Abstract
Tissue culture is very important for identifying the gene function of Camellia sinensis (L.) and exploiting novel germplasm through transgenic technology. Regeneration system of tea plant has been explored but not been well established since the molecular mechanism of tea plant regeneration is not clear yet. In this study, transcriptomic analysis was performed in the initial explants of tea plant and their dedifferentiated and redifferentiated tissues. A total of 93,607 unigenes were obtained through de novo assembly, and 7,193 differentially expressed genes (DEGs) were screened out from the 42,417 annotated unigenes. Much more DEGs were observed during phase transition rather than at growth stages of callus. Our KOG and KEGG analysis, and qPCR results confirmed that phase transition of tea plant was closely related to the mechanism that regulate expression of genes encoding the auxin- and cytokinin-responsive proteins, transcription factor MYB15 and ethylene-responsive transcription factor ERF RAP2-12. These findings provide a reliable foundation for elucidating the mechanism of the phase transition and may help to optimize the regeneration system by regulating the gene expression pattern.
Introduction
Tea plant (Camellia sinensis (L.) O. Kuntzes) is one of the most important woody crops worldwide and getting popular because of the health benefit effect of the non-alcoholic beverage made from its tender shoot. It is very difficult to elucidate the genetic bases of the main economic traits and shorten the time for cultivar improvement since self-incompatibility and long cycle of seed to seed of the tea plant. Transgenetic technology is a useful tool to understand genetic mechanisms of the traits and accelerate innovation of the germplasms. Unfortunately, there are many obstacles in application of transgenic technologies in understanding development mechanism of the tea plant such as low transformation frequency and absence of a robust plant regeneration system. Calli, buds and embryoids have been successfully induced from the cotyledons1, cotyledon petioles, hypocotyls, immature embryos, axillary buds and immature leaves2, as well as, from stems and anthers3. Although plantlets had also been regenerated through organogenesis and somatic embryogenesis, a significant difference in regeneration frequency was observed from various explants4, and very low frequency was usually witnessed during induction of many explants. Studies on mechanism of dedifferentiation and redifferentiation during tissue culture may help to optimize high-frequency regeneration system of the tea plant.
Phase transition, a term widely used in plant development and cell cycle, has also been extendedly used in tissue culture and micropropagation to express the change among the stage of explants, dedifferentiated and redifferentiated tissues5–8. Phase transition during tissue culture is remarkably influenced by many factors, especially by plant hormones. Auxins and cytokinins (CKs), as important plant hormones, play crucial roles in DNA duplication, metabolism of nucleic acids, synthesis of various proteins, as well as mitosis and cytokinesis9,10. In addition to being considered as the essential elements for plant tissue culture, auxins and CKs have been used for the induction of calli and plantlet regeneration. Many studies in model plants revealed that hormones influence several levels of regulation, such as signal transduction and gene expression regulation. During root induction of Arabidopsis by auxin, the signal could be transmitted through interactions among exogenous hormone and the key regulators ARF6 (AUXIN RESPONSE FACTOR 6) and ARF8 (AUXIN RESPONSE FACTOR 8)11–13, and WOX11/12 (WUSCHEL-RELATED HOMEOBOX 11 and 12) which were up-regulated by the hormone from competent cells to root founder cells14. However, considering tea plant regeneration system, most studies mainly focused on optimization of the culture formulae and conditions, whilst few have been carried out to elucidate the regulatory effect of auxins and CKs on the dedifferentiation and redifferentiation of tea plant at molecular level.
Transcriptome analysis can reveal the presence and quantity of mRNA in a biological sample at a given moment and provide the profile of gene expression under a certain condition through ribonucleic acid sequencing (RNA-Seq) technology, providing an important way for mapping metabolic pathways and distinguishing functional genes15. This technology has the advantages of high throughput, low cost, high sensitivity and wide application to the species with unknown genomic sequence. Transcriptome analysis has been applied in tea plant for revealing key genes involved in response against stress, such as drought16, cold17, aluminum toxicity18 and disease19, as well as mapping the metabolic pathways including theanine biosynthesis20, ascorbic acid biosynthesis21 and nitrogen assimilation22. In the present study, difference of gene expression pattern in the induced primary calli, calli as well as redifferentiated roots was investigated during tissue culture of tea plant, and the cell division and redifferentiation-related regulating pathways triggered by auxins and CKs were also discussed.
Results
Morphological change during phase transition
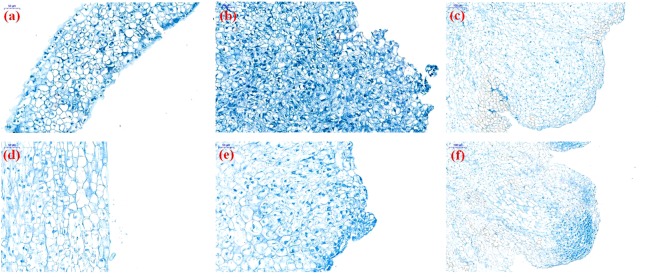
When the stem and leaf were incubated on the callus inducing medium, the cells around the wound began to become competent; many white tiny cell clusters, the primary calli, could be seen through naked eye after incubation for 10–12 days, and rapidly divided cells with relative big nucleus could be easily observed through optical microscope at this stage (Fig. 1a,d). One week later, massive cell clusters appeared around explant23,24 and calli formed (Fig. 1b,e). After the callus was incubated on root induced medium for more than 15 days, the root primordium began to appear, and the vascular connection was established between callus and the root primordium (Fig. 1c,f)25–27.
Figure 1.
Microscopic observation of leaf- and stem- derived tissue culture samples. (a) stem-derived primary callus, (b) stem-derived callus, and (c) longitudinal section of regenerated root from stem-derived callus; (d) leaf-derived primary callus, (e) leaf-derived callus, and (f) longitudinal section of regenerated root from leaf-derived callus.
De novo assembly and functional annotation
Transcripts of the explants (leaf and stem), primary calli, calli and redifferentiated roots were sequenced on Illumina Hiseq 2500 platform and average of the obtained clean data for each sample exceeded 2GB. A total of 93,607 unigenes was de novo assembled from the transcription data of these samples, with 784 bp in average length and 1,018 bp in N50 length; and 19,380 unigenes had a length of above 1000 bp, accounting for 20.60% of the total sequence number (Table 1). All the unigenes were compared with the reference sequences in the Nr, Swiss-Prot, KEGG, COG, KOG, GO and Pfam databases. 51,190 unigenes (54.7%) did not show significant similarity with known genes, while 42,417 unigenes (45.3%) were annotated at least in one database, of which 16,120 sequences were above 1000 bp in length (Table 2).
Table 1.
The obtained unigene library of C. sinensis cultivar Jinxuan.
| Length range | Total number | Percentage (%) |
|---|---|---|
| 0–300 | — | — |
| 300–500 | 47,544 | 50.79 |
| 500–1000 | 26,783 | 28.61 |
| 1000–2000 | 12,844 | 13.72 |
| 2000+ | 6,436 | 6.88 |
| Total number | 93,607 | / |
| Total length | 73,398,653 | / |
| N50 length | 1,018 | / |
| Mean length | 784 | / |
Table 2.
Summary of the unigene annotation in different databases.
| Database | Annotated number | 300 ≤ length < 1000 | length ≥ 1000 |
|---|---|---|---|
| COG | 11,881 | 5,944 | 5,937 |
| GO | 23,477 | 13,938 | 9,539 |
| KEGG | 15,076 | 9,252 | 5,824 |
| KOG | 25,132 | 15,300 | 9,832 |
| Pfam | 28,279 | 14,668 | 13,611 |
| Swissprot | 25,852 | 14,578 | 11,274 |
| Nr | 40,131 | 24,215 | 15,916 |
| All | 42,417 | 26,297 | 16,120 |
Differentially expressed genes during phase transition
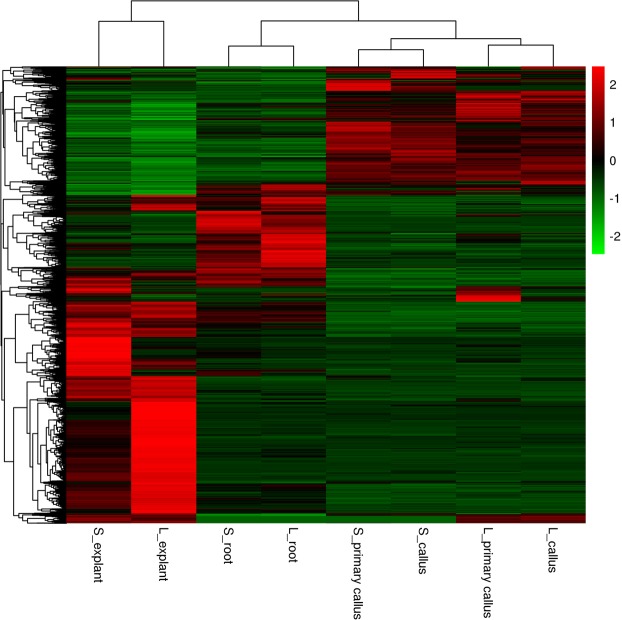
Analysis showed that 7,193 differentially expressed genes (DEGs) were obtained after comparison of the expression level between these samples according to the threshold of q value < 0.005 & |log2 (fold change)| > 1. Although the calli were derived from different explants, these tissues were clustered into a branch according to the gene expression behavior; similarly, the redifferentiated roots derived from stem and leaf were also clustered together (Fig. 2). More than 3,000 DEGs were observed during the phase change from stem explant to stem-derived primary callus (3,014 DEGs) and from leaf explant to leaf-derived primary callus (3,495 DEGs), respectively (Fig. 3). Among them, much more genes were down-regulated (2,133 down vs. 881 up in stem-derived tissues and 2,574 down vs. 921 up in leaf-derived tissues) during these phase changes. Similarly, a large number of DEGs were also found during the phase transition from stem-derived callus to its regenerated root (2,610) and from leaf-derived callus to its regenerated root (2,744), however, more genes were up-regulated (723 down vs. 1,887 up in stem-derived tissues and 937 down vs. 1,807 up in leaf-derived tissues) during root redifferentiation. Meanwhile, relatively fewer DEGs were found during the growth stage from stem-derived primary callus to stem-derived callus (100 down vs. 101 up) and from leaf-derived primary callus to leaf-derived callus (227 down vs. 200 up). It was clear that much more genes were required to change their expression patterns during the phase transition such as dedifferentiation and redifferentiation, while fewer genes changed their expressions at various growth periods. Similar change trends of up- and down-regulated DEGs were obtained from different initial explants (stem and leaf) during dedifferentiation and redifferentiation, indicating that the various phases or statuses of tissue culture could be determined by a set of DEGs and epigenetic variation of these genes might profoundly impact on the fate of the regenerated cells. Interestingly, the number of the down- and up-regulated genes changed reversely during dedifferentiation and redifferentiation, indicating differentiated cells required to activate much more genes for exerting special function of the cells.
Figure 2.
The heat-map of 7,193 DEGs shared in the 8 samples, based on Z-score normalized FPKM values in eight internode segments. S and L represented the stem and leaf. The callus was induced from the stem and leaf on the MS medium supplemented with 2,4-D (2.0 mg/L) and BAP (0.4 mg/L), the root was regenerated from the stem and leaf derived callus on 1/2 MS medium supplemented with NAA (1.5 mg/L).
Figure 3.
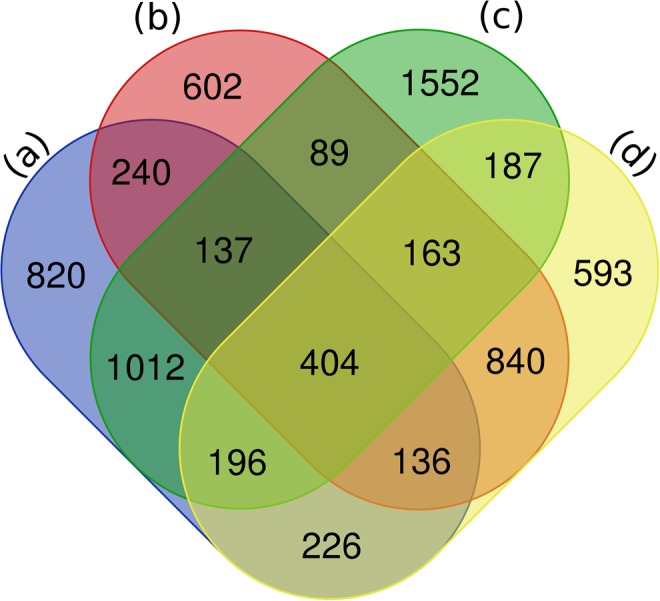
Venn diagram showed the number of DEGs observed between different samples. (a) DEGs between the stem explant and the callus, (b) DEGs between the stem derived callus and the regenerated root, (c) DEGs between the leaf explant and the callus, (d) DEGs between leaf derived callus and the regenerated root.
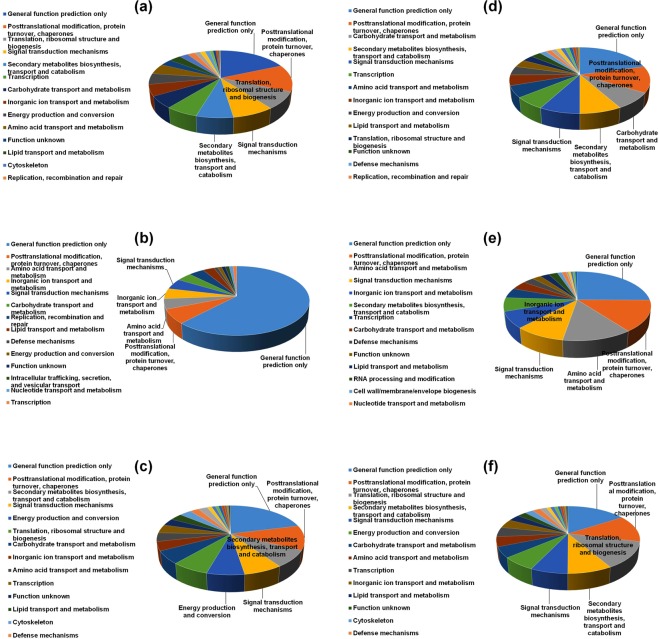
KOG classification of the DEGs
KOG analysis showed that around 80% DEGs were mainly categorized into the classes of the “general function prediction only”, “posttranslational modification, protein turnover, chaperones”, “signal transduction mechanisms”, “secondary metabolites biosynthesis, transport and catabolism”, “carbohydrate transport and metabolism”, “transcription”, “inorganic ion transport and metabolism”, “energy production and conversion”, “amino acid transport and metabolism”, and “translation, ribosomal structure and biogenesis”, although number of the DEGs among the samples with various tissue culture status was different in these classes (Fig. 4), indicating that the expression change of the genes associated with these classes might be very important for phase transition during tissue culture since levels in the secondary metabolites, proteins, carbohydrates, inorganic ions, energetic metabolism and amino acids are likely quite different between differentiated tissues (such as leaf, stem and root) and dedifferentiated ones.
Figure 4.
KOG annotation of the DEGs. (a) stem explant vs. stem-derived primary callus, (b) stem-derived primary callus vs. stem-derived callus, and (c) stem-derived callus vs. regenerated root; (d) leaf explants vs. leaf-derived primary callus, (e) leaf-derived primary callus vs. leaf-derived callus, and (f) leaf-derived callus vs. regenerated root.
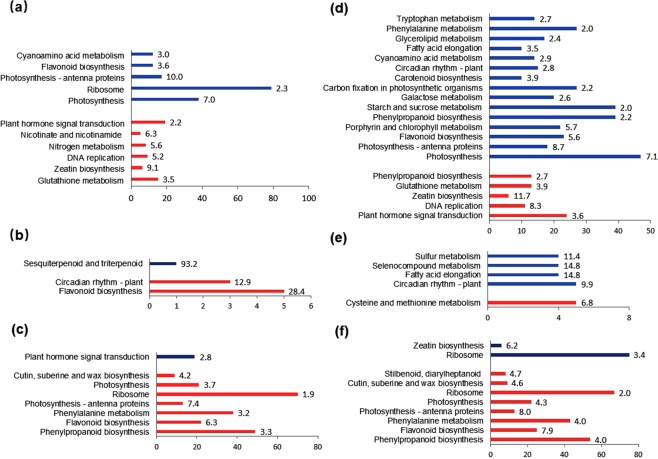
KEGG pathway enrichment of the DEGs
When the primary callus was formed from the stem explant, the up-regulated DEGs significantly enriched in the KEGG item of “glutathione metabolism”, “zeatin biosynthesis”, “DNA replication”, “nitrogen metabolism”, “nicotinate and nicotinamide metabolism”, and “plant hormone signal transduction”; while the down-regulated DEGs did in the item of “photosynthesis”, “ribosome”, “photosynthesis-antenna proteins”, “flavonoid biosynthesis”, and “cyanoamino acid metabolism” (Fig. 5). When the root was regenerated from the stem-derived callus, the enriched items of the up-regulated DEGs were “phenylpropanoid biosynthesis”, “flavonoid biosynthesis”, “phenylalanine metabolism”, “photosynthesis-antenna proteins”, “ribosome”, “photosynthesis”, as well as “cutin, suberine and wax biosynthesis”; the significantly enriched item of the down-regulated DEGs was “plant hormone signal transduction”. During the phase transition from leaf explant to primary callus, the pathway of “plant hormone signal transduction”, “DNA replication”, “zeatin biosynthesis”, and “glutathione metabolism” was significantly up-regulated, while pathway of “photosynthesis”, “photosynthesis-antenna proteins”, “flavonoid biosynthesis”, “porphyrin and chlorophyll metabolism”, “starch and sucrose metabolism”, “galactose metabolism”, and “carbon fixation in photosynthetic organisms” was significantly down-regulated, besides the remarkable change of the “phenylpropanoid biosynthesis”. Compared with the leaf-derived callus, the pathway of “phenylpropanoid biosynthesis”, “flavonoid biosynthesis”, “phenylalanine metabolism”, “photosynthesis - antenna proteins”, “photosynthesis”, “cutin, suberine and wax biosynthesis”, as well as “stilbenoid, diarylheptanoid and gingerol biosynthesis” was up-regulated in the regenerated root, while the pathway of “zeatin biosynthesis” was down-regulated significantly, besides remarkable change of the pathway of “ribosome”. It was apparent that up-regulation of “plant hormone signal transduction”, “zeatin biosynthesis”, “DNA replication” and “glutathione metabolism” pathways, as well as down-regulation of the photosynthesis- and secondary metabolism- related pathways were necessary for dedifferentiation of the explants although change of pathways might also be influenced by the various initial explants28–32. Meanwhile, root redifferentitation requires the slowdown of cell division through deactivating the signal transduction pathway, and activating the pathway related to biosynthesis of the cutin, suberine, wax, and phenylpropanoid33. Plastid might also be rebuilt in the rededifferentiated cell through up-regulating the photosynthesis-related pathways.
Figure 5.
KEGG pathway analysis of the DEGs. (a) Stem explant vs. stem-derived primary callus, (b) stem-derived primary callus vs. stem-derived callus, and (c) stem-derived callus vs. regenerated root; (d) leaf explants vs. leaf-derived primary callus, (e) leaf-derived primary callus vs. leaf-derived callus, and (f) leaf-derived callus vs. regenerated root. Blue and red bar indicated the enriched gene number of the KEGG pathway in down- and up-regulated DEGs respectively; the number in front of the bar indicated the enrich factor of the KEGG pathway in down- and up-regulated DEGs (q < 0.1).
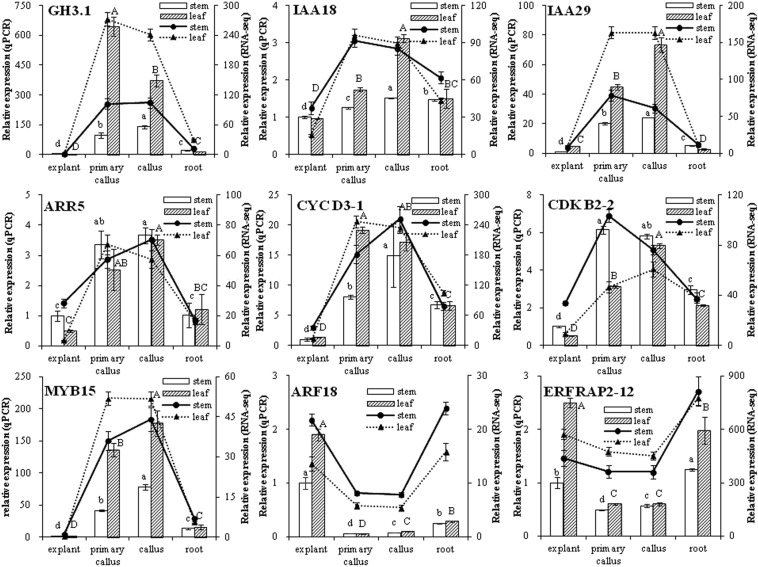
Expression verification of auxins and CKs regulation-related genes
As the pathway related with plant hormone signal transduction was significantly fluctuated during dedifferentiation and redifferentiation, nine DEGs, being considered to be closely related to regulation of the auxins and CKs during phase transition, were screened out (Table 3), namely indole-3-acetic acid-amido synthetase GH3.1 (GH3.1, GenBank accession No. MH051721), auxin-responsive protein IAA18 –like (IAA18, GenBank accession No. MH051728), auxin-responsive protein IAA29 (IAA29, GenBank accession No. MH051727) and auxin-responsive factor 18-like (ARF18, GenBank accession No. MH051722); two-component response regulator ARR5-like (ARR5, GenBank accession No. MH051725); transcription factor MYB15 (MYB15, GenBank accession No. MH051730), ethylene-responsive transcription factor ERF RAP2–12 (ERF RAP2-12, GenBank accession No. MH051724), Cyclin D3-1 (CYCD3-1, GenBank accession No. MH051723) and cyclin-dependent kinase B2-2 (CDKB2-2, GenBank accession No. MH051726). The expression pattern of these genes during phase transition was verified by qPCR (Fig. 6). The result showed that expression profiles of these 9 genes in the dedifferentiated and redifferentiated tissues derived from initial stem and leaf explants were quite similar to those obtained from the transcriptomic analysis, with a correlation coefficient being 0.8745 (p < 0.01) (Fig. 7). Among these 9 genes, ARR5, GH3.1, IAA18, IAA29, CYCD3-1, CDKB2-2 and MYB15 were up-regulated in primary callus and callus then down-regulated in the regenerated root, while the expressions of ARF18 and ERF RAP2-12 were down-regulated in primary callus and callus but up-regulated in the regenerated roots. These validations indicated that transcriptome analysis faithfully revealed the gene modulation mechanism during the phase changes.
Table 3.
Auxin and cytokinins regulation-related DEGs for qPCR validation.
| Gene | GenBank accession No. | Annotation | Primer pair |
|---|---|---|---|
| GH3.1 | MH051721 | Indole-3-acetic acid-amido synthetase GH3.1 | GH3.1 F 5′-GGTATACCGACAAGGCCGAG |
| GH3.1 R 5′-GCTCCTCAAAGGTCCCACTC | |||
| ARF18 | MH051722 | Auxin-responsive factor 18-like | ARF18F 5′-ACCATTCTGGAGAACCGCTG |
| ARF18R 5′-ACCATTCTGGAGAACCGCTG | |||
| CYCD3-1 | MH051723 | Cyclin D3-1 | CYC-D3-1F 5′-CAAGTCACGGGTCGGTAGAG |
| CYC-D3-1R 5′-GTTGTACCCGAGTGTCCTCC | |||
| RAP2-12 | MH051724 | Ethylene-responsive transcription factor ERF RAP2-12 | ERF RAP2-12F 5′-TGACTCGGACACACTCTCCT |
| ERF RAP2-12R 5′-TTTGGGGGTGAGACCTTTGG | |||
| ARR5 | MH051725 | Two-component response regulator ARR5-like | ARR5F 5′-GGCATCGGAAAAACGGGTTG |
| ARR5R 5′-TCAGTGCCATCAGACGAAGG | |||
| CDKB2-2 | MH051726 | Cyclin-dependent kinase B2-2 | CDKsF 5′-TGCACTTCCAACGCACAATC |
| CDKsR 5′-GCCTCATCAAAACCTCGCTTC | |||
| IAA29 | MH051727 | Auxin-responsive protein IAA29 | IAA29F 5′-TGCACTTCCAACGCACAATC |
| IAA29R 5′-GCCTCATCAAAACCTCGCTTC | |||
| IAA18 | MH051728 | Auxin-responsive protein IAA18 | IAA18F 5′-CCCCAACAAGCATCCTGTCT |
| IAA18R 5′-ATCCAGAACAAGCACGAGGG | |||
| MYB15 | MH051730 | Transcription factor MYB15 | MYB15F 5′-CTCCTCTTCATTGGCAGGTCC |
| MYB15F 5′-CGAACCGACAACGAGATCAA |
Figure 6.
Expression validation of the auxins and cytokinins regulation -related genes by qPCR. Relative expression levels obtained from qPCR and RNA-Seq were shown as column and line respectively, and different letters (lower case for stem-derived samples, upper case for leaf-derived samples) indicated the significant difference at p < 0.05.
Figure 7.
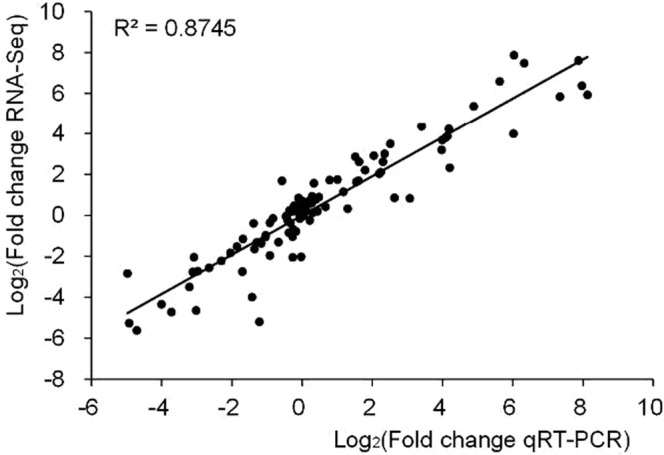
Correlation of the gene expression obtained from qPCR and RNA-seq.
Discussion
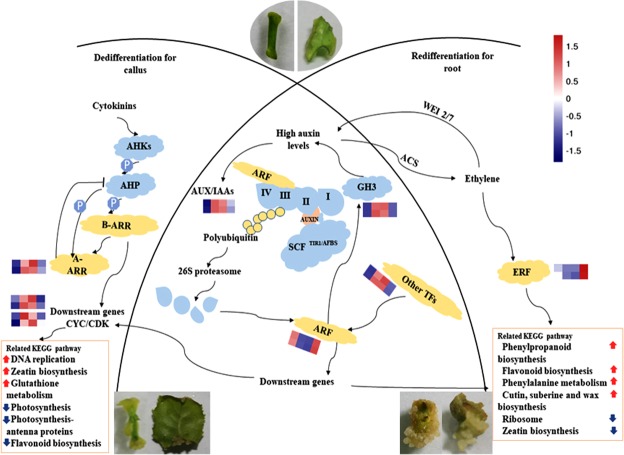
Exogenous plant growth regulators are usually applied to induce specific cell types and/or organs during dedifferentiation and redifferentiation of tissue culture. In this study, 2,4-dichlorophenoxyacetic acid (2,4-D, belonging to auxins) and 6-benzylaminopurine (BAP, belonging to CKs) were used for inducing callus from stems and leaves of tea plant. The transcriptome and qPCR results revealed that many DEGs, especially the hormone-related genes, were significantly triggered by the growth regulators treatment, and finally initiated the dedifferentiation process. As shown in Fig. 8, the cell division and callus formation might be induced and facilitated through combination of the two regulating pathways. One was auxin-related pathway, in which the expression of auxin-responsive protein encoding gene IAA18 and IAA29 were up-regulated by addition of exogenous 2,4-D, the increased gene products of the IAA18 and IAA29 would then accelerate the release of the ARFs through combination with different TIR1/AFB (transport inhibitor response 1/auxin-related F-box protein) and ARF proteins and activating the ubiquitinoylation, then the excited ARFs would up-regulate the expression of the downstream gene CDK B2-234–36. The previous reports concerning early gene response to auxin treatment also showed that Aux/IAA are short-lived nuclear proteins and mostly have four conserved domains, where the domain III and IV are responsible for dimerization with other Aux/IAA or heterodimerization with auxin responsive factors (ARFs), and the domain II contains the degron motif and can interact directly with the TIR1/AFB and auxin, leading to the ubiquitination and degradation via 26 S proteasome at higher auxin levels and consequently releasing ARFs to regulate the expression of downstream genes37,38. Another was CK-related pathway, in which the expression of response regulator gene B-ARRs (B-type Arabidopsis response regulators) and A-ARR5 were all phosphorylated through AHKs (Arabidopsis histidine kinases) → AHPs (Arabidopsis histidine-containing phosphotransfer proteins) phosphorelay signaling way39, and cyclin gene CYC D3-1 was up-regulated as the downstream gene of the phosphorylated B-ARRs, due to the addition of BAP. Our result confirmed that dedifferentiation process is also closely related to the CKs modulation pathway which has been reported in Arabidopsis. When the cell is contacted with CKs, transmembrane receptors, AHKs will transmit signals to the nucleus via the phosphorelay pathway, leading to phosphorylation and activation of transcription factors B-ARRs40, B-type ARRs then sequentially induce the expression of some response genes, including A-ARRs41 and cyclin (CYC)42. In addition, extremely up-regulated transcription factor MYB15 was also observed, indicating this gene might also be involved in modulation of the dedifferentiation because MYB15 could modulate the expression of auxin-inducible genes by interacting with ARFs43 and affect the plant growth and development34. Our study also revealed that a GH3 family protein encoding gene GH3.1 was significantly up-regulated in callus, which was consistent with other previous finding that GH3 can maintain auxin homeostasis by conjugating excessive indole-3-acetic acid to amino acids in the presence of a higher level auxin44. The up-regulated expression of the MYB15, IAA18 and IAA29 could promote auxin signaling pathways through interactions with related ARFs; up-regulating CDK B2-2 and CYC D3-1, involved in auxin and CKs signaling pathways, might be necessary for callus induction of tea plant. The up-regulation of GH3.1 and A-ARR5 might be a response of tissues against high hormonal levels. After the genes related to signaling transduction changed their expression, many downstream pathways including DNA replication, zeatin biosynthesis, glutathione metabolism and photosynthesis were also be modulated. Zeatin biosynthesis and DNA replication were modified for initiating cell division to form callus28–30, while photosynthesis was changed due to dedifferentiation of chloroplast. Initiation and proliferation of the callus require the up-regulation of glutathione metabolism to deal with the antioxidant defense, nutrient requisition, and regulation of cellular events31,32.
Figure 8.
The proposed hormone-related gene modulation during dedifferentiation and redifferentiation of tea plant. AHKs: Arabidopsis histidine kinases; AHPs: Arabidopsis histidine-containing phosphotransfer proteins; A-ARR: A-type Arabidopsis response regulator; B-ARR: B-type Arabidopsis response regulator; CYC/CDK: cyclin/yclin-dependent kinase; AUX/IAAs: auxin/indole-3-acetic acid family proteins; SCF: S-phase kinase-associated protein- cullin-F box; TIR1/AFB: transport inhibitor response 1/auxin-related F-box; ARF: auxin responsive factor; GH3: Gretchen Hagen 3; Other TFs: transcription factors including MYB15; WEI2/7: weak ethylene insensitive 2/7; ACS: 1-aminocyclopropane- 1-carboxylate synthase; ERF: ethylene-responsive transcription factor. Expression levels in stem/leaf-derived tissues of tea plant obtained from RNA-seq were indicated as color bar and normalized by z-score processing.
When the root was regenerated from the callus in the 1/2 MS medium supplemented with 1.5 mg/L naphthalene acetic acid (NAA), the expression level of IAA18 and IAA29 were quite lower than that in callus stage, but similar to that in initial explants; while the genes encoding the auxin-responsive factor ARF18 and ethylene-responsive transcription factor ERF RAP2-12 were remarkably up-regulated compared with the callus stage. According to the published reports, auxin application could promote expression of the ACS4 gene encoding 1-aminocyclopropane-1-carboxylate (ACC) synthase to induce ethylene biosynthesis45, leading to enhanced ethylene level and up-regulating expression of ethylene-responsive transcription factor. Furthermore, ethylene could influence auxin level and further facilitate auxin signaling pathway46 through modulating the expression of the weak ethylene insensitive 2 and 7 (WEI2 and WEI7) which encoded the rate-limiting enzyme anthranilate synthase in the tryptophan biosynthesis47. Thus, up-regulating expression of ARF18 and ERF RAP2-12, and down-regulating expression of IAA18 and IAA29 might be essential for root induction, as well as changes in gene expression of some downstream pathways, such as cutin, suberine, wax, and phenylpropanoid metabolism which is required to form the specialized cell wall through activating the pathway related to biosynthesis of the cutin, suberine, wax, and phenylpropanoid33.
Methods and Materials
Preparation of tea plant samples
Seedlings of Camellia sinensis cultivar ‘Jinxuan’ derived through in-vitro technique were micropropagated and maintained on the Murashige and Skoog (MS) medium with addition of 2 mg/L 6-benzylaminopurine (BAP), 0.1 mg/L naphthalene acetic acid (NAA), 30 g/L sucrose and 9 g/L agar through single-node culture. A callus inducing medium (CIM) was prepared and autoclaved at 121 °C for 20 min after adding 2.0 mg/L 2,4-dichlorophenoxyacetic acid (2,4-D), 0.4 mg/L BAP, 30 g/L sucrose and 9 g/L agar into MS medium, and a root inducing medium (RIM) was also prepared and autoclaved after adding 1.5 mg/L NAA, 30 g/L sucrose and 9 g/L agar into 1/2 MS medium. pH value of the all the media was adjusted to pH5.8.
The stems and leaves were disassociated from the seedlings and cut into small pieces (~0.5 cm for stem and ~0.5 cm2 for leaf) and used as explants. The explants were inoculated onto the CIM and cultivated in tissue culture room at 24 ± 1 °C under the dark condition. The callus began to appear from the cuts of the stem explant (S-explant) after 10 days, and then surrounded the explant after 16 days. Sampling was conducted on the 10th day and 16th day, designated as S-primary callus and S-callus, respectively. The stem-derived callus was then inoculated onto the RIM, the root was induced after 15 days. Sampling was carried out and designated as S-root. Similarly, the leaf-derived primary callus and callus (L-primary callus and L-callus) were also obtained after cultivating the leaf explant (L-explant) on the CIM for 12 and 20 days, and redifferentiated root (L-root) was sampled after inducing the L-callus on the RIM for 20 days. The obtained samples were immediately frozen in liquid nitrogen and stored at −80 °C for further use. All the tests were conducted in biological triplicates.
Observation of the callus and regenerated root
In order to confirm the phase transition of the tissue culture, samples were embedded in paraffin and stained with aniline blue solution48,49. Observation was performed on a Nikon Eclipse E100 (Nikon Co., Ltd, Japan) with a Nikon DS-U3 image capture.
RNA extraction
RNA extraction was carried out with RNAprep Pure Plant Kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s instruction. The purity of RNA was checked by NanoPhotometer® spectrophotometer (Implen, CA, USA). The concentration of RNA was measured on Qubit® 2.0 Flurometer (Life Technologies, CA, USA) by using Qubit® RNA Assay Kit. The integrity of RNA was assessed on Bioanalyzer 2100 system (Agilent Technologies, CA, USA) by using RNA Nano6000 Assay Kit.
Transcriptome analysis
The mRNAs were enriched by using Oligo (dT) magnetic beads from the 3 μg total RNA for each sample. Sequencing libraries were generated using NEBNext®Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA) according to the manufacturer’s recommendations and index codes were added to attribute sequences to each sample. The clustering of the index-coded samples was performed on a cBot Cluster Generation System using TruSeq PE Cluster Kit v3-cBot-HS (Illumia, NEB, USA) according to the manufacturer’s instructions. After cluster generation, the libraries were sequenced on an Illumina Hiseq 2500 platform and paired-end reads were generated. Clean data were obtained by removing reads containing adapter, reads containing ploy-N and low quality reads from raw data. Transcriptome assembly was accomplished from the clean data using Trinity software. Gene function of all non-redundant transcripts was annotated based on the database of the NCBI nonredundant protein sequences (Nr), Protein family (Pfam), Eukaryotic Orthologous Group/Clusters of Orthologous Groups of proteins (KOG/COG), a manually annotated and reviewed protein sequences (Swiss-Prot), Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) by using BLASTALL package with the significant threshold of E-value ≤ 10−5. FPKM was used as expression strength of the gene, and differential expression analysis of two samples was performed using the DEGseq R package (ver. 2.1.0). p value was adjusted using q value and q value < 0.005 & |log2 (fold change)| > 1 was set as the threshold for significantly differential expression. Samples of two biological replicates at each culture stage were used for transcriptome analysis.
Quantitative real-time PCR analysis
To validate the gene expression results of transcriptome, qRT-PCR was employed to determine the expression of 9 genes related to auxins and cytokinins regulation (Table 3) on Applied Biosystems™ StepOnePlus™ Real-Time PCR System (ABI, Carlsbad, CA, USA) by using SYBR Premix Ex TaqTM II (TaKaRa Biotechnology Co., Ltd., Dalian, China) according to the previous paper50. PCR primers were designed by primer-blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) with default parameters except the product size (100–250 bp), and the primers were ordered from Sangon Biotech (Shanghai) Co., Ltd. The PCR cycling conditions were: 40 cycles of 95 °C for 30 s, 55 °C for 5 s and 60 °C for 30 s. The melting-curves were analyzed during the reactions to ensure reaction specificity. The relative expression levels of the different genes were calculated by the 2−ΔΔCt method51 by using β-actin gene as control. qPCR analysis was performed in three biological replicates with 3 technical replicates for each biological replicate.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 31470687).
Author Contributions
Ying Gao: mainly conducted the experiments, drafted the article; Min Zhao: conducted the experiments, analysis; Da Li: conducted the experiments; Xiao-Han Wu: conducted the experiments; Devajit Borthakur, review and polish the article; Jian-Hui Ye: interpretation of the results; Xin-Qiang Zheng: interpretation of the results; Jian-Liang Lu: design of the study, preparation of the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the correspondingauthor on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying Gao and Min Zhao contributed equally.
References
- 1.Bano Z, Rajarathnam S, Mohanty BD. Somatic embryogenesis in cotyledon cultures of tea (Thea sinensis L.) J. Hortic. Sci. 1991;66:465–470. doi: 10.1080/00221589.1991.11516175. [DOI] [Google Scholar]
- 2.Kato M. Somatic embryogenesis from immature leaves of in vitro grown tea shoots. Plant Cell Rep. 1996;15:920–923. doi: 10.1007/BF00231588. [DOI] [PubMed] [Google Scholar]
- 3.Seran TH, Hirimburegama K, Hirimburegama WK, Shanmugarajah V. J. Natl. Sci. Found. Sri. 1999. Callus formation in anther culture of tea clones, Camellia sinensis (L.) Kuntze; pp. 165–175. [Google Scholar]
- 4.Mondal TK, Bhattacharya A, Ahuja PS, Chand PK. Transgenic tea [Camellia sinensis (L.) O. Kuntze cv. Kangra Jat] plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep. 2001;20:712–720. doi: 10.1007/s002990100382. [DOI] [Google Scholar]
- 5.Gianquinto G. Morphological and physiological aspects of phase transition in radicchio (Cichorium intybus L. var. silvestre Bisch.): influence of daylength and its interaction with low temperature. Sci. Hortic. 1997;71:13–26. doi: 10.1016/S0304-4238(97)00058-7. [DOI] [Google Scholar]
- 6.Honda H. In situ regeneration of rice (Oryza sativa L.) callus immobilized in polyurethane foam. J. Biosci. Bioeng. 2001;91:76–80. doi: 10.1016/S1389-1723(01)80115-8. [DOI] [PubMed] [Google Scholar]
- 7.She W, et al. Chromatin reprogramming during the somatic-to-reproductive cell fate transition in plants. Development. 2013;140:4008–4019. doi: 10.1242/dev.095034. [DOI] [PubMed] [Google Scholar]
- 8.Garciajove Navarro M, et al. Api5 contributes to E2F1 control of the G1/S cell cycle phase transition. Plos One. 2013;8:e71443. doi: 10.1371/journal.pone.0071443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stals H, Inzé D. When plant cells decide to divide. Trends Plant Sci. 2001;6:359–364. doi: 10.1016/S1360-1385(01)02016-7. [DOI] [PubMed] [Google Scholar]
- 10.Roudier F, Kondorosi E. The Medicago species A2-type cyclin is auxin regulated and involved in meristem formation but dispensable for endoreduplication-associated developmental programs. Plant Physiol. 2003;131:1091–1103. doi: 10.1104/pp.102.011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutierrez L, et al. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell. 2009;21:3119–3132. doi: 10.1105/tpc.108.064758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavenus J, et al. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 2013;18:450–458. doi: 10.1016/j.tplants.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Li K, et al. Transcriptome analysis reveals multiple hormones, wounding and sugar signaling pathways mediate adventitious root formation in apple rootstock. Int. J. Mol. Sci. 2018;19:2201. doi: 10.3390/ijms19082201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu X, Xu L. Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiol. 2016;172:2363–2373. doi: 10.1104/pp.16.01067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangwan RS, Tripathi S, Singh J, Narnoliya LK, Sangwan NS. De novo sequencing and assembly of Centella asiatica leaf transcriptome for mapping of structural, functional and regulatory genes with special reference to secondary metabolism. Gene. 2013;525:58–76. doi: 10.1016/j.gene.2013.04.057. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, et al. Transcriptomic analysis reveals the molecular mechanisms of drought-stress-induced decreases in Camellia sinensis leaf quality. Front. Plant Sci. 2016;7:385. doi: 10.3389/fpls.2016.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng C, et al. Integrated RNA-seq and sRNA-seq analysis identifies chilling and freezing responsive key molecular players and pathways in tea plant (Camellia sinensis) Plos One. 2015;10:e0125031. doi: 10.1371/journal.pone.0125031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, et al. An RNA-seq transcriptome analysis revealing novel insights into aluminum tolerance and accumulation in tea plant. Planta. 2017;246:91–103. doi: 10.1007/s00425-017-2688-6. [DOI] [PubMed] [Google Scholar]
- 19.Jayaswall K, et al. Transcriptome analysis reveals candidate genes involved in blister blight defense in tea (Camellia sinensis (L) kuntze) Sci. Rep. 2016;6:30412. doi: 10.1038/srep30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi CY, et al. Deep sequencing of the Camellia sinensis transcriptome revealed candidate genes for major metabolic pathways of tea-specific compounds. BMC Genomics. 2011;12:1471–2164. doi: 10.1186/1471-2164-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, et al. Transcriptomic analysis of the biosynthesis, recycling, and distribution of ascorbic acid during leaf development in tea plant (Camellia sinensis (L.) O. kuntze) Sci. Rep. 2017;7:46212. doi: 10.1038/srep46212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, et al. Transcriptome and metabolite analysis identifies nitrogen utilization genes in tea plant (Camellia sinensis) Sci. Rep. 2017;7:1693. doi: 10.1038/s41598-017-01949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt ED, Guzzo F, Toonen MA, Vries SCDA. leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- 24.Rocha DI, et al. Cellular and molecular changes associated with competence acquisition during passion fruit somatic embryogenesis: ultrastructural characterization and analysis of SERK gene expression. Protoplasma. 2015;253:1–15. doi: 10.1007/s00709-015-0837-y. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, et al. Wox11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell. 2014;26:1081–1093. doi: 10.1105/tpc.114.122887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montain CR, Haissig BE, Curtis JD. Differentiation of adventitious root primordia in callus of Pinus banksiana. Can. J. Forest Res. 1983;13:195–200. doi: 10.1139/x83-028. [DOI] [Google Scholar]
- 27.Klerk GJD, Arnholdt-Schmitt B, Lieberei R, Neumann KH. Regeneration of roots, shoots and embryos: physiological, biochemical and molecular aspects. Bio. Plantarum. 1997;39:53–66. doi: 10.1023/A:1000304922507. [DOI] [Google Scholar]
- 28.Pasternak TP, et al. The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of Alfalfa. Plant Physiol. 2002;129:1807–1819. doi: 10.1104/pp.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda-Iwai M, Umehara M, Satoh S, Kamada H. Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2010;34:107–114. doi: 10.1046/j.1365-313X.2003.01702.x. [DOI] [PubMed] [Google Scholar]
- 30.Panda BB, Sekhar S, Dash SK, Behera L, Shaw BP. Biochemical and molecular characterisation of exogenous cytokinin application on grain filling in rice. BMC Plant Biol. 2018;18:89. doi: 10.1186/s12870-018-1279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shohael AM, Ali MB, Hahn EJ, Paek KY. Glutathione metabolism and antioxidant responses during eleutherococcus senticosus, somatic embryo development in a bioreactor. Plant Cell Tiss. Org. 2007;89:121–129. doi: 10.1007/s11240-007-9220-9. [DOI] [Google Scholar]
- 32.Verma SK, Das AK, Cingoz GS, Uslu E, Gurel E. Influence of nutrient media on callus induction, somatic embryogenesis and plant regeneration in selected Turkish crocus species. Biotechnol. Rep. 2016;10:66–74. doi: 10.1016/j.btre.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li SW, Shi RF, Leng Y. De novo characterization of the mung bean transcriptome and transcriptomic analysis of adventitious rooting in seedlings using RNA-seq. Plos One. 2015;10:e0132969. doi: 10.1371/journal.pone.0132969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salehin M, Bagchi R, Estelle M. SCFTIR1/AFB -based auxin perception: mechanism and role in plant growth and development. Plant Cell. 2015;27:9–19. doi: 10.1105/tpc.114.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dindas J, et al. AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nat. Commun. 2018;9:1174. doi: 10.1038/s41467-018-03582-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takatsuka H, Umeda M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014;65:2633–2643. doi: 10.1093/jxb/ert485. [DOI] [PubMed] [Google Scholar]
- 37.Abel S, Theologis A. Early genes and auxin action. Plant Physiol. 1996;111:9–17. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulmasov T, Hagen G, Guilfoyle TJ. ARF1, a transcription factor that binds to auxin response elements. Science. 1997;276:1865–1868. doi: 10.1126/science.276.5320.1865. [DOI] [PubMed] [Google Scholar]
- 39.To JPC, et al. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell. 2004;16:658–671. doi: 10.1105/tpc.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mason MG, et al. 2005. Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell. 2005;17:3007–3018. doi: 10.1105/tpc.105.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.To JPC, Kieber JJ. Cytokinin signaling: two-components and more. Trends Plant Sci. 2008;13:85–92. doi: 10.1016/j.tplants.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Riou-khamlichi C, Huntley R, Jacqmard A, Murray JA. Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science. 1999;283:1541–1544. doi: 10.1126/science.283.5407.1541. [DOI] [PubMed] [Google Scholar]
- 43.Shin R, et al. The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell. 2007;19:2440–2453. doi: 10.1105/tpc.107.050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hagen G, Guilfoyle T. Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol. Biol. 2002;49:373–385. doi: 10.1023/A:1015207114117. [DOI] [PubMed] [Google Scholar]
- 45.Abel S, Nguyen MD, Chow W, Theologis A. ACS4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. J. Biol. Chem. 1995;270:19093–19099. doi: 10.1074/jbc.270.32.19093. [DOI] [PubMed] [Google Scholar]
- 46.Růzicka K, et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM. A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell. 2005;17:2230–2242. doi: 10.1105/tpc.105.033365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, et al. Late-acting self-incompatibility in tea plant (Camellia sinensis) Biologia. 2012;67:347–351. [Google Scholar]
- 49.Liu J, Cheng Y, Yan K, Liu Q, Wang Z. The relationship between reproductive growth and blank fruit formation in Corylus heterophylla Fisch. Sci. Hortic. 2012;136:128–134. doi: 10.1016/j.scienta.2012.01.008. [DOI] [Google Scholar]
- 50.Li QS, et al. Effect of fluoride treatment on gene expression in tea plant (Camellia sinensis) Sci. Rep. 2017;7:9847. doi: 10.1038/s41598-017-08587-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the correspondingauthor on reasonable request.