Abstract
Background.
Cognitive impairment is common in patients with end-stage renal disease and is associated with poor outcomes on dialysis. We hypothesized that cognitive impairment might be associated with an increased risk of all-cause graft loss (ACGL) in kidney transplant (KT) recipients.
Methods.
Using the Modified Mini-Mental State (3MS) examination, we measured global cognitive function at KT hospital admission in a prospective, two-center cohort of 864 KT candidates (8/2009–7/2016). We estimated the association between pre-KT cognitive impairment and ACGL using Cox regression, adjusting for recipient, donor, and transplant factors.
Results.
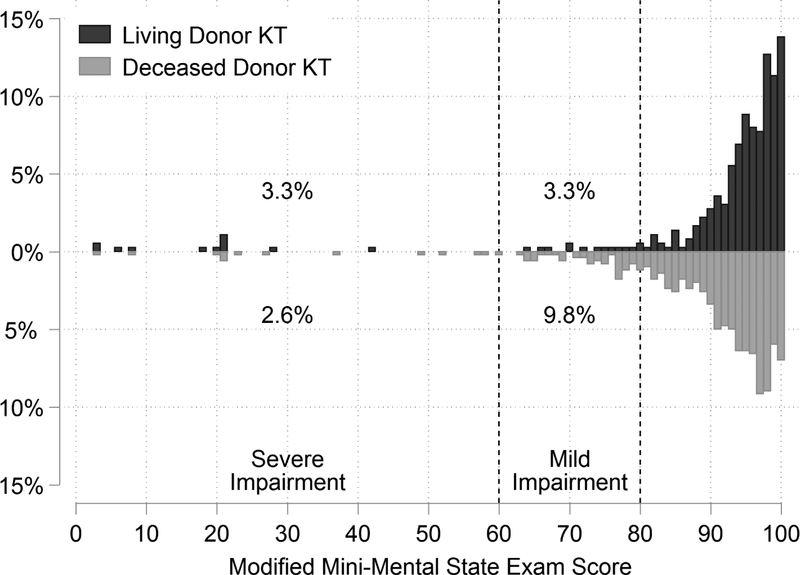
In living donor KT (LDKT) recipients, the prevalence was 3.3% for mild impairment (60≤3MS<80) and 3.3% for severe impairment (3MS<60). In deceased donor KT (DDKT) recipients, the prevalence was 9.8% for mild impairment and 2.6% for severe impairment. LDKT recipients with cognitive impairment had substantially higher ACGL risk than unimpaired recipients (5-year ACGL: 45.5% vs 10.6%, p<0.01; aHR any impairment: 5.40 (95% CI: 1.78–16.34), p<0.01; aHR severe impairment: 5.57 (95% CI: 1.29–24.00), p=0.02). Similarly, DDKT recipients with severe impairment had higher ACGL risk than recipients without severe impairment (5-year ACGL: 53.0% vs 24.2%, p=0.04; aHR severe impairment: 2.92 (95% CI: 1.13–7.50), p=0.03).
Conclusions.
Given the elevated risk of ACGL among KT recipients with cognitive impairment observed in this two-center cohort, research efforts should explore the mechanisms of graft loss and mortality associated with cognitive impairment and identify potential interventions to improve posttransplant survival.
Keywords: Cognition, Kidney Transplantation, Graft Loss, Survival Analysis, Transplant Recipients
INTRODUCTION
Dementia is a relative contraindication to transplantation; however, there are no guidelines for evaluating cognitive impairment in transplant candidates when the degree of cognitive impairment does not fulfill the diagnostic criteria for dementia. Cognitive impairment is increasingly recognized as a risk factor for adverse outcomes in older adults, including adults reaching end-stage renal disease (ESRD) (1). In the community-dwelling geriatric population, cognitive impairment is associated with an increased risk of mortality (2, 3), hospitalization (4), admission to an intensive care unit (5), and discharge to a nursing home following hospitalization (4, 6). We previously reported that incident dementia and Alzheimer’s disease are associated with an elevated risk of graft loss and mortality in older adults following kidney transplantation (KT) (7). Cognitive impairment, which often proceeds dementia, detected prior to KT has not been well characterized and might also be independently associated with graft loss and mortality in KT recipients.
Studies of chronic kidney disease (CKD) patients have shown that cognitive impairment increases in prevalence and severity as kidney function declines (8–13). Cognitive changes across multiple domains, including executive function and memory, begin early in CKD progression and continue after reaching ESRD (13). As a result, patients with ESRD have twice the prevalence of moderate to severe cognitive impairment as the general population (14, 15). Additionally, dialysis initiation has been associated with a decrease in executive function in a multi-center cohort study (16). Cognitive impairment in dialysis patients has been independently associated with an elevated risk of mortality (1, 17, 18), emphasizing the importance of transplantation in this population.
Since the prevalence of cognitive impairment in ESRD patients is high, cognitive impairment is likely also prevalent but underdiagnosed in KT recipients. We hypothesized that baseline cognitive impairment might be independently associated with graft loss and mortality after KT. Using a prospective, longitudinal, two-center cohort of KT recipients, we measured global cognitive function and estimated the association between cognitive impairment and all-cause graft loss, adjusting for recipient, donor, and transplant characteristics.
MATERIALS AND METHODS
Prospective Cohort Data Source
This study used data from a prospective, longitudinal two-center cohort study at the Johns Hopkins Hospital (N=798), Baltimore, Maryland and the University of Michigan Hospital (N=66), Ann Arbor, Michigan, which has been described elsewhere (19–22). Briefly, study participants were enrolled prior to KT and consented to medical record abstraction to allow for the identification of demographics and co-morbidities. All study participants underwent cognitive testing including the Modified Mini-Mental State (3MS) exam upon admission for KT. The clinical and research activities being reported are consistent with the Declaration of Helsinki and Declaration of Istanbul. The Institutional Review Boards of Johns Hopkins Hospital and the University of Michigan approved this study, and all participants provided written informed consent.
National Registry Data Source
This study also used data from the Scientific Registry of Transplant Recipients (SRTR) external release made available in September 2017. The SRTR data system includes data on all donors, waitlist candidates, and transplant recipients in the United States (US), submitted by members of the Organ Procurement and Transplantation Network (OPTN), and has been previously described (23). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Using SRTR, we identified 101 718 adults (age≥18) recipients who underwent KT between August 2009 and July 2016. We cross-validated key recipient, donor, and transplant factors in the linkage between our prospective cohort data source and the SRTR. All recipients were successfully linked with SRTR based on age, sex, unique transplant ID, and date of transplant. We rely on the national registry’s capture of data for all analytic variables and outcomes with the exception of cognitive impairment and additional comorbidity statuses (described below).
Global Cognitive Function
The 3MS examination, a validated assessment of global cognitive function (24), was administered to study participants at admission for KT, which was a median (interquartile range [IQR]) 1 (0–1) days prior to KT. Scores for the 3MS examination range between 0–100 (lower scores indicate worse cognition) based on responses to 15 exam components including temporal and spatial orientation, multi-stage commands, and recall. While there are no standardized thresholds for cognitive impairment in the KT candidate population, it is common in the literature to use standard deviation (SD) thresholds to define cognitive impairment in novel populations (24, 25). Consistent with this convention, we defined any cognitive impairment as a 3MS score<80 (−1 SD) and severe cognitive impairment as a 3MS score<60 (−2 SD). By definition, participants with severe cognitive impairment were a subset of those with cognitive impairment and were therefore included in both groups for the purposes of analyses. In sensitivity analyses, we considered cut-offs for cognitive impairment stratified by age and educational attainment based on normative data from an external population of community-dwelling adults (26) (SDC, Materials and Methods).
Model Variable Selection
We used Cox proportional hazards regression to assess the independent association between cognitive impairment and all-cause graft loss (ACGL), defined as graft loss or mortality. The independent association between cognitive impairment and death-censored graft failure (DCGF) and mortality, evaluated separately, are presented in supplemental materials using Cox proportional hazards regression (Tables S1, S2). All analyses were stratified by living or deceased donor status, given the differences in the relevant risk factors. For example, cold ischemia time (CIT) is known to be an important risk factor for graft failure among deceased donor recipients, but CIT is not associated with graft failure in living donor recipients (27). Stratification is also consistent with the SRTR risk-adjustment models (28). In addition to traditional Cox regression, we also present results from hybrid registry-augmented Cox regression models, a statistically efficient method that brings precisely estimated coefficients from the national registry model into the prospective cohort model (29, 30).
Potential confounders were identified using the SRTR risk-adjustment models (28). Covariates included in the final multivariable models were selected to optimize goodness-of-fit as assessed by the log-likelihood test. For living donor kidney transplant (LDKT) recipients, we adjusted for recipient characteristics (age, Black race, Hispanic ethnicity, years on dialysis, diabetes status, panel reactive antibody [PRA] at transplant, college education, employment status, public insurance status, Hepatitis C Virus [HCV] infection, body mass index [BMI], hypertension status, history of transplantation, and Charlson Comorbidity Index [CCI]), donor characteristics (age, BMI), and transplant characteristics (recipient and donor both male, zero HLA mismatches, blood type incompatibility, and transplant date) (31). For deceased donor kidney transplant (DDKT) recipients, we adjusted for recipient factors (age, sex, Black race, Hispanic ethnicity, years on dialysis, diabetes status, PRA at transplant, college education, BMI, hypertension status, history of transplantation, and CCI), donor factors (kidney donor profile index [KDPI]) (32), and transplant factors (CIT and transplant date).
Handling of Missingness
Variable missingness in the national registry data was quite low: CIT (5.2%), education (4.6%), PRA at transplant (2.8%), recipient BMI (1.6%), donor BMI (1.1%), time on dialysis (0.6%), HLA mismatch (0.2%), all other variables <0.1%. We handled missing covariate values using multiple imputation by chained equations (MICE) (SDC, Materials and Methods).
Statistical Analysis
For participants in the prospective cohort, differences in recipient, donor, and transplant characteristics by cognitive impairment were assessed using the χ2 (categorical variables) and Mann-Whitney rank-sum (continuous variables) tests. We report frailty as measured by Fried (33); recipients were classified as frail if they had at least 3 of the 5 frailty components as we have previously published (34–37). Low functional status captures recipients unable to perform normal activities. Functional status is reported to the OPTN on a percent scale; we classified low functional status as 70% or lower, which is the point at which a patient is unable to perform normal activity. We classified induction agents as antibody depleting (muromonab-CD3, equine anti-lymphocyte globulin, lymphocyte immune globulin, thymoglobulin, rabbit anti-thymocyte globulin, alemtuzumab) or nonantibody depleting (daclizumab, basiliximab, and rituximab). Differences in the survivor function were assessed using the log-rank test. Functional forms of continuous variables were empirically derived using Martingale residuals. Proportional hazards were confirmed visually by graphing the log-log plot of survival and statistically using Schoenfeld residuals. We used a two-sided α of 0.05 to indicate a statistically significant difference. We report adjusted hazard ratio (aHR) 95% confidence intervals for as per the method of Louis and Zeger (38). This method shows the lower 95% confidence interval first as a subscript, then the point estimate, and finally the upper 95% confidence interval as a subscript. All analyses were performed using Stata 15/MP for Linux (College Station, Texas).
RESULTS
Prospective Cohort Study Population
Participants in our prospective, longitudinal two-center cohort were followed for a median (IQR) 3.3 (2.0–5.2) years and contributed a total of 2640 person-years at risk. Among the 864 KT recipients in our cohort, 362 underwent LDKT and 502 underwent DDKT. There were a total of 134 ACGL events (40 among LDKT recipients and 94 among DDKT recipients). Median (IQR) recipient age was 53 (42–63) years, median (IQR) BMI was 28.0 (24.0–32.0), median (IQR) time on dialysis prior to KT was 1.9 (0.2–4.2) years, 39.4% were female, 39.1% were Black, 2.3% were Hispanic, 65.8% were college educated, 46.6% were employed, 50.7% had public insurance, 7.1% were positive for HCV, 17.4% had a history of diabetes, 29.8% had a history of hypertension, 21.4% had a history of previous transplant, and 10.5% had a PRA > 80 at the time of transplant. Median (IQR) donor age was 41 (29–51) years, median (IQR) BMI was 26.9 (23.8–30.6), 50.3% were female, 18.1% were Black, and 4.1% were Hispanic. Deceased KT donors had a median (IQR) KDPI of 43.5 (27.2–64.5) and a median (IQR) CIT of 11.8 (2.0–26.4) hours. There were 5.7% recipient-donor pairs with zero HLA mismatches. Characteristics of the registry study population can be found in supplemental materials (SDC, Materials and Methods).
Prevalence of Cognitive Impairment in Prospective Cohort
LDKT recipients in our prospective cohort had a median (IQR) 3MS score of 96 (92–99) and DDKT recipients had a median (IQR) 3MS score of 94 (87–97) (Figure 1). The overall prevalence of cognitive impairment was 10.0% (6.6% in LDKT recipients and 12.4% in DDKT recipients), and the prevalence of severe cognitive impairment was 2.9% (3.3% in LDKT recipients and 2.6% in DDKT recipients). LDKT recipients with cognitive impairment were younger (p=0.03) and had lower BMI (p<0.01) than those without cognitive impairment (Table 1). DDKT recipients with cognitive impairment were more likely to be Black (p=0.04), older (p=0.03), and have diabetes (p=0.04) and less likely to be college educated (p<0.001) than those without cognitive impairment. DDKT recipients with cognitive impairment received kidneys with a higher median KDPI (p=0.049) (Table 2).
Figure 1. Distribution of Modified Mini-Mental State Exam (3MS) scores by donor type.
Lower 3MS scores indicate worse global cognition and a 3MS score<80 indicates cognitive impairment. The distribution of 3MS scores were left-skewed for both living donor kidney transplant (LDKT) and deceased donor kidney transplant (DDKT) recipients. The median (IQR) 3MS score was 96 (92–99) for LDKT recipients and 94 (87–97) for DDKT recipients (p<0.001). A larger portion of DDKT recipients were below the threshold for cognitive impairment than LDKT recipients (p<0.001).
Table 1.
Characteristics of 362 living donor kidney transplant (KT) candidates by pre-KT cognitive impairment status.
| No Impairment | Mild Impairment | Severe Impairment | |
|---|---|---|---|
| N | 338 (93.4%) | 12 (3.3%) | 12 (3.3%) |
| Recipient Characteristics1 | |||
| Female | 150 (44.4%) | 4 (33.3%) | 9 (75.0%) |
| Black | 65 (19.2%) | 1 (8.3%) | 2 (16.7%) |
| Hispanic | 7 (2.1%) | 0 (0.0%) | 1 (8.3%) |
| Age | 49.0 (36.0–59.0) | 50.0 (30.0–60.0) | 33.5 (23.5–43.0) |
| Years on Dialysis | 0.7 (0.0–2.4) | 0.4 (0.0–2.0) | 0.5 (0.0–1.9) |
| BMI | 27.1 (23.0–30.9) | 24.3 (22.2–28.3) | 22.6 (22.0–24.4) |
| HCV | 3 (0.9%) | 0 (0.0%) | 1 (8.3%) |
| College Educated | 251 (76.1%) | 7 (58.3%) | 8 (72.7%) |
| Employed | 204 (60.7%) | 4 (33.3%) | 6 (54.5%) |
| Public Insurance | 123 (36.4%) | 4 (33.3%) | 3 (25.0%) |
| Diabetes | 43 (12.7%) | 2 (16.7%) | 0 (0.0%) |
| Hypertension | 63 (18.6%) | 3 (25.0%) | 1 (8.3%) |
| Previous Transplant | 100 (29.6%) | 3 (25.0%) | 0 (0.0%) |
| PRA>80 at Transplant | 26 (7.7%) | 1 (8.3%) | 1 (8.3%) |
| Fried Frailty | 48 (14.2%) | 2 (16.7%) | 1 (8.3%) |
| Low Functional Status | 9 (2.7%) | 0 (0.0%) | 0 (0.0%) |
| EPTS Score | 34.2 (13.5–57.3) | 10.5 (1.2–35.3) | 5.8 (1.1–17.0) |
| Serum Albumin (g/dL) | 4.2 (3.9–4.6) | 4.6 (4.3–4.7) | 4.5 (4.0–4.9) |
| Donor Characteristics | |||
| Female | 211 (62.4%) | 7 (58.3%) | 8 (66.7% |
| Black | 46 (13.6%) | 1 (8.3%) | 1 (8.3% |
| Hispanic | 10 (3.0%) | 1 (8.3%) | 0 (0.0% |
| Age | 46.0 (37.0–54.0) | 45.5 (39.5–51.0) | 49.0 (38.5–53.0) |
| BMI | 26.6 (23.7–29.2) | 27.7 (25.5–29.1) | 24.0 (21.9–30.7) |
| eGFR | 100.1 (85.2–113.4) | 106.0 (90.2–125.5) | 97.3 (82.8–108.1) |
| Biologically Related | 122 (36.1%) | 7 (58.3%) | 5 (41.7% |
| Transplant Characteristics | |||
| ABO Incompatible | 43 (12.7%) | 1 (8.3%) | 1 (8.3%) |
| Zero HLA mismatch | 21 (6.3%) | 1 (8.3%) | 0 (0.0%) |
| Cold Ischemia Time | 1.5 (1.0–3.0) | 1.5 (1.0–2.0) | 1.5 (0.8–7.0) |
| Antibody Depleting Induction | 283 (83.7%) | 9 (75.0%) | 11 (91.7%) |
| Nonantibody Depleting Induction | 65 (19.2%) | 2 (16.7%) | 1 (8.3%) |
Characteristics are presented as percentages for binary variables and median (interquartile range) for continuous variables.
Table 2.
Characteristics of 502 deceased donor kidney transplant (KT) candidates by pre-KT cognitive impairment status.
| No Impairment | Mild Impairment | Severe Impairment | |
|---|---|---|---|
| N | 440 (87.6%) | 49 (9.8%) | 13 (2.6%) |
| Recipient Characteristics1 | |||
| Female | 153 (34.8%) | 17 (34.7%) | 7 (53.9%) |
| Black | 229 (52.1%) | 33 (67.4%) | 8 (61.5%) |
| Hispanic | 10 (2.3%) | 2 (4.1%) | 0 (0.0%) |
| Age | 56.0 (46.0–64.5) | 62.0 (54.0–70.0) | 57.0 (52.0–67.0) |
| Years on Dialysis | 2.9 (1.0–5.1) | 2.8 (1.0–4.5) | 2.8 (0.0–4.3) |
| BMI | 28.7 (24.9–32.9) | 30.3 (25.4–33.6) | 29.4 (24.4–30.9) |
| HCV | 53 (12.1%) | 4 (8.2%) | 0 (0.0%) |
| College Educated | 267 (61.8%) | 17 (35.4%) | 7 (53.9%) |
| Employed | 165 (37.8%) | 15 (31.3%) | 5 (38.5%) |
| Public Insurance | 269 (61.1%) | 31 (63.3%) | 8 (61.5%) |
| Diabetes | 86 (19.6%) | 15 (30.6%) | 4 (30.8%) |
| Hypertension | 166 (37.7%) | 22 (44.9%) | 2 (15.4%) |
| Previous Transplant | 76 (17.3%) | 5 (10.2%) | 1 (7.7%) |
| PRA>80 at Transplant | 54 (12.3%) | 3 (6.2%) | 0 (0.0%) |
| Fried Frailty | 78 (17.7%) | 13 (26.5%) | 2 (15.4%) |
| Low Functional Status | 12 (2.7%) | 3 (6.1%) | 0 (0.0%) |
| EPTS Score | 55.0 (31.4–76.0) | 70.1 (34.8–85.4) | 69.5 (29.4–90.0) |
| Serum Albumin (g/dL) | 4.3 (4.0–4.6) | 4.1 (3.9–4.3) | 4.2 (4.0–4.6) |
| Donor Characteristics | |||
| Female | 187 (42.5%) | 14 (28.6%) | 8 (61.5%) |
| Black | 91 (20.7%) | 14 (28.6%) | 3 (23.1%) |
| Hispanic | 21 (4.8%) | 2 (4.1%) | 1 (7.7%) |
| Age | 35.0 (26.0–48.0) | 41.0 (31.0–50.0) | 37.0 (22.0–48.0) |
| BMI | 27.7 (23.8–32.3) | 27.5 (24.5–32.7) | 27.2 (21.5–34.0) |
| KDPI | 43.0 (26.5–64.1) | 49.9 (34.1–69.7) | 46.0 (29.1–66.5) |
| Transplant Characteristics | |||
| Zero HLA mismatch | 25 (5.7%) | 1 (2.0%) | 1 (7.7%) |
| Cold Ischemia Time | 25.0 (17.1–32.0) | 25.5 (15.4–33.4) | 22.5 (17.0–30.1) |
| Antibody Depleting Induction | 395 (89.8%) | 42 (85.7%) | 11 (84.6%) |
| Nonantibody Depleting Induction | 43 (9.7%) | 4 (8.1%) | 1 (7.7%) |
Characteristics are presented as percentages for binary variables and median (interquartile range) for continuous variables.
Post-KT Outcomes, LDKT Recipients
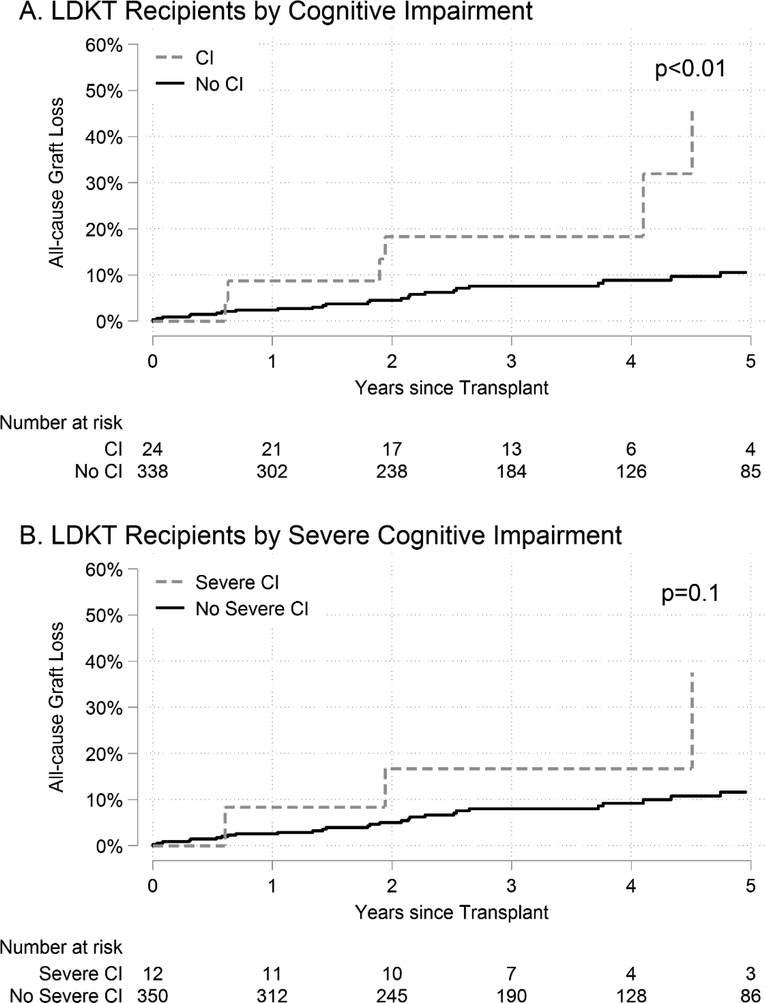
LDKT recipients with any cognitive impairment had higher unadjusted rates of graft loss compared to those without cognitive impairment (p<0.01); however, there were no detectable differences by severe cognitive impairment status in LDKT recipients in unadjusted analyses (p=0.1) (Figure 2). ACGL for LDKT recipients with any cognitive impairment was 45.5% at 5 years versus 10.6% at 5 years for LDKT recipients without cognitive impairment. ACGL for LDKT recipients with severe cognitive impairment was 37.5% at 5 years versus 11.6% at 5 years for LDKT recipients without severe cognitive impairment (Table 3). After adjusting for recipient, donor, and transplant factors using Cox regression, any cognitive impairment in LDKT recipients was associated with a 5.40-fold increased risk of ACGL (aHR: 1.785.4016.34, p<0.01) and severe cognitive impairment was associated with a 5.57-fold increased risk of ACGL (aHR: 1.295.5724.00, p=0.02). After adjusting for recipient, donor, and transplant factors using hybrid registry-augmented regression (Table S6), any cognitive impairment in LDKT recipients was associated with a 3.22-fold increased risk of ACGL (aHR: 1.223.228.50, p=0.02); however, there was no statistically significant difference by severe cognitive impairment (aHR: 0.843.2212.36, p=0.09) (Table 4). In sensitivity analyses where cognitive impairment was defined based on an external population, the magnitudes of the associations between cognitive impairment and ACGL increased in LDKT recipients, but our inferences were unchanged (Table S3).
Figure 2. Cumulative incidence of all-cause graft loss (ACGL) in living donor kidney transplant (LDKT) recipients.
LDKT recipients with (A) cognitive impairment (CI) and (B) severe CI.
Table 3.
Unadjusted Risk of all-cause graft loss (ACGL) for kidney transplant (KT) recipients with cognitive impairment.
| % 1-Year ACGL1 | % 3-Year ACGL | % 5-Year ACGL | |
|---|---|---|---|
| Living Donor KT | |||
| No Impairment | 2.4 (1.2–4.7) | 7.6 (5.0–11.5) | 10.6 (7.1–15.7) |
| Cognitive Impairment | 8.7 (2.3–30.5) | 18.3 (7.3–41.8) | 45.5 (20.3–80.4) |
| No Severe Impairment | 2.6 (1.4–4.9) | 8.0 (5.4–11.9) | 11.6 (7.9–16.9) |
| Severe Impairment | 8.3 (1.2–46.1) | 16.7 (4.5–51.8) | 37.5 (11.8–82.8) |
| Deceased Donor KT | |||
| No Impairment | 6.3 (4.3–9.0) | 17.7 (13.4–22.6) | 24.9 (19.6–31.3) |
| Cognitive Impairment | 9.7 (4.5–20.3) | 18.6 (10.3–32.3) | 26.8 (15.3–44.5) |
| No Severe Impairment | 6.5 (4.6–9.0) | 17.3 (13.6–21.8) | 24.2 (19.3–30.2) |
| Severe Impairment | 15.4 (4.1–48.8) | 37.3 (15.3–73.3) | 53.0 (24.5–86.8) |
ACGL estimated by the Kaplan-Meier method with 95% confidence intervals shown in parentheses.
Table 4.
Risk of all-cause graft loss (ACGL) for kidney transplant (KT) recipients with cognitive impairment. The risk of ACGL was assessed using hybrid registry-augmented Cox proportional hazards (PH) regression. The exposure of interest, cognitive impairment, and the Charleston Comorbidity Index (CCI) were estimated in the prospective cohort model. All other confounders were estimated using the SRTR study population. Recipients with cognitive impairment were compared to recipients without cognitive impairment and recipients with severe cognitive impairment were compared to recipients without severe cognitive impairment.
| Cox PH | Hybrid Registry-Augmented Cox PH | |||
|---|---|---|---|---|
| Exposure | aHR ACGL | P value | aHR ACGL | P value |
| LDKT1 | ||||
| Any Cognitive Impariment2 | 1.785.4016.34 | <0.01 | 1.223.228.50 | 0.02 |
| Severe Cognitive Impairment3 | 1.295.5724.00 | 0.02 | 0.843.2212.36 | 0.09 |
| DDKT4 | ||||
| Any Cognitive Impairment | 0.581.051.89 | 0.9 | 0.591.041.84 | 0.9 |
| Severe Cognitive Impairment | 1.132.927.50 | 0.03 | 1.352.936.35 | <0.01 |
LDKT Models adjusted for CCI and confounders from SRTR data: recipient characteristics (continuous age with knots at 35 and 65, Black race, Hispanic ethnicity, years on dialysis, diabetes status, PRA at transplant, college education, employment, public insurance status, HCV status, BMI, hypertension status, and history of transplantation), donor characteristics (age, BMI), and transplant characteristics (recipient and donor both male sex, zero HLA mismatches, blood type incompatibility, and date of transplant).
3MS Score<80.
3MS Score<60.
DDKT Models adjusted for CCI and confounders from SRTR data: recipient factors (continuous age with knots at 35 and 65, sex, Black race, Hispanic ethnicity, years on dialysis, diabetes status, PRA at transplant, college education, employment, public insurance status, HCV status, BMI, hypertension status, and history of transplantation), donor factors (KDPI), and transplant factors (CIT and date of transplant).
In sensitivity analyses, any cognitive impairment was associated with a 3.01-fold increased risk of mortality among LDKT recipients (aHR: 1.333.016.80, p<0.01) after adjusting for recipient, donor and transplant factors using hybrid registry augmented regression. We did not detect an association between severe impairment and mortality among LDKT recipients (Table S1). When cognitive impairment was defined based on an external population of community dwelling older adults, the association between any cognitive impairment and mortality was slightly attenuated (aHR: 1.062.757.11, p=0.04) (Table S4). We did not detect an association between cognitive impairment and death-censored graft failure among LDKT recipients using hybrid registry augmented regression with either internally or externally defined cognitive impairment (Tables S2, S5).
Post-KT Outcomes, DDKT Recipients
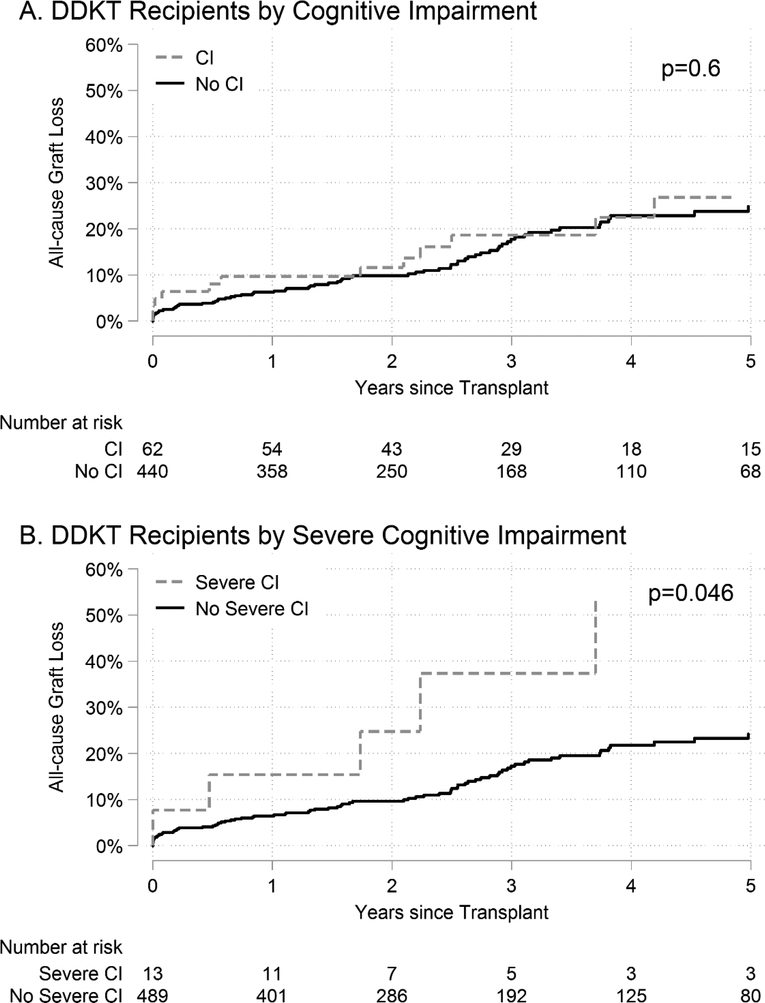
DDKT recipients with any cognitive impairment had similar rates of graft loss as individuals without cognitive impairment (p=0.6); however, DDKT recipients with severe cognitive impairment had higher rates of graft loss, compared to DDKT recipients without severe cognitive impairment (p=0.046) (Figure 3). ACGL for DDKT recipients with any cognitive impairment was 26.8% at 5 years versus 24.9% at 5 years for DDKT recipients without cognitive impairment. ACGL for DDKT recipients with severe cognitive impairment was 53.0% at 5 years versus 24.2% at 5 years for DDKT recipients without severe cognitive impairment (Table 3). After adjusting for recipient, donor, and transplant factors using Cox regression, severe cognitive impairment in DDKT recipients was associated with a 2.92-fold increased risk of ACGL (aHR: 1.132.927.50, p=0.03); however, there was no statistically significant difference by any cognitive impairment (aHR: 0.581.051.89, p=0.9). After adjusting for recipient, donor, and transplant factors in DDKT recipients using hybrid registry-augmented regression (Table S7), severe cognitive impairment in DDKT recipients was associated with a 2.93-fold increased risk of ACGL (aHR: 1.352.936.35, p<0.01); however, there was no statistically significant difference by any cognitive impairment (aHR: 0.591.041.84, p=0.9) (Table 4). In sensitivity analyses where cognitive impairment was defined based on an external population, the magnitude of the associations between cognitive impairment and ACGL increased in DDKT recipients, but our inferences were unchanged (Table S3).
Figure 3. Cumulative incidence of all-cause graft loss (ACGL) in deceased donor kidney transplant (DDKT) recipients.
DDKT recipients with (A) cognitive impairment (CI) and (B) severe CI.
In sensitivity analyses, we did not detect an association between cognitive impairment and mortality or death-censored graft failure among DDKT recipients (Tables S1, S2). However, in sensitivity analyses where cognitive impairment was defined based on an external population, there was an association between severe cognitive impairment and mortality (aHR: 1.0032.265.09, p=0.049) (Table S4). We did not detect an association between cognitive impairment and death-censored graft failure using cognitive impairment definitions defined in the external population (Table S5).
DISCUSSION
In this prospective, longitudinal two-center cohort study of 864 KT recipients, we found that pretransplant cognitive impairment was common and was associated with an elevated risk of all-cause graft loss (a composite of graft loss and mortality). The prevalence of cognitive impairment in our study population was 10.0%. Any cognitive impairment was associated with a 5.40-fold higher risk of ACGL in LDKT recipients (aHR: 1.785.4016.34). Severe cognitive impairment was associated with a 5.57-fold higher risk of ACGL in LDKT recipients (aHR: 1.295.5724.00) and with a 2.92-fold higher risk of ACGL in DDKT recipients (aHR: 1.132.927.50).
A nationally representative survey in the US that estimated that the prevalence of cognitive impairment was 16% among adults without dementia age 71–79 (39). In our two-center cohort, the prevalence of cognitive impairment was 22.9% among adults aged 71–79 (N=61), possibly pointing to the increased risk of cognitive impairment among patients with chronic kidney disease. While older age was associated with cognitive impairment in our study, the prevalence of cognitive impairment across the study population (median age 53; IQR: 42–63) was noteworthy. Previous studies have identified an association between dialysis initiation and duration with progressively worsening cognitive function (15, 16, 18, 40) likely through the buildup of uremic toxins, inflammation, and cerebral hypotension and hypoxia during dialysis sessions (41, 42). These mechanisms lead to a higher risk of cognitive impairment in kidney transplant candidates of all ages, as we observed in our study. This underscores the need to consider screening for cognitive impairment in KT candidates, even those who would not otherwise be considered at risk due to age alone.
Our observed association of higher graft loss in kidney transplant recipients with cognitive impairment is consistent with prior studies that have shown cognitive impairment to be associated with inferior medical outcomes. One possible mechanism that might explain their elevated risk of all-cause graft loss is poorer medication adherence among recipients with cognitive impairment. Since adherence to the immunosuppressive medication regimen affects the longevity and function of the transplanted allograft, cognitive impairment might indirectly cause inferior posttransplant outcomes. The level of cognitive impairment observed in these studies is severe enough to interfere with adherence to medication and treatment regimens as seen in studies of dialysis patients (14) and nontransplant surgical patients (43–45).This potential pathway warrants further study, as it may represent a target for intervention to improve KT outcomes in this vulnerable population (46).
A limitation of this study was the relatively small sample size of our two-center cohort compared to national transplant recipient datasets. In order to address this limitation, we were able to adjust for important donor, recipient, and transplant characteristics using hybrid registry-augmented regression; however, future multi-center cohort studies will be necessary to improve the generalizability of our findings. Our study used 3MS thresholds based on algorithmically defined cut-offs for cognitive impairment. Given the lack of normative data on 3MS scores in KT candidates, cognitive impairment status might have been misclassified. In sensitivity analyses, we used 3MS thresholds based on an external population with detailed normative data and also stratified by educational attainment and age and found that our inferences did not change (Table S3). Another notable limitation of this study is that we were only able to identify an association between cognitive impairment and all-cause graft loss, not causation. However, we believe that the relationship between cognitive impairment and inferior outcomes is plausible and warrants further study. While we present results for the association of cognitive impairment and mortality and death-censored graft failure (evaluated separately) in the supplemental material, these analyses were likely underpowered based on the sample size and number of events. We were not able to account for social support or other important elements of social context. Strengths of this study include the prospective measurement of global cognitive impairment using a validated instrument (3MS) and reliable ascertainment of posttransplant outcomes using the national registry. Using prospectively collected data, we were also able to adjust for a wider range of comorbidities than is available in the national registry.
In summary, we found that cognitive impairment was common in KT recipients and was associated with an elevated risk of a composite outcome of graft loss and mortality. These findings underscore the importance of elucidating potential mechanisms underlying the relationship between cognitive impairment and inferior posttransplant outcomes, such as medication nonadherence, and emphasize the need for designing interventions to improve outcomes. Transplant centers may consider screening for cognitive impairment to identify higher-risk KT recipients and to inform pre and posttransplant clinical management of these patients. Screening processes might combine a test of global function, like 3MS, with other validated tools specific to the ESRD population, such as the Kidney Disease Quality of Life Cognitive Function scale (47). Further study of the effect of pretransplant interventions to preserve or improve cognitive function, such as cognitive or exercise training, is also warranted.
Supplementary Material
Acknowledgments
Funding/Support: Funding for this study was provided in part by the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK); National Heart, Lung, and Blood Institute (NHBLI); and the National Institute on Aging (NIA); grant numbers T32HL007055 (Alvin Thomas), K01AG043501 (PI: Mara McAdams-DeMarco), R01AG055781 (PI: Mara McAdams-DeMarco), R01DK114074 (PI: Mara McAdams-DeMarco), F30DK116658 (PI: Ashton Shaffer), F32AG053025 (PI: Christine Haugen), K01AG050699 (PI: Alden Gross), K24DK101828 (PI: Dorry Segev), and R01DK096008 (PI: Dorry Segev). Additionally, Jessica Ruck and Dorry Segev are supported by a Doris Duke Charitable Foundation Clinical Research Mentorship grant.
ABBREVIATIONS
- 3MS
modified mini-mental status
- ACGL
all-cause graft loss
- aHR
adjusted hazard ratio
- CCI
Charlson Comorbidity Index
- CIT
cold ischemia time
- CKD
chronic kidney disease
- DDKT
deceased donor kidney transplant(ation)
- ESRD
end-stage renal disease
- HCV
Hepatitis C virus
- HRSA
Health Resources and Services Administration
- IQR
interquartile range
- LDKT
living donor kidney transplant(ation)
- KDPI
kidney donor profile index
- KT
kidney transplant(ation)
- MICE
multiple imputation by chained equations
- OPTN
Organ Procurement and Transplantation Network
- PPM
predictive mean matching
- SRTR
Scientific Registry of Transplant Recipients
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Mr. Thomas, Dr. Ruck, Ms. Shaffer, Dr. Haugen, Dr. Segev, and Dr. McAdams-DeMarco reported institutional grant support from the National Institutes of Health. Dr. Ruck and Dr. Segev also report institutional grant support from the Doris Duke Charitable Foundation. No other disclosures were reported.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Study concept and design: Thomas, Ruck, Shaffer, McAdams-DeMarco. Acquisition of data: Thomas, Ying, Norman, Segev, McAdams-DeMarco. Analysis and interpretation of data: Thomas, Segev, McAdams-DeMarco. Drafting of the manuscript: Thomas, Ruck, Shaffer, Haugen, McAdams-DeMarco. Critical revision of the manuscript for important intellectual content: Thomas, Ruck, Shaffer, Haugen, Ying, Warsame, Carlson, Gross, Norman, Segev, McAdams-DeMarco. Statistical analysis: Thomas, McAdams-DeMarco. Obtained funding: Thomas, Ruck, Shaffer, Haugen, Segev, McAdams-DeMarco. Administrative, technical, and material support: Chu, Carlson, Gross, Segev, McAdams-DeMarco. Study supervision: McAdams-DeMarco.
REFERENCES
- 1.Kallenberg MH, Kleinveld HA, Dekker FW, et al. Functional and Cognitive Impairment, Frailty, and Adverse Health Outcomes in Older Patients Reaching ESRD—A Systematic Review. Clin J Am Soc Nephrol. 2016;11:1624–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. [DOI] [PubMed] [Google Scholar]
- 3.Bassuk SS, Wypij D, Berkman LF. Cognitive impairment and mortality in the community-dwelling elderly. Am J Epidemiol. 2000;151:676–688. [DOI] [PubMed] [Google Scholar]
- 4.Xiang Xiaoling, An Ruopeng. The Impact of Cognitive Impairment and Comorbid Depression on Disability, Health Care Utilization, and Costs. Psychiatr Serv. 2015;66:1245–1248. [DOI] [PubMed] [Google Scholar]
- 5.Teeters DA, Moua T, Li G, et al. Mild Cognitive Impairment and Risk of Critical Illness*. Crit Care Med. 2016;44:2045–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helvik A-S, Skancke RH, Selbæk G, Engedal K: Nursing home admission during the first year after hospitalization - the contribution of cognitive impairment. PLoS One. 2014. pp e86116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAdams-DeMarco MA, Bae S, Chu N,et al. Dementia and Alzheimer’s Disease among Older Kidney Transplant Recipients. J Am Soc Nephrol. 2017;28:1575–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurella M, Chtertow GM, Fried LF, et al. Chronic Kidney Disease and Cognitive Impairment in the Elderly: The Health, Aging, and Body Composition Study. J Am Soc Nephrol. 2005;16:2127–2133. [DOI] [PubMed] [Google Scholar]
- 9.Yaffe K, Ackerson L, Tamura MK, et al. Chronic Kidney Disease and Cognitive Function in Older Adults: Findings from the Chronic Renal Insufficiency Cohort Cognitive Study. J Am Geriatr Soc. 2010;58: 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anand S, Johansen KL, Kurella Tamura M. Aging and chronic kidney disease: the impact on physical function and cognition. J Gerontol A Biol Sci Med Sci. 2014;69: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray AM, Bell EJ, Tupper DE, et al. The Brain in Kidney Disease (BRINK) Cohort Study: Design and Baseline Cognitive Function. Am J Kidney Dis. 2016;67:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner DE, Gaussoin SA, Nord J, et al. Cognitive Function and Kidney Disease: Baseline Data From the Systolic Blood Pressure Intervention Trial (SPRINT). Am J Kidney Dis. 2017;70:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger I, Wu S, Masson P, Kelly PJ, Duthie FA, Whiteley W, Parker D, Gillespie D, Webster AC: Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med, 14: 206, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalirao P, Pederson S, Foley RN, et al. Cognitive Impairment in Peritoneal Dialysis Patients. Am J Kidney Dis. 2011;57:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurella Tamura M, Yaffe K: Dementia and cognitive impairment in ESRD: diagnostic and therapeutic strategies. Kidney Int. 2011;79:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurella Tamura M, Vittinghoff E, Hsu C-y, et al. Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney Int. 2017;91:948–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griva K, Stygall J, Hankins M, Davenport A, Harrison M, Newman SP. Cognitive Impairment and 7-Year Mortality in Dialysis Patients. Am J Kidney Dis. 2010;56:693–703. [DOI] [PubMed] [Google Scholar]
- 18.Drew DA, Weiner DE, Tighiouart H, et al. Cognitive Function and All-Cause Mortality in Maintenance Hemodialysis Patients. Am J Kidney Dis. 2015;65:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McAdams-DeMarco MA, Law A, Tan J, et al. Frailty, Mycophenolate Reduction, and Graft Loss in Kidney Transplant Recipients. Transplantation. 2014;99:805–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAdams-DeMarco MA, Ying H, Olorundare I, et al. Individual Frailty Components and Mortality In Kidney Transplant Recipients. Transplantation. 2016;101:2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty and early hospital readmission after kidney transplantation. Am J Transplant. 2013;13:2091–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nastasi A, McAdams-DeMarco M, Schrack J, et al. Pre-Kidney Transplant Lower Extremity Impairment and Post-Transplant Mortality. Am J Transplant. 2017;17: 81–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massie AB, Kuricka LM, Segev DL. Big Data in Organ Transplantation: Registries and Administrative Claims. Am J Transplant. 2014;14:1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teng E, Chui H. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 25.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia – meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. [DOI] [PubMed] [Google Scholar]
- 26.Tombaugh TN, McDowell I, Kristjansson B, Hubley AM. Mini-Mental State Examination (MMSE) and the modified MMSE (3MS): A psychometric comparison and normative data. Psychol Assessment. 1996;8:48–59. [Google Scholar]
- 27.Treat E, Chow EKH, Peipert JD, et al. Shipping living donor kidneys and transplant recipient outcomes. Am J Transplant. 2018;18:632–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder JJ, Salkowski N, Kim SJ, et al. Developing Statistical Models to Assess Transplant Outcomes Using National Registries: The Process in the United States. Transplantation. 2016;100:288–294. [DOI] [PubMed] [Google Scholar]
- 29.McAdams-DeMarco MA, King EA, Luo X, et al. Frailty, Length of Stay, and Mortality in Kidney Transplant Recipients: A National Registry and Prospective Cohort Study. Ann Surg. 2016;266:1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McAdams-DeMarco MA, Law A, King E, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Manen JG, Korevaar JC, Dekker FW, Boeschoten EW, Bossuyt PMM, Krediet RT. How to adjust for comorbidity in survival studies in ESRD patients: A comparison of different indices. Am J Kidney Dis. 2002;40:82–89. [DOI] [PubMed] [Google Scholar]
- 32.Rao PS, Schaubel DE, Guidinger MK, et al. A Comprehensive Risk Quantification Score for Deceased Donor Kidneys: The Kidney Donor Risk Index. Transplantation. 2009;88:231–236. [DOI] [PubMed] [Google Scholar]
- 33.Fried LP, Tangen CM, Walston J, et al. Cardiovascular Health Study Collaborative Research G: Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. [DOI] [PubMed] [Google Scholar]
- 34.Fitzpatrick J, Sozio SM, Jaar BG, et al. Frailty, body composition and the risk of mortality in incident hemodialysis patients: the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease study. Nephrol Dial Transplant. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haugen CE, Mountford A, Warsame F, et al. Incidence, Risk Factors, and Sequelae of Post-kidney Transplant Delirium. J Am Soc Nephrol. 2018;29:1752–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAdams-DeMarco MA, Ying H, Thomas AG, e al. Frailty, Inflammatory Markers, and Waitlist Mortality Among Patients with End-Stage Renal Disease in a Prospective Cohort Study. Transplantation. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAdams-DeMarco MA, Olorundare IO, Ying H, et al. Frailty and Postkidney Transplant Health-Related Quality of Life. Transplantation. 2018;102:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009;10:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iyasere O, Brown EA. Cognitive function before and after dialysis initiation in adults with chronic kidney disease—a new perspective on an old problem? Kidney Int. 2017;91:784–786. [DOI] [PubMed] [Google Scholar]
- 41.Matta SMd, Janaina Matos M, Kummer AMe, Barbosa IG, Teixeira AL, Silva ACSe. Alterações cognitivas na doença renal crônica: uma atualização. J Bras Nefrol. 2014;36:241–245.25055365 [Google Scholar]
- 42.Hermann DM, Kribben A, Bruck H. Cognitive impairment in chronic kidney disease: clinical findings, risk factors and consequences for patient care. J Neural Transm. 2014;121:627–632. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs Ú, De Castro MS, Fuchs FD, Ferreira MBC. The influence of cognition, anxiety and psychiatric disorders over treatment adherence in uncontrolled hypertensive patients. PLoS One. 2011;6:e22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Partridge JSL, Dhesi JK, Cross JD, et al. The prevalence and impact of undiagnosed cognitive impairment in older vascular surgical patients. J Vasc Surg. 2014;60:1002–1011. [DOI] [PubMed] [Google Scholar]
- 45.Jefferis JM, Taylor J-P, Clarke MP. Does cognitive impairment influence outcomes from cataract surgery? Results from a 1-year follow-up cohort study. Br J Ophthalmol. 2015;99:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAdams-DeMarco MA, Konel J, Warsame F, et al. Intradialytic Cognitive and Exercise Training May Preserve Cognitive Function. Kidney Int Rep. 2018;3:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurella M, Luan J, Yaffe K, Chertow GM. Validation of the Kidney Disease Quality of Life (KDQOL) Cognitive Function subscale. Kid Int. 2004;66:2361–2367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.