Abstract
Avian coccidiosis is an economically important disease in the poultry industry. In view of the disadvantages of anti-coccidial drugs in chickens, edible plants and their compounds are re-emerging as an alternative strategy to combat this disease. A previous publication reported that the edible plant B. pilosa showed promise for use against coccidiosis. Here, we first investigated into the anti-coccidial effects of B. pilosa. We found that B. pilosa at 100 ppm or more significantly suppressed E. tenella as evidenced by reduction in mortality rate, oocyst excretion and gut pathological severity in chickens and its minimum prophylactic duration was 3 days. Next, we explored the mode of action of anti-coccidial mechanism of B. pilosa. The E. tenella oocysts were not directly killed by B. pilosa; however, administration of the plant suppressed oocyst sporulation, sporozoite invasion, and schizonts in the life cycle of E. tenella. Besides, B. pilosa boosted T cell-mediated immunity. Finally, we characterized the related anti-coccidial phytochemicals and their mode of action. One of three potent polyynes present in B. pilsoa, Compound 1 (cytopiloyne), acted against coccidiosis in chickens in a similar manner to B. pilosa. These data illustrate the anti-coccidial potency and mechanism of B. pilosa and one of its active compounds, and provide a cornerstone for development of novel herbal remedies for avian coccidiosis.
Introduction
It is estimated that 50 billion chickens are raised annually worldwide. The parasitic disease coccidiosis costs the poultry industry an estimated 3 billion US dollars per year due to high mortality, poor growth and high medical costs1–3. Coccidiosis in chickens (and other animals) is caused by protozoa from the Eimeria genus (from the subclass Coccidia). Due to low efficiency and the disadvantages of current anti-coccidial drugs and vaccines4–6, edible plants and/or natural products are being considered as possible viable alternative substituents. However, despite considerable progress over recent years, safety, efficacy, and the mechanisms of the modes of action of edible plants and their compounds still require further study if they are to be considered a viable alternative to current anti-coccidial approaches7.
It has been reported that over 1200 plants have anti-protozoal activity8,9. So far, only about 20 herbal plants have been studied for anti-coccidial activities4,10–18. Among these, members of the B. pilosa (Asteraceae family) are used as foods and medicines worldwide19. The Food and Agriculture Organization of the United Nations and the Taiwan government list B. pilosa as a food staple20. We previously reported that B. pilosa manifests high anti-coccidial activity and low induction of drug resistance in Eimeria parasites18,21. However, the anti-coccidial mechanism underlying B. pilosa is not clear. Further, despite the discovery of over 200 compounds in B. pilosa19, the identities of its anti-coccidial compounds are unknown, which currently limits the commercial use of B. pilosa in the poultry industry.
In this study, we first tested the efficacy of B. pilosa against coccidiosis in chickens. Next, using a bioactivity-directed fractionation and isolation procedure, we identified the anti-coccidial compounds from this plant. In addition, we explored the mode of action of B. pilosa and its bioactive compounds using in vitro co-incubation with E. tenella oocysts and sporozoites. Finally, we also confirmed the anti-coccidal action of its bioactive compounds in chickens.
Results
Prophylactic efficacy of B. pilosa in chicken coccidiosis
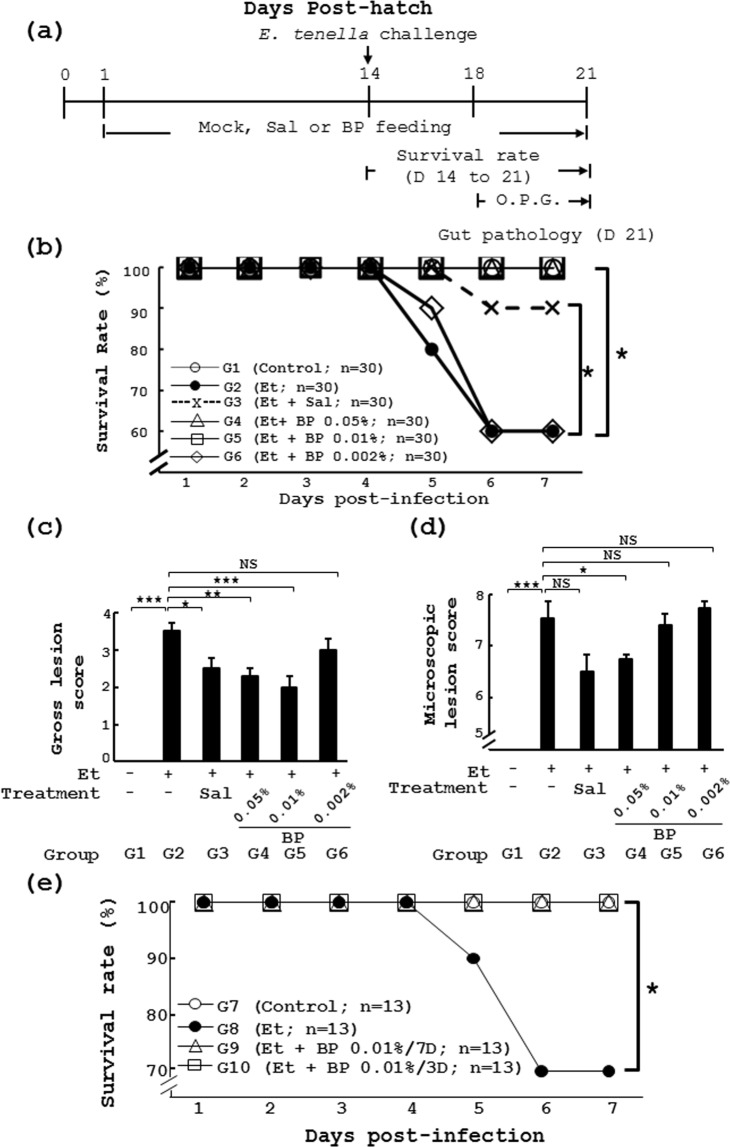
Our previous publication showed that B. pilosa could protect chickens from Eimeria tenella infection18. With an eye to development of B. pilosa as a feed additive to prevent coccidiosis in chickens, here we explored its in vivo efficacy as measured by the minimum effective dose and minimum prophylactic duration. First, chickens were fed daily standard chicken feed from day 1 to day 21. The feed contains the commercial anti-coccidial chemical salinomycin or 0.05%, 0.01% and 0.002% B. pilosa powder as described in Fig. 1a. After challenging with E. tenella, chickens with standard feed had lower survival rate (60% in Group 2 (Et)) compared to the control group (100% in Group 1 (CTR)) (Fig. 1b). The challenged chickens with feed containing salinomycin had 90% survival rate in Group 3 (Et + Sal, Fig. 1b) as we expected. In contrast, the survival rates were 100%, 100%, and 60% for infected chickens with the feed containing B. pilosa at the doses of 0.05%, 0.01% and 0.002% (Groups 4 (Et + BP 0.05%), 5 (Et + BP 0.01%) and 6 (Et + BP 0.002%), Fig. 1b), respectively. Consistently, B. pilosa improved the body weight loss in chickens challenged with E. tenella (Table 1).The data suggest that the minimum effective dose of B. pilosa is 0.01% (100 ppm).
Figure 1.
Preventive effect and minimum prophylactic duration of B. pilosa on coccidiosis in chickens. (a) The experimental protocol of the study. (b–d) Effect of B. pilosa on survival rate of chickens given E. tenella challenge. In Experiment 1, 6 groups of chicks had daily access to a diet containing vehicle, salinomycin (Sal) or different doses of B. pilosa (BP 0.05%, BP 0.01% and BP 0.002%). On day 14, chickens were administered with PBS or E. tenella sporulated oocysts (Et) by gavage. Survival rate was measured daily from day 1 to 7 post infection (b). Gross lesion score (c) and microscopic lesion (d) score were obtained from the grading of the cecal lesions of the same chicks as in Figure 1b. (e) In Experiment 2, 4 groups of chickens were used for the study. The chickens in Group 7 were fed with the standard diet from days 1 to 21 with E. tenella infection. Chicks were pre-administered the diet (Et, Group 8), from days 1 to 21, and the diet containing B. pilosa powder (0.01%), from days 11 to 14, for 3 days (Et + BP/3D, Group 9), and, from days 11 to 18, for 7 days (Et + BP/7D, Group 10), respectively. On day 14, the birds were orally infected with PBS or sporulated oocysts of E. tenella. The survival of the chicks was monitored from days 14 to 21. The number (n) of chicks in each group is indicated. P < 0.05 (*) was considered to be statistically significant.
Table 1.
Body weight gain (BWG) of chickens given standard diet with or without salinomycin and different doses of B. pilosa from days 1 to 21.
| Group cage no. (chickens) | BWG(g) | P-valuea | P-valueb | BWG(g) | P-valuea | P-valueb |
|---|---|---|---|---|---|---|
| Day 14-1 | Day 14-1 | Day 14-1 | Day 21-1 | Day 21-1 | Day 21-1 | |
| G1 (n = 10) 3 (3, 3, 4) | 126.73 ± 3.82 | 234.72 ± 3.4 | ||||
| G2 (n = 10) 3 (3, 3, 4) | 127.14 ± 3.21 | >0.05 | 169.6 ± 8.81 | <0.05 | ||
| G3 (n = 10) 3 (3, 3, 4) | 126.35 ± 3.57 | >0.05 | >0.05 | 200.01 ± 5.91 | <0.05 | <0.05 |
| G4 (n = 10) 3 (3, 3, 4) | 120.66 ± 2.14 | >0.05 | >0.05 | 217.78 ± 5.37 | <0.05 | <0.05 |
| G5 (n = 10) 3 (3, 3, 4) | 125.51 ± 5.44 | >0.05 | >0.05 | 215.77 ± 6.88 | <0.05 | <0.05 |
| G6 (n = 10) 3 (3, 3, 4) | 130.93 ± 2.02 | >0.05 | >0.05 | 161.35 ± 4.53 | <0.05 | >0.05 |
The chickens in Experiment 1 were divided into Groups 1 to 6. Group 1 (uninfected unmedicated control, CTR) and Group 2 (infected unmedicated control, Et) were given daily standard chicken diet from day 1 to day 21. Group 3 (Et + Sal) had daily access to a diet with salinomycin (Sal, 100 mg/kg diet). Group 4 (Et + BP 0.05%), Group 5 (Et + BP 0.01%), and Group 6 (Et + BP 0.002%) were fed daily with the diet containing B. pilosa powder at the indicated doses. The number (n) of chickens and cage number in each group and number of chickens in each cage are shown. Body weight gain (BWG): body weight on day T (14 or 21) – body weight on day 1. aNested ANOVA was used to determine the difference in chicken body weight gain (g) between infected groups (Groups 2–6) and uninfected unmedicated group (Group 1) and the data are presented by P value. bNested ANOVA was used to determine the difference in chicken body weight gain (g) between infected medicated groups (Groups 3–6) and infected unmedicated group (Group 2) and the data are presented by P value.
Consistently, the oocyst excretion from the chickens, expressed as oocysts per gram of feces (OPG), an indicator of Eimeria multiplication, was also evaluated. There were no oocysts in the feces of the unchallenged controls without medication (Group 1, Table 2). After E. tenella infection, the fecal oocyst excretion from days 4 to 7 was measured in all infected groups. The OPG in the infected unmedicated birds was between 4.18 × 104 and 8.28 × 104 (days 4 to 7 post-infection) (Group 2, Table 2). As expected, the salinomycin-fed chickens with infection in Group 3 (Table 2) had significantly lower OPG than those in Group 2. Similarly, the B. pilosa-fed chickens with infection in Group 4 (Et + 0.05% BP, Table 2) and Group 5 (Et + 0.01% BP, Table 2) had significantly fewer OPG than those in Group 2 as shown in Table 2. However, the chickens in Group 6 (Et + 0.002% BP, Table 2) had similar OPG to those in Group 2 (Table 2).
Table 2.
Fecal oocyst excretion of chickens given standard diet with or without salinomycin and different doses of B. pilosa 4 to 7 days after challenge with E. tenella.
| Group | Days post-infection | ||||
|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | ||
| Ln (OPG + 1) | Ln (OPG + 11) | Ln (OPG + 11) | Ln (OPG + 11) | ||
| CTR | G1 (n = 9) | 0 | 0 | 0 | 0 |
| Et | G2 (n = 9) | 0 | 10.64 ± 7.65a | 11.32 ± 9.16a | 10.98 ± 10.07a |
| Et + Sal | G3 (n = 9) | 0 | 8.98 ± 6.26a,b | 9.58 ± 7.99a,b | 9.23 ± 7.87a,b |
| Et + BP 0.05% | G4 (n = 9) | 0 | 6.40 ± 4.76a,b | 10.74 ± 8.06a,b | 10.13 ± 8.40a,b |
| Et + BP 0.01% | G5 (n = 9) | 0 | 6.15 ± 4.90a,b | 10.85 ± 9.03a,b | 10.36 ± 8.58a,b |
| Et + BP 0.002% | G6 (n = 9) | 0 | 10.57 ± 7.99a | 11.24 ± 9.69a | 10.84 ± 9.63a |
After challenge with E. tenella from day 3 to day 7, the oocysts per gram feces (OPG) of the same chickens from Table 1 in Experiment 1 were measured. The values (×104) of chicken OPG in all the groups were transformed into Ln(OPG + 1) and the data was evaluated by ANOVA using the GLM procedure of the SAS system under a normal distribution. The number (n) of chickens in all the groups is shown.
aThe P value (<0.05) is statistically significant in the chicken OPG between the infected groups (G2–6) and uninfected unmedicated group (G1) on the presented days.
bThe P value (<0.05) is statistically significant in the chicken OPG between the infected medicated groups (G3–6) and infected unmedicated group (G2) on the presented days.
In parallel, the gross cecal lesion in the chickens with different diets was examined at post-infection day 7. Gross cecal lesion score is shown in Fig. 1c. The uninfected control chickens without medication (Group 1, Fig. 1c) had no lesions in the ceca (score = 0). In contrast, the chickens without medication had more gross cecal lesions in gut 7 days after infection, as evidenced by a lesion score close to 4 (Group 2, Fig. 1c). Like salinomycin (Group 3, Fig. 1c), B. pilosa at doses of 0.05% and 0.01%, but not 0.002%, significantly reduced cecal damage in challenged chickens (Groups 4 to 6, Fig. 1c) as shown by the gross lesion scores of 2.0 to 3.0 and microscopic lesion score of 6.8 to 7.7 (Groups 4 to 6, Fig. 1d).
Further, we tried out the minimum prophylactic duration of B. pilosa in chickens. We found that the preventive use of B. pilosa at the dose of 0.01%, once a day for 3 and 7 days, could fully protect chickens from coccidiosis as evidenced by survival rate of chickens (Groups 7 to 10, Fig. 1e). These data suggest that the minimum prophylactic duration of B. pilosa is as short as 3 days.
Overall, B. pilosa showed a high level of anti-coccidial efficacy, superior to that of the commercial anti-coccidial chemical, salinomycin.
B. pilosa suppresses sporulation and invasion of E. tenella
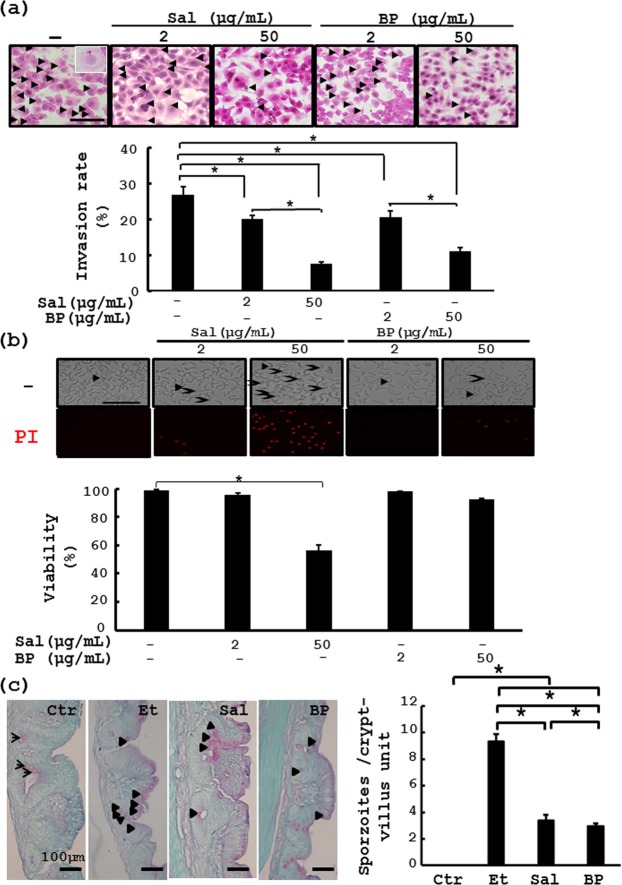
To tease out the mode of action of B. pilosa on coccidiosis, we first examined the direct killing activity of B. pilosa in E. tenella oocysts. As expected, boiling treatment, as a positive control, could effectively kill the oocysts as demonstrated by PI staining (Fig. 2). However, B. pilosa at high doses (5% and 0.5%) failed to kill the oocysts (Fig. 2). Next, we tested the effect of B. pilosa on the sporulation of E. tenella oocysts. Seventy percent of the oocysts were able to sporulate in the in vitro culture (PBS, Fig. 2c). However, boiling treatment completely stopped this sporulation (Boiling, Fig. 2c). In sharp contrast, in the presence of B. pilosa at 0.5% to 5%, less than 20% of the oocysts sporulated (BP, Fig. 2c). Finally, we checked the effect of B. pilosa on the entry of E. tenella sporozoites into MDBK cells. As reported in a previous publication22, the sporozoites could invade into 27% of the cells (Fig. 3a). Salinomycin at the doses of 2 and 50 μg/ml, decreased this invasion to 20% and 8%, respectively. In contrast, B. pilosa, at the doses of 2 and 50 μg/ml, also reduced the invasion to 21% and 11%, respectively (Fig. 3a). In contrast, viability assay showed that salinomycin induced dose-dependent death of the sporozoites (Fig. 3b). However, B. pilosa, failed to induce death of the sporozoites or MDBK cells at the indicated dosages (Figs 3b and S1). These data demonstrate that, unlike salinomycin, B. pilosa inhibited oocyst sporulation and sporozoite invasion but did not directly kill oocysts and sporozoites. Moreover, the histochemical data on the ceca of chickens infected with E. tenella sporozoites which were pre-treated with salinomycin and B. pilosa showed that like in vitro invasion assay, B. pilosa inhibited the in vivo entry of the sporozoites into gut cells in chickens (Fig. 3c). Consistently, we also found that B. pilosa, reduced the percentage and size of the second-generation schizonts (Fig. S2a–c) and the number of fecal oocysts (Fig. S2d). Collectively, these data clearly demonstrate that B. pilosa interfered with the life cycle of E. tenella at oocyst sporulation, sporozoite invasion and schizont maturation.
Figure 2.
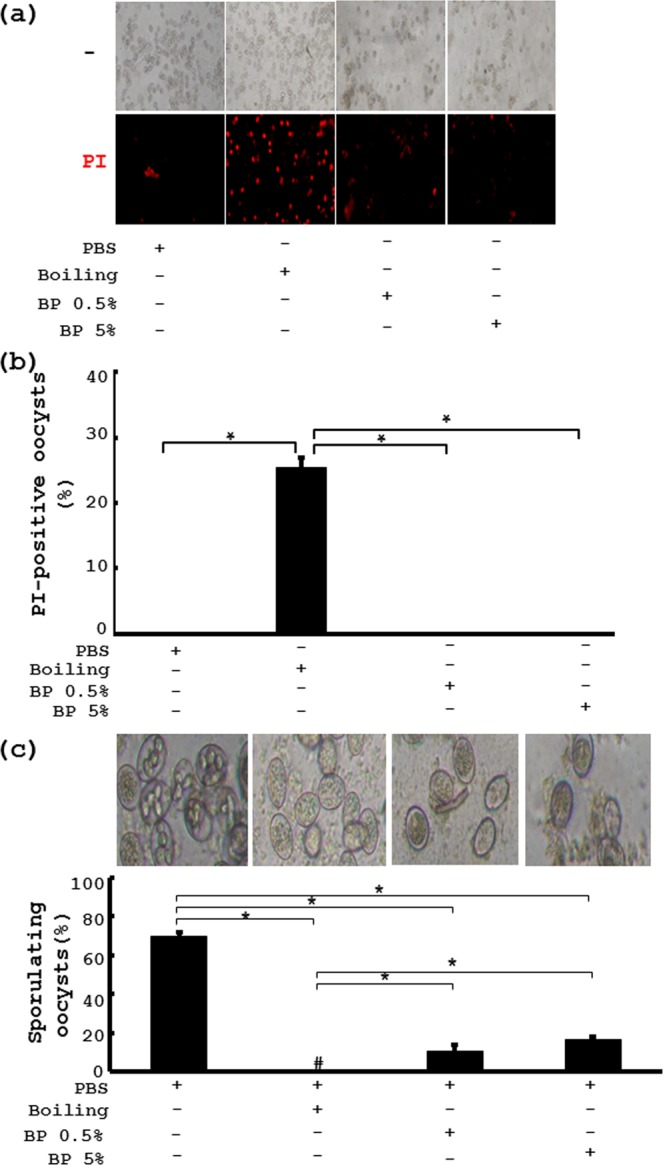
In vitro effect of B. pilosa on E. tenella oocyst viability and sporulation. (a) The oocysts were pre-treated with PBS, boiling and B. pilosa at 5% and 0.5% for 48 h. After PI staining, the oocyst viability was examined using a microscope. (b) Percentage of PI-positive oocysts, presented as mean ± SE, was plotted into bar graphs. (c) The oocysts were induced to sporulate by potassium dichromate for 2 days. The percentage of sporulating oocysts was counted using microscopy (top panel) and plotted into bar graphs (bottom panel).
Figure 3.
In vitro and in vivo effect of B. pilosa on E. tenella sporozoite invasion and viability. (a) MDBK cells were incubated with PBS vehicle, salinomycin (Sal) and B. pilosa powder (BP) at the indicated doses for 0.5 h. The sporozoites were added to the cells for an additional 4 h. After extensive washing, the cells were stained with hematoxylin and eosin and counted (top panel). The invasion percentage (%) was plotted into bar graphs (bottom panel). (b) The sporozoites were incubated with PBS, salinomycin (Sal) and B. pilosa powder (BP) at the indicated doses for 4.5 h. Following propidium iodide (PI) staining, the cells were photographed (top panel) and the viability (%) of the sporozoites was determined and plotted into bar graphs (bottom panel). (c) The in vivo entry of E. tenella sporozoites into chicken ceca in the chickens of Group 17 (CTR), Group 18 (Et), Group 19 (Et + Sal), Group 20 (Et + 0.01% BP) in Experiment 4 were analyzed. The number of the sporozoites per crypt-villus unit in chicken ceca was counted. Goblet cells (arrows) and sporozoites (arrow heads). P < 0.05 (*) was considered to be statistically significant.
Cytopiloyne, the most active compound present in B. pilosa, suppresses sporozoite invasion and coccidiosis in chickens
To better understand the anti-coccidial mechanism of B. pilosa, we next turned our attention to identifying the anti-coccidial compounds present in B. pilosa, and their anti-coccidial action. First, we combined invasion assays and phytochemistry to identify active phytochemicals from B. pilosa based on a bioactivity-guided fractionation and isolation strategy (Fig. S3). Three polynes, 2-β-D-glucopyranosyloxy-1-hydroxytrideca-5,7,9,11-tetrayne (Compound 1, also named cytopiloyne, 0.021%), 2-β-D-glucopyranosyloxy-1-hydroxy-5(E)-tridecene-7,9,11-triyne (Compound 2, 0.018%), and 3-β-D-glucopyranosyloxy-1-hydroxy-6(E)-tetradecene-8,10,12-triyne (Compound 3, 0.013%) were identified from this plant. Their structures were elucidated and confirmed using a UV spectrophotometer (Fig. S4), mass spectroscope (Fig. S5) and nuclear magnetic resonance instrument (data not shown).
In parallel, invasion assays were conducted to evaluate the anti-coccdial activity of the 3 polyynes. As expected, salonomycin, used as a positive control, dose-dependently inhibited the invasion of E. tenella spopozoites into MDBK cells (Sal, Fig. 4a). A phenolic compound, chlorogenic acid (CA, Fig. 4a), used as a negative control, did not affect this invasion. In contrast, cytopiloyne (CPD 1, Fig. 4a) exhibited the most potent inhibition of the entry of sporozoites into MDBK cells in comparison with the other two polyynes (CPD 2 and 3, Fig. 4a) and an inactive phenolic, chlorogenic acid (CA, Fig. 4a). This inhibition was not due to the cytotoxicity of cytopiloyne since cytopiloyne failed to kill sporozoites in a direct way (Fig. 4b). Similar to the anti-coccidial mechanism of B. pilosa, the action of cytopiloyne against E. tenella could be attributed to the sporozoite invasion into gut cells, but not direct killing of sporozoites (Fig. 4b) nor suppression of oocyst sporulation (data not shown). All these polyynes and cholorogenic acid did not affect MDBK cell viability (Fig. S1).
Figure 4.
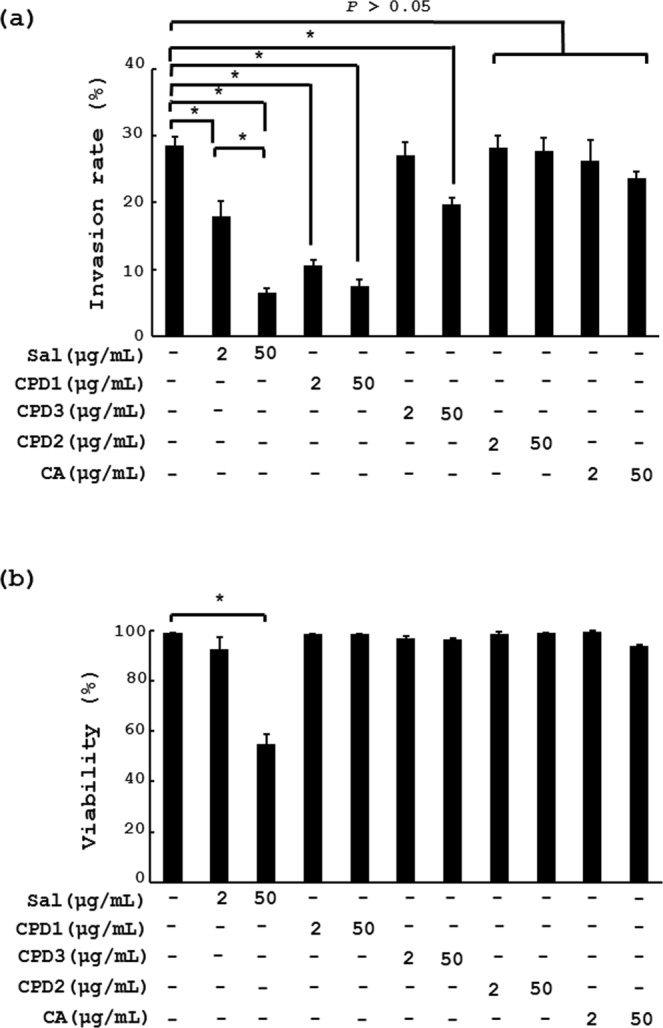
In vitro effect of phytochemicals extracted from B. pilosa on E. tenella sporozoite invasion and viability. (a) MDBK cells were pre-incubated with PBS vehicle, salinomycin (Sal), 3 polyynes (CPD1, CPD2 and CPD3) and chlorogenic acid (CA) at the indicated doses for 0.5 h, followed by additional incubation with sporozoites using the same procedure as in Fig. 3. The invasion percentage (%) is presented on a bar graph. (b) The same sporozoites as (a) were examined for viability. Their viability (%) was determined and plotted into a bar graph (b). P < 0.05 (*) was considered to be statistically significant.
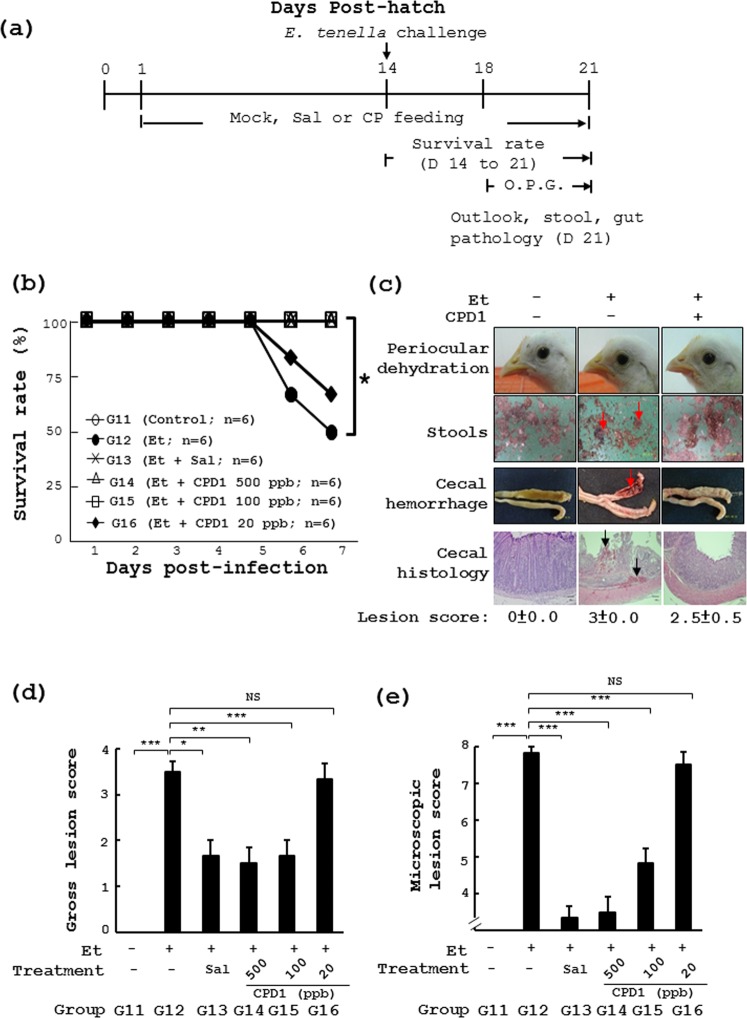
We also checked the anti-coccidial effect of the most active polyyne, cytopiloyne, in chickens. Chickens received daily standard chicken feed (day 1 to day 21) containing salinomycin (Sal, Fig. 5a,b) or cytopilloyne at 500 ppb, 100 ppb and 20 ppb (CPD1, Fig. 5a,b). After E. tenella challenge, the survival rate dropped from 100% (Group 11) to ~50% (Group 12) in the chickens with standard feed (Fig. 5a,b). However, the survival rate of infected chickens with feed containing cytopiloyne at 500 ppb, 100 ppb and 20 ppb was 100%, 100% and 67%, respectively (Groups 14 to 16, Fig. 5b). In addition, chickens infected with E. tenella also showed periocular dehydration, bloody stools, and cecal bleeding/damage (Group 12, Fig. 5c). In sharp contrast, similar to the uninfected controls (Group 11, Fig. 5c), birds fed cytopiloyne at 500 ppb showed no sick bird signs (periocular dehydration, Fig. 5c) or bloody stools (Fig. 5c). Accordingly, cytopiloyne dose-dependently reduced cecal bleeding (Fig. 5c) and damage (Fig. 5c–e) and fecal oocyst counts (OPG, Table 3). Taking these results together, we conclude that cytopiloyne exerted great anti-coccidial activity in chickens via regulation of sporozoite sporulation and invasion.
Figure 5.
Preventive effect of cytopiloyne on coccidiosis in vivo. (a,b) The experimental protocol of the study (a). The same procedure as Fig. 1 except that cytopiloyne (CPD1) was used in the study (b). In Experiment 3, 6 groups of chicks had daily access to the standard diet (CTR) or a diet containing cytopiloyne (Et + CPD1) at the indicated dose. On day 14, chickens were infected with PBS or sporulated E. tenella oocysts (Et) by gavage. Survival rate was measured daily from day 1 to 7 post infection. (c) Periocular dehydration, stools and gut pathology were measured. The number (n) of chicks in each group is indicated. (d,e) Gross lesion score and microscopic lesion score were obtained from the grading of the cecal lesions of the same chicks as in (b). The number (n) of chicks in each group is indicated. P < 0.05 (*) was considered to be statistically significant.
Table 3.
Fecal oocyst excretion of chickens given standard diet with or without salinomycin and different doses of cytopiloyne (CPD1) 4 to7 days after challenge with E. tenella.
| Group | Day post-infection | ||||
|---|---|---|---|---|---|
| 4 | 5 | 6 | 7 | ||
| Ln (OPG+1) | Ln (OPG+1) | Ln (OPG+1) | Ln (OPG+1) | ||
| CTR | G11 (n = 3) | 0 | 0 | 0 | 0 |
| Et | G12 (n = 3) | 0 | 8.03 ± 4.21a | 11.45 ± 9.37a | 11.38±9.56a |
| Et+Sal | G13 (n = 3) | 0 | 6.91 ± 5.30a,b | 9.69 ± 9.55a,b | 9.32 ± 9.19a,b |
| Et+CPD1 500 ppb | G14 (n = 3) | 0 | 6.40 ± 4.76a,b | 9.69 ± 9.55a,b | 9.33 ± 9.19a,b |
| Et+CPD1 100 ppb | G15 (n = 3) | 0 | 6.15 ± 4.90a,b | 10.60 ± 7.48a,b | 10.53 ± 7.35a,b |
| Et+CPD1 20 ppb | G16 (n = 3) | 0 | 8.01 ± 4.76a | 10.48 ± 10.34a | 10.30 ± 10.19a |
After challenge with E. tenella from day 3 to day 7, the oocysts per gram feces (OPG) of the same chickens from Fig. 3c in Experiment 3 were measured. The values (×104) of chicken OPG in all the groups were transformed into Ln(OPG + 1) and the data was evaluated by ANOVA using the GLM procedure of the SAS system under a normal distribution. The number (n) of chickens in all the groups is shown.
aThe P value (<0.05) is statistically significant in the chicken OPG between the infected groups (G12–16) and uninfected unmedicated group (G11) on the presented days.
bThe P value (<0.05) is statistically significant in the chicken OPG between the infected medicated groups (G13–16) and infected unmedicated group (G12) on the presented days.
Discussion
Coccidiosis is a bane to the poultry industry causing considerable economic loss. Misuse and abuse of current anti-coccidial drugs in poultry farming has raised public concerns about food safety. Edible herbs are emerging as an alternative approach to treat coccidiosis in chickens4,8,17. However, the use of medicinal herbs in coccidiosis is limited by the complexity of constituent phytochemicals and unknown mechanisms. Our previous publication demonstrated that B. pilosa has promising efficacy and safety18,23. Here, we extended our study to explore the minimum effective dose and prophylactic duration, identify the active compound(s) and elucidate the mechanism of B. pilosa and its active compounds. The results of this study will aid the development B. pilosa as an anti-coccidial phytogenic and medicine prior to commercial use in chickens.
In terms of anti-coccidial efficacy, we proved that the effective dose of B. pilosa could be as low as 0.01% under our experimental conditions (Fig. 1). In addition, 3-day administration of 0.01% B. pilosa was good enough to achieve anti-coccidial prevention (Fig. 1e). Of note, some parameters may affect the efficacy of B. pilosa; combinatorial infection with different Eimeria species, titer and virulence of Eimeria species, and chicken genetics24,25.
Eimeria species have a complex life cycle that starts when the sporulated oocysts are swallowed by chickens. The grinding action of the gizzard coupled to the enzymatic action in the gut lead to sporozoite release. The sporozoites develop into merizoites, followed by gametocytes, zygocytes and oocytes26. In this work, we illustrated that B. pilosa interfered with oocyst sporulation (Fig. 2c) and sporozoite invasion into cells (Fig. 3a) but not the viability of oocysts (Fig. 2a,b) and sporozoites (Fig. 3b). The histochemical staining of chicken ceca also showed that B. pilosa decreased the percentage of schizonts and their size (Fig. S2a–c) and the number of fecal oocysts (Fig. S2d), leading to production of precocious oocysts. All these data support the notion that B. pilosa interfered with the life cycle of E. tenella at the stages of sporogony, merogony, and, probably, gametogony. This anti-coccidial mode of action has an advantage over chemical anti-coccidials. Namely that B. pilosa may impair but not completely kill Eimeria progeny which, may in turn serve as a vaccine to boost host immunity to coccidiosis. Besides, the data on the intervention of sporozoite invasion by B. pilosa are consistent with a decrease in the shedding of fecal oocysts and survival rate in experimental chickens (Table 2). This work also demonstrates the feasibility of B. pilosa as a veterinary medicine for controlling coccidiosis in chickens.
Identification of active compound(s) from plants is a key challenge to developing herbal applications for medical purposes. Using a bioactivity-directed strategy, here we found that cytopiloyne inhibited the entry of sporozoites into cells more effectively than salinomycin, Compound 3 and Compound 2 (Fig. 4a). This result confirmed that cytopiloyne is the most active polyyne against coccidiosis. Of note, B. pilosa at 100 ppm and cytopilyne at 100 ppb fully protected against coccidiosis in chickens (Figs 1 and 5), suggesting that cytopiloyne was 1000 times more effective against coccidiosis than B. pilosa. Coincidently, the percentage of three polyynes in B. pilosa was 0.52‰, implying that polyynes are the major active phytochemicals of B. pilosa, although we cannot rule out the existence of other active compound(s) (Figs 4, 5 and S3). In a similar manner to B. pilosa extract (Figs 1–3), cytopiloyne exerted its anti-coccidial activities via suppression of sporozoite sporulation (data not shown) and invasion into cells (Figs 3c and 4a,b). Obviously, B. pilosa suppresses coccidiosis in chickens via interference with the life cycle of Eimeria (Figs 3, 4 and S2), but not via direct chemical destruction (Figs 3b and 4b). Therefore, this study provides the first evidence of the mechanism of B. pilosa in control of coccidiosis, a key step in research and development of in-feed additives and medicines against coccidiosis.
B. pilosa has been reported to modulate immune responses in animals27–33. As far as chicken immunity to coccidiosis is concerned, intestinal T cells have been reported to play a major role in host protection against coccidiosis in chickens34. We examined the impact of B. pilosa on T cells using a chicken Affymetrix genechip. The genome-wide study found that B. pilosa influenced the expression of 540 genes with a more than 1.5-fold increase (176 genes) or 2-fold decrease (364 genes) in T cells (data not shown). Among 540 genes, 100 genes were functionally known and selected for heatmap analysis (Fig. S2a). IFNγ, an anti-coccidial immunomodulator, was up-regulated by B. pilosa during E. tenella infection (Fig. S6b). Under non-infection conditions, B. pilosa did not boost IFNγ production (Fig. S6b). The data are consistent with the literature stating that IFN-γ expression was significantly increased in cecal tonsils which are an important component of the host immunity against coccidiosis35,36. However, whether the polyynes can increase IFN-γ needs to be ascertained.
Here, we assessed the efficacy, minimum effective dose and minimum prophylactic duration of B. pilosa for treating coccidiosis in chickens as evidenced by reducing mortality, oocyst excretion, intestinal lesions and body weight gain. In parallel, we identified three polyynes as active compounds present in B. pilosa using a bioactivity-guided approach. Among the polyynes, cytopiloyne was the most active compound in B. pilosa. Furthermore, we demonstrated that B. pilosa and cytopiloyne exert their anti-coccidial action via intervention with the protozoan life cycle and augmenting chicken immunity. In conclusion, this study demonstrates the anti-coccidial effects and mechanism of B. pilosa and its active compounds in chickens.
Methods
Preparation and analysis of B. pilosa and polyynes
The processing and analysis of B. pilosa were performed as previously published18. Briefly, the whole plant was authenticated by Dr. Kuo-Fang Chung (Academia Sinica Herbarium), collected and pulverized. For compound isolation and identification, B. pilosa was extracted with methanol and partitioned into different fractions, followed by compound isolation and identification using high pressure liquid chromatography37 unless indicated otherwise. Using an invasion assay-guided fractionation and isolation strategy, active polyynes were isolated and identified by spectroscopic methods as described elsewhere.
Preparation and sporulation of E. tenella oocysts
As previously described18, the E. tenella strain Et C1 was amplified and used throughout the study. The oocysts were collected from fresh feces of chickens, followed by sporulation with potassium dichromate.
Poultry husbandry, feed formula and oral infection of E. tenella
One-day-old uninfected Lohmann female chicks were obtained from a local hatchery. For efficacy study of B. pilosa, the chickens were randomly divided into 6 groups. They had ad libitum access to diets and water in the experiments. In Experiment 1, Group 1 (uninfected unmedicated control, CTR) and Group 2 (infected unmedicated control, Et) received daily standard chicken diet from day 1 to day 21. Group 3 (Et + Sal) were given a daily diet containing salinomycin (Sal, 60 mg/kg diet). Group 4 (Et + BP 0.05%), Group 5 (Et + BP 0.01%), and Group 6 (Et + BP 0.002%) were fed daily with a diet containing B. pilosa powder at the dose of 0.05% (0.5 g BP/kg diet), 0.01% (0.1 g BP/kg diet) or 0.002% (0.02 g BP/kg diet), respectively. In Experiment 2, to test the minimum prophylactic duration of B. pilosa powder, 4 groups of chickens (Groups 7 to 10) were fed with a standard diet or a diet containing B. pilosa powder (0.01%) for the indicated time periods prior to E. tenella challenge, on day 14. In Experiment 3, which was an efficacy study of Compound 1 (cytopiloyne, CP), the chickens were randomly divided into 6 groups. The chickens in Group 11 (CTR), Group 12 (Et), Group 13 (Et + Sal), Group 14 (Et + 500 ppb CP), Group 15 (Et + 100 ppb CP) and Group 16 (Et + 20 ppb CP) were fed daily with a standard diet and a diet containing salinomycin (Sal, 60 mg/kg diet) and CP (500, 100 and 20 μg/kg diet).
Chickens were challenged with E. tenella on day 14. Control chickens in Groups 1, 7 and 11 were given 2 ml of phosphate buffered saline (PBS) and those in Groups 2 to 6, 8 to 10, and 12 to 16 were challenged with E. tenella sporulated oocysts (1 × 104) on day 14. Survival rate, gut pathology, stool, and/or sick bird appearance were observed daily unless indicated otherwise in each group. Based on the study by Daszak et al., initial invasion of the fold tip of cecum occurs at ~4 hr post infection, when sporozites of E. tenella invade enterocytes and migrate through the connective tissue into the crypt epithelium38. In Experiment 4, to test the entry of E. tenella sporozoites into chicken guts, the chickens were randomly divided into 4 groups. The chickens in Group 17 (CTR), Group 18 (Et), Group 19 (Et + Sal), Group 20 (Et + 0.01% BP) were fed daily with a standard diet. On day 14, the chickens in Group 17 (CTR) were given 2 ml PBS and those in Group 18, 19 and 20 were challenged with a dose (1 × 104) of E. tenella and E. tenella-treated with salinomycin and 0.01% B. pilosa powder (0.1 g BP/kg diet). The chickens were sacrificed 4 hr post infection. The chicken ceca were fixed with formaldehyde, microtomized and stained with a periodic acid-Schieff kit as published39. All chickens in the study were complied with according to the guidelines and were approved by Institutional Animal Care and Use Committee (IACUC) of the National Chung Hsing University (permit number: 100–60).
Evaluation of survival rate, oocyst numbers, and gut lesions in animals
Survival rate and chicken appearance were observed daily as described previously18,23. The body weight of all the birds in the cages were measured on days 1, 7, 14 and 21 after hatching. Fecal samples were collected daily, from day 3 to 7 post infection, weighed and counted. Fecal oocyst number, expressed as oocysts per gram of feces (OPG), was obtained from the average of 3 counts of each sample. On day 14 post infection, each group of chickens was sacrificed and their ceca were collected. Macroscopic (gross) and microscopic lesion scores were calculated as described in our previous publication18,23.
Invasion assay, viability test and propidium iodide (PI) staining of E. tenella sporozoites
Madin–Darby bovine kidney (MDBK, ATCC CCL-22) cells were grown in DMEM containing 10% fetal bovine serum and supplements. The cells were seeded at a density of 2 × 105 cells/well onto glass cover slips in 24 wells. One day later, the cells were incubated with DMEM medium containing salinomycin (Fluka), plant extracts and phytochemicals at the indicated doses for 0.5 h. Fresh sporozoites (2 × 105) were added to the cells for an additional 4 h. After extensive PBS washing, the cells were fixed and stained with hematoxylin and eosin (Sigma). Photographs were taken with a microscope. Invasion percentage (%) was obtained by the formula, 100% × (the number of cells invaded by sporozoites/total cell number)22. For the viability test, the E. tenella sporozoites were incubated with plant extract, phytochemicals and salinomycin for 4.5 h. Microscopy was used to distinguish life and death in sporozoites. Survival rate (%) was obtained by the normalization of the dead cell number by total cell number multiplied by 100%. For sporulation assay, the E. tenella oocysts were pre-treated with PBS, boiling (100 °C for 30 min) and plant extracts at the indicated doses for 48 h. The oocysts were incubated with 2% potassium dichromate for 2 days before sporulation. The percentage of sporulating oocysts (%) was counted. For PI staining, the E. tenella oocysts underwent PBS (1 h), boiling treatment (100 °C for 30 min) or incubation with B. pilosa extracts at the indicated doses for 1 h. The oocysts were stained with PI. After PBS washing, the oocysts were examined using a microscope15.
Statistical analysis
Data from each group of chickens are presented as mean ± standard error (SE). The survival rate between treatment groups and control groups were analyzed using Pearson’s chi square test. The body weight gain of the factors group and cage group were analyzed by two way ANOVA using the GLM procedure of the SAS System. Data of the excreted oocyst was transformed into ln(x + 1) and subjected to ANOVA using the GLM procedure of the SAS System under a normal distribution. Chi-square test was used to value lesion scores after multinomial transformation. Actual P values of all experiments are presented.
Supplementary information
Acknowledgements
The authors thank all the members of WCY and CLTC laboratories for their technical assistance and Ms. M. Loney for editing the manuscript. They also thank the Metabolomics Core facilities of the Agricultural Biotechnology Research Center, Academia Sinica for their technical assistance. National Science Council of Taiwan (MOST 106-3114-B-005-001 and 106-2313-B-005-045-), Council of Agriculture of Taiwan (106AS-1.3.2-AD-U1 and 107AS-22.1.2-LI-L1), and Innovative Translational Agricultural Research Program of Academia Sinica (PRE04) grants supported this work.
Author Contributions
C.L.-T.C. conceptualized and supervised this study. W.-C.Y., C.-Y.Y., Y.-C.L., C.-W.Y., W.-Q.L., C.-Y.C., M.-T.Y. and T.-F.K., C.-F.L., C.-L.L. and C.L.-T.C. designed and performed experiments, analyzed data, interpreted results, and wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen-Chin Yang and Cheng-Ying Yang contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39194-2.
References
- 1.Faber TA, Dilger RN, Hopkins AC, Price NP, Fahey GC., Jr. The effects of a galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex in broiler chicks challenged with Eimeria acervulina. Poultry science. 2012;91:1089–1096. doi: 10.3382/ps.2011-019933. [DOI] [PubMed] [Google Scholar]
- 2.Williams RB. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. International journal for parasitology. 1999;29:1209–1229. doi: 10.1016/S0020-7519(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 3.Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert review of vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Orengo J, et al. Evaluating the efficacy of cinnamaldehyde and Echinacea purpurea plant extract in broilers against Eimeria acervulina. Veterinary parasitology. 2012;185:158–163. doi: 10.1016/j.vetpar.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 5.McDonald V, Shirley MW. Past and future: vaccination against Eimeria. Parasitology. 2009;136:1477–1489. doi: 10.1017/S0031182009006349. [DOI] [PubMed] [Google Scholar]
- 6.Chapman HD. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian pathology: journal of the W.V.P.A. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- 7.Sharman PA, Smith NC, Wallach MG, Katrib M. Chasing the golden egg: vaccination against poultry coccidiosis. Parasite Immunol. 2010;32:590–598. doi: 10.1111/j.1365-3024.2010.01209.x. [DOI] [PubMed] [Google Scholar]
- 8.Muthamilselvan, T., Kuo, T. F., Wu, Y. C. & Yang, W. C. Herbal Remedies for Coccidiosis Control: A Review of Plants, Compounds, and Anticoccidial Actions. Evid-Based Compl Alt, doi:Artn265798110.1155/2016/2657981 (2016). [DOI] [PMC free article] [PubMed]
- 9.Willcox ML, Bodeker G. Traditional herbal medicines for malaria. Bmj. 2004;329:1156–1159. doi: 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Youn HJ, Noh JW. Screening of the anticoccidial effects of herb extracts against Eimeria tenella. Veterinary parasitology. 2001;96:257–263. doi: 10.1016/S0304-4017(01)00385-5. [DOI] [PubMed] [Google Scholar]
- 11.Naidoo V, McGaw LJ, Bisschop SP, Duncan N, Eloff JN. The value of plant extracts with antioxidant activity in attenuating coccidiosis in broiler chickens. Veterinary parasitology. 2008;153:214–219. doi: 10.1016/j.vetpar.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Akhtar M, et al. Immunostimulatory and protective effects of Aloe vera against coccidiosis in industrial broiler chickens. Veterinary parasitology. 2012;186:170–177. doi: 10.1016/j.vetpar.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 13.Allen PC. Dietary supplementation with Echinacea and development of immunity to challenge infection with coccidia. Parasitol Res. 2003;91:74–78. doi: 10.1007/s00436-003-0938-y. [DOI] [PubMed] [Google Scholar]
- 14.Lee SH, et al. Effects of dietary supplementation with phytonutrients on vaccine-stimulated immunity against infection with Eimeria tenella. Veterinary parasitology. 2011;181:97–105. doi: 10.1016/j.vetpar.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.del Cacho E, Gallego M, Francesch M, Quilez J, Sanchez-Acedo C. Effect of artemisinin on oocyst wall formation and sporulation during Eimeria tenella infection. Parasitol Int. 2010;59:506–511. doi: 10.1016/j.parint.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Allen PC, Lydon J, Danforth HD. Effects of components of Artemisia annua on coccidia infections in chickens. Poultry science. 1997;76:1156–1163. doi: 10.1093/ps/76.8.1156. [DOI] [PubMed] [Google Scholar]
- 17.Remmal A, Achahbar S, Bouddine L, Chami N, Chami F. In vitro destruction of Eimeria oocysts by essential oils. Veterinary parasitology. 2011;182:121–126. doi: 10.1016/j.vetpar.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Yang WC, et al. Effect of Bidens pilosa on infection and drug resistance of Eimeria in chickens. Research in veterinary science. 2015;98:74–81. doi: 10.1016/j.rvsc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Bartolome AP, Villasenor IM, Yang WC. Bidens pilosa L. (Asteraceae): Botanical Properties, Traditional Uses, Phytochemistry, and Pharmacology. Evid Based Complement Alternat Med. 2013;2013:340215. doi: 10.1155/2013/340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young PH, Hsu YJ, Yang WC. Bidens pilosa and its medicinal use. Recent Progress in Medicial Plants/Drug plants II. 2010;28:411–426. doi: 10.3109/13880200903485729. [DOI] [Google Scholar]
- 21.Chang, C. L. T., Yang, C. Y., Muthamilselvan, T. & Yang, W. C. Field trial of medicinal plant, Bidens pilosa, against eimeriosis in broilers. Sci Rep-Uk 6, doi:Artn2469210.1038/Srep24692 (2016). [DOI] [PMC free article] [PubMed]
- 22.Burt SA, Tersteeg-Zijderveld MH, Jongerius-Gortemaker BG, Vervelde L, Vernooij JC. In vitro inhibition of Eimeria tenella invasion of epithelial cells by phytochemicals. Veterinary parasitology. 2013;191:374–378. doi: 10.1016/j.vetpar.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Chang CL, Yang CY, Muthamilselvan T, Yang WC. Field trial of medicinal plant, Bidens pilosa, against eimeriosis in broilers. Sci Rep. 2016;6:24692. doi: 10.1038/srep24692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abu-Akkada SS, Awad AM. Isolation, propagation, identification and comparative pathogenicity of five Egyptian field strains of Eimeria tenella from broiler chickens in five different provinces in Egypt. Research in veterinary science. 2012;92:92–95. doi: 10.1016/j.rvsc.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Bumstead N, Millard B. Genetics of resistance to coccidiosis: response of inbred chicken lines to infection by Eimeria tenella and Eimeria maxima. British poultry science. 1987;28:705–715. doi: 10.1080/00071668708417006. [DOI] [PubMed] [Google Scholar]
- 26.Price KR. Use of live vaccines for coccidiosis control in replacement layer pullets. Journal of Applied Poultry Research. 2012;21:679–692. doi: 10.1016/j.vetpar.2015.01.009. [DOI] [Google Scholar]
- 27.Chung CY, et al. Data on the effect of Cytopiloyne against Listeria monocytogenes infection in mice. Data in brief. 2016;7:995–998. doi: 10.1016/j.dib.2016.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung CY, et al. Cytopiloyne, a polyacetylenic glucoside from Bidens pilosa, acts as a novel anticandidal agent via regulation of macrophages. Journal of ethnopharmacology. 2016;184:72–80. doi: 10.1016/j.jep.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 29.Chang CL, et al. Cytopiloyne, a polyacetylenic glucoside, prevents type 1 diabetes in nonobese diabetic mice. Journal of immunology. 2007;178:6984–6993. doi: 10.4049/jimmunol.178.11.6984. [DOI] [PubMed] [Google Scholar]
- 30.Chang CL, et al. The distinct effects of a butanol fraction of Bidens pilosa plant extract on the development of Th1-mediated diabetes and Th2-mediated airway inflammation in mice. J Biomed Sci. 2005;12:79–89. doi: 10.1007/s11373-004-8172-x. [DOI] [PubMed] [Google Scholar]
- 31.Chang SL, et al. Polyacetylenic compounds and butanol fraction from Bidens pilosa can modulate the differentiation of helper T cells and prevent autoimmune diabetes in non-obese diabetic mice. Planta Med. 2004;70:1045–1051. doi: 10.1055/s-2004-832645. [DOI] [PubMed] [Google Scholar]
- 32.Chang SL, et al. Flavonoids, centaurein and centaureidin, from Bidens pilosa, stimulate IFN-gamma expression. Journal of ethnopharmacology. 2007;112:232–236. doi: 10.1016/j.jep.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Chang SL, et al. The effect of centaurein on interferon-gamma expression and Listeria infection in mice. Toxicol Appl Pharmacol. 2007;219:54–61. doi: 10.1016/j.taap.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Lillehoj HS, Trout JM. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clinical microbiology reviews. 1996;9:349–360. doi: 10.1128/CMR.9.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yun CH, Lillehoj HS, Choi KD. Eimeria tenella infection induces local gamma interferon production and intestinal lymphocyte subpopulation changes. Infection and immunity. 2000;68:1282–1288. doi: 10.1128/IAI.68.3.1282-1288.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lillehoj HS, Choi KD. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian diseases. 1998;42:307–314. doi: 10.2307/1592481. [DOI] [PubMed] [Google Scholar]
- 37.Chien SC, et al. Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry. 2009;70:1246–1254. doi: 10.1016/j.phytochem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Daszak P. Zoite migration during infection: parasite adaptation to host defences. Parasitology today. 1999;15:67–72. doi: 10.1016/S0169-4758(98)01379-9. [DOI] [PubMed] [Google Scholar]
- 39.Nakai Y, Ogimoto K. Relationship between amylopectin and infectivity of Eimeria tenella sporozoite. Nihon juigaku zasshi. The Japanese journal of veterinary science. 1987;49:447–452. doi: 10.1016/S0169-4758(98)01379-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.