Abstract
Studies examining associations between purine metabolites and type 2 diabetes (T2D) are limited. We prospectively examined associations between plasma levels of purine metabolites with T2D risk and the modifying effects of transcription factor-7-like-2 (TCF7L2) rs7903146 polymorphism on these associations. This is a case-cohort design study within the PREDIMED study, with 251 incident T2D cases and a random sample of 694 participants (641 non-cases and 53 overlapping cases) without T2D at baseline (median follow-up: 3.8 years). Metabolites were semi-quantitatively profiled with LC-MS/MS. Cox regression analysis revealed that high plasma allantoin levels, including allantoin-to-uric acid ratio and high xanthine-to-hypoxanthine ratio were inversely and positively associated with T2D risk, respectively, independently of classical risk factors. Elevated plasma xanthine and inosine levels were associated with a higher T2D risk in homozygous carriers of the TCF7L2-rs7903146 T-allele. The potential mechanisms linking the aforementioned purine metabolites and T2D risk must be also further investigated.
Introduction
Purines are constituent parts of nucleotides and nucleic acids, playing several roles in human physiology, affecting tissue function, cell integrity, and oxidation. In catabolism, their monophosphate forms are converted to inosine and guanosine; purine nucleoside phosphorylase converts them to hypoxanthine and guanine, respectively. Xanthine oxidase (XO), and guanine deaminase, convert them to xanthine. Finally, xanthine is again oxidized by XO to uric acid1.
Blood levels of uric acid are well regulated in healthy subjects but when hyperuricemia occurs, its chronicity has been associated with gout, muscular pain, cardiovascular or/and renal disease and type 2 diabetes (T2D)2. Concerning other purine metabolites, elevated plasma allantoin concentrations (8-fold higher) were reported in patients with diabetes, compared to healthy controls3, indicating an increased oxidative stress in this metabolic condition. In addition, studies in erythrocytes of patients with diabetes4 indicated increased adenine and guanine de-phosphorylation, as well as increased adenosine, inosine, guanosine, and hypoxanthine concentrations, together with a higher turnover ratio of nucleotides and hyper-metabolism, when compared to that in healthy subjects.
In regard to the enzymes involved, compared to healthy controls, serum XO activity was found increased in patients with T2D5. Similarly, in patients with metabolic syndrome, XO activity was increased and associated with inflammatory or oxidative status markers6. In addition, the activity of adenosine deaminase (ADA), an enzyme that converts adenosine into inosine, was found increased significantly in T2D patients, correlating positively with blood glucose levels7. Since adenosine increases glucose uptake into cells, its deamination in insulin-sensitive tissues may be considered a homeostatic cell-protecting adaptation8.
Studies that examine cross-sectional or/and longitudinal associations among peripheral levels of uric acid and other purine metabolites, diabetes risk factors, and diabetes incidence are limited9. Likewise, although the transcription factor-7-like-2 (TCF7L2) rs7903146 polymorphism is one of the main genes associated with increased T2D risk, its interactions with purine metabolites overall10, or involvement in genetically linking T2D with gout development11, remain unknown. Therefore, in the present prospective study, nested in the framework of the PREDIMED trial, we examined associations between plasma levels of purine-catabolism metabolites or relevant ratios (precursor-product) and T2D risk. Furthermore, we examined whether the TCF7L2-rs7903146 polymorphism modifies these associations.
Methods
Study design and population
This study was nested, as an unstratified case-cohort study, within the PREDIMED study (ISRCTN35739639), a cardiovascular primary-prevention trial conducted in Spanish primary healthcare centers. The methods and design of the PREDIMED trial has been described in detail elsewhere12. The present case-cohort study comprises a random selection of 694 non-diabetic participants (approximately 20%) from the eligible subjects of the PREDIMED cohort without T2D at study inception (3,541 participants were free of T2D at baseline) and with available blood samples, together with all incident cases of T2D that occurred during the follow-up with available plasma samples (251 out of the 273 incident cases). Of the 892 participants included in our analyses, 641 were in the sub-cohort (including 53 overlapping cases) and 198 were the rest of the T2D incident cases, giving a total of 251 cases (Supplemental Fig. 1). Of these, 686 participants out of the 892 had available samples after 1-year of follow-up and were included in the 1-year changes analyses (Supplemental Fig. 1). The Research Ethics Committees (RECs) for each of the recruitment centers approved the study protocol, and participants provided written informed consent. All analyses were performed in accordance with the relevant guidelines and regulations. The RECs were: del Hospital Clinic de Barcelona, del Hospital Universitari Sant Joan de Reus, de la universidad de Valencia, de la Universidad de Navarra, de la Facultad de Medicina, de la Universidad de Málaga, de Euskadi, del Complejo Hospitalario Universitario Insular- Materno Infantil (CHUIMI) del Servicio Canario de Salud, Las Palmas de Gran Canaria, del Parc de Salut Mar, de les Balearic Islands.
Study samples and metabolite profiling
Fasting (for ≥8 hours) plasma EDTA samples (baseline and at the end of 1-year of follow-up) were collected from subjects and stored at −80 °C. In June 2015, pairs of samples for each participant were randomly ordered and shipped on dry ice to the Broad Institute (Boston, Massachusets, USA) for metabolomics assays. Metabolites related to purine catabolism, including uric acid, allantoin, xanthine, hypoxanthine, inosine, adenosine and guanosine, were semi-quantitatively profiled using liquid chromatography-tandem mass spectrometry (LC-MS) on a system comprised of a Shimadzu Nexera X2 U-HPLC (Shimadzu Corp., Marlborough, MA, USA), coupled to a Q Exactive hybrid quadrupoleorbitrap mass spectrometer (Thermo Fisher Scientific)13. Metabolite identities were confirmed using authentic reference standards. Raw data were processed using TraceFinder software (Thermo Fisher Scientific) and Progenesis QI (Nonlinear Dynamics; Newcastle upon Tyne, UK).
DNA Extraction and Genotyping
Genomic DNA was extracted from buffy-coat and the TCF7L2-rs7903146 was genotyped in the whole cohort on a 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using a fluorescent allelic discrimination TaqManTM assay as previously described14.
Ascertainment of T2D cases
T2D was a pre-specified secondary endpoint of the PREDIMED trial and it was identified by clinical diagnosis and/or use of antidiabetic medication. Information was collected through contact with participants and primary health care physicians, annual follow-up visits, yearly ad-hoc reviews of medical charts and annual consultation of the National Death Index, as it has been described elsewhere15. The American Diabetes Association criteria16, namely two confirmations of fasting plasma glucose ≥7.0 mmol/L or 2-h plasma glucose ≥11.1 mmol/L, after a 75-g oral glucose load were used to adjudicate incident cases.
Assessment of covariates and other variables
At baseline and at yearly follow-up visits, a 47-item questionnaire about lifestyle variables, smoking status, medical history and medication use was administered. Physical activity was assessed using a validated Spanish version of the Minnesota Leisure Time Physical Activity Questionnaire17. To assess the degree of adherence to Mediterranean diet (MedDiet), a 14-item validated questionnaire was filled in for each participant18. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Participants’ triacylglycerol (TAG), total cholesterol, high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels were measured using fasting plasma at baseline. Blood glucose and insulin levels were centrally assessed at baseline and at the end of 1-year of follow-up. Insulin resistance was estimated by the HOMA method using the following equation: HOMA-IR was calculated using the following equation: HOMA-IR = [fasting insulin (μIU/mL) × fasting glucose (mmol/L)]/22.5.
Statistical analysis
Baseline characteristics of cases and non-cases were described as means and standard deviations (SD) for quantitative variables, and percentages or numbers for categorical variables. We applied a natural logarithmic transformation to approximate a normal distribution of metabolites levels. We also examined product-to-precursor ratios of metabolites, as described in the pathway of purine catabolism, as a metabolite trait (by dividing the raw values and then taking natural logarithmic transformations). Person-time of follow-up was calculated as the interval between the randomization date and date of T2D event, death, or date of the last contact, whichever came first. We used Cox proportional hazard models, with Barlow weights (to account for the over-representation of cases), to estimate hazard ratios (HRs) and their 95% confidence intervals (CIs) for risk of T2D. A crude model and two multivariable-adjusted Cox regression models were fitted as follows: a) multivariable model 1 (MV1), adjusted for age (years), sex (male, female), body mass index (kg/m2), intervention group and baseline fasting glucose (mg/dl) (adding a quadratic term to account for the departure from linearity; b) MV2, additionally adjusted for the TCF7L2-rs7903146 polymorphism (assuming an additive genetic model), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), baseline dyslipidaemia (yes/no), and hypertension (yes/no). We stratified the models according to recruitment center. Baseline metabolites levels and their ratios were analysed as both continuous variables (1-SD increment in their transformed levels) and using quartiles (using cut-points defined among non-cases). To appraise the linear trend across quartiles, the median metabolites levels and their ratios within each quartile was included in the Cox regression models as a continuous variable. To account for multiple testing, we adjusted p-values of the multivariable adjusted associations between quartiles or 1-SD increment in metabolites levels and T2D risk, using Benjamin-Hochberg false discovery rate (FDR) procedure19. A FDR-p-value < 0.05 was considered to be statistically significant. We also examined the associations of 1-year changes in metabolites and their ratios with T2D risk. We used the same models as in the baseline analyses but further adjusted for baseline metabolites levels or their ratios. With respect to metabolites or their ratios, we first calculated the ratio between 1-year and baseline levels and then normalized this ratio with the natural logarithmic transformation. To test the robustness of our findings we conducted one sensitivity analysis on the associations of baseline metabolites or their ratios with risk for T2D by including two variables (HDL and TAG) in the multivariable model 2 that were significantly different between cases and non-cases, leaving 71 incident cases due to the high percentage of missing values (HDL: 41%, TAG: 33%). To examine whether the aforementioned genotypes modified the association between metabolites or relevant ratios and T2D incidence, interactions were tested by including a multiplicative interaction term (metabolite x TCF7L2-rs7903146 genotypes) or (ratio x TCF7L2-rs7903146 genotypes) and also a TCF7L2-rs7903146 genotypes main effect in the MV2. Stratified analyses by TCF7L2-rs7903146 genotypes were also carried out assuming an additive genetic model. The associations were adjusted for multiple testing as described above. We also estimated the joint association of plasma metabolites levels and rs7903146 polymorphisms (TT or CC genotypes) with T2D. We considered as the reference group those participants with CC genotype and metabolites levels (lower than the median). Statistical analyses were performed using Stata 13.1 (Stata Corp., College Station, Texas, USA). A two-sided p value less than 0.05 was considered significant.
Results
Participants’ characteristics
The median follow-up of the study population was 3.8 years. A total of 251 incident cases and 641 control participants were included in the study. Participants’ characteristics are summarized in Table 1. The mean age of participants at baseline was 66.5 years and the mean BMI was 30.1 (3.5) kg/m2. Briefly, a higher proportion of incident cases were men and current smokers compared to non-cases. As compared with non-cases, those participants who developed T2D were also more likely to have a higher prevalence of hypertension and dyslipidaemia in addition to higher BMI, fasting glucose and triacylglycerol levels while lower HDL cholesterol levels (Table 1).
Table 1.
Baseline characteristics of the study population.
| Total | Cases | Non-cases | p value | |
|---|---|---|---|---|
| n | 892 | 251 | 641 | |
| Age (years) | 66.5 (5.7) | 66.4 (5.7) | 66.5 (5.7) | 0.781 |
| Sex (% Women) | 61.2 | 55.0 | 63.6 | 0.017 |
| Body mass index, kg/m2 | 30.1 (3.5) | 30.8 (3.3) | 29.8 (3.6) | <0.001 |
| Physical activity, METs/d | 240.7 (234.6) | 249.2 (233.5) | 237.4 (235.1) | 0.500 |
| Intervention group, % | ||||
| MedDiet + EVOO | 30.6 | 29.9 | 30.9 | 0.425 |
| MedDiet + Nuts | 36.3 | 37.2 | 33.8 | |
| Control group | 33.1 | 36.3 | 31.8 | |
| Hypertension, % | 91.7 | 96.0 | 90.0 | 0.003 |
| Dyslipidaemia, % | 84.3 | 79.7 | 86.1 | 0.018 |
| Smoking, % | ||||
| Never | 59.0 | 52.6 | 61.5 | 0.006 |
| Former | 22.4 | 22.3 | 22.5 | |
| Current | 18.6 | 25.1 | 16.0 | |
| Score for adherence to Mediterranean dieta | 8.5 (2.0) | 8.4 (2.0) | 8.6 (1.9) | 0.186 |
| Fasting blood glucose, mg/dl | 103.3 (17.6) | 118.6 (18.0) | 97.8 (13.8) | <0.001 |
| Total cholesterol, mg/dl | 222.2 (39.3) | 221.7 (42.3) | 222.4 (38.1) | 0.846 |
| HDL cholesterol, mg/dL | 54.7 (13.0) | 52.6 (12.7) | 55.7 (13.1) | 0.014 |
| LDL cholesterol, mg/dL | 139.7 (32.9) | 137.0 (31.6) | 141.0 (33.3) | 0.201 |
| Triacylglycerol, mg/dL | 140.1 (85.0) | 169.0 (121.0) | 128.6 (62.0) | <0.001 |
Data are mean (SD) or percentage. aThis score is based on the 14-item dietary screener. The x2 test was used for comparison of categorical variables and Student’s t-test was used for comparison of continuous variables. MedDiet, Mediterranean diet; EVOO, Extra-virgin olive oil; MET, metabolic equivalent, HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Associations of baseline metabolites and relevant ratios (precursor-product) with risk for T2D
Table 2 shows the associations of baseline levels of individual metabolites and relevant ratios with T2D risk. In fully-adjusted model (MV2), allantoin when modelled as quartile, was significantly associated with lower T2D risk [HR in the highest versus lowest quartile was 0.44 (95% CI 0.25–0.80). Regarding ratios of allantoin-to-uric acid and xanthine-to-hypoxanthine, the estimated HR for incident T2D in the highest versus lowest quartile was 0.42 (95% CI 0.22–0.82) and 2.53 (95% CI 1.40–4.58), respectively. These associations remained significant after accounting for multiple comparisons. There were no significant associations between other baseline metabolites or ratios and incident T2D, either they were modelled continuously (per 1 SD) or as quartiles.
Table 2.
Associations of baseline individual metabolites levels and relevant ratios (precursor-product) with the risk of type 2 diabetes in the PREDIMED study, 2003–2010. Overall group.
| Quartiles of plasma metabolite levels | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Metabolite | Q1 | Q2 | Q3 | Q4 | p trend | FDR-Adjusted p value (Q4 vs. Q1) | HR per 1 SD increment | p value | FDR-Adjusted p value |
| Uric Acid | |||||||||
| Cases | 47 | 60 | 58 | 85 | |||||
| Crude model | Ref. | 1.18 (0.73, 1.91) | 1.01 (0.62, 1.64) | 1.55 (0.98, 2.45) | 0.091 | 1.16 (0.97, 1.38) | 0.098 | ||
| MV1 | Ref. | 1.20 (0.70, 2.05) | 0.97 (0.54, 1.74) | 1.35 (0.78, 2.35) | 0.373 | 1.09 (0.90, 1.31) | 0.363 | ||
| MV2 | Ref. | 1.20 (0.68, 2.11) | 0.94 (0.52, 1.71) | 1.36 (0.77, 2.40) | 0.412 | 0.637 | 1.08 (0.88, 1.31) | 0.446 | 0.559 |
| Allantoin | |||||||||
| Cases | 72 | 63 | 72 | 43 | |||||
| Crude model | Ref. | 0.65 (0.42, 1.02) | 0.89 (0.58, 1.37) | 0.60 (0.36, 1.01) | 0.142 | 0.97 (0.83, 1.15) | 0.787 | ||
| MV1 | Ref. | 0.70 (0.44, 1.13) | 0.79 (0.49, 1.28) | 0.48 (0.27, 0.85) | 0.020 | 0.89 (0.75, 1.06) | 0.193 | ||
| MV2 | Ref. | 0.66 (0.40, 1.08) | 0.73 (0.44, 1.22) | 0.44 (0.25, 0.80) | 0.011 | 0.045 | 0.86 (0.73, 1.02) | 0.094 | 0.407 |
| Xanthine | |||||||||
| Cases | 56 | 60 | 64 | 69 | |||||
| Crude model | Ref. | 1.09 (0.68, 1.76) | 1.17 (0.72, 1.90) | 1.47 (0.91, 2.37) | 0.095 | 1.21 (1.04, 1.41) | 0.015 | ||
| MV1 | Ref. | 1.23 (0.73, 2.07) | 1.19 (0.71, 2.00) | 1.07 (0.61, 1.88) | 0.901 | 1.08 (0.89, 1.33) | 0.408 | ||
| MV2 | Ref. | 1.15 (0.67, 1.99) | 1.22 (0.71, 2.09) | 1.04 (0.58, 1.87) | 0.929 | 0.886 | 1.13 (0.92, 1.38) | 0.243 | 0.451 |
| Hypoxanthine | |||||||||
| Cases | 61 | 77 | 55 | 57 | |||||
| Crude model | Ref. | 1.33 (0.86, 2.06) | 0.93 (0.58, 1.48) | 1.02 (0.63, 1.66) | 0.738 | 1.00 (0.83, 1.20) | 0.977 | ||
| MV1 | Ref. | 1.44 (0.91, 2.29) | 0.93 (0.55, 1.57) | 0.85 (0.49, 1.47) | 0.296 | 0.93 (0.76, 1.13) | 0.472 | ||
| MV2 | Ref. | 1.30 (0.80, 2.11) | 0.82 (0.48, 1.42) | 0.75 (0.42, 1.33) | 0.145 | 0.637 | 0.89 (0.72, 1.10) | 0.280 | 0.455 |
| Inosine | |||||||||
| Cases | 67 | 48 | 75 | 56 | |||||
| Crude model | Ref. | 0.73 (0.45, 1.18) | 1.02 (0.65, 1.62) | 1.02 (0.62, 1.68) | 0.613 | 1.11 (0.91 1.34) | 0.286 | ||
| MV1 | Ref. | 0.73 (0.42, 1.27) | 0.90 (0.54, 1.48) | 0.92 (0.54, 1.57) | 0.995 | 1.06 (0.86, 1.31) | 0.542 | ||
| MV2 | Ref. | 0.75 (0.42, 1.33) | 0.98 (0.58, 1.63) | 0.89 (0.50, 1.58) | 0.939 | 0.798 | 1.05 (0.84, 1.31) | 0.646 | 0.699 |
| Adenosine | |||||||||
| Cases | 42 | 62 | 82 | 62 | |||||
| Crude model | Ref. | 1.06 (0.64, 1.76) | 1.35 (0.84, 2.19) | 0.90 (0.54, 1.48) | 0.774 | 1.03 (0.88, 1.21) | 0.700 | ||
| MV1 | Ref. | 0.98 (0.56, 1.71) | 1.34 (0.80, 2.25) | 0.72 (0.40, 1.31) | 0.374 | 0.89 (0.72, 1.09) | 0.275 | ||
| MV2 | Ref. | 0.88 (0.50, 1.55) | 1.21 (0.72, 2.05) | 0.64 (0.34, 1.19) | 0.224 | 0.516 | 0.85 (0.68, 1.06) | 0.162 | 0.451 |
| Guanosine | |||||||||
| Cases | 44 | 57 | 71 | 77 | |||||
| Crude model | Ref. | 0.92 (0.56, 1.51) | 1.04 (0.64, 1.69) | 1.15 (0.71, 1.88) | 0.461 | 1.00 (0.80, 1.24) | 0.992 | ||
| MV1 | Ref. | 0.93 (0.51, 1.70) | 1.09 (0.60, 1.95) | 1.22 (0.67, 2.22) | 0.412 | 1.12 (0.88, 1.42) | 0.361 | ||
| MV2 | Ref. | 0.95 (0.50, 1.78) | 1.06 (0.58, 1.94) | 1.34 (0.73, 2.46) | 0.281 | 0.637 | 1.17 (0.89, 1.54) | 0.239 | 0.451 |
| Ratio of metabolites | |||||||||
| Inosine-to-Adenosine Ratio | |||||||||
| Cases | 54 | 61 | 44 | 58 | |||||
| Crude model | Ref. | 1.04 (0.64, 1.68) | 0.79 (0.47, 1.32) | 1.04 (0.62, 1.76) | 0.821 | 0.84 (0.68, 1.03) | 0.104 | ||
| MV1 | Ref. | 1.13 (0.66, 1.92) | 0.95 (0.55, 1.64) | 1.15 (0.65, 2.02) | 0.761 | 0.86 (0.68, 1.09) | 0.221 | ||
| MV2 | Ref. | 1.17 (0.66, 2.07) | 0.93 (0.51, 1.70) | 1.20 (0.66, 2.21) | 0.712 | 0.787 | 0.89 (0.71, 1.13) | 0.349 | 0.504 |
| Uric Acid-to-Xanthine Ratio | |||||||||
| Cases | 56 | 59 | 71 | 65 | |||||
| Crude model | Ref. | 0.74 (0.47, 1.17) | 0.91 (0.57, 1.45) | 0.83 (0.52, 1.31) | 0.586 | 0.88 (0.74, 1.05) | 0.161 | ||
| MV1 | Ref. | 0.94 (0.53, 1.66) | 1.13 (0.67, 1.89) | 0.99 (0.58, 1.70) | 0.886 | 0.96 (0.77, 1.19) | 0.706 | ||
| MV2 | Ref. | 0.87 (0.49, 1.56) | 1.16 (0.68, 1.98) | 0.83 (0.47, 1.47) | 0.731 | 0.787 | 0.92 (0.73, 1.15) | 0.473 | 0.559 |
| Allantoin-to-Uric Acid Ratio | |||||||||
| Cases | 73 | 70 | 69 | 37 | |||||
| Crude model | Ref. | 0.78 (0.50, 1.20) | 0.79 (0.52, 1.22) | 0.57 (0.33, 0.98) | 0.052 | 0.91 (0.78, 1.07) | 0.283 | ||
| MV1 | Ref. | 0.77 (0.48, 1.25) | 0.89 (0.56, 1.42) | 0.43 (0.22, 0.82) | 0.023 | 0.85 (0.72, 1.01) | 0.074 | ||
| MV2 | Ref. | 0.77 (0.46, 1.27) | 0.91 (0.57, 1.45) | 0.42 (0.22, 0.82) | 0.023 | 0.047 | 0.84 (0.71, 0.98) | 0.035 | 0.234 |
| Xanthine-to-Guanosine Ratio | |||||||||
| Cases | 62 | 54 | 77 | 55 | |||||
| Crude model | Ref. | 0.97 (0.61, 1.57) | 1.31 (0.84, 2.06) | 1.42 (0.88, 2.30) | 0.077 | 1.22 (1.04, 1.43) | 0.013 | ||
| MV1 | Ref. | 0.96 (0.58, 1.60) | 1.16 (0.71, 1.91) | 0.87 (0.49, 1.55) | 0.762 | 1.02 (0.84, 1.25) | 0.803 | ||
| MV2 | Ref. | 0.98 (0.58, 1.66) | 1.22 (0.72, 2.06) | 0.87 (0.48, 1.58) | 0.742 | 0.798 | 1.03 (0.84, 1.29) | 0.727 | 0.727 |
| Xanthine-to-Hypoxanthine Ratio | |||||||||
| Cases | 43 | 70 | 57 | 80 | |||||
| Crude model | Ref. | 1.21 (0.75, 1.96) | 1.06 (0.64, 1.76) | 1.86 (1.14, 3.02) | 0.290 | 1.21 (1.03, 1.42) | 0.017 | ||
| MV1 | Ref. | 1.01 (0.59, 1.72) | 1.07 (0.61, 1.91) | 1.84 (1.08, 3.15) | 0.095 | 1.14 (0.95, 1.38) | 0.157 | ||
| MV2 | Ref. | 1.16 (0.65, 2.07) | 1.32 (0.70, 2.47) | 2.53 (1.40, 4.58) | 0.085 | 0.026 | 1.23 (1.01, 1.50) | 0.036 | 0.234 |
| Hypoxanthine-to-Inosine Ratio | |||||||||
| Cases | 72 | 57 | 54 | 66 | |||||
| Crude model | Ref. | 0.61 (0.39, 0.96) | 0.63 (0.39, 1.01) | 0.77 (0.48, 1.24) | 0.301 | 0.89 (0.73, 1.08) | 0.244 | ||
| MV1 | Ref. | 0.68 (0.42, 1.10) | 0.58 (0.33, 1.00) | 0.93 (0.56, 1.55) | 0.561 | 0.87 (0.70, 1.08) | 0.212 | ||
| MV2 | Ref. | 0.69 (0.41, 1.13) | 0.63 (0.35, 1.11) | 0.91 (0.53, 1.55) | 0.552 | 0.798 | 0.86 (0.69, 1.08) | 0.201 | 0.451 |
Abbreviations: MV, multivariable model; SD, standard deviation. A natural logarithmic transformation was applied to the raw value of individual metabolites. In the case of ratios of metabolites characterised by a precursor-product relationship, their raw values underwent natural logarithmic transformation. Cox regression analysis. MV1: Adjusted for age (years), sex (male, female), body mass index (kg/m2), intervention group (MedDiet + EVOO, MedDiet + nuts) and baseline fasting glucose (mg/dl) (centered on the sample mean and adding quadratic term). MV2: additionally adjusted for TCF7L2-rs7903146 genotype (assuming an additive genetic model), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia and hypertension. False discovery rate (FDR) controlling adjustments were conducted by applying the method of Benjamini and Hochberg. MV, multivariable model.
One year changes in levels of metabolites, relevant ratios (precursor-product) and risk of T2D
Associations between 1-year changes in metabolites levels and relevant ratios across quartiles with the risk of T2D are shown in Supplemental Table 1. In the highest quartile of increase in inosine-to-adenosine and xanthine-to-guanosine ratio a significant inverse association with T2D risk was found, with HR 0.43 (95% CI 0.19–0.95), and HR 0.42 (95% CI 0.20–0.88), respectively. We repeated the analyses using 1-SD increment in 1-year changes of metabolite levels and relevant ratios and found that per 1-SD increase in xanthine-to-guanosine ratio, the risk of T2D was associated with a decrease of 22%, with HR 0.78 (95% CI 0.62–0.98) (Supplemental Table 2). After adjusting for multiple testing, none of these associations remained statistically significant.
Sensitivity analysis
Supplemental Table 3 shows the associations of baseline levels of individual metabolites and relevant ratios with T2D risk after including HDL cholesterol and TAG in the fully adjusted model. The associations between allantoin and allantoin-to-uric acid ratio with T2D risk remained significant.
Spearman’s correlation analysis
The correlations between baseline metabolites, relevant ratios and baseline HOMA-IR are presented in Table 3. Several metabolites including uric acid, guanosine, xanthine and adenosine were found to be positively correlated with HOMA-IR (p < 0.05). The ratio of xanthine-to-hypoxanthine was also positively correlated with HOMA-IR (r = 0.09, p = 0.015). On the other hand, inosine-to-adenosine and allantoin-to-uric acid ratios were negatively correlated with HOMA-IR (r = −0.09, p = 0.013; r = −0.07, p = 0.037). Correlation analysis between 1-year changes in levels of metabolites, relevant ratios and 1-year changes in HOMA-IR (increase in HOMA-IR levels) revealed significant positive correlations for uric acid (r = 0.14, p = 0.001), allantoin (r = 0.09, p = 0.026), guanosine (r = 0.13, p = 0.002) and xanthine-to-hypoxanthine ratio (r = 0.10, p = 0.024).
Table 3.
Spearman’s correlation analysis between baseline metabolites levels, relevant ratios and baseline HOMA-IR.
| Spearman r | p value | |
|---|---|---|
| Uric Acid | 0.22 | <0.001 |
| Allantoin | 0.01 | 0.682 |
| Xanthine | 0.13 | 0.001 |
| Hypoxanthine | 0.02 | 0.644 |
| Inosine | 0.01 | 0.719 |
| Adenosine | 0.11 | 0.003 |
| Guanosine | 0.16 | <0.001 |
| Inosine-to-Adenosine | −0.09 | 0.013 |
| Uric Acid-to-Xanthine | 0.04 | 0.214 |
| Allantoin-to-Uric Acid | −0.07 | 0.037 |
| Xanthine-to-Guanosine | 0.04 | 0.266 |
| Xanthine-to-Hypoxanthine | 0.09 | 0.015 |
| Hypoxanthine-to-Inosine | 0.01 | 0.821 |
Interaction between the TCF7L2-rs7903146 polymorphism and metabolites on T2D risk
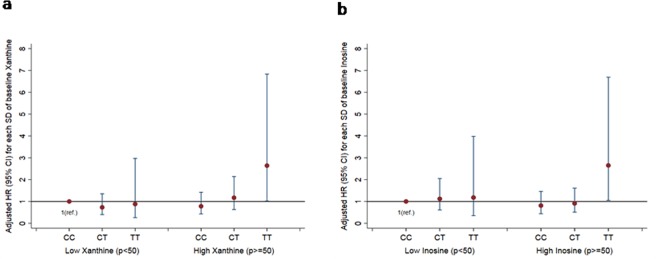
As expected, TT individuals had a higher risk for T2D compared with CC homozygotes, with HR 2.03 (95% CI 1.12–3.70), p = 0.020, after controlling for several potential confounding factors. No excess risk was conferred by the heterozygous genotype [CT vs. CC: HR 1.19 (95% CI 0.79–1.79), p = 0.387]. We found statistically significant interactions between metabolites levels, a relevant ratio and the TCF7L2-rs7903146 polymorphism in determining T2D risk in an additive model (Table 4). We found that per 1-SD increase in plasma xanthine and inosine levels, the risk of T2D significantly increased in TT individuals, with HR 2.34 (95% CI 1.23–4.45), and HR 3.09 (95% CI 1.23–7.73), respectively. On the other hand, per 1-SD increase in hypoxanthine-to-inosine ratio, a 74% lower risk of T2D was found in TT subjects [HR 0.26 (95% CI 0.10–0.70)]. After adjustment for multiple comparisons the associations between these metabolites, the ratio and T2D risk remained statistically significant. When the joint effects were examined, individuals with TT genotype and plasma xanthine levels above/equal to the median value had significantly higher risk of T2D [HR 2.64 (95% CI 1.02–6.83)] than those with CC genotype and xanthine levels below the median (reference) (Fig. 1a). Similarly, higher risk was found in TT individuals when levels of inosine were higher or equal to the median [HR 2.65 (95% CI 1.05–6.69)] (Fig. 1b). The levels of the aforementioned metabolites did not significantly differ across the genotypes in the additive model.
Table 4.
Associations of baseline individual metabolites levels and relevant ratios (precursor-product) with the risk of type 2 diabetes by transcription factor-7-like 2 (TCF7L2) rs7903146 genotype (additive model: TT & CT & CC).
| CC | CT | TT | FDR-Adjusted p value* | p interaction | |
|---|---|---|---|---|---|
| n | 382 | 383 | 104 | ||
| Cases, n | 106 | 103 | 35 | ||
| Per SD of change | HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Metabolite | |||||
| Uric Acid | 1.08 (0.82, 1.42) | 0.97 (0.71, 1.32) | 1.35 (0.48, 3.84) | 0.668 | 0.540 |
| Allantoin | 0.92 (0.73, 1.15) | 0.92 (0.63, 1.34) | 0.74 (0.58, 0.93) | 0.026 | 0.581 |
| Xanthine | 1.01 (0.73, 1.40) | 1.37 (0.99, 1.89) | 2.34 (1.23, 4.45) | 0.026 | 0.009 |
| Hypoxanthine | 0.95 (0.69, 1.31) | 0.83 (0.59, 1.17) | 1.23 (0.29, 5.27) | 0.842 | 0.238 |
| Inosine | 0.89 (0.61, 1.28) | 1.05 (0.78, 1.41) | 3.09 (1.23, 7.73) | 0.029 | 0.009 |
| Adenosine | 0.95 (0.71, 1.27) | 0.70 (0.47, 1.03) | 0.32 (0.04, 2.62) | 0.371 | 0.190 |
| Guanosine | 0.97 (0.78, 1.20) | 1.75 (1.07, 2.87) | 1.02 (0.26, 3.92) | 0.978 | <0.001 |
| Ratio of metabolites | |||||
| Inosine-to-Adenosine Ratio | 0.99 (0.71, 1.39) | 0.87 (0.58, 1.30) | 0.33 (0.14, 0.78) | 0.026 | 0.083 |
| Uric Acid-to-Xanthine Ratio | 1.04 (0.70, 1.55) | 0.74 (0.54, 1.02) | 0.46 (0.19, 1.14) | 0.135 | 0.032 |
| Allantoin-to-Uric Acid Ratio | 0.87 (0.69, 1.11) | 0.94 (0.64, 1.38) | 0.74 (0.57, 0.95) | 0.032 | 0.520 |
| Xanthine-to-Guanosine Ratio | 1.04 (0.76, 1.42) | 1.03 (0.69, 1.52) | 2.91 (1.42, 5.97) | 0.026 | 0.068 |
| Xanthine-to-Hypoxanthine Ratio | 1.05 (0.76, 1.46) | 1.55 (1.12, 2.14) | 2.84 (1.26, 6.38) | 0.026 | 0.372 |
| Hypoxanthine-to-Inosine Ratio | 1.15 (0.78, 1.71) | 0.83 (0.62, 1.11) | 0.26 (0.10, 0.70) | 0.026 | 0.004 |
Product (TCF7L2 × metabolite or ratio) interaction term was included into the model. The PREDIMED study, 2003–2010.
Abbreviations: SD, standard deviation.
aA natural logarithmic transformation was applied to the raw values individual metabolites. In the case of ratios of metabolites characterised by a precursor-product relationship, their raw values underwent natural logarithmic transformation. Cox regression analysis.
bStratified by recruitment center; cAdjusted for baseline fasting glucose (mg/dl) (centered on the sample mean and adding quadratic term), age (years), sex (male, female), intervention group (MedDiet + EVOO, MedDiet + nuts), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia and hypertension. False discovery rate (FDR) controlling adjustments were conducted by applying the method of Benjamini and Hochberg.
*Homozygous carriers of the TCF7L2-rs7903146 T-allele.
Figure 1.
(a) Joint association of plasma xanthine levels and TCF7L2-rs7903146 genotypes in relation to type 2 diabetes risk. The number 50 corresponds to 50th percentile; median. (b) Joint association of plasma inosine levels and TCF7L2-rs7903146 genotypes in relation to type 2 diabetes risk. The number 50 corresponds to 50th percentile; median.
Discussion
In the present case-cohort design study within the PREDIMED trial, after adjusting for recognized T2D risk-factors and multiple testing, we found inverse and positive associations between high baseline levels of allantoin, including allantoin-to-uric acid ratio and high xanthine-to-hypoxanthine ratio with T2D risk, respectively. We also found that elevated plasma xanthine and inosine levels were associated with a higher T2D risk in individuals with TT alleles on rs7903146. This is the first time, as far as we know10,11, that this genetic variant of the TCF7L2 gene, which mediates high susceptibility to T2D, is reported to interact with purine-catabolism metabolites.
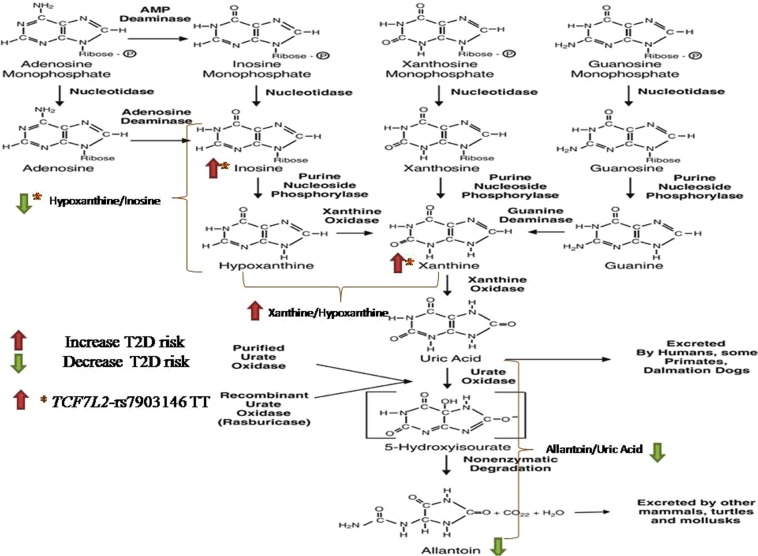
Allantoin is produced from the non-enzymatic oxidation of uric acid (Fig. 2) in humans and is considered to be a specific biomarker of oxidative stress3,20. We found that higher allantoin levels were associated with lower risk of T2D incidence as was the allantoin-to-uric acid ratio. This ratio was also negatively correlated with HOMA-IR at baseline. Our results are in accordance with the literature. When administered through injection, allantoin decreased blood glucose levels and increased blood insulin in a dose-dependent manner, in normal rats21. Allantoin has also been found to lower plasma glucose in diabetic rats by activating imidazoline-receptors I2 or I321, which improve insulin action. Furthermore, in diabetic rats, allantoin offered through diet had antidiabetic effects by modulating antioxidant activities and lipid profiles, promoting glucagon-like peptide-1 (GLP-1) release, thereby facilitating β-cells to maintain insulin and glucose levels22. Finally, in a cohort study, allantoin measured in urine of humans was also inversely associated with the risk of T2D20.
Figure 2.
Purine Catabolism Pathway. Reproduced and modified from41.
Uric acid is the end product of purine metabolism in humans. In our study, plasma uric acid was not associated with future risk of T2D, but correlated positively both with HOMA-IR at baseline and at 1-year follow-up. The causal association between serum uric acid levels and T2D remains controversial23. Uric acid is generally considered to be a feature of hyperinsulinemia or/and insulin resistance23; however, in patients with diabetes, its level may be low, due to increased urate clearance that can be associated with glycosuria, increased sodium (Na) secretion, and overall decreased metabolic control24.
Uric acid can be produced from xanthine via the enzymatic action of XO, which also participates in the conversion of hypoxanthine-to-xanthine (Fig. 2). In our study, baseline xanthine levels and xanthine-to-hypoxanthine ratio were correlated with higher HOMA-IR levels. In addition, we found that this ratio was associated with increased risk of T2D. An increase in this ratio may reflect higher XO activity, which is involved in free radical production25 and has been found, increased in T2D patients5. Whether xanthine production from hypoxanthine, plays a role in T2D development due to increased XO activity or due to xanthine production in parallel with the consequent decrease in hypoxanthine, requires further investigation.
In the present study, guanosine was also positively correlated to HOMA-IR. Cyclic Guanosine monophosphate (cGMP) is a second messenger that mediates incretin effects; potentiates glucose-stimulated insulin secretion; promotes proper beta-cells differentiation, and prevents beta-cells apoptosis, cooperating with biotin26. In addition, cGMP is involved in various signal-transduction pathways, mediating messages of insulin itself27. The interactions between the extracellular and intracellular guanosine metabolites, or/and the possible modulations of the latter in T2D, remain an open issue.
Inosine, a precursor of xanthine that was not associated to diabetes incidence in the overall population of our study, has notable anti-inflammatory effects, which may be mediated, at least in part, by activation of the adenosine A2a receptor28. Both, adenosine and inosine have been suggested to play a protective role against diabetes development28. We observed that inosine-to-adenosine ratio was negatively correlated with baseline HOMA-IR. A working hypothesis is that the production of inosine is more favourable in improving glucose homeostasis, as compared to adenosine.
The TCF7L2-rs7903146 polymorphism is one of the strongest and most widely replicated locus associated with T2D29, with the homozygous individuals (TT) being those who present a higher prevalence and fasting glucose; the mechanism has yet to be determined14, although various hypotheses, affecting regulation of the Wnt signalling-pathway30 have been proposed. The key effector of Wnt pathway, the bipartite transcription factor β-cat/TCF, is formed by free β-catenin (β-cat) and a TCF protein, including TCF7L231. Wnt signalling and TCF7L2 appear to exercise a very complex effect in metabolic homeostasis, affecting not only the pancreatic islets, but also other organs (liver gluconeogenesis), and crosstalk with stress, aging as well as tumorigenesis signalling pathways/cascades31. Several signalling components of the Wnt signal transduction pathway have been identified but a clear understanding of the Wnt signalling’s diverse function, integration and specificity is lacking. On the other hand, there is strong evidence for a direct association between dysregulated Wnt signalling and chronic diseases32. In due course, choosing the rs7903146-allele, was an innovative approach in our study.
In this study, using a case-cohort design, we confirmed the association of the TCF7L2-rs7903146 TT genotype, with the risk of T2D in PREDIMED study participants14. Changes in the blood of TT homozygotes reported until now mainly concerned increased levels of plasma glucose in response to a meal challenge of proinsulin and elevated glucose-dependent insulinotropic peptide secretion33; reduced secretion of insulin/glucagon, and reduced insulinotropic effect of incretin hormones34; altered postprandial triglyceride response, mainly influencing VLDL and HDL subclasses35, as well as non-significant increase of plasma sphingomyelins, phosphatidylcholines and lyso-phosphatidylcholines species36. To our knowledge, this is the first study to examine the interaction between plasma purine-catabolism metabolites levels and the TCF7L2-rs7903146 genetic variation, focusing on T2D risk.
We observed that xanthine and inosine were associated with increased T2D risk only in individuals with TT alleles on rs7903146. Moreover, the combination of TT genotype and high plasma xanthine and inosine levels was associated with a higher risk of T2D than the combined CC genotype and their low levels, which confirmed that both the rs7903146 T allele and increased plasma xanthine and inosine are associated with T2D. The protective role of inosine is well-documented37, while xanthine may simply increase due to increased XO activity in the blood38. A working hypothesis is that xanthine and inosine play a compensatory role, competing or cooperating; since in both, when their levels were low, no significant association was found between TT and T2D, as compared to their high levels (see joint analysis). Among purine-catabolism related ratios, only hypoxanthine-to-inosine ratio was inversely associated with T2D risk in individuals with TT alleles on rs7903146 with a significant test of interaction. Whether hypoxanthine production from inosine confers higher protection from T2D in individuals carrying these polymorphisms, by inhibiting the activation of poly(ADP-ribose) polymerase39 and thus increasing TCF7L2-mediated GLP-1 production and activity40, needs to be further explored.
The results of the present study should be interpreted in the context of its limitations and strengths. First, participants were elderly Mediterranean individuals at high cardiovascular risk and this may limit the generalizability of the findings to other age-groups or populations. Second, even though we adjusted for several potential confounders, residual confounding may exist. Regarding strengths, the prospective evaluation of the association between metabolites levels and well-documented incident T2D, in the frame of a case-cohort design, minimizes biases that can affect case-control studies.
In conclusion, our prospective study documented, for the first time, an inverse and a positive association between high plasma allantoin levels, including allantoin-to-uric acid ratio and high xanthine-to-hypoxanthine ratio with incident T2D risk, respectively, in an elderly population at high cardiovascular risk, independently of the TCF7L2-rs7903146 polymorphism. Elevated plasma levels of xanthine and inosine appeared to be associated with higher T2D risk only in TT individuals. These results must be interpreted cautiously and need to be replicated in other populations. The potential mechanisms linking the aforementioned purine metabolites and T2D risk must be also further investigated.
Supplementary information
Acknowledgements
This study was supported by research grant R01-DK-102896 from the National Institutes of Health. The Prevención con DietaMediterránea (PREDIMED) trial was supported by the official funding agency for biomedical research of the Spanish government, the Instituto de Salud Carlos III, through grants provided to research networks specifically developed for the trial [grant RTIC G03/140 (to Ramón Estruch); grant RTIC RD 06/0045 (to Miguel A. Martínez-González)] and through the Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición and by grants from Centro Nacional de Investigaciones Cardiovasculares (grant CNIC 06/2007), the Fondo de Investigación Sanitaria Fondo Europeo de Desarrollo Regional (grants PI04–2239, PI 05/2584, CP06/00100, PI07/0240, PI07/1138, PI07/0954, PI 07/0473, PI10/01407, PI10/02658, PI11/01647, P11/02505, and PI13/00462), the Ministerio de Ciencia e Innovación (grants AGL-2009–13906-C02 and AGL2010–22319-C03), the Fundación Mapfre 2010, Consejería de Salud de la Junta de Andalucía (grant PI0105/2007), the Public Health Division of the Department of Health of the Autonomous Government of Catalonia, Generalitat Valenciana (grants ACOMP06109, GVA-COMP2010–181, GVACOMP2011–151, CS2010-AP-111, and CS2011-AP-042), and the Regional Government of Navarra (grant P27/2011). Genotyping of the TCF7L2-rs7903146 polymorphism was supported by PROMETEO17/2017 from the Generalitat Valenciana, and 538/U/2016 from Fundacio la Marato-TV3. Dr. Christopher Papandreou was supported by a postdoctoral fellowship granted by the Autonomous Government of Catalonia (PERIS 2016-2020 INCORPORACIÓ DE CIENTÍFICAS I TECNÒLEGS, SLT002/0016/00428). Dr Marta Guasch-Ferré was supported by a postdoctoral fellowship granted by the Lilly Foundation European Association of Diabetes (EASD) through the Institut d’Investigacions Sanitàries Pere i Virgili (IISPV), Tarragona, Spain. The authors are indebted to George A. Fragkiadakis (Department of Nutrition & Dietetics, Technological Education Institute of Crete, Greece) for his intellectual contributions to this manuscript.
Author Contributions
F.B.H., J.S.-S. and M.M.-G. designed research; C.P., M.B., Y.Z., M.R.-C., E.Y., M.G.-F., C.R., D.C., R.E., E.R., M.F., F.A., M.F.I.O.L., J.L., L.S.-M., M.M.-G. and J.S.-S. conducted research; D.C., R.E., M.F., F.A., M.F.I.O.L., J.L., L.S.-M., M.M.-G. and J.S.-S. were the coordinators of subject recruitment at the outpatient clinics; C.P. and M.B. analyzed the data; C.P., M.B., F.B.H., J.S.-S. interpreted statistical analysis and data; C.C. analyzed metabolomics data; C.P. drafted the paper; F.B.H. and J.S.-S. supervised the study and C.P., M.B. and J.S.-S. had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. All authors revised the manuscript for important intellectual content, read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mònica Bulló, Email: monica.bullo@urv.cat.
Jordi Salas-Salvadó, Email: jordi.salas@urv.cat.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39441-6.
References
- 1.Maiuolo J, Oppedisano F, Gratteri S, Muscoli CMV. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 2.Ekpenyong CAE. Abnormal Serum Uric Acid Levels in Health and Disease: A Double-Edged Sword. Am. J. Intern. Med. 2014;2(6):113–130. [Google Scholar]
- 3.Chung WYBI. Plasma allantoin measurement by isocratic liquid chromatography with tandem mass spectrometry: method evaluation and application in oxidative stress biomonitoring. Clin. Chim. Acta. 2013;424:237–244. doi: 10.1016/j.cca.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Dudzinska W. Purine nucleotides and their metabolites in patients with type 1 and 2 diabetes mellitus. JBiSE. 2014;7:38–44. doi: 10.4236/jbise.2014.71006. [DOI] [Google Scholar]
- 5.Kuppusamy UR, Indran MRP. Glycaemic control in relation to xanthine oxidase and antioxidant indices in Malaysian Type 2 diabetes patients. Diabet. Med. 2005;22(10):1343–1346. doi: 10.1111/j.1464-5491.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 6.Feoli AM, Macagnan FE, Piovesan CH, Bodanese LC, Siqueria IR. Xanthine oxidase activity is associated with risk factors for cardiovascular disease and inflammatory and oxidative status markers in metabolic syndrome: effects of a single exercise session. Oxid. Med. Cell. Longev. 2014;2014:587083. doi: 10.1155/2014/587083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J-G, et al. Changes in Adenosine Deaminase Activity in Patients with Type 2 Diabetes Mellitus and Effect of DPP-4 Inhibitor Treatment on ADA Activity. Diabetes Metab. J. 2011;35(2):149–158. doi: 10.4093/dmj.2011.35.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kather H. Pathways of purine metabolism in human adipocytes. Further evidence against a role of adenosine as an endogenous regulator of human fat cell function. J. Biol. Chem. 1990;265:96–102. [PubMed] [Google Scholar]
- 9.Dille, R. Serum Uric Acid and Type 2 Diabetes (2013).
- 10.Dong Z, et al. Effects of multiple genetic loci on the pathogenesis from serum urate to gout. Sci. Rep. 2017;7:43614. doi: 10.1038/srep43614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altaf, S. Investigation of the genetic link between the metabolic diseases gout and type 2 diabetes (Thesis, Doctor of Philosophy). (2014).
- 12.Estruch R, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018;378(25):e34. doi: 10.1056/NEJMoa1800389. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corella D, et al. Mediterranean diet reduces the adverse effect of the TCF7L2-rs7903146 polymorphism on cardiovascular risk factors and stroke incidence: a randomized controlled trial in a high-cardiovascular-risk population. Diabetes Care. 2013;201336(11):3803–3811. doi: 10.2337/dc13-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salas-Salvadó J, et al. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann. Intern. Med. 2014;160(1):1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31:S55–S56. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 17.Elosua R, Marrugat J, Molina L, Pons SPE. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish men. The MARATHOM Investigators. Am. J. Epidemiol. 1994;139(12):1197–1209. doi: 10.1093/oxfordjournals.aje.a116966. [DOI] [PubMed] [Google Scholar]
- 18.Schröder H, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011;141(6):1140–1145. doi: 10.3945/jn.110.135566. [DOI] [PubMed] [Google Scholar]
- 19.Benjamini YHY. Controlling the False Discovery Rate: A Practical and Powerful Approach to MultipleTesting. J. R. Stat. Soc. Ser B. 1995;57(1):289–300. [Google Scholar]
- 20.Il’yasova D, et al. Urinary F2-isoprostanes as a biomarker of reduced risk of type 2 diabetes. Diabetes Care. 2012;35(1):173–174. doi: 10.2337/dc11-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai CC, et al. Allantoin activates imidazoline I-3 receptors to enhance insulin secretion in pancreatic β-cells. Nutr. Metab. (Lond) 2014;11:41. doi: 10.1186/1743-7075-11-41. [DOI] [Google Scholar]
- 22.Go HK, et al. Antidiabetic Effects of Yam (Dioscoreabatatas) and Its Active Constituent, Allantoin, in a Rat Model of Streptozotocin-Induced Diabetes. Nutrients. 2015;7(10):8532–8544. doi: 10.3390/nu7105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013;25(2):210–216. doi: 10.1097/BOR.0b013e32835d951e. [DOI] [PubMed] [Google Scholar]
- 24.Andrade JAM, Kang HC, Greffin S, Garcia Rosa ML, Lugon JR. Serum uric acid and disorders of glucose metabolism: the role of glycosuria. Braz. J. Med. Biol. Res. 2014;47:917–923. doi: 10.1590/1414-431X20143878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beckman, J. A. Pathophysiology of Vascular Dysfunction in Diabetes. Cardiol. Rounds8(10) (2004).
- 26.McCarthy MF. cGMP may have trophic effects on beta cell function comparable to those of cAMP, implying a role for high-dose biotin in prevention/treatment of diabetes. Med. Hypotheses. 2006;66(2):323–328. doi: 10.1016/j.mehy.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Suslova TE, et al. Platelet hemostasis in patients with metabolic syndrome and type 2 diabetes mellitus: cGMP- and NO-dependent mechanisms in the insulin-mediated platelet aggregation. Front. Physiol. 2015;5:501. doi: 10.3389/fphys.2014.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haskó G, et al. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J. Immunol. 2000;164(2):1013–1019. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- 29.Grant SF, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006;38(3):320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 30.Shao W, et al. The Wnt signaling pathway effector TCF7L2 controls gut and brain proglucagon gene expression and glucose homeostasis. Diabetes. 2013;62(3):789–800. doi: 10.2337/db12-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ip W, Chiang YT, Jin T. The involvement of the wnt signaling pathway and TCF7L2 in diabetes mellitus: The current understanding, dispute, and perspective. Cell Biosci. 2012;2(1):28. doi: 10.1186/2045-3701-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gjesing AP, et al. Carriers of the TCF7L2 rs7903146 TT genotype have elevated levels of plasma glucose, serum proinsulin and plasma gastric inhibitory polypeptide (GIP) during a meal test. Diabetologia. 2011;54(1):103–110. doi: 10.1007/s00125-010-1940-4. [DOI] [PubMed] [Google Scholar]
- 34.Pilgaard K, et al. The T allele of rs7903146 TCF7L2 is associated with impaired insulinotropic action of incretin hormones, reduced 24 h profiles of plasma insulin and glucagon, and increased hepatic glucose production in young healthy men. Diabetologia. 2009;52(7):1298–1307. doi: 10.1007/s00125-009-1307-x. [DOI] [PubMed] [Google Scholar]
- 35.Engelbrechtsen L, et al. Homozygous carriers of the TCF7L2 rs7903146 T-allele show altered postprandial response in triglycerides and triglyceride-rich lipoproteins. Sci. Rep. 2017;7:43128. doi: 10.1038/srep43128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Then C, et al. Plasma metabolomics reveal alterations of sphingo- and glycerophospholipid levels in non-diabetic carriers of the transcription factor 7-like 2 polymorphism rs7903146. PLoS One. 2013;8(10):e78430. doi: 10.1371/journal.pone.0078430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haskó G, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14(13):2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 38.Miric DJ, et al. Xanthine Oxidase Activity in Type 2 Diabetes Mellitus Patients with and without Diabetic Peripheral Neuropathy. J. Diabetes Res. 2016;2016:4370490. doi: 10.1155/2016/4370490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Virág L. S.C. Purines inhibit poly(ADP-ribose) polymerase activation and modulate oxidant-induced cell death. FASEB J. 2001;15(1):99–107. doi: 10.1096/fj.00-0299com. [DOI] [PubMed] [Google Scholar]
- 40.Xia Q, et al. PARP-1 inhibition rescues short lifespan in hyperglycemic C. elegans and improves GLP-1 secretion in human cells. Aging Dis. 2018;9(1):17–30. doi: 10.14336/AD.2017.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navolanic PM, et al. Elitek-rasburicase: an effective means to prevent and treat hyperuricemia associated with tumor lysis syndrome, a Meeting Report, Dallas, Texas, January 2002. Leukemia. 2003;17(3):499–514. doi: 10.1038/sj.leu.2402847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.