Abstract
Objective:
To determine the relationship between serum S100A8/A9 and S100A12 and the maintenance of clinical inactive disease (CID) in patients with polyarticular forms of juvenile idiopathic arthritis (PF-JIA) while on anti-tumor necrosis factor therapy (anti-TNF) and disease flare following withdrawal of anti-TNF.
Methods:
In this prospective, multicenter study, 137 patients with PF-JIA were enrolled while in CID on anti-TNF. Patients were observed for the initial 6 months. Those who maintained CID had anti-TNF withdrawn and were followed for 8 months to assess for flare. Serum S100 levels were measured at baseline and when anti-TNF was withdrawn. Rank correlation, Mann-Whitney test, Kruskal-Wallis test, receiver-operating-characteristic (ROC) curve and Kaplan-Meier survival analysis were used to assess the relationship between S100 levels and maintenance of CID and disease flare.
Results:
Twenty-four out of 130 (18%) evaluable patients lost CID while on anti-TNF, and 39 of 106 (37%) evaluable patients flared following anti-TNF withdrawal. S100A8/A9 and S100A12 levels were elevated in up to 45% of patients. In the ROC analysis, S100 levels did not predict maintenance of CID and disease flare. Elevated S100A8/A9 levels did not predict disease flare within 30, 60, 90 days or 8 months following anti-TNF withdrawal, and S100A12 had modest predictive ability for flare within 30, 60, and 90 days. S100A12 levels at time of withdrawal and time to disease flare was inversely correlated (r=−0.36).
Conclusion:
Serum S100 levels did not predict maintenance of CID or disease flare, with S100A12 levels only moderately correlating inversely with time to disease flare.
Most children with polyarticular forms of juvenile idiopathic arthritis (PF-JIA), i.e. extended oligoarthritis and polyarthritis, achieve clinical inactive disease (CID) while on contemporary treatment (1, 2). Anti-tumor necrosis factor (TNF) therapy has markedly improved the ability to achieve CID and favorable long-term outcomes in patients with PF-JIA (3–7). Therefore, anti-TNF therapy is used in up to 60% of patients with PF-JIA (2, 8). Still, anti-TNF therapy has potential side effects, is costly, and patients often desire discontinuation of therapy if possible (9, 10). It is well known that some patients can maintain CID after discontinuation of anti-TNF therapy, hence achieve clinical remission off therapy (1). A large proportion of patients, however, will experience a disease flare or lose the CID state after discontinuation of anti-rheumatic therapy, possibly even more so after discontinuation of anti-TNF therapy (11). So far, specific prognostic factors to identify patients who may safely discontinue anti-TNF therapy without subsequent disease flare have been elusive.
The S100A8/A9 complex (also known as myeloid-related peptide [MRP] 8/14 or calprotectin) and the S100A12 protein, are members of the calgranulin family and released from inflammatory cells of the myeloid lineage (12). It has been speculated that elevated serum levels of the S100A8/A9 and S100A12 may be indicators of a state of subclinical inflammation. Subclinical inflammation is considered to be undetectable on clinical examination or by measuring conventional inflammatory markers, for example, C-reactive protein (CRP) or erythrocyte sedimentation rate (13, 14). Furthermore, subclinical inflammation maybe present in patients with PF-JIA in CID while on treatment. Subclinical inflammation is likely a risk factor for recurrence of clinically overt signs and symptoms of JIA once anti-rheumatic therapy is decreased or stopped (15). In line with this hypothesis there is initial evidence that elevated serum S100 levels while on therapy, at the time of withdrawal, indicate an increased risk for loss of CID after treatment withdrawal (16, 17). In another study, comparing the biomarkers S100A8/A9, S100A12 and high-sensitivity CRP, S100A12 was the single most accurate biomarker in predicting loss of CID within 6 months after discontinuation of methotrexate (MTX) (18).
The clinical characteristics of this multicenter study cohort and clinical factors relating to the maintenance of CID while on treatment and the occurrence of disease flare after anti-TNF withdrawal have been identified and reported separately (19).
The objectives of this prospective study were to determine the performance of the serum biomarker S100A8/A9 and S100A12 at baseline and their relation to maintenance of CID, and their level at the time of anti-TNF therapy withdrawal and their relation to disease flare in patients with PF-JIA.
PATIENTS AND METHODS
Study design:
Details of the study design have been reported elsewhere (19). One hundred thirty-seven patients with PF-JIA, i.e. extended oligoarthritis, rheumatoid factor (RF) positive polyarthritis and RF negative polyarthritis while on anti-TNF treatment and in CID were enrolled in 16 tertiary pediatric centers in the US. The study consisted of two phases. First, there was an initial 6-month phase during which anti-TNF therapy was continued and which consisted of quarterly monitoring to assess maintenance of CID. This was followed by anti-TNF withdrawal and a second phase during which patients were observed monthly over 8 months for disease flare, the primary outcome of the study. Patients were only allowed to enter the second phase of the study if CID was maintained during the first phase of the study.
Definitions:
CID was defined according to the American College of Rheumatology provisional criteria (20). Disease flare was defined according to the stringent preliminary flare criteria (21).
Inclusion and exclusion criteria:
The detailed inclusion and exclusion criteria are reported elsewhere (22). In brief, patients with PF-JIA were between 4 and 20 years of age, were in a state of CID while receiving anti-TNF therapy (adalimumab [ADA], etanercept [ETN], or infliximab [IFX]) and all food and drug administration label exclusions had to be absent. Patients were excluded if they were diagnosed with other acute or chronic inflammatory illnesses, were previously treated with rituximab, received concurrent treatment with another biologic agent or more than low-dose corticosteroids (prednisone-equivalent of greater than 0.2 mg/kg/day, or greater than 10 mg/day).
Anti-TNF therapy:
Allowable anti-TNF therapy included ADA, ETN and IFX. Patients could receive therapy with nonsteroidal anti-inflammatory drugs (NSAIDs), MTX or other non-biological disease-modifying anti-rheumatic drugs (DMARDs), and low-dose corticosteroid (prednisone-equivalent up to 0.2 mg/kg/days); those treatments were to remain unchanged during the study.
Serum samples and S100 protein measurement:
S100 proteins were measured twice during the course of the study, at baseline, i.e. at the beginning of the first phase, and at the time of anti-TNF withdrawal. Serum was separated within two hours of blood sampling. Serum samples were immediately frozen and stored at −80°C. The serum samples were subsequently shipped frozen. Concentrations of S100A8/A9 and S100A12 were determined by a double sandwich ELISA system established in our laboratory, as previously described (23, 24). All samples were diluted to the linear range of the assay. The readers of the laboratory assays were blinded to the disease course. For comparison with earlier studies, internal control sera were included in all ELISA studies.
Statistical analysis:
Microsoft Excel (Redmond, WA, USA) and GraphPad Prism 6 (La Jolla, CA, USA) were used for data analysis. Since the S100 levels were not normally distributed, summary measures were reported as medians (range). Rank correlation analyses between different parameters were performed in order to obtain the Spearman correlation coefficient. Between and among group comparisons of the serum S100 levels were done by the Mann-Whitney U test (in case of two groups) and the Kruskal-Wallis test (in case of more than two groups), respectively. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive accuracy of serum S100 levels in regards to maintenance of CID (phase 1) and occurrence of disease flare (phase 2). For the ROC curve analysis the area-under-the-curve (AUC) and 95% confidence interval was calculated (25). Optimal threshold levels for S100A8/A9 and S100A12 were identified by the levels that resulted in the highest likelihood ratio (sensitivity/[1-specificity]). If optimal levels could not be identified this way, previously identified threshold levels were applied (18). The Youden index (sensitivity + specificity −1) was calculated (range −1 to +1), where +1 represents a perfect test and zero represents a non-discriminatory test. Furthermore, Kaplan-Meier analysis was performed to estimate flare-free survival in patients with lower vs. elevated serum S100 levels. Distributions were compared via chi square test. Discrepancy between S100A8/A9 and S100A12 was defined by the presence of an above-threshold S100A8/A9 and a below-threshold S100A12 level or vice versa.
RESULTS
Patient characteristics and overall distribution of serum S100 levels:
Clinical characteristics are shown in Table 1. Of the initially 137 patients enrolled, 7 patients dropped out (6 due to loss of follow-up or non-compliance and 1 due to a change in diagnosis to psoriatic arthritis), resulting in 130 evaluable patients. Serum S100A8/A9 and S100A12 levels at the baseline visit were not significantly different among the 3 different categories of PF-JIA, between patients taking or not taking MTX nor between the different anti-TNF agents.
Table 1:
Patient characteristics at the baseline study visit
| Characteristic | Evaluable cohort (n=130) | |
|---|---|---|
| Age – mean (standard deviation) | 11.2 (4.5) years | |
| Sex – n (%) | 97 (74.6%) female | |
| Category – n (%) | ||
| Extended oligoarthritis | 18 (13.8%) | |
| Seronegative polyarthritis | 97 (74.6%) | |
| Seropositive polyarthritis | 15 (11.5%) | |
| On methotrexate therapy | 54 (41.5%) | |
| Type of anti-TNF therapy – n (%) | ||
| Adalimumab | 20 (15.4%) | |
| Etanercept | 104 (80.0%) | |
| Infliximab | 6 (4.6%) | |
| Disease duration – mean (standard deviation) | 5.0 (3.6) years | |
| Duration of CID – mean (standard deviation) | 1.2 (1.8) years | |
| Baseline S100 protein level – median (range) | S100A8/A9 [ng/ml] | S100A12 [ng/ml] |
| All patients | 652 (49–3892) | 93 (0–1558) |
| According to category | ||
| Extended oligoarthritis | 817 (158–2070) | 91 (0–890) |
| Seronegative polyarthritis | 610 (49–3892) | 93 (11–1558) |
| Seropositive polyarthritis | 820 (200–1890) | 114 (16–566) |
| According to MTX therapy | ||
| On MTX therapy | 718 (94–3000) | 84 (11–1558) |
| Not on MTX therapy | 617 (49–3892) | 95 (0–1029) |
| According to anti-TNF agent | ||
| Adalimumab | 875 (133–2180) | 115 (14–479) |
| Etanercept | 595 (49–3892) | 90 (0–1558) |
| Infliximab | 900 (180–2070) | 121 (21–890) |
anti-TNF, anti-tumor necrosis factor; CID, clinically inactive disease; MTX, methotrexate
Correlation of serum S100 levels
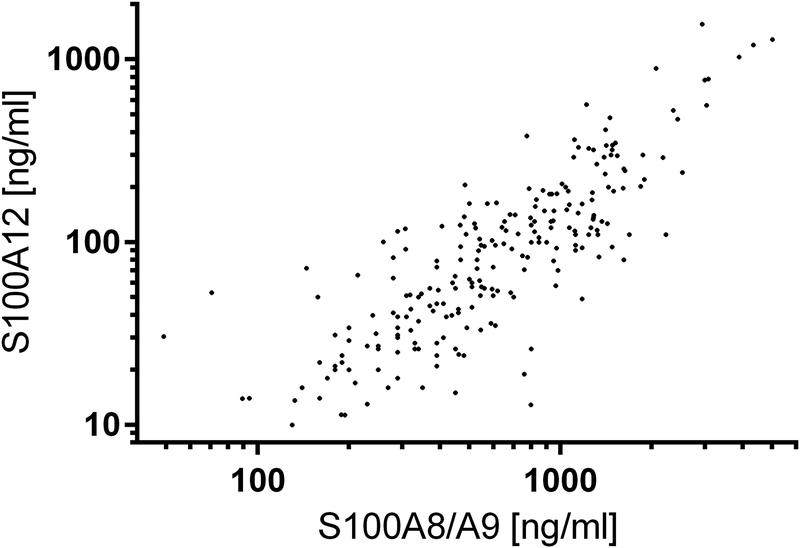
There was only a moderate correlation between S100 protein levels at the baseline visit and the end of the first phase, i.e. immediately prior to discontinuation of TNF blockade even though patients were in CID at both time points (for S100A8/A9: Spearman correlation coefficient of r = 0.36; for S100A12: r = 0.45). There was a very strong correlation between S100A8/A9 and S100A12, taking into account measurements both at baseline and at the end of the first phase (r = 0.82) (Figure 1). There was at most a weak inverse correlation between baseline S100A8/A9 levels and age (r=−0.19), weight (r=−0.17), and duration of CID (r=−0.22) and no correlation with disease duration. There was no significant association between baseline serum S100A12 levels and age, weight, disease duration and duration of CID, respectively.
Figure 1:
Correlation between individual subject’s S100A8/A9 and S100A12 levels both at baseline and at the end of the first phase (Spearman correlation coefficient 0.82).
Serum S100 levels and maintenance of clinical inactive disease on anti-TNF therapy
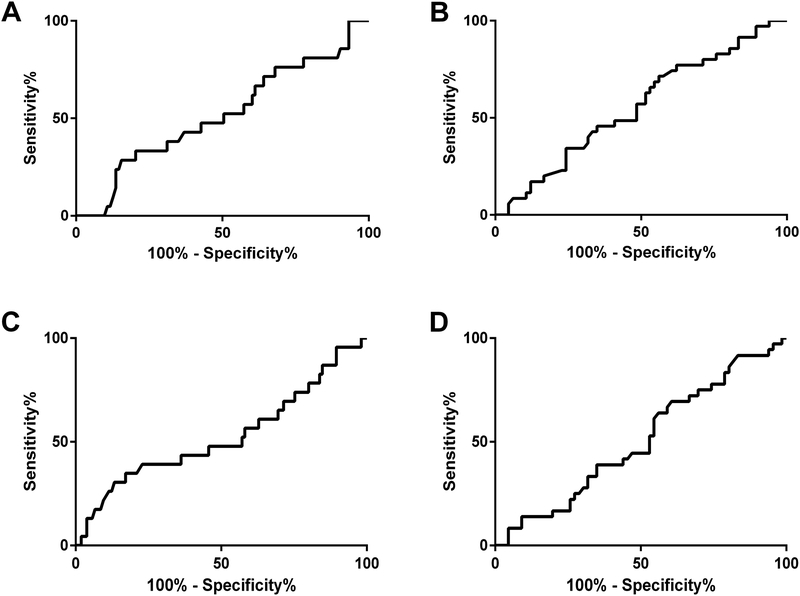
Comparison of S100A8/A9 and S100A12 levels between patients who maintained CID (n=106) and those who did not did (n=24) not reveal significant differences when taking into account the different JIA categories, the specific anti-TNF agent or accompanying MTX therapy (Table 2 and Supplementary Figure 1). ROC curve analysis suggested that neither serum S100A8/A9 nor S100A12 level at baseline predicted maintenance of CID throughout the 6 months of the first phase (for S100A8/A9: AUC 0.52, 95% confidence interval [CI] 0.38–0.65; for S100A12: AUC 0.53, CI 0.38–0.67) (Figure 2A and Figure 2C).
Table 2:
Serum S100A8/A9 and S100A12 levels according to disease course
| First Phase (n=130) | Second Phase (n=106) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CID maintained | CID lost before withdrawal | P value* | No flare within 8 months | Disease flare | P value* | |||||
| S100A8/A9 | n | S100A8/A9 [ng/ml] | n | S100A8/A9 [ng/ml] | n | S100A8/A9 [ng/ml] | n | S100A8/A9 [ng/ml] | ||
| All patients | 106 | 650 (49–3892) | 24 | 654 (200–2070) | 0.82 | 67 | 584 (71–5009) | 39 | 544 (140–2361) | 0.36 |
| Per JIA category | ||||||||||
| Extended oligoarthritis | 17 | 760 (158–1621) | 1 | 2070 (N/A) | N/A | 9 | 800 (130–4339) | 8 | 500 (160–1276) | 0.55 |
| Seronegative polyarthritis | 78 | 614 (49–3892) | 17 | 528 (230–1839) | 0.25 | 50 | 595 (71–5009) | 30 | 505 (140–2361) | 0.29 |
| Seropositive polyarthritis | 8 | 510 (210–1260) | 1 | 1140 (200–1890) | 0.31 | 8 | 430 (180–950) | 1 | 1541 (N/A) | N/A |
| Per anti-TNF agent | ||||||||||
| Adalimumab | 16 | 930 (133–2180) | 4 | 350 (290–1240) | 0.38 | 9 | 490 (280–1633) | 7 | 307 (140–1276) | 0.17 |
| Etanercept | 86 | 571 (49–3892) | 18 | 654 (200–1890) | 0.92 | 55 | 618 (71–5009) | 31 | 560 (145–2361) | 0.75 |
| Infliximab | 4 | 805 (180–1404) | 2 | 2070 (N/A) | N/A | 3 | 460 (290–1070) | 1 | 281 (N/A) | N/A |
| Per MTX therapy | ||||||||||
| On MTX | 41 | 668 (94–3000) | 13 | 832 (250–2070) | 0.63 | 28 | 920 (160–5009) | 13 | 750 (145–1541) | 0.64 |
| Not on MTX | 65 | 620 (49–3892) | 11 | 482 (200–1890) | 0.28 | 39 | 468 (71–2530) | 26 | 440 (140–2361) | 0.57 |
| S100A12 | n | S100A12 [ng/ml] | n | S100A12 [ng/ml] | n | S100A12 [ng/ml] | n | S100A12 [ng/ml] | ||
| All patients | 106 | 93 (0–1558) | 24 | 100 (13–890) | 0.69 | 67 | 92 (14–1558) | 39 | 94 (0–479) | 0.39 |
| Per JIA category | ||||||||||
| Extended oligoarthritis | 17 | 90 (0–339) | 1 | 890 (N/A) | N/A | 9 | 70 (10–1197) | 8 | 56 (14–186) | 0.70 |
| Seronegative polyarthritis | 78 | 93 (11–1558) | 17 | 91 (13–202) | 0.45 | 50 | 92 (14–1283) | 30 | 73 (11–526) | 0.91 |
| Seropositive polyarthritis | 8 | 114 (17–566) | 1 | 121 (16–326) | 0.91 | 8 | 51 (31–183) |
1 | 297 (N/A) | N/A |
| Per anti-TNF agent | ||||||||||
| Adalimumab | 16 | 115 (14–479) | 4 | 115 (16–326) | 0.80 | 9 | 70 (34–300) | 7 | 66 (16–186) | 0.96 |
| Etanercept | 86 | 90 (0–1558) | 18 | 91 (13–320) | 0.73 | 55 | 93 (10–1283) | 31 | 73 (11–526) | 0.92 |
| Infliximab | 4 | 121 (21–236) | 2 | 457 (24–890) | 0.80 | 3 | 41 (34–120) | 1 | 64 (N/A) | N/A |
| Per MTX therapy | ||||||||||
| On MTX | 41 | 84 (11–1558) | 13 | 83 (16–890) | 0.99 | 28 | 110 (18–1283) | 13 | 62 (11–364) | 0.57 |
| Not on MTX | 65 | 94 (0–1029) | 11 | 103 (13–220) | 0.53 | 39 | 52 (10–246) | 26 | 73 (14–526) | 0.38 |
Mann-Whitney test
CID, clinically inactive disease; MTX, methotrexate; N/A, not applicable; TNF, tumor necrosis factor-alpha
Figure 2:
Receiver operating curve (ROC) curve characteristics of serum S100 levels: (A) S100A8/A9 at baseline and the occurrence of loss of clinically inactive disease during six months of anti-TNF continuation (area-under the curve [AUC] 0.52, 95% confidence interval [CI] 0.38–0.65), (B) S100A8/A9 at the time of anti-TNF agent withdrawal and the occurrence of disease flare within eight months following anti-TNF withdrawal (AUC 0.56, CI 0.44–0.67), (C)) S100A12 at baseline and the occurrence of loss of clinically inactive disease during six months of anti-TNF continuation (AUC 0.53, CI 0.38–0.67), (B) S100A12 at the time of anti-TNF agent withdrawal and the occurrence of disease flare within eight months following anti-TNF withdrawal (AUC 0.51, CI 0.39–0.62).
Serum S100 levels and disease flare after anti-TNF withdrawal
Comparison of S100A8/A9 and S100A12 levels between patients who flared (n=39) and those who did not flare (n=67) did not reveal significant differences when taking into account the different JIA categories, the specific anti-TNF agent or accompanying MTX therapy (Table 2 and Supplementary Figure 1). ROC curve analysis of the serum S100 level at the time of anti-TNF withdrawal and its relation to disease flare or no flare during the eight months of the second phase indicated that neither serum S100A8/A9 nor S100A12 levels predicted disease flare (Figure 2B and Figure 2D). ROC analysis of the serum S100A12 levels and its relation to flare within 30, 60 and 90 days after anti-TNF withdrawal indicated an AUC (95% CI) of 0.64 (0.50–0.77), 0.66 (0.54–0.79) and 0.64 (0.51–0.77), indicating, at best, poor prediction of disease flare (Table 3). The same analysis for S100A8A9 did not yield significant results (AUC [95% CI] 0.51 [0.31–0.70], 0.51 [0.33–0.69] and 0.51 [0.34–0.67], respectively). An ideal threshold level for the prediction of flare could not be determined for S100A8/A9 for any time frame since the ROC curve was very close to the line of no-discrimination. For S100A12, the optimal threshold level for prediction of flare at 30, 60, and 90 days was 120 ng/ml (positive likelihood ratio between 1.94 and 1.86).
Table 3:
Diagnostic accuracy of serum S100A8/A9 and S100A12 levels at the time of anti-TNF withdrawal regarding the prediction of flare within 30 days, 60 days, 90 days or 8 months.
| S100A8/A9 (cut-off 690 ng/ml) | S100A12 (cut-off 120 ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| 30 days | 60 days | 90 days | 8 months | 30 days | 60 days | 90 days | 8 months | |
| Sensitivity | 67% | 38% | 40% | 66% | 56% | 54% | 53% | 33% |
| Specificity | 42% | 58% | 58% | 45% | 70% | 70% | 71% | 68% |
| Positive likelihood ratio | 1.16 | 0.91 | 0.95 | 1.20 | 1.85 | 1.84 | 1.86 | 1.05 |
| Youden index | 0.09 | −0.04 | −0.02 | 0.11 | 0.25 | 0.24 | 0.25 | 0.02 |
| Hazard ratio*(95% CI), P | 0.68 (0.18–2.58), 0.57 | 0.83 (0.28–2.52), 0.75 | 0.89 (0.32–2.49), 0.82 | 0.72 (0.36–1.42), 0.34 | 2.83 (0.70; 11.49), 0.15 | 2.63 (0.82; 8.43), 0.10 | 2.59 (0.87; 7.67), 0.09 | 1.13 (0.55; 2.33), 0.74 |
| AUC (95% CI) | 0.51 (0.31; 0.70) | 0.51 (0.33; 0.69) | 0.51 (0.34; 0.67) | 0.56 (0.44; 0.67) | 0.64 (0.50; 0.77) | 0.66 (0.54; 0.79) | 0.64 (0.51; 0.77) | 0.51 (0.39; 0.62) |
hazard ratio for the occurrence of disease flare if level is above threshold
AUC, area under the receiver operating characteristic curve; CI, confidence interval
The Youden index is calculated as follows: sensitivity + specificity - 1
Based on an established threshold level for serum S100A8/A9 of 690 ng/ml for the prediction of flare after MTX withdrawal with the same assay (18), a large proportion of patients had elevated S100A8/A9 levels at baseline (59 of 130 patients [45.4%]) and at the time of anti-TNF withdrawal (43 of 106 patients [40.6%]). Based on a threshold level for serum S100A12 of 120 ng/ml which is both the upper limit of normal in healthy controls in our laboratory (mean plus two standard deviations) and the optimal threshold level based on the ROC analysis (see above), the level was elevated at baseline in 46 of 130 patients [35.4%] and at the time of anti-TNF withdrawal in 35 of 106 patients [33.0%]. MTX background therapy did not affect the distribution of patients with elevated levels, except for the S100A8/A9 levels at the time of anti-TNF withdrawal (elevated in 24 of 41 [58.5%] of patients on MTX compared to 19 of 65 [29.2%] not on MTX; chi square test, p<0.01).
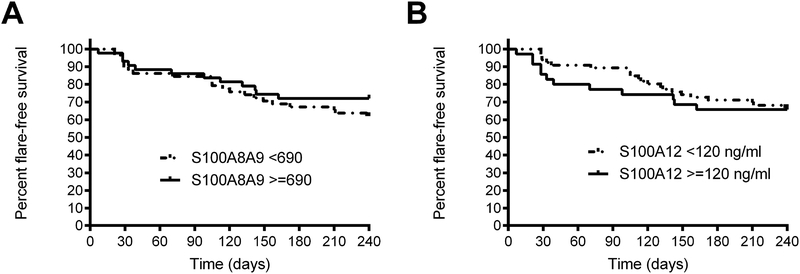
Kaplan-Meier survival analysis comparing disease flare within 8 months, 90 days, 60 days and 30 days after anti-TNF withdrawal did not demonstrate significant differences between patients with elevated vs. normal S100 protein levels (Figure 3).
Figure 3:
Kaplan-Meier survival analysis comparing flare-free survival throughout the eight-month phase following anti-TNF withdrawal according to elevated vs. normal (A) serum S100A8/A9 and (B) serum S100A12 levels at the time of anti-TNF withdrawal (Hazard ratio per logrank test for S100A8/A9 >690 ng/ml 0.72 (95% CI 0.36–1.42) and for S100A12 >120 ng/ml 1.13 [95% CI 0.55–2.33])
There was no correlation between time to disease flare and S100A8/A9 levels at time of anti-TNF withdrawal (Spearman rank coefficient r=−0.16, p=0.35), and moderate inverse correlation for S100A12 (r=−0.36; p=0.04) (Supplementary Table 2).
Discrepancies between serum S100A8/A9 and S100A12 levels
Since there was at least some difference in the prognostic accuracy between S100A8/A9 and S100A12 levels, we investigated discrepant values. Among the 39 patients who developed a disease flare following anti-TNF withdrawal, 4 patients (10.3%) had discrepant values (2 had an elevated S100A8/A9 and a normal S100A12, and 2 had a normal S100A8/A9 and an elevated S100A12). Among the 67 patients without disease flare following anti-TNF withdrawal, 13 patients (19.4%) had discrepant values (11 had an elevated S100A8/A9 and a normal S100A12, and two had a normal S100A8/A9 and an elevated S100A12).
DISCUSSION
In this prospective study of patients with PF-JIA and CID, serum S100 levels obtained while on anti-TNF therapy predicted neither maintenance of CID nor the occurrence of flare during an 8-month anti-TNF withdrawal period.
These findings are in contrast with previously published data. In another large-scale, prospective treatment withdrawal study of 364 patients with JIA in CID on MTX therapy, S100A8/A9 levels were measured in 188 patients and elevated S100A8/A9 levels, identified by post hoc analysis, were predictive of disease flare within 12 months of observation (hazard ratio 2.24, 95% CI 1.39–3.62) (17). Samples from the same study were re-analyzed to demonstrate that S100A12 had an even better predictive value in that cohort (hazard ratio 2.81, 95% CI 1.70–4.65) (18). In a retrospective, exploratory analysis of samples of patients with JIA in CID on ETN therapy within 2 separate registries, elevated S100A8A9 levels, again identified by post hoc analysis, at the time of ETN withdrawal indicated an increased risk of disease flare within six months (positive likelihood ratio 3.5, ROC 0.75 with 95% CI 0.55–0.95) (16). In these studies, findings were derived post hoc, subjecting them to bias from de facto multiple hypothesis testing which may in part account for the divergence in cut-off values across studies. Currently, these is a trial prospectively studying antirheumatic drug withdrawal based on biomarker levels (ISRCTN69963079).
Several aspects need to be considered, including substantial differences between those previous studies and this study, and factors affecting sensitivity, specificity or both. For example, the primary outcome in another study was loss of CID (rather than disease flare by the more stringent flare criteria used in this study), and sampling of S100 protein levels was incomplete in that study (17, 18). Furthermore, a retrospective study on anti-TNF withdrawal reported a much higher rate of disease flare (54% in an 8-month period) but this was a small study and, thus, selection bias may have occurred (16). An additional difference between our study and previous studies is that previous studies addressed patients who had all anti-rheumatic drugs withdrawn, whereas in our study only the anti-TNF therapy was discontinued. Of note, concomitant MTX therapy did not affect flare rate or S100 protein levels in this study.
Sensitivity of the biomarkers may be affected by several issues. The disease in patients with PF-JIA in CID on anti-TNF therapy may be intrinsically more unstable than PF-JIA in CID on MTX therapy. This may also be indicated by others’ findings on a high risk of disease relapse after discontinuation of anti-TNF therapy vs. discontinuation of MTX therapy (11). In fact, it appears that serum S100A12 levels were perhaps slightly better at predicting flare within 30, 60 or 90 days of anti-TNF withdrawal than over the entire 8 months of the second phase in this study; however, this effect was not seen for S100A8/A9. However, the study is underpowered to determine whether elevated serum S100A12 levels may predict a higher risk of flare within this time frame (due to the rare number of events). Furthermore, S100 protein kinetics may play a role. While the precise elimination half-life of S100A12 is unknown, it is presumably rather short (in the order of hours rather than days), similar to the half-life of the related S100A8/A9 proteins (26, 27). In addition, TNF is a known stimulus for S100A8/A9 and S100A12 secretion by inflammatory cells and anti-TNF therapy down-regulates S100 protein expression (27, 28). Anti-TNF agents are typically applied in intervals that range from 1 week (ETN), to 2 weeks (ADA) and to 8 weeks (IFX), and the half-lives range from 3 days (for the fusion molecule ETN) to 10–20 days (for the monoclonal antibodies ADA and IFX) (29–31). Furthermore, mathematic modeling indicates that free TNF-alpha levels drop rapidly (within hours) following administration of anti-TNF agents (32). Therefore, the timing of serum sampling in regards to anti-TNF application may be critical when assessing serum S100 protein levels. We are unable to account for this factor since the dates of the anti-TNF administration were not recorded during this study.
Finally, the specificity of the biomarkers may be limited as well. Other conditions, especially infections, may affect serum S100 protein levels substantially, even in minor infections which occur frequently in children (33–35). The source of elevated serum S100 protein levels therefore is difficult to determine individually and may be prone to misinterpretation, issues that are difficult to overcome.
Slight differences in diagnostic accuracy between S100A8/A9 levels and S100A12 levels may exist because S100A8/A9 is predominantly produced by monocytes whereas S100A12 is mainly produced by neutrophils (36). One may speculate that TNF differentially affects monocytes and neutrophils (37), thus affecting the pattern of S100 protein secretion during anti-TNF therapy, more so than during MTX therapy (which may target lymphocytes more prominently), for example (38).
Furthermore, we cannot exclude that technical issues may contribute to the marked variability and lack of predictive value. For this study, we have used an in-house ELISA using polyclonal S100A8/A9 and S100A12 antibodies. In the meantime, we have developed a more robust assay using monoclonal S100A12 antibody which is being used for current studies (unpublished data). Of note, several (including commercial) assays exist both for S100A8/A9 and for S100A12; while each of these assays may deliver consistent results, absolute reported values differ substantially (39). Therefore, caution should be applied when interpreting S100A8/A9 or S100A12 levels.
It may be speculated that further studies on the concept of subclinical arthritis and its relation to maintenance of CID and disease flare should account for these issues and incorporate, for example, standardized sampling conditions (as it relates to anti-TNF dosing), excluding sampling at the time of obvious infection, and incorporate further measures, such as imaging studies to assess for evidence of joint-related subclinical inflammation, e.g. joint ultrasound (40, 41). In the long-term, the goal will be to guide clinicians’ decisions on when or when not to withdraw anti-rheumatic therapies in individual patients. The utility of a biomarker-supported withdrawal strategy is currently being tested in a prospective clinical trial (ISRCTN69963079) (18). Furthermore, more studies are needed to identify potential novel biomarkers of subclinical active disease.
In summary, analysis of the serum biomarkers S100A8/A9 and S100A12 in a prospective cohort study of patients with PF-JIA who were closely monitored in CID and had anti-TNF therapy withdrawn, indicated that S100 protein levels did not predict maintenance of CID or the occurrence of disease flare over an 8-month period following the withdrawal. Further studies on the concept of subclinical inflammation in JIA should possibly include more stringent handling of the biomarker sampling and possibly also include imaging studies.
Supplementary Material
Supplementary figure 1: Patient-level data from the onset of the first phase are comparing patients receiving tumor necrosis factor inhibition who maintained clinical inactive disease (CID) with those who did not for (A) S100A8/A9 and for (B) S100A12. Furthermore, data are shown comparing patient-level data between patients who flared during the second phase (after withdrawal of tumor necrosis factor inhibition) compared to those who did not for (C) S100A8/A9 and (D) S100A12. The dashed grid lines represent the cut-off for healthy normal controls established in our laboratory.
Supplementary figure 2: (A) Time to flare according to S100A8/A9 levels at the beginning of the second phase, and (B) time to flare according to S100A12 levels at the beginning of phase 2.
Acknowledgments
GRANTS, FINANCIAL SUPPORT:
This work was sponsored by the NIH (NIAMS, Grant No. 2P60AR047784–06A2). The grant paid for all aspects of the study and supported the work of the Data Safety and Monitoring Board.
INVESTIGATOR DISCLOSURES: Dr. Hinze has received consultancies, speaking fees, or honoraria (less than $10,000 each) from Novartis. Dr. Foell has received consultancies, speaking fees, or honoraria (less than $10,000 each) from Chugai Pharma/Roche, Novartis and Pfizer. Dr. Gottlieb has received consultancies (less than $10,000 each) from Medac Pharmaceutical. Dr. Kimura has received consultancies, speaking fees, or honoraria (less than $10,000 each) from Novartis and Sobi. Dr. Grom has received consultancies, speaking fees, or honoraria (less than $10,000 each) from Novartis. Dr. Shishov has received honoraria (less than $10,000 each) from Novartis. Dr. Dare has received research grants from AbbVie, AstraZeneca, Bristol-Myers Squibb, Horizon Pharma, Medac, Pfizer, Roche and UCB. Dr. Ede has received consultancies, speaking fees, or honoraria (less than $10,000 each) from AbbVie and Novartis. Dr. Beukelman has received consultancies (less than $10,000 each) from Novartis and UCB. Dr. Lovell has received consultancies, speaking fees, or honoraria (less than $10,000 each) from Abbott, AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Forest Research, GSK, Hoffmann-La Roche, Janssen, Novartis, Pfizer, Roche, Takeda, UBC and Wyeth Pharma.
CLINICALTRIALS.GOV IDENTIFIER: NCT 00792233
REFERENCES
- 1.Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Annals of the rheumatic diseases. 2015;74(10):1854–60. [DOI] [PubMed] [Google Scholar]
- 2.Klotsche J, Raab A, Niewerth M, Sengler C, Ganser G, Kallinich T, et al. Outcome and Trends in Treatment of Systemic Juvenile Idiopathic Arthritis in the German National Pediatric Rheumatologic Database, 2000–2013. Arthritis Rheumatol. 2016;68(12):3023–34. [DOI] [PubMed] [Google Scholar]
- 3.Ruperto N, Lovell DJ, Cuttica R, Wilkinson N, Woo P, Espada G, et al. A randomized, placebo-controlled trial of infliximab plus methotrexate for the treatment of polyarticular-course juvenile rheumatoid arthritis. Arthritis and rheumatism. 2007;56(9):3096–106. [DOI] [PubMed] [Google Scholar]
- 4.Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. The New England journal of medicine. 2008;359(8):810–20. [DOI] [PubMed] [Google Scholar]
- 5.Lovell DJ, Reiff A, Ilowite NT, Wallace CA, Chon Y, Lin SL, et al. Safety and efficacy of up to eight years of continuous etanercept therapy in patients with juvenile rheumatoid arthritis. Arthritis and rheumatism. 2008;58(5):1496–504. [DOI] [PubMed] [Google Scholar]
- 6.Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. The New England journal of medicine. 2000;342(11):763–9. [DOI] [PubMed] [Google Scholar]
- 7.Giannini EH, Ilowite NT, Lovell DJ, Wallace CA, Rabinovich CE, Reiff A, et al. Effects of long-term etanercept treatment on growth in children with selected categories of juvenile idiopathic arthritis. Arthritis and rheumatism. 2010;62(11):3259–64. [DOI] [PubMed] [Google Scholar]
- 8.Beukelman T, Ringold S, Davis TE, DeWitt EM, Pelajo CF, Weiss PF, et al. Disease-modifying antirheumatic drug use in the treatment of juvenile idiopathic arthritis: a cross-sectional analysis of the CARRA Registry. The Journal of rheumatology. 2012;39(9):1867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett HF, Regier DA, Feldman BM, Miller FA, Ungar WJ. Parents’ preferences for drug treatments in juvenile idiopathic arthritis: a discrete choice experiment. Arthritis care & research. 2012;64(9):1382–91. [DOI] [PubMed] [Google Scholar]
- 10.Ungar WJ, Costa V, Hancock-Howard R, Feldman BM, Laxer RM. Cost-effectiveness of biologics in polyarticular-course juvenile idiopathic arthritis patients unresponsive to disease-modifying antirheumatic drugs. Arthritis Care Res (Hoboken). 2011;63(1):111–9. [DOI] [PubMed] [Google Scholar]
- 11.Chang CY, Meyer RM, Reiff AO. Impact of medication withdrawal method on flare-free survival in patients with juvenile idiopathic arthritis on combination therapy. Arthritis care & research. 2015;67(5):658–66. [DOI] [PubMed] [Google Scholar]
- 12.Vogl T, Propper C, Hartmann M, Strey A, Strupat K, van den Bos C, et al. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. The Journal of biological chemistry. 1999;274(36):25291–6. [DOI] [PubMed] [Google Scholar]
- 13.Holzinger D, Foll D. [Biomarkers for chronic inflammatory diseases]. Z Rheumatol. 2015;74(10):887–96; quiz 97. [DOI] [PubMed] [Google Scholar]
- 14.Lavric M, Miranda-Garcia MA, Holzinger D, Foell D, Wittkowski H. Alarmins firing arthritis: Helpful diagnostic tools and promising therapeutic targets. Joint Bone Spine. 2016. [DOI] [PubMed] [Google Scholar]
- 15.Hinze C, Gohar F, Foell D. Management of juvenile idiopathic arthritis: hitting the target. Nat Rev Rheumatol. 2015;11(5):290–300. [DOI] [PubMed] [Google Scholar]
- 16.Anink J, Van Suijlekom-Smit LW, Otten MH, Prince FH, van Rossum MA, Dolman KM, et al. MRP8/14 serum levels as a predictor of response to starting and stopping anti-TNF treatment in juvenile idiopathic arthritis. Arthritis Res Ther. 2015;17:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foell D, Wulffraat N, Wedderburn LR, Wittkowski H, Frosch M, Gerss J, et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA. 2010;303(13):1266–73. [DOI] [PubMed] [Google Scholar]
- 18.Gerss J, Roth J, Holzinger D, Ruperto N, Wittkowski H, Frosch M, et al. Phagocyte-specific S100 proteins and high-sensitivity C reactive protein as biomarkers for a risk-adapted treatment to maintain remission in juvenile idiopathic arthritis: a comparative study. Ann Rheum Dis. 2012;71(12):1991–7. [DOI] [PubMed] [Google Scholar]
- 19.Lovell DJ, Johnson AL, Huang B, Gottlieb BS, Morris PW, Kimura Y, et al. Risk, Timing and Predictors of Disease Flare after Discontinuation of Anti-Tumor Necrosis Factor (TNF) Therapy in Children with Polyarticular Forms of Juvenile Idiopathic Arthritis (JIA) in Clinical Inactive Disease. Arthritis Rheumatol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N, Childhood Arthritis Rheumatology Research A, et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis care & research. 2011;63(7):929–36. [DOI] [PubMed] [Google Scholar]
- 21.Brunner HI, Lovell DJ, Finck BK, Giannini EH. Preliminary definition of disease flare in juvenile rheumatoid arthritis. The Journal of rheumatology. 2002;29(5):1058–64. [PubMed] [Google Scholar]
- 22.Lovell DJ, Johnson A, Kimura Y, Spalding SJ, Morris PW, Gottlieb BS, et al. A20: Understanding the Use and Biology of TNF Therapy in JIA—Clinical Outcomes. Arthritis & Rheumatology. 2014;66:S31–S2. [Google Scholar]
- 23.Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52(6):847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frosch M, Strey A, Vogl T, Wulffraat NM, Kuis W, Sunderkotter C, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43(3):628–37. [DOI] [PubMed] [Google Scholar]
- 25.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 26.van Zoelen MA, Vogl T, Foell D, Van Veen SQ, van Till JW, Florquin S, et al. Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. American journal of respiratory and critical care medicine. 2009;180(11):1098–106. [DOI] [PubMed] [Google Scholar]
- 27.Foell D, Wittkowski H, Kessel C, Luken A, Weinhage T, Varga G, et al. Proinflammatory S100A12 can activate human monocytes via Toll-like receptor 4. Am J Respir Crit Care Med. 2013;187(12):1324–34. [DOI] [PubMed] [Google Scholar]
- 28.Xu K, Geczy CL. IFN-gamma and TNF regulate macrophage expression of the chemotactic S100 protein S100A8. Journal of immunology. 2000;164(9):4916–23. [DOI] [PubMed] [Google Scholar]
- 29.Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000;34(2):161–4. [DOI] [PubMed] [Google Scholar]
- 30.den Broeder A, van de Putte L, Rau R, Schattenkirchner M, Van Riel P, Sander O, et al. A single dose, placebo controlled study of the fully human anti-tumor necrosis factor-alpha antibody adalimumab (D2E7) in patients with rheumatoid arthritis. J Rheumatol. 2002;29(11):2288–98. [PubMed] [Google Scholar]
- 31.Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet. 2007;46(8):645–60. [DOI] [PubMed] [Google Scholar]
- 32.Jit M, Henderson B, Stevens M, Seymour RM. TNF-alpha neutralization in cytokine-driven diseases: a mathematical model to account for therapeutic success in rheumatoid arthritis but therapeutic failure in systemic inflammatory response syndrome. Rheumatology (Oxford). 2005;44(3):323–31. [DOI] [PubMed] [Google Scholar]
- 33.Wittkowski H, Frosch M, Wulffraat N, Goldbach-Mansky R, Kallinich T, Kuemmerle-Deschner J, et al. S100A12 is a novel molecular marker differentiating systemic-onset juvenile idiopathic arthritis from other causes of fever of unknown origin. Arthritis Rheum. 2008;58(12):3924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu K, Pichichero ME. Clinical significance of serum S100A12 in acute otitis media in young children. Pediatr Infect Dis J. 2012;31(3):e56–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gruber C, Keil T, Kulig M, Roll S, Wahn U, Wahn V, et al. History of respiratory infections in the first 12 yr among children from a birth cohort. Pediatr Allergy Immunol. 2008;19(6):505–12. [DOI] [PubMed] [Google Scholar]
- 36.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81(1):28–37. [DOI] [PubMed] [Google Scholar]
- 37.Hubl W, Wolfbauer G, Streicher J, Andert S, Stanek G, Fitzal S, et al. Differential expression of tumor necrosis factor receptor subtypes on leukocytes in systemic inflammatory response syndrome. Critical care medicine. 1999;27(2):319–24. [DOI] [PubMed] [Google Scholar]
- 38.Cronstein BN. Molecular therapeutics. Methotrexate and its mechanism of action. Arthritis Rheum. 1996;39(12):1951–60. [DOI] [PubMed] [Google Scholar]
- 39.Rothmund F, Gerss J, Ruperto N, Dabritz J, Wittkowski H, Frosch M, et al. Validation of relapse risk biomarkers for routine use in patients with juvenile idiopathic arthritis. Arthritis care & research. 2014;66(6):949–55. [DOI] [PubMed] [Google Scholar]
- 40.Magni-Manzoni S, Epis O, Ravelli A, Klersy C, Veisconti C, Lanni S, et al. Comparison of clinical versus ultrasound-determined synovitis in juvenile idiopathic arthritis. Arthritis Rheum. 2009;61(11):1497–504. [DOI] [PubMed] [Google Scholar]
- 41.Haslam KE, McCann LJ, Wyatt S, Wakefield RJ. The detection of subclinical synovitis by ultrasound in oligoarticular juvenile idiopathic arthritis: a pilot study. Rheumatology (Oxford). 2010;49(1):123–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: Patient-level data from the onset of the first phase are comparing patients receiving tumor necrosis factor inhibition who maintained clinical inactive disease (CID) with those who did not for (A) S100A8/A9 and for (B) S100A12. Furthermore, data are shown comparing patient-level data between patients who flared during the second phase (after withdrawal of tumor necrosis factor inhibition) compared to those who did not for (C) S100A8/A9 and (D) S100A12. The dashed grid lines represent the cut-off for healthy normal controls established in our laboratory.
Supplementary figure 2: (A) Time to flare according to S100A8/A9 levels at the beginning of the second phase, and (B) time to flare according to S100A12 levels at the beginning of phase 2.