffmann MB, Sharpe LT & Wandell BA (2001) Abnormal retinotopic representations in human visual cortex revealed by fMRI. Acta Psychol (Amst), 107, 229–247. [DOI] [PubMed] [Google Scholar]
- Muller JL (2001) Johann Bernhard Aloys von Gudden (1824–1886): not only the King of Bavaria’s psychiatrist. Int J Psychiatry Clin Pract, 5, 135–139. [DOI] [PubMed] [Google Scholar]
- Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB & Thiele EA Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology, 76, 981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat F, Oglu MGI & Cavdar S (2018) Thalamic branches of corticofugal axons from view of a critical eye and great mentor, Ray Guillery. The European Journal of Neuroscience. [DOI] [PubMed] [Google Scholar]
- Poduri A, Evrony GD, Cai X, Elhosary PC, Beroukhim R, Lehtinen MK, Hills LB, Heinzen EL, Hill A, Hill RS, Barry BJ, Bourgeois BF, Riviello JJ, Barkovich AJ, Black PM, Ligon KL & Walsh CA (2012) Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron, 74, 41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley EH & Walsh C (1984) A technique for flat embedding and en face sectioning of the mammalian retina for autoradiography. J Neurosci Methods, 12, 57–64. [DOI] [PubMed] [Google Scholar]
- Reid CB, Tavazoie SF & Walsh CA (1997) Clonal dispersion and evidence for asymmetric cell division in ferret cortex. Development, 124, 2441–2450. [DOI] [PubMed] [Google Scholar]
- Shafi MM, Vernet M, Klooster D, Chu CJ, Boric K, Barnard ME, Romatoski K, Westover MB, Christodoulou JA, Gabrieli JD, Whitfield-Gabrieli S, Pascual-Leone A & Chang BS (2015) Physiological consequences of abnormal connectivity in a developmental epilepsy. Ann Neurol, 77, 487–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen VL, Dixon PH, Fox JW, Hong SE, Kinton L, Sisodiya SM, Duncan JS, Dubeau F, Scheffer IE, Schachter SC, Wilner A, Henchy R, Crino P, Kamuro K, DiMario F, Berg M, Kuzniecky R, Cole AJ, Bromfield E, Biber M, Schomer D, Wheless J, Silver K, Mochida GH, Berkovic SF, Andermann F, Andermann E, Dobyns WB, Wood NW & Walsh CA (2001) Mutations in the X-linked filamin 1 gene cause periventricular nodular heterotopia in males as well as in females. Hum Mol Genet, 10, 1775–1783. [DOI] [PubMed] [Google Scholar]
- Sherman SM & Guillery RW (1996) Functional organization of thalamocortical relays. J Neurophysiol, 76, 1367–1395. [DOI] [PubMed] [Google Scholar]
- Sherman SM & Guillery RW (2001) Exploring the Thalamus. Academic Press, San Diego. [Google Scholar]
- Sherman SM, Mason CA, Atabay KD, Kaas JH, LaMantia AS, Mitchell A & Walsh C (2017) Rainer (Ray) W. Guillery 28 August 1929–7 April 2017. Eur J Neurosci, 46, 1933–1936. [Google Scholar]
- Stent GS (1981) Strength and weakness of the genetic approach to the development of the nervous system. Ann Rev Neurosci, 4, 163–194. [DOI] [PubMed] [Google Scholar]
- Taylor J (2018) Studies with Ray Guillery on the early development of the visual pathways: eye cup, optic nerve, chiasm and optic tract. The European Journal of Neuroscience. [DOI] [PubMed] [Google Scholar]
- Torrealba F, Guillery RW, Polley EH & Mason CA (1981) A demonstration of several independent, partially overlapping, retinotopic maps in the optic tract of the cat. Brain Res, 219, 428–432. [DOI] [PubMed] [Google Scholar]
- von dem Hagen EA, Houston GC, Hoffmann MB, Jeffery G & Morland AB (2005) Retinal abnormalities in human albinism translate into a reduction of grey matter in the occipital cortex. Eur J Neurosci, 22, 2475–2480. [DOI] [PubMed] [Google Scholar]
- Walsh C (1986) Age-related fiber order in the ferret’s optic nerve and optic chiasm. J Neurosci, 6, 1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C & Cepko CL (1988) Clonally related neurons show several patterns of migration in cerebral cortex. Science (New York, N.Y, 255, 1342–1345. [DOI] [PubMed] [Google Scholar]
- Walsh C & Cepko CL (1992) Widespread dispersion of neuronal clones across functional regions of the cerebral cortex. Science (New York, N.Y, 255, 434–440. [DOI] [PubMed] [Google Scholar]
- Walsh C & Cepko CL (1993) Widespread clonal dispersion in proliferative layers of cerebral cortex. Nature, 362, 632–635. [DOI] [PubMed] [Google Scholar]
- Walsh C & Guillery RW (1985) Age-related fiber order in the optic tract of the ferret. J Neurosci, 5, 3061–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C & Polley EH (1985) The topography of ganglion cell production in the cat’s retina. J Neurosci, 5, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C, Polley EH, Hickey TL & Guillery RW (1983) Generation of cat retinal ganglion cells in relation to central pathways. Nature, 302, 611–614. [DOI] [PubMed] [Google Scholar]
- Ware ML, Tavazoie SF, Reid CB & Walsh CA (1999) Coexistence of widespread clones and large radial clones in early embryonic ferret cortex. Cereb Cortex, 9, 636–645. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, Platt OS, Ruderfer DM, Walsh CA, Altshuler D, Chakravarti A, Tanzi RE, Stefansson K, Santangelo SL, Gusella JF, Sklar P, Wu BL & Daly MJ (2008) Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med, 358, 667–675. [DOI] [PubMed] [Google Scholar]


