Abstract
Cocaine, a known psychostimulant, results in oxidative stress and inflammation. Recent studies from our group have shown that cocaine induces inflammation in glial cells. Our current study was aimed at investigating whether cocaine exposure could also induce inflammation in non-glial cells such as the pericytes with a focus on the endoplasmic reticulum (ER) stress/autophagy axis. Our in vitro findings demonstrated that exposure of pericytes to cocaine resulted in upregulation of the pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in both the intracellular as well as extracellular compartments, thus underpinning pericytes as yet another source of neuroinflammation. Cocaine exposure of pericytes resulted in increased formation of autophagosomes as demonstrated by a time-dependent increase of autophagy markers, with a concomitant defect in the fusion of the autophagosome with the lysosomes. Pharmacological blocking of the sigma 1 receptor underscored its role in cocaine-mediated activation of pericytes. Furthermore, it was also demonstrated that cocaine-mediated dysregulation of autophagy involved upstream activation of the ER stress pathways, with a subsequent downstream production of pro-inflammatory cytokines in pericytes. These findings were also validated in an in vivo model wherein pericytes in the isolated brain microvessels of cocaine injected mice (7-days) exhibited increased expression of both the autophagy marker - LC3 as well as the pro-inflammatory cytokine, IL-6. This is the first report describing the role of pericytes in cocaine-mediated neuroinflammation. Interventions aimed at blocking either the sigma-1 receptor or the upstream ER stress mediators could likely be envisioned as promising therapeutic targets for abrogating cocaine-mediated inflammation in pericytes.
Keywords: Pericytes, dysregulated autophagy, ER stress, cocaine, neuroinflammation, microvessels
Introduction
Cocaine, one of the most commonly used street drugs, is a powerful addictive psychostimulant which activates the brain reward pathway [1,2]. According to Drug Abuse Warning Network (DAWN) report in 2011, cocaine abuse accounted for 40% of drug misuse or abuse-related emergency department visits [3]. Both cocaine and HIV can affect the central nervous system (CNS). Cocaine, that can cross the blood-brain barrier (BBB), has also been shown to result in BBB dysfunction while also exerting its effects on multiple cells of the CNS. In the setting of HIV-1 infection, cocaine-mediated neuroinflammation and increased transmigration of infected/activated leukocytes from the periphery into the brain can ultimately lead to exacerbated neurodegeneration [4–7].
The brain neurovascular unit is mainly comprised of the brain endothelial cells, pericytes, neurons and glial cells, all of which are essential for the maintenance and integrity of the CNS [8]. Endothelial cells, are the most widely studied cell type related with BBB permeability. Numerous studies have suggested that cocaine exposure leads to breach of BBB permeability via downregulated expression of tight junction proteins as well as disruption of F-actin in the endothelial cells [4,9–11]. Cocaine has also been shown to impair neuronal morphology and survival while also inducing glial activation, which together could contribute to increased neuroinflammation and cognitive impairment observed in cocaine addicts [12,13]. The role of non-glial cell types such as the pericytes, which also are important cellular constituents of the neurovascular unit, however, has received much less attention in the context of neuroinflammation. More specifically, the role of pericytes in cocaine-mediated neuroinflammation has not been reported earlier and constituted the focus of our study.
Pericytes are vascular mural cells that play a vital role in the functioning of the neurovascular unit [8]. More recently, these cells have gained attention based on their proximity and contact with other cells of the neurovascular unit such as the endothelial cells, the astrocyte end feet, perivascular microglia and neurons [14–18]. Pericytes play critical roles in the maintenance of BBB integrity, regulation of angiogenesis, control of cerebral blood flow (CBF), neuroinflammation as well as stem cell activity [19–22]. Additionally, pericytes also play a vital role in regulating the immunological response via modulation of the peripheral immune cell transmigration across the BBB. It has been suggested that the cell adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) and the vascular cell adhesion molecules (VCAM-1), expressed on the pericytes allow leukocyte rolling and adhesion [23]. Pericytes are also known to secrete inflammatory mediators, which in turn, can recruit circulating leukocytes to the site of inflammation [24]. Furthermore, elegant work by Nakagawa et. al. (2012) has also shown that pericytes are susceptible to infection by both X4-tropic NL4–3 as well as R5-tropic JR-CSF HIV-1 strains, which subsequently leads to breach of the BBB integrity [15,25,26]. Other novel studies by Persidsky et.al. (2012) have also demonstrated that exposure of pericytes to various cytokines such as TNF-α/ IL-1β can result in the dysfunction of pericytes, leading subsequently to damage of the BBB [27]. Recently, it has also been observed that brain pericytes shows properties of immune regulation, including responding to and expressing inflammatory molecules, presenting antigen, and displaying phagocytic ability [14]. Taken together these findings underpin pericytes as key mediators of regulation of BBB as well as neuroinflammation.
Sigma-1 receptor (σ−1R) is an endoplasmic reticulum membrane protein that has been shown to directly bind with psychostimulants such as cocaine [28]. Previous studies have indicated a role for σ−1R in cocaine addiction[29], cocaine-mediated neuroinflammation [30,31], as well as cocaine-induced BBB damage [4,10]. Interestingly, σ−1R is believed to be a potential therapeutic target owing to its involvement in cocaine-mediated neuropathology [30,32]. The role of σ−1R in cocaine induced neuroinflammation mediated by the pericytes however, remains elusive.
Autophagy, a cellular self-degradative process, delivers cytoplasmic constituents to the lysosome, which is necessary for maintaining the cellular homeostasis and functions [33]. Autophagy has been implicated in neuroinflammation and neurodegenerative disorders, including Alzheimer’s disease, Amyotrophic lateral sclerosis, Parkinson’s disease and HIV associated-neurocognitive disorders [14,34–37]. Our previous studies demonstrated the role of dysregulated autophagy in cocaine-mediated induction of inflammatory mediators such as TNF-α, IL-1β, IL-6 and CCL-2 in both the astrocytes and microglia [13,38].
The present study was undertaken to assess the role of non-glial cells such as pericytes as contributors of cocaine-mediated neuroinflammation, with the involvement of ER stress-autophagy pathway. In this report, we demonstrated for the first time that exposure of primary human brain vascular pericytes (HBVP) to cocaine induced ER stress-mediated formation of autophagosomes, with a concomitant block in the fusion of autophagosomes with the lysosomes, resulting, in turn, to increased activation of pericytes with upregulated expression of TNF-α, IL-1β and IL-6. These findings were further validated in an in vivo model of cocaine addiction. Taken together these findings underpin the role of pericytes in cocaine-mediated neuroinflammation. Interventions aimed at targeting the upstream components of the ER-stress-autophagy pathway could thus be developed as therapeutic strategies to dampen cocaine-mediated neuroinflammation in cocaine addicts and/or in HIV-infected cocaine abusers.
Materials and Methods
Animals
C57BL/6 male mice were purchased from Charles River Laboratories (Wilmington, MA, USA). All animals were housed under conditions of constant temperature and humidity on a 12-hour light/12-hour dark cycle, with lights on at 7:00 AM. Food and water were available ad libitum. All animal procedures were performed according to the protocols approved by the Institutional Animal Care and Use Committee at the University of Nebraska Medical Center. αAnimals were divided into two groups (n=6/group): (1) saline, (2) cocaine. Cocaine was injected once daily at a dose of 20 mg/kg intraperitoneally for 7 days. One hour after the last injection animals were sacrificed and subjected to transcranial perfusion with saline to remove blood from tissue and blood vessels, followed by the microvessel isolation [39].
Cell culture
Primary human brain vascular pericytes (HBVPs) were purchased from ScienCell Research Laboratories (1800) (Carlsbad, CA, USA) and cultured in the pericyte medium (ScienCell) with 2% FBS (ScienCell Research Laboratories, 0010), 1% pericyte growth supplement (ScienCell Research Laboratories, 1252) and 1% penicillin-streptomycin solution (ScienCell Research Laboratories, 0503) in a 5% CO2-humidified incubator at 37°C. Cell culture dishes were coated with poly-L-lysine (2 μg/cm2, ScienCell) and cells were used in passages 2–5. Pericytes were serum starved for 12 h before exposure to cocaine.
Reagents
Cocaine hydrochloride (C5776), Σ−1R(σ−1 R) antagonist BD1047 (B8562), autophagy inhibitors wortmannin (W3144) and 3-MA (M9281) were purchased from Sigma-Aldrich (St Louis, MO, USA). The ER stress inhibitor 4-PBA (567616) was obtained from EMD Millipore Corporation (Billerica, MA, USA). Autophagosome-lysosome fusion inhibitor bafilomycin A1 (BAF. A1). The concentrations of these inhibitors were based on our previous reports [33,34][33,34][33,34][32,33][32,33][32,33][32,33][31,32][30,31][29,30][28,29].
The primary antibodies (2:10,000) used were: BECN1 (sc-11427), ATF6 (sc-22799), p-EIF2α (Cell Sig9721), EIF2α (Cell Sig 9722), p-PERK (ab192591), PERK (ab 79483), IRE 1α (sc-20790) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA); LC3 (NB100–2220) was from Novus Biological Company (Littleton, CO, USA); BiP (610979) was from BD Biosciences (San Jose, CA, USA) and p62 (PM045) was from MBL International (Woburn, MA, USA); IL-6 (1:100), Desmin (1:50), PDGFRβ (1:100) antibodies were obtained from Abcam (Cambridge, MA, USA); PDGFRβ (1:50) antibodies were ordered from Thermo Fisher Scientific (Waltham, MA, USA); β-actin was purchased from Sigma-Aldrich (St Louis, MO, USA). The secondary antibodies were alkaline phosphatase conjugated to goat anti mouse/rabbit IgG, or rabbit anti Goat IgG (Jackson ImmunoResearch Labs, West Grove, PA, USA). The secondary antibodies for immunostaining were AlexaFluor 594–conjugated anti– mouse (1:100), AlexaFluor 488–conjugated anti–rabbit (1:200), and AlexaFluor 594–conjugated anti–rat (1:100), AlexaFluor 594–conjugated anti–rabbit (1:200) immunoglobulin G (Invitrogen, Carlsbad, CA, USA).
Western blot
Treated cells were lysed using the Mammalian Cell Lysis kit (Sigma-Aldrich, MCL1–1KT) as described previously [40]. Cell lysates were centrifuged at 12000 ×g for 10 min at 4°C, the protein content of the supernatant was quantified by a BCA assay using Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, 23227) according to the manufacturer’s protocol. Equal amounts of protein were electrophoresed in a sodium dodecyl sulfate-polyacrylamide gel under reducing conditions followed by transfer to polyvinylidene fluoride membrane (Millipore, IPVH00010) membranes. Blots were blocked with 5% milk in TBST and western blots were probed with antibodies specific for BiP, ATF6, IRE 1α, p-PERK/PERK, p-EIF2α/ EIF2α, BECN1, LC3B-II, p62 and β-actin. Signals were detected by Super Signal West Pico (Thermo Fisher Scientific, 34078) or Dura Chemiluminescent Substrate (Thermo Fisher Scientific, 34076). Image J (v1.4.3.67; NIH, Bethesda, MD) software was used for quantification.Normalization was done with β-actin, an internal control and the fold change was obtained. All experiments were repeated at least six times and representative blots are presented in the figures.
Enzyme linked immune absorbent assay (ELISA)
Cytokine assay: Human primary pericytes (3×105 cells per well) were seeded in 6 well plates and incubated overnight at 37°C in a humidified, 5% CO2 incubator. After overnight serum starvation, cells were treated with different concentrations (1, 10, 100 μM) of cocaine followed by collection of the cell supernatants. For time course study cells were exposed to a fixed concentration of cocaine (10 μM) for varying time points (0, 1, 3, 6, 12, 24 h) and culture fluids collected. The cell supernatant fluids were used for IL-6 detection by ELISA using a human IL 6 ELISA kit (ab46027; Abcam), TNF-α detection by Human TNF alpha ELISA Kit (ab181421; Abcam) and IL 1β detection by Human IL-1 beta ELISA Kit (Interleukin-1 beta) (ab108865; Abcam) according to the manufacturer’s instructions. Three independent replicates per sample and six separate sets of experiments for each study points were analyzed.
Reverse transcription and real-time qPCR
The conditions for RT and real-time PCR assays have been described previously [38]. Real-Time customized PCR Taqman primers for human TNFα (Hs00174128 m1), IL-1β (Hs01555410 m1), IL-6 (Hs00174131 m1) and GAPDH (Hs02786624 g1) were obtained from ThermoFisher (4331182). Total RNA was extracted with the Quick-RNA Miniprep kit according to the manufacturer’s instructions from ZYMO Research (Irvine, CA, USA). Quantitative analyses of RNA were conducted using NanoDrop (Thermo Fisher Scientific). Real-time PCR amplifications were carried out for 40 cycles (denaturation: 30 secs at 95°C; annealing: 1 min at 60°C).
Autophagosome-lysosome fusion staining
Human pericytes were seeded in a 24-well plate containing sterile glass coverslips (11 mm) at a density of 5×104 cells per well at 37°C in a humidified, 5% CO2 incubator for 24 h. The cells were transfected with the RFP-GFP-LC3B plasmid (ptfLC3; Addgene plasmid # 21074) [41] using Lipofectamine® 2000 Reagent, according to the manufacturer’s protocol, for 10 h following which the culture medium was replaced with the respective 10% heat-inactivated FBS-DMEM and the astrocytes were then treated with different agents. Thereafter, the pericytes were rinsed 2 times with phosphate-buffered saline (PBS; Hyclone Laboratories, SH3025801) and fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, followed by rinsing thrice with PBS. Subsequently, the coverslips were mounted on glass slides with ProLong Gold Antifade Reagent with DAPI (Molecular Probes, P36935). Fluorescent images were taken on a Zeiss Observer using a Z1 inverted microscope (Carl Zeiss, Thornwood, NY, USA) and the acquired images were analyzed using the AxioVs 40 Version 4.8.0.0 software (Carl Zeiss MicroImaging GmbH).
LC3B-II turnover and p62 degradation assay
Human pericytes were seeded in a 6-well plate at a density of 3×105 cells per well at 37°C in a humidified, 5% CO2 incubator for 24 h. Next day cells were treated with either 500 nM of cocaine or left untreated (control). Four hours before harvesting, the cells were treated with BAF (400 nM) (in control and cocaine-exposed cells). These cells were processed further for western blot analysis of LC3B-II and p62.
Isolation of brain microvessels
Brain microvessels were isolated as described previously [4]. Briefly, the brains of mice administered either saline or cocaine were removed and immediately immersed in ice-cold isolation buffer A (103 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4,1.2 mM MgSO4, and 15 mM HEPES, pH 7.4). The choroid plexus, meninges, cerebellum and brain stem were removed followed by homogenization of the brain in 2.5 ml of isolation buffer B (25 mM NaHCO3, 10 mM glucose, 1 mM Na+ pyruvate, and10 g/L dextran, pH 7.4) with complete protease inhibitors. Six ml of Dextran (26%) was then added to the homogenates followed by centrifugation at 5,800 g for 20 minutes. Cell pellets were resuspended in isolation buffer B and filtered through a 70 μm mesh filter (Becton Dickinson, Franklin Lakes, NJ, USA). Filtered homogenates were re-pelleted by centrifugation and was used for staining by smearing on glass slides.
Immunofluorescence staining
Brain microvessels smeared on glass slides and HBVPS seeded on glass coverslips were fixed with 4% formaldehyde in PBS for 20 minutes at room temperature (RT) followed by three washes with PBS, permeabilized with 0.3% Triton X-100 for 30 minutes, rewashed thrice, and blocked in 10% goat serum in PBS for 2h at RT. The following antibodies were used for immunostaining: rabbit LC3 antibodies, rat PDGFRβ antibodies, rabbit IL-6 antibodies, mouse Desmin antibodies. Both, PDGFRβ and Desmin were used to stain the pericytes for confirmation. The slides were incubated with primary antibodies overnight at 4 °C, followed by 3 times PBS washing and incubated with AlexaFluor 594–conjugated anti–mouse, AlexaFluor 488–conjugated anti–rabbit, AlexaFluor 594-conjugated, anti–rabbit or AlexaFluor 594-conjugated anti-rat immunoglobulin G (IgG) for 1h at RT. After a final wash with PBS, the slides/coverslips were mounted with mounting medium (Prolong Gold Anti-fade Reagent; Invitrogen, Grand Island, NY, USA). Fluorescent images were acquired at RT on a Zeiss Observer using a Z1 inverted microscope with a (40×/0.3) or (63×/0.3) oil objective. Images were processed using the AxioVs 40 Version 4.8.0.0 software (Carl Zeiss MicroImaging GmbH). Photographs were acquired with an AxioCam MRm digital camera and were analyzed with Image J software (v1.4.3.67; NIH, Bethesda, MD).
Statistical analysis
The data are represented as mean ± SEM. Statistical analysis was performed using one way ANOVA followed by Bonferroni post hoc test for multiple groups or student t-test for two groups using the GraphPad Prism software (Version 5). Statistical analysis where probability levels were less than 0.05 were considered statistically significant.
Results
Cocaine increases the expression of pro-inflammatory cytokines in human primary pericytes
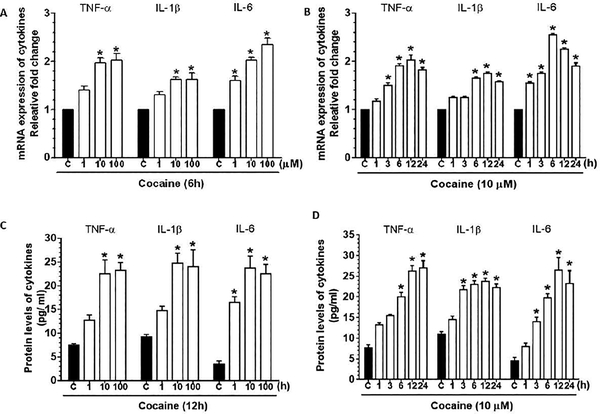
We initially sought to determine whether exposure of human brain primary pericytes (HBVP) to cocaine could result in production of pro-inflammatory cytokines. Cells were exposed to varying concentrations of cocaine (1, 10 and 100 μM) for 6h. As shown in Fig. 1A, cocaine significantly (P<0.05) increased the mRNA expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in a dose-dependent manner with a maximal expression at a dose of 10 μM. The dose of 10 μM dose was thus chosen for all the subsequent experiments. It should be noted that this concentration of cocaine is in keeping with the cocaine concentrations in postmortem brains of chronic cocaine users, which are found to be >100 μM[42]. We next sought to determine the optimal time of cocaine exposure for the induction of pro-inflammatory cytokines. As shown in Fig. 1B, cocaine (10 μM) exposure resulted in a significant increase in the mRNA expression of proinflammatory cytokines (P<0.05), with maximal expression at 12h following cocaine exposure. From the dose- and time-course studies, the optimal dose and time of cocaine for pericytes to elicit an inflammatory response was 10 μM at 12h. This dose and time of cocaine exposure was thus maintained for all subsequent experiments. ELISA data also showed significant (P<0.05) dose- and time-dependent upregulation of the expression of pro-inflammatory cytokines in the supernatants of pericytes exposed to cocaine (Figs. 1C and D). For HBVP purity, cultures were stained for pericyte markers – PDGFR-β and Desmin (Supplementary Fig. 1).
Figure 1.
Cocaine-mediated secretion of proinflammatory cytokines in human pericytes. qPCR analysis showing the dose- (A) and time-dependent (B) upregulation of proinflammatory cytokines, such as TNF-α, IL-1β and IL 6 in human primary pericytes exposed to cocaine. ELISA showing dose- (C) and time-dependent (D) upregulated expression of the cytokines -TNF-α, IL 1β and IL 6 in human primary pericytes exposed to cocaine. GAPDH was used as an internal control for mRNA expression of cytokines. Data are presented as mean ± SEM; the mean is derived from 6 independent experiments (n = 6). One-way ANOVA followed by Bonferroni post hoc test was used to determine the statistical significance between multiple groups: *, P < 0.05 vs. control.
Cocaine induces ER stress and autophagy initiation in human primary pericytes
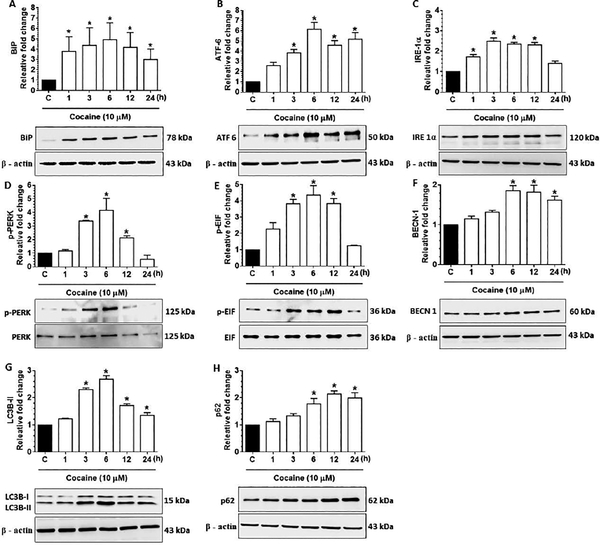
Several studies have demonstrated the role of ER stress and autophagy in neuroinflammation [13,38] [40]. Additionally, cocaine exposure has also been reported to induce autophagy in both microglia and astrocytes [13] [38]. We thus next sought to examine whether exposure of HBVPs to cocaine could lead to induction of ER stress (BiP, ATF 6, IRE 1α, p-PERK, PERK, p-EIF 2α and EIF 2α) as well as autophagy mediators (BECN 1, LC3B-II, p62). As shown in Figs. 2A-2E, cocaine exposure significantly (P<0.05) upregulated the expression of ER stress markers BiP (Fig. 2A), ATF 6 (Fig. 2B), IRE 1α (Fig. 2C), p-PERK/ PERK (Fig. 2D), p-EIF 2α/ EIF 2α (Fig. 2E) as well as the autophagy markers BECN1 (Fig. 2F), LC3B-II (Fig. 2G) thus implicating cocaine mediated induction of both ER stress and autophagy in HBVPs. Intriguingly, expression of p62, a marker for autophagy flux was found to increase time-dependently in HBVPs following cocaine exposure, thereby indicating a defective autophagic flux in cells following cocaine exposure (Fig. 2H).
Figure 2.
Cocaine-mediated initiation of autophagy and ER stress in human primary pericytes (HBVP). Representative western blots showing time-dependent upregulation of BiP (A), ATF 6 (B), IRE 1α (C), p-PERK/ PERK (D), p-EIF/ EIF (E), BECN1 (F), LC3B-II (G) and p62 (H) in HBVP exposed to cocaine (10 μM) for the indicated time points. β-actin was used as a loading control for all experiments. Data are presented as mean ± SEM; the mean is derived from 6 independent experiments (n = 6). One-way ANOVA followed by Bonferroni post hoc test was used to determine the statistical significance between multiple groups: *, P < 0.05 vs. control.
Cocaine inhibits autophagic flux in human primary pericytes
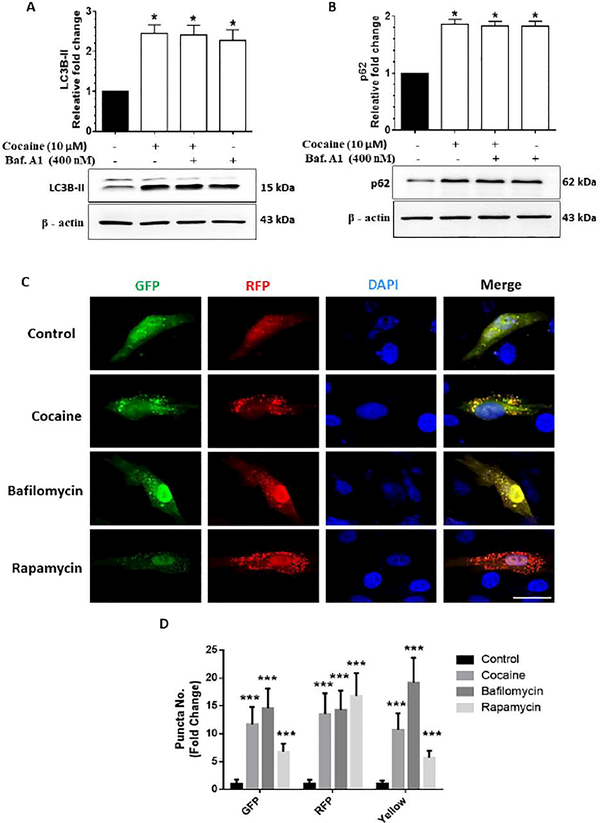
Based on our findings in Fig. 2, demonstrating cocaine-mediated induction of autophagosomes and a concomitant defect in autopahgic flux in HBVPs, we next sought to further validate the mechanism(s) involved in the autophagosome-lysosomal fusion defect. For this we next assessed the autophagic flux using the LC3B-II turnover assay as well as the p62 degradation assay in pericytes exposed to cocaine. The ubiquitin-binding adaptor protein p62 gets selectively integrated into the phagophores by directly binding to the LC3B-II protein which aids in the turnover of ubiquitinated proteins, while itself getting degraded by autophagy. Accumulation of p62 protein can thus be considered as an indicator of autophagic flux inhibition. HBVPs were exposed to cocaine (10 μM) followed by exposure of cells to bafilomycin A1 (BAF) - an inhibitor of autophagosome-lysosome fusion, that was added at a saturating concentration of 400 nM during the last 4 h prior to cell harvesting followed by assessing the accumulation of LC3B-II and p62 proteins. As shown in Figs. 3A and B, exposure of HBVPs to cocaine followed by BAF exposure resulted in significantly (P<0.05) increased accumulation of LC3B-II/p62 proteins compared with cells not exposed to cocaine. Interestingly, similar to BAF-treated HBVPs, there was also an increased accumulation of LC3B-II/ p62 in HBVPs exposed to cocaine alone, likely underscoring cocaine-mediated inhibition of the autophagic flux.
Figure 3.
Cocaine-mediated dysregulated autophagy in human primary pericytes (HBVP). Representative western blots showing protein levels of LC3B-II (A) and p62 (B) in human HBVP exposed to cocaine (10 μM) for 12 h followed by treatment with 400 nM BAF, which was added in the last 4 h of the 12 h treatment period. (C) Representative fluorescent photomicrographs showing the LC3B-II puncta formation in HBVP cells transfected with tandem fluorescent-tagged LC3B-II plasmid and treated with 10 μM cocaine for 12 h, 400 nM BAF - last 4 h of the 12 h treatment period and 10 nM rapamycin for 24 h. Quantitative analyses of yellow, red and green puncta (D) formation in different experimental groups of tandem fluorescent-tagged LC3B-II plasmid transfected HBVP. Scale bar: 10 μm. β-actin was used as a loading control for all experiments. Data are presented as mean ± SEM; the mean is derived from 6 independent experiments (n = 6). Abbreviation: Baf. A1: Bafilomycin A1. One-way ANOVA followed by Bonferroni post hoc test was used to determine the statistical significance. *, P<0.05 vs control.
We next wanted to validate cocaine-mediated inhibition of autophagic flux in HBVPs using the tandem fluorescent-tagged LC3B reporter plasmid transfection. Cells that exhibit normal autophagosome formation express both RFP (red) and GFP (green) tagged LC3B, while upon fusion with the lysosomes, (due to the acidic pH) the GFP degrades, and RFP is more predominant (Fig. 3C). HBVPs were transfected with a tandem fluorescently-tagged LC3B plasmid, followed by exposure of cells to either 10 μM cocaine or BAF (400 nM) or 10 nM rapamycin (inducer of autophagy), and subsequently assessed for the presence of red and green fluorescent LC3B puncta by immunofluorescence imaging. Interestingly, and as expected, cocaine exposure significantly (P<0.05) increased the formation of both red and green LC3B puncta formation (yellow puncta for the merged) in HBVP (Fig. 3D) compared to control cells. Compared to control cells, BAF exposure resulted in the formation of yellow puncta, which was comparable to the cocaine alone group. On the other hand, rapamycin (a known inducer of autophagy) exposed HBVPs showed significantly (P<0.05) increased red puncta, indicating the effective fusion of autophagosomes with the lysosomes. These data underscore cocaine-mediated initiation of autophagosome formation, followed by defects in fusion of autophagosome with the lysosome in pericytes.
Cocaine-mediated initiation of autophagy involves upstream activation of ER stress in human primary pericytes
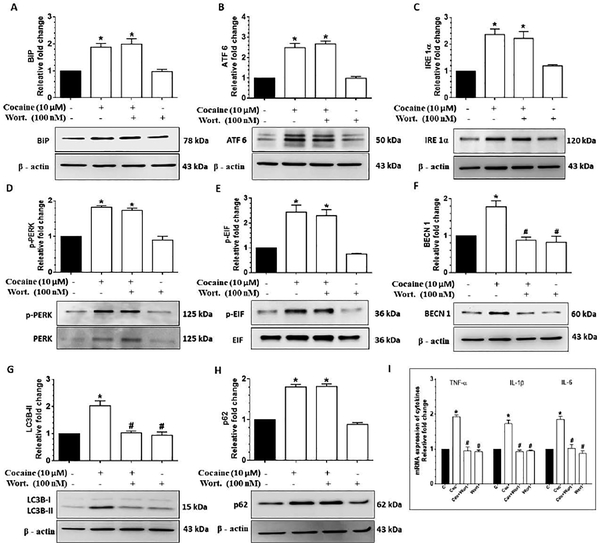
Next we sought to determine whether there existed a link between ER stress and the activation of autophagy in cocaine-exposed HBVPs. Herein HBVPs were either pretreated with wortmannin (100 nM), a pharmacological inhibitor of autophagy (involving inhibition of the PI3K signaling), for 1h followed by exposure of cells to 10 μM cocaine. Wortmannin pre-treatment in cocaine exposed cells resulted in a significant decrease (P<0.05) in the expression of autophagy markers - BECN1 and LC3B-II, (p62 remaining unchanged), while the expression of the ER stress markers (BiP, ATF 6, IRE 1α, p-PERK/ PERK, p-EIF 2α/ EIF 2α) remained unchanged compared with the cocaine exposed group (Fig. 4A-4H), thereby indicating that activation of ER stress was upstream of autophagy signaling. Additionally, pre-treatment of wortmannin also resulted in significant reduction (P<0.05) of expression of proinflammatory cytokine (TNF-α, IL 1β and IL-6) mRNAs (Fig. 4I), thus underpinning the involvement of upstream autophagy signaling in the regulation of inflammation in HBVP.
Figure 4.
Cocaine-mediated dysregulated autophagy involves upstream activation of ER stress in HBVP. Representative western blots showing the protein levels of BiP (A), ATF 6 (B), IRE 1α (C), p-PERK/ PERK (D), p-EIF/ EIF (E), BECN1 (F), LC3B-II (G) and p62 (H) in HBVP pretreated with 100 nM wortmannin for 1 h followed by 10 μM cocaine exposure for 12 h. (I) RT-qPCR showing relative expression of proinflammatory cytokines such as TNF-α, IL 1β and IL 6 mRNA in HBVP pretreated with 100 nM wortmannin for 1 h followed by 10 μM cocaine exposure for 6 h. Data are presented as mean ± SEM; the mean is derived from 6 independent experiments (n = 6). Abbreviations: Coc: Cocaine, Wort.: wortmannin. One-way ANOVA followed by Bonferroni post hoc test was used to determine the statistical significance: *, P<0.05 vs. control; #, P<0.05 vs. cocaine.
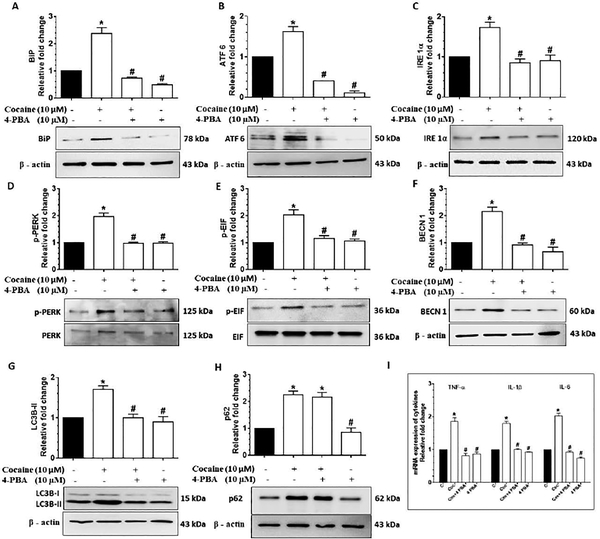
Based on our findings that cocaine exposure resulted in the induction of ER stress mediated autophagy, we next sought to validate our findings using the pharmacological approach. For this cells were pretreated with the pharmacological inhibitor of ER stress -sodium 4-phenylbutyrate (4-PBA; 10 μM for 1h) followed by exposure of cells to cocaine and assessment of ER stress and autophagy mediators as well as expression of cytokines. In cells pretreated with 4-PBA, cocaine exposure resulted in significant inhibition (P<0.05) of expression of the ER stress mediators (BiP, ATF 6, IRE 1α, p-PERK/ PERK, p-EIF 2α/ EIF 2α) (Figs. 5A-5E) as well as the autophagy mediators (BECN1, LC3B-II) – with no change in the expression of p62 (Figs. 5F-H). Similar to these findings, expression of proinflammatory cytokine mRNA was also inhibited (Fig. 5I), thereby confirming cocaine mediated activation of the ER stress pathways, which in turn, initiates autophagy, followed by defective autophagosome - lysosome fusion, leading to induction of proinflammatory cytokines in HBVP.
Figure 5.
Cocaine-mediated dysregulated autophagy involves upstream activation of ER stress in HBVP. Representative western blots showing the protein levels of BiP (A), ATF 6 (B), IRE 1α (C), p-PERK/ PERK (D), p-EIF/ EIF (E), BECN1 (F), LC3B-II (G) and p62 (H) in HBVP pretreated with 10 μM 4- PBA for 1 h followed by 10 μM cocaine exposure for 12 h. (I) RT-qPCR showing relative expression of proinflammatory cytokines such as TNF-α, IL 1β and IL 6 mRNA in HBVP pretreated with 10 μM 4- PBA for 1 h followed by 10 μM cocaine exposure for 6 h. Data are presented as mean ± SEM; the mean is derived from 6 independent experiments (n = 6).Abbreviation: Coc: Cocaine. One-way ANOVA followed by Bonferroni post hoc test was used to determine the statistical significance: *, P<0.05 vs. control; #, P<0.05 vs. cocaine.
Cocaine-mediated defective autophagy in human primary pericytes involves σ−1R
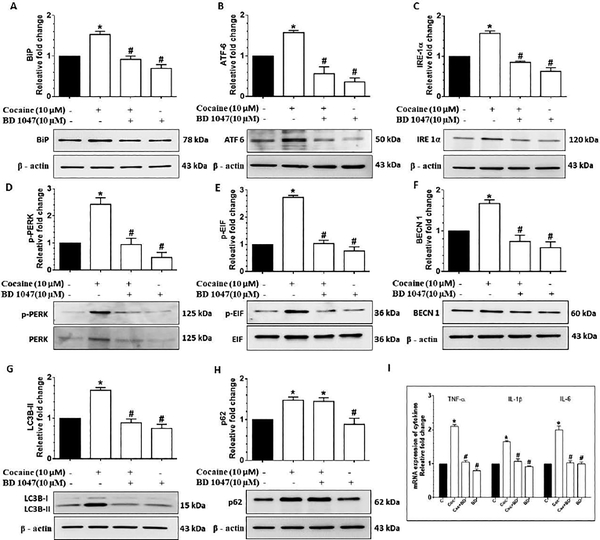
Next, we sought to determine whether cocaine mediated induction of cytokines via the ER stress-impaired autophagy signaling involved activation of σ−1R. Pretreatment of HBVPs with the sigma receptor antagonist -BD 1047 (for 1 h) followed by cocaine exposure (10 μM) resulted in a significant (P<0.05) decrease in the expression of ER stress mediators (BiP, ATF 6, IRE 1α, p-PERK/ PERK, p-EIF 2α/ EIF 2α) as shown in Fig. 6A-E as well as a decrease in autophagy mediators - BECN1 and LC3B-II (Fig. 6F-G), and a decrease in the expression of proinflammatory cytokine mRNAs (Fig.6I), compared with pericytes exposed to cocaine alone. The expression of p62 on the hand, remained unchanged in cells pretreated with BD1047 (Fig. 6H), Overall, these findings underscore the upstream involvement of σ−1Rin cocaine induced ER stress, impaired autophagy and generation of proinflammatory cytokines.
Figure 6.
Cocaine-mediated ER stress-dysregulated autophagy mediated inflammation involves σ−1Rin HBVP. Representative western blots showing the protein levels of BiP (A), ATF 6 (B), IRE 1α (C), p-PERK/ PERK (D), p-EIF/ EIF (E), BECN1 (F), LC3B-II (G) and p62 (H) in HBVP pretreated with 10 μM BD 1047 for 1 h followed by 10 μM cocaine exposure for 12h. (I) RT-qPCR showing relative expression of proinflammatory cytokines such as TNF-α, IL 1β and IL 6 mRNA in HBVP pretreated with 10 μM BD 1047 for 1 h followed by 10 μM cocaine exposure for 6 h. Data are presented as mean ± SEM; the mean is derived from 6 independent experiments (n = 6). Abbreviation: Coc: Cocaine. One-way ANOVA followed by Bonferroni post hoc test was used to determine the statistical significance: *, P<0.05 vs. control; #, P<0.05 vs. cocaine.
Cocaine-dependent mice exhibit impaired autophagy and inflammation in the pericytes of isolated brain microvessels
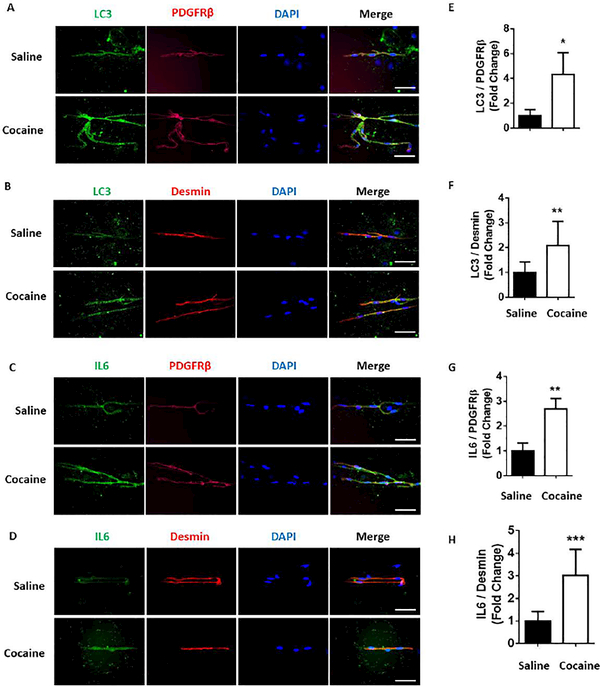
To validate our in vitro findings demonstrating the role of the ER stress-autophagy axis in cocaine-mediated induction of proinflammatory cytokines in pericytes, we next sought to assess the expression of autophagy marker LC3 as well as the pro inflammatory cytokine IL-6 in the pericytes present in the microvessels isolated from the brains of cocaine injected mice (20 mg/ kg/ day, i.p. for 7 days). Our findings demonstrated a significant (P<0.05) upregulation of LC 3 puncta formation (Fig. 7A, B, E, F) in the pericytes (in brain microvessels) of cocaine administered mice, along with the upregulation of the proinflammatory cytokine IL-6 in the pericytes (Fig. 7C, D, G, H) compared with the saline injected mice. These in vivo data validated our cell culture findings implying cocaine-mediated activation of autophagy and generation of proinflammatory cytokines in the pericytes.
Figure 7.
Cocaine-dependent mice express autophagy and inflammatory markers in isolated brain microvessels. Representative immunohistochemistry images demonstrating the expression of LC3 (A and B) and IL 6 (C and D) in the microvessels of cocaine administered mice. Scale bar: 50 μm. Quantitative expression of LC3 (E and F) and IL 6 (G and H) in the microvessels of cocaine administered mice. Data are presented as mean ± SEM; the mean is derived from 6 independent experiments (n = 6). Student’s t test was used to determine the statistical significance: *, P<0.05 vs. saline.
Discussion
Cocaine abuse leads to psychostimulation [43] resulting in altered functioning of the CNS) [44]. Intermittent cocaine use is also known to exert several adverse effects in the CNS including, but not limited, to inhibition of monoamine reuptake, particularly through binding of dopamine transporter [2], neuronal death in mice cortical neurons [45] and enhanced BBB permeability [46], thereby contributing to enhanced entry of peripheral cells into the CNS [46]. Additionally, cocaine abuse has also been considered to be an important comorbidity for the progression of HIV-associated neurological disorders [7,9,47]. Cocaine has also been well documented to be a potent mediator of oxidative stress and inflammation [13,38,48], resulting in glial cell activation leading, in turn, to neuroinflammation [13,38,49,50]. Pericytes, one of the important components of BBB have gained recent attention for their vital roles in the maintenance of the BBB[51–53]. While pericytes have been implicated as a source of neuroinflammation [14], their activation by drugs of abuse such as cocaine has never been explored before. The goal of the current work was to elucidate the molecular mechanism(s) by which cocaine induces neuroinflammation mediated by pericytes.
In line with the previous findings from our lab in glial cells, our current findings demonstrated that cocaine exposure resulted in induced expression of proinflammatory cytokines both in vitro as well as in vivo in pericytes, thereby implicating these cells as yet another important source of neuroinflammation in the CNS. Pericytes are key players in the maintenance and integrity of the BBB and blood spinal cord barrier (BSB). It is likely that cocaine mediated generation of inflammatory mediators by pericytes, could in turn, dysregulate the pericyte-endothelial interactions, resulting in breach of the BBB as well as the BSB [14].
Various reports implicate the role of defective autophagy as an upstream signaling pathway in the generation of inflammatory response. For example, there are reports indicating that macrophages of Atg16l1 knockout mice produced more IL-1β following stimulation with LPS and, this was attributed to exaggerated activation of caspase1 in the Atg16l 1-deficient mice [54]. Furthermore, genetic association studies have also suggested that defects in autophagy can confer susceptibility to several autoimmune and inflammatory disorders, particularly the inflammatory bowel disease [55]. Cocaine exposure has been shown to induce autophagy in microglia [38], astrocytes [13], neurons [56] as well as in vivo in the striatum of mice brain [13]. Our previous findings have also demonstrated that cocaine exposure resulted in neuroinflammation via the ER stress-autophagy axis [13,38]. The present study demonstrated that cocaine exposure resulted in initiation of autophagy signaling with the formation of autophagosomes in pericytes with a subsequent block in the fusion of autophagosomes with the lysosomes. These findings were also confirmed using the LC3B-II turnover assay as well as the p62 degradation assay in pericytes. Our findings are in agreement with the reports in other cells demonstrating the ability of cocaine to inhibit autophagic fusion [13,49], which then culminates into neuroinflammation [13,38].
Several studies have demonstrated the regulatory role of ER stress pathways in regulating the induction of autophagy [57–61]. In the present study we also demonstrated that in pericytes cocaine-mediated impaired autophagy was linked to activation of the ER stress pathways and, this was further validated using the pharmacological inhibition approach with 4-PBA - a pharmacological inhibitor of ER stress, as well as wortmannin - a pharmacological inhibitor of autophagy signaling, both of which resulted in abrogation of cocaine-mediated autophagy and induction of pro-inflammatory cytokines, with no effect on the expression of upstream ER stress mediators. These findings are in agreement with our previous report in glial cells demonstrating the role of cocaine-mediated induction of ER stress/autophagy axis in inflammation [13,38]. Furthermore, the present study also validated the role of σ−1R in cocaine mediated induction of ER stress/ autophagy and inflammation. Role of σ−1R in cocaine mediated effects is in agreement with previously published findings [5,12,31,62–65].
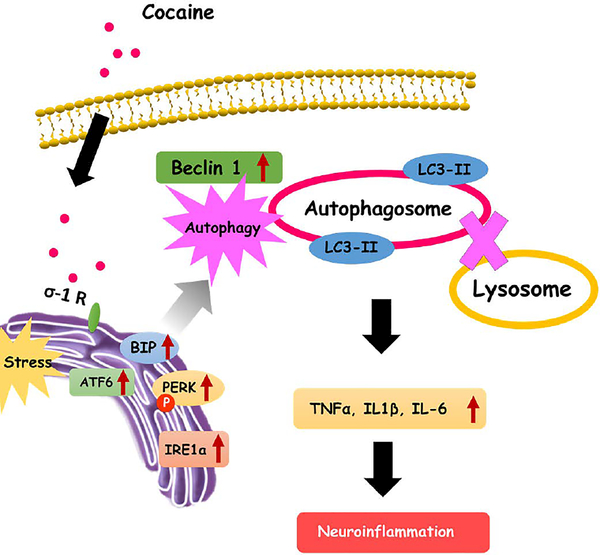
We also validated our cell culture findings in microvessels isolated from cocaine administered mice brain. Our in vivo study demonstrated that that cocaine administered mice exhibited increased expression of the autophagy marker LC3 which, in turn, colocalized with pericytes in the isolated microvessels. Interestingly, pericytes in the isolated microvessels also expressed increased levels of the cytokine IL-6. These in vivo findings validate the role of pericytes as yet another source of neuroinflmamatory cytokines in the context of cocaine abuse. Figure 8 summarizes our findings on the mechanism(s) by which cocaine induces neuroinflammation mediated by ER stress - dysregulated autophagic pathway via the
Figure 8.
Schematic representation of cocaine induced pericyte mediated inflammation involving the ER stress-autophagy axis via the sigma-1 receptor. Cocaine binds to the σ−1Rin the pericytes and activates the various ER stress pathways. Activation of ER stress in turn, induces the formation of autophagosomes while blocking the fusion of autophagosomes with the lysosomes, resulting in dysregulated autophagy. Dysregulated autophagy, in turn, leads to generation and secretion of proinflammatory cytokines thereby contributing to neuroinflammation.
While much attention is garnered on the glial cells in the context of cocaine addiction [13,38,49], based on the current findings it can be envisioned that pericyte activation could also contribute to future development of drug addiction. These novel findings for the first time implicate pericytes as a source of neuroinflamamtion in the brain both via direct interactions with cocaine, and likely via their deleterious indirect effects on the endothelium, which, in turn, could lead to an exacerbated influx of peripheral leukocytes into the CNS. Taken together, disruption of the cerebrovascular unit by pericytes could further catalyze neurodegeneration via cascading effect of the cytokine storm as well as via the influx of inflammatory cells into the brain, thereby contributing to disease severity. Interventions aimed at blocking pathway(s) involved in cocaine-mediated activation of pericytes, could thus be considered as future therapeutic strategies for dampening cocaine-mediated neuroinflammation.
Supplementary Material
(A) Immunostaining of HBVPs using specific pericyte marker - PDGFRβ. Scale bar: 50 μm. (B) Immunostaining of HBVPs using specific pericyte marker - Desmin. Scale bar: 50 μm.
Acknowledgements
This work was supported by grants DA035203, DA040397, DA041751, DA044586 (SB), DA043138 (SB & GH) from the National Institutes of Health and 2P30MH062261 from National Institute of Mental Health,. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The support by Nebraska Center for Substance Abuse Research is acknowledged. We are grateful to Drs. Guoku Hu, Minglei Guo, Ernest Chivero, Annadurai Thangaraj and Ashutosh Tripathi for their useful discussions.
Footnotes
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References:
- 1.Wolf ME (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17 (6):351–365. doi: 10.1038/nrn.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett G, Hawks R, Resnick R (1981) Cocaine pharmacokinetics in humans. Journal of ethnopharmacology 3 (2–3):353–366 [DOI] [PubMed] [Google Scholar]
- 3.CBHSQ CfBHSaQ (2011) Drug Abuse Warning Network: 2011: Selected Tables of National Estimates of Drug-Related Emergency Department Visits. Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- 4.Yao H, Duan M, Buch S (2011) Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood 117 (8):2538–2547. doi: 10.1182/blood-2010-10-313593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, Prat A, Wang J, Su TP, Buch S (2011) Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci 31 (16):5942–5955. doi: 10.1523/JNEUROSCI.5618-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buch S, Yao H, Guo M, Mori T, Su TP, Wang J (2011) Cocaine and HIV-1 interplay: molecular mechanisms of action and addiction. J Neuroimmune Pharmacol 6 (4):503–515. doi: 10.1007/s11481-011-9297-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dash S, Balasubramaniam M, Villalta F, Dash C, Pandhare J (2015) Impact of cocaine abuse on HIV pathogenesis. Front Microbiol 6:1111. doi: 10.3389/fmicb.2015.01111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkins BT, Davis TP (2005) The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57 (2):173–185. doi: 10.1124/pr.57.2.4 [DOI] [PubMed] [Google Scholar]
- 9.Dhillon NK, Peng F, Bokhari S, Callen S, Shin SH, Zhu X, Kim KJ, Buch SJ (2008) Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. J Neuroimmune Pharmacol 3 (1):52–56. doi: 10.1007/s11481-007-9091-1 [DOI] [PubMed] [Google Scholar]
- 10.Kolodgie FD, Wilson PS, Mergner WJ, Virmani R (1999) Cocaine-induced increase in the permeability function of human vascular endothelial cell monolayers. Exp Mol Pathol 66 (2):109–122. doi: 10.1006/exmp.1999.2253 [DOI] [PubMed] [Google Scholar]
- 11.Sharma HS, Muresanu D, Sharma A, Patnaik R (2009) Cocaine-induced breakdown of the blood-brain barrier and neurotoxicity. Int Rev Neurobiol 88:297–334. doi: 10.1016/S0074-7742(09)88011-2 [DOI] [PubMed] [Google Scholar]
- 12.Ka M, Kook YH, Liao K, Buch S, Kim WY (2016) Transactivation of TrkB by Sigma-1 receptor mediates cocaine-induced changes in dendritic spine density and morphology in hippocampal and cortical neurons. Cell Death Dis 7 (10):e2414. doi: 10.1038/cddis.2016.319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Periyasamy P, Guo ML, Buch S (2016) Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy 12 (8):1310–1329. doi: 10.1080/15548627.2016.1183844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rustenhoven J, Jansson D, Smyth LC, Dragunow M (2017) Brain Pericytes As Mediators of Neuroinflammation. Trends Pharmacol Sci 38 (3):291–304. doi: 10.1016/j.tips.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa S, Castro V, Toborek M (2012) Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med 16 (12):2950–2957. doi: 10.1111/j.1582-4934.2012.01622.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsumoto J, Dohgu S, Takata F, Machida T, Bolukbasi Hatip FF, Hatip-Al-Khatib I, Yamauchi A, Kataoka Y (2018) TNF-alpha-sensitive brain pericytes activate microglia by releasing IL-6 through cooperation between IkappaB-NFkappaB and JAK-STAT3 pathways. Brain Res 1692:34–44. doi: 10.1016/j.brainres.2018.04.023 [DOI] [PubMed] [Google Scholar]
- 17.Liang XG, Tan C, Wang CK, Tao RR, Huang YJ, Ma KF, Fukunaga K, Huang MZ, Han F (2018) Myt1l induced direct reprogramming of pericytes into cholinergic neurons. CNS Neurosci Ther. doi: 10.1111/cns.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banks WA, Kovac A, Morofuji Y (2018) Neurovascular unit crosstalk: Pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J Cereb Blood Flow Metab 38 (6):1104–1118. doi: 10.1177/0271678X17740793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney MD, Ayyadurai S, Zlokovic BV (2016) Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 19 (6):771–783. doi: 10.1038/nn.4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Lucas-Osma AM, Black S, Bandet MV, Stephens MJ, Vavrek R, Sanelli L, Fenrich KK, Di Narzo AF, Dracheva S, Winship IR, Fouad K, Bennett DJ (2017) Pericytes impair capillary blood flow and motor function after chronic spinal cord injury. Nat Med 23 (6):733–741. doi: 10.1038/nm.4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Silva Meirelles L, Bellagamba BC, Camassola M, Nardi NB (2016) Mesenchymal stem cells and their relationship to pericytes. Front Biosci (Landmark Ed) 21:130–156 [DOI] [PubMed] [Google Scholar]
- 22.Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL (2014) Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res 51 (3):163–174. doi: 10.1159/000362276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S (2012) Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 209 (6):1219–1234. doi: 10.1084/jem.20111622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro R, Compte M, Alvarez-Vallina L, Sanz L (2016) Immune Regulation by Pericytes: Modulating Innate and Adaptive Immunity. Front Immunol 7:480. doi: 10.3389/fimmu.2016.00480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro V, Skowronska M, Lombardi J, He J, Seth N, Velichkovska M, Toborek M (2018) Occludin regulates glucose uptake and ATP production in pericytes by influencing AMP-activated protein kinase activity. J Cereb Blood Flow Metab 38 (2):317–332. doi: 10.1177/0271678X17720816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho HJ, Kuo AM, Bertrand L, Toborek M (2017) HIV Alters Gap Junction-Mediated Intercellular Communication in Human Brain Pericytes. Front Mol Neurosci 10:410. doi: 10.3389/fnmol.2017.00410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persidsky Y, Hill J, Zhang M, Dykstra H, Winfield M, Reichenbach NL, Potula R, Mukherjee A, Ramirez SH, Rom S (2016) Dysfunction of brain pericytes in chronic neuroinflammation. J Cereb Blood Flow Metab 36 (4):794–807. doi: 10.1177/0271678X15606149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi T, Su TP (2003) Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108–15 cells. J Pharmacol Exp Ther 306 (2):726–733. doi: 10.1124/jpet.103.051292 [DOI] [PubMed] [Google Scholar]
- 29.Romieu P, Phan VL, Martin-Fardon R, Maurice T (2002) Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology 26 (4):444–455. doi: 10.1016/S0893-133X(01)00391-8 [DOI] [PubMed] [Google Scholar]
- 30.Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR (2002) Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev 26 (4):499–527 [DOI] [PubMed] [Google Scholar]
- 31.Yao H, Yang Y, Kim KJ, Bethel-Brown C, Gong N, Funa K, Gendelman HE, Su TP, Wang JQ, Buch S (2010) Molecular mechanisms involving sigma receptor-mediated induction of MCP-1: implication for increased monocyte transmigration. Blood 115 (23):4951–4962. doi: 10.1182/blood-2010-01-266221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto RR (2009) Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol 2 (4):351–358. doi: 10.1586/ecp.09.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu L, Chen Y, Tooze SA (2018) Autophagy pathway: Cellular and molecular mechanisms. Autophagy 14 (2):207–215. doi: 10.1080/15548627.2017.1378838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fields J, Dumaop W, Eleuteri S, Campos S, Serger E, Trejo M, Kosberg K, Adame A, Spencer B, Rockenstein E, He JJ, Masliah E (2015) HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci 35 (5):1921–1938. doi: 10.1523/JNEUROSCI.3207-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho MH, Cho K, Kang HJ, Jeon EY, Kim HS, Kwon HJ, Kim HM, Kim DH, Yoon SY (2014) Autophagy in microglia degrades extracellular beta-amyloid fibrils and regulates the NLRP3 inflammasome. Autophagy 10 (10):1761–1775. doi: 10.4161/auto.29647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S (2016) Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy 12 (2):225–244. doi: 10.1080/15548627.2015.1121360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winslow AR, Rubinsztein DC (2011) The Parkinson disease protein alpha-synuclein inhibits autophagy. Autophagy 7 (4):429–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S (2015) Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 11 (7):995–1009. doi: 10.1080/15548627.2015.1052205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niu F, Yao H, Zhang W, Sutliff RL, Buch S (2014) Tat 101-mediated enhancement of brain pericyte migration involves platelet-derived growth factor subunit B homodimer: implications for human immunodeficiency virus-associated neurocognitive disorders. J Neurosci 34 (35):11812–11825. doi: 10.1523/JNEUROSCI.1139-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sil S, Periyasamy P, Guo ML, Callen S, Buch S (2018) Morphine-Mediated Brain Region-Specific Astrocytosis Involves the ER Stress-Autophagy Axis. Molecular neurobiology. doi: 10.1007/s12035-018-0878-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kimura S, Noda T, Yoshimori T (2007) Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3 (5):452–460 [DOI] [PubMed] [Google Scholar]
- 42.Kalasinsky KS, Bosy TZ, Schmunk GA, Ang L, Adams V, Gore SB, Smialek J, Furukawa Y, Guttman M, Kish SJ (2000) Regional distribution of cocaine in postmortem brain of chronic human cocaine users. J Forensic Sci 45 (5):1041–1048 [PubMed] [Google Scholar]
- 43.Kalivas PW (2007) Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues in clinical neuroscience 9 (4):389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prakash A, Das G (1993) Cocaine and the nervous system. International journal of clinical pharmacology, therapy, and toxicology 31 (12):575–581 [PubMed] [Google Scholar]
- 45.Nassogne MC, Louahed J, Evrard P, Courtoy PJ (1997) Cocaine induces apoptosis in cortical neurons of fetal mice. Journal of neurochemistry 68 (6):2442–2450 [DOI] [PubMed] [Google Scholar]
- 46.Kousik SM, Napier TC, Carvey PM (2012) The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Frontiers in pharmacology 3:121. doi: 10.3389/fphar.2012.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dhillon NK, Williams R, Peng F, Tsai YJ, Dhillon S, Nicolay B, Gadgil M, Kumar A, Buch SJ (2007) Cocaine-mediated enhancement of virus replication in macrophages: implications for human immunodeficiency virus-associated dementia. Journal of neurovirology 13 (6):483–495. doi: 10.1080/13550280701528684 [DOI] [PubMed] [Google Scholar]
- 48.Poon HF, Abdullah L, Mullan MA, Mullan MJ, Crawford FC (2007) Cocaine-induced oxidative stress precedes cell death in human neuronal progenitor cells. Neurochemistry international 50 (1):69–73. doi: 10.1016/j.neuint.2006.06.012 [DOI] [PubMed] [Google Scholar]
- 49.Cao L, Walker MP, Vaidya NK, Fu M, Kumar S, Kumar A (2016) Cocaine-Mediated Autophagy in Astrocytes Involves Sigma 1 Receptor, PI3K, mTOR, Atg5/7, Beclin-1 and Induces Type II Programed Cell Death. Mol Neurobiol 53 (7):4417–4430. doi: 10.1007/s12035-015-9377-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark KH, Wiley CA, Bradberry CW (2013) Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotoxicity research 23 (2):174–188. doi: 10.1007/s12640-012-9334-7 [DOI] [PubMed] [Google Scholar]
- 51.Daneman R, Zhou L, Kebede AA, Barres BA (2010) Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468 (7323):562–566. doi: 10.1038/nature09513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C (2010) Pericytes regulate the blood-brain barrier. Nature 468 (7323):557–561 doi: 10.1038/nature09522 [DOI] [PubMed] [Google Scholar]
- 53.Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7 (4):452–464. doi: 10.1215/S1152851705000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, Tanaka K, Kawai T, Tsujimura T, Takeuchi O, Yoshimori T, Akira S (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456 (7219):264–268. doi: 10.1038/nature07383 [DOI] [PubMed] [Google Scholar]
- 55.Jones SA, Mills KH, Harris J (2013) Autophagy and inflammatory diseases. Immunology and cell biology 91 (3):250–258. doi: 10.1038/icb.2012.82 [DOI] [PubMed] [Google Scholar]
- 56.Guha P, Harraz MM, Snyder SH (2016) Cocaine elicits autophagic cytotoxicity via a nitric oxide-GAPDH signaling cascade. Proceedings of the National Academy of Sciences of the United States of America 113 (5):1417–1422. doi: 10.1073/pnas.1524860113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Funakoshi-Hirose I, Aki T, Unuma K, Funakoshi T, Noritake K, Uemura K (2013) Distinct effects of methamphetamine on autophagy-lysosome and ubiquitin-proteasome systems in HL-1 cultured mouse atrial cardiomyocytes. Toxicology 312:74–82. doi: 10.1016/j.tox.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 58.Matus S, Glimcher LH, Hetz C (2011) Protein folding stress in neurodegenerative diseases: a glimpse into the ER. Current opinion in cell biology 23 (2):239–252. doi: 10.1016/j.ceb.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 59.Doyle KM, Kennedy D, Gorman AM, Gupta S, Healy SJ, Samali A (2011) Unfolded proteins and endoplasmic reticulum stress in neurodegenerative disorders. Journal of cellular and molecular medicine 15 (10):2025–2039. doi: 10.1111/j.1582-4934.2011.01374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ciechomska IA, Gabrusiewicz K, Szczepankiewicz AA, Kaminska B (2013) Endoplasmic reticulum stress triggers autophagy in malignant glioma cells undergoing cyclosporine a-induced cell death. Oncogene 32 (12):1518–1529. doi: 10.1038/onc.2012.174 [DOI] [PubMed] [Google Scholar]
- 61.Buch S, Yao H, Guo M, Mori T, Mathias-Costa B, Singh V, Seth P, Wang J, Su TP (2012) Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Current HIV research 10 (5):425–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang L, Yao H, Chen X, Cai Y, Callen S, Buch S (2016) Role of Sigma Receptor in Cocaine-Mediated Induction of Glial Fibrillary Acidic Protein: Implications for HAND. Molecular neurobiology 53 (2):1329–1342. doi: 10.1007/s12035-015-9094-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cai Y, Yang L, Niu F, Liao K, Buch S (2017) Role of Sigma-1 Receptor in Cocaine Abuse and Neurodegenerative Disease. Advances in experimental medicine and biology 964:163–175. doi: 10.1007/978-3-319-50174-1_12 [DOI] [PubMed] [Google Scholar]
- 64.Tsai SY, Chuang JY, Tsai MS, Wang XF, Xi ZX, Hung JJ, Chang WC, Bonci A, Su TP (2015) Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proceedings of the National Academy of Sciences of the United States of America 112 (47):E6562–6570. doi: 10.1073/pnas.1518894112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nixon CC, Schwartz BH, Dixit D, Zack JA, Vatakis DN (2015) Cocaine exposure impairs multilineage hematopoiesis of human hematopoietic progenitor cells mediated by the sigma-1 receptor [corrected]. Scientific reports 5:8670. doi: 10.1038/srep08670 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Immunostaining of HBVPs using specific pericyte marker - PDGFRβ. Scale bar: 50 μm. (B) Immunostaining of HBVPs using specific pericyte marker - Desmin. Scale bar: 50 μm.