Abstract
Breast cancer metastasis accounts for most of the deaths from breast cancer. Since epithelial-mesenchymal transition (EMT) plays an important role in promoting metastasis of cancer, many mechanisms regarding EMT have been studied. We previously showed that Ribonucleic acid export 1 (RAE1) is dysregulated in breast cancer and its overexpression leads to aggressive breast cancer phenotypes by inducing EMT. Here, we evaluated the functional capacity of RAE1 in breast cancer metastasis by using a three-dimensional (3D) culture system and xenograft models. Furthermore, to investigate the mechanisms of RAE1-driven EMT, in vitro studies were carried out. The induction of EMT with RAE1-overexpression was confirmed under the 3D culture system and in vivo system. Importantly, RAE1 mediates upregulation of an EMT marker ZEB1, by binding to the promoter region of ZEB1. Knockdown of ZEB1 in RAE1-overexpressing cells suppressed invasive and migratory behaviors, accompanied by an increase in epithelial and a decrease in mesenchymal markers. Taken together, these data demonstrate that RAE1 contributes to breast cancer metastasis by regulating a key EMT-inducing factor ZEB1 expression, suggesting its potential as a therapeutic target.
Introduction
Breast cancer is one of the most commonly occurring cancers in women worldwide1. The main reason for death of breast cancer patients is metastasis2. The epithelial-mesenchymal transition (EMT), a process that is typically induced by interruption of intracellular tight junctions and loss of cell-cell contacts, is a key step in cancer metastasis1,3,4. During EMT, morphological changes from cobblestone-like to spindle-shaped cells are accompanied by a marked reduction in E-cadherin and increase in mesenchymal markers, such as Vimentin and N-cadherin5–7. Furthermore, EMT has been highlighted in breast cancer resistance to chemotherapy and/or target therapies8–11. Because of its importance, numerous studies have focused on these phenomena to explain and discover new mechanisms involved in breast cancer progression and metastasis; however, further studies of the regulation of EMT are required.
Ribonucleic acid export 1 (Rae1) was originally reported as a nucleocytoplasmic transport factor in yeast12. Since then, human RAE1, a homologue of the yeast Rae113, was discovered as tone component of nuclear pore complexes (NPCs)14 and as a mitotic checkpoint regulator15–17. Recently, several studies demonstrated that RAE1 expression was dysregulated in breast cancer18–20. Furthermore, mRNA expression of RAE1 was found positively correlated to gene copy number19. Among genes that were amplified and overexpressed in breast cancer, several genes, such as FGFR1, IKBKB, and ERBB2, were especially considered as potential therapeutic target21,22. With the expectation that RAE1 would also be a useful target for cancer therapy, we carried out functional studies in breast cancer cell lines and found that RAE1 contributes to aggressive cancer cell phenotype and induces EMT18. Furthermore, the expression level of RAE1 was positively correlated with the histologic grading in breast cancer patients with invasive ductal carcinoma18. Elevated RAE1 expression indicated a poor outcome in breast cancer patients18,20.
In this study, we investigated how RAE1 contributes to invasion and metastasis of breast cancer, three-dimensional (3D) culture system and xenograft models. In addition, our in vitro studies have revealed that RAE1 induces EMT by enhancing the expression of transcription factor ZEB1. Considering that EMT enhances the metastatic potential of breast cancer, our results support the relationship between RAE1 activity and breast cancer aggressiveness.
Results
RAE1 overexpression enhances cell spreading in 3D culture systems and metastasis in mouse xenograft models
To investigate the precise effects of RAE1 overexpression in breast cancer, we carried out 3D cell culture analysis with stable MCF7 cell lines overexpressing RAE1 (MCF7:RAE1 #1, 2, and 3) and empty vector (MCF7:emp vec #1, and 2). The Matrigel-embedded 3D culture system is more appropriate for structural and functional studies than the 2D culture system23. The results of phalloidin and DAPI staining at day 10 showed that MCF7 cells stably overexpressing RAE1 spread outwards along the extracellular matrix, whereas the control MCF7 cell lines maintained a spherical morphology without extending along the bottom line of the 3D culture vessel (Fig. 1A). In addition, confocal images representing a cross-section of the colony revealed that RAE1-overexpressing MCF7 cells were dispersed towards the outside, while control MCF7 cells gathered near the center (Fig. 1B). Serial confocal transverse section images of each stable cell line are provided in Fig. S1.
Figure 1.
Effects of RAE1 overexpression in 3D in vitro culture system. (A,B) Confocal microscopy images of MCF7 cells in 3D culture system at day 10. Control (MCF7:empty vec #1 and 2) and RAE1-overexpressing MCF7 (MCF7:RAE1 #1, 2, and 3) cells were cultured in DMEM containing 4% Matrigel in a vessel coated with absolute Matrigel. Structures were stained with DAPI (blue) and phalloidin (red). The migrating features were observed in the cross-section images of control and RAE1-overexpressing MCF7 cell lines (A) and in the total colony structures (B).
To further explore the functional role of RAE1 in breast cancer progression in vivo, we evaluated the effects of RAE1 on the metastasis in a breast cancer xenograft model. MDA-MB-231 cells were used in this study because of their high metastatic potential. Tumors were monitored over a period of 11 weeks, and then the distance between the injection site and final position was measured as shown in Fig. 2A. Short-term xenograft (6 hrs–1 week) did not show significant migration, while long-term xenograft (7–11 weeks) showed significant migratory abilities by RAE1-overexpressing cells. The investigation of tumor cell spreading from the primary tumor cells to other sites at 11 weeks showed that xenograft mice injected with control cells formed primary tumors at the injection site (2 out of 4 mice) but did not form metastatic tumors at any other organs throughout the body except the liver (Fig. 2B,C). On the other hand, xenograft mice with RAE1-overexpressing cells formed primary tumors at the injection site (4 out of 4 mice) and metastatic tumors at the fat pad opposite to the injection site (3 out of 4 mice) (Fig. 2B,C). Although the metastatic tumors were not found during this period of time in distal organs such as kidney and lung in both groups, all mice injected with RAE1-overexpressing cells showed a stronger signal for liver metastases (Fig. 2C). Together, these results support that RAE1 accelerates tumor metastasis in vivo.
Figure 2.
Effects of RAE1 overexpression in xenograft tumor progression. (A,B) In vivo xenograft models of breast cancer metastasis. Three cancer cell lines (MDA-MB-231, MDA-MB-231:empty vec, and MDA-MB-231:RAE1) were injected into the fat pads of nude mice. Four nude mice were used for each cell line. (A) Migration distance from 6 hrs to 11 weeks after injection. **P < 0.01, ***P < 0.001. (B) Measurement of metastatic spread of cancer cells to other organs (liver, pancreas, spleen, kidney, lung, and heart) at 11 weeks after injection. Yellow arrow heads indicate tumors formed at the injection site and black arrow heads indicate tumors formed at the fat pad opposite to the injection site. (C) Quantification analysis of signals in liver metastasis and tumors on fat pads of Fig. 1B shows average radiant efficiency. Each blue circle (injection of control cells) and red triangle (injection of RAE1 overexpressing cells) represents an individual xenograft, and empty circles and triangles indicate the no tumor has developed in the organ. In the graph, the horizontal lines represent the average value of each experimental group. *P < 0.05.
Upregulation of RAE1 enhances the expression of ZEB1 by binding to the promoter region
To investigate the molecular mechanisms underlying the role of RAE1 in mediating cancer metastasis, we performed gain of function studies using in vitro models. Among various breast cancer cell lines, we found that RAE1 is expressed highly in BT474, but it is expressed relatively low in MDA-MB-453, T47D, and MDA-MB-231 (Fig. S2A,B). We confirmed the subcellular localization of endogenous and exogenous RAE1 in several different cell lines (Fig. S2C,D) and concluded that forced expression of RAE1 does not lead to mislocalization of abnormal protein product. Recent studies on the NPC components and their association with gene expression regulation suggest that high concentration of RAE1 at the peripheral portion of the nucleus may play a role as a transcription regulator24–26. As RAE1 has been shown to induce EMT signals and promote invasion and migration abilities, we determined the expression levels of several EMT-associated transcription factors (Fig. S3) and found that ZEB1 mRNA levels were significantly upregulated by RAE1 overexpression (Fig. 3A). Furthermore, in order to confirm the positive correlation between RAE1 and ZEB1 in an in vivo system, IHC was performed with anti-ZEB1 antibody in tumor tissues retrieved from the xenograft experiment. In the MDA-MB-231 xenograft tumor tissues, ZEB1 was expressed mainly in the nucleus. The number of ZEB1-positive cells decreased from 129.5 ± 4.42 to 44.6 ± 11.45 in RAE1-knockdowned tumors, but increased from 126.3 ± 2.80 to 199.6 ± 9.03 in RAE1-overexpressing tumors. This may be an indirect evidence for the altered expression of ZEB1 through RAE1 regulation (Fig. 3B,C).
Figure 3.
Positive correlation of RAE1 and ZEB1 in vitro and in vivo. (A) RAE1 and ZEB1 mRNA expression levels in RAE1-overexpressing MCF7 cells and control cells. (B) Immunohistochemistry of tumor tissues from xenograft-bearing mice with RAE1-manupulation to display the distribution of ZEB1 in the tumor sections using anti-ZEB1 antibody. (C) Quantification of the ZEB1-positive cells was performed. ZEB1-positive cells were measured in 10 frames each experiment. (D) Map of the ZEB1 promoter region and gene desert. Green bar indicates the CpG islands and gray box shows ChIP amplicons (pZEB1 #1: −881 to −574, #2: −537 to −165 and #3: −164 to +64). H3K4Me3 and Pol2 signals were derived from ENCODE (https://genome.ucsc.edu). (E) Quantitative interpretation of ChIP-qPCR data. Chromatin was extracted from MCF7 cells stably overexpressing RAE1 and control cells. ChIP products were used in qPCR for pZEB1 #1, #2, and #3. An amplicon for a gene desert was included as a negative control. Data are shown as % of input, after normalization with IgG. (F) Luciferase activity of control and ZEB1 promoter construct, with and without transfected RAE1 plasmid in HEK293T cells. Relative luciferase units (RLU) were measured and normalized against internal control (Renilla) luciferase activity. All experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001.
To determine whether RAE1 may regulate for ZEB1 expression, the ability of RAE1 to bind the ZEB1 promoter was determined by ChIP assay. PCR amplicon sites were designed near the putative promoter region of ZEB1 (Fig. 3D). ChIP-qPCR data showed that overexpression of RAE1 led to increased binding of RAE1 in the −880 to −157 bp and −164 to +64 bp amplicon sites (pZEB1 #1 and #3) (Fig. 3E). To further delineate the effects of RAE1 on ZEB1 transcriptional activity, we performed dual luciferase assay by cloning ZEB1 promoter region (from −881 up to + 64 bp downstream of the TSS) and negative control (from −1500 up to −888 bp downstream of the TSS) into the luciferase vector. The ZEB1 promoter activity was increased by overexpression of RAE1 compared to negative control in HEK293T cells (Fig. 3F). Collectively, these results suggest RAE1 positively regulates ZEB1 expression during cancer progression.
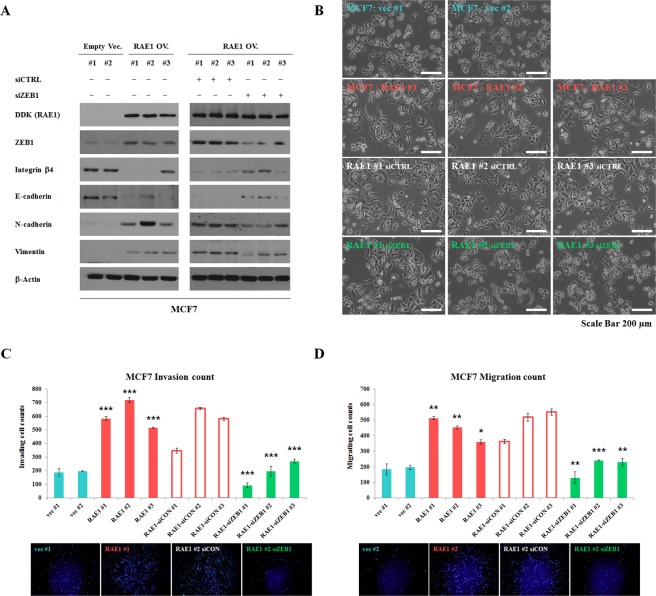
ZEB1 is a mediator for RAE1-induced EMT, invasion and migration in breast cancer
In a previous study, we have shown that overexpression of RAE1 in epithelial-like MCF7 and T47D induces EMT-like morphological changes and EMT marker expression18. In an opposite way, knockdown of RAE1 in mesenchymal-like MDA-MB-231 cells reduced cancer cell invasion and migration18. Here, to examine whether RAE1-induced metastatic capability was mediated by ZEB1, siRNA was used to silence ZEB1 gene expression. We have previously shown that overexpression of RAE1 in MCF7 cells also altered EMT-related marker levels and showed more distinct spindle-shape morphology (Fig. 4A, left 6 lanes; Fig. 4B, rows 1 and 2). Knockdown of ZEB1 in RAE1-overexpressing MCF7 cells promoted a reversal of EMT by increasing the expression of epithelial markers (Integrin β4 and E-cadherin) and decreasing the levels of mesenchymal markers (N-cadherin and Vimentin) (Fig. 4A, right 6 lanes; Fig. 4B, rows 3 and 4). In addition, silencing ZEB1 repressed RAE1-driven invasion and migration abilities (Fig. 4C,D). The effect of ZEB1 knockdown on the morphological and molecular changes was similarly observed in RAE1-overexpressing T47D and MDA-MB-231cells (Figs S5 and S6). Together, these data suggest that ZEB1 functions as a key component of RAE1-mediated EMT in breast cancer.
Figure 4.
ZEB1 mediates RAE1-induced EMT and invasion/migration abilities. (A) Western blotting analysis for epithelial and mesenchymal markers in stable RAE1-overexpressing MCF7 cells, with treatment of siCTRL or siZEB1. Full-length blots are presented in Fig. S4. (B) Effect of ZEB1 knockdown on cell morphological changes. For these experiments, 3.5 × 105 cells were seeded onto 6-well plates, transfected with siZEB1 or siCTRL for 48 hrs, and then imaged using a microscope for any morphological changes. Scale bar = 200 μm. (C,D) Matrigel invasion and migration assay in stable RAE1-overexpressing MCF7 cells, with treatment of siCTRL or siZEB1. For these experiments, 5 × 104 cells were placed in each chamber. After incubation for 72 hrs, invading or migrating cells were stained with DAPI and analyzed via fluorescent microscopy. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, we evaluated the metastatic properties of RAE1-overexpressing breast cancer cells both in 3D culture systems and in vivo xenograft models, and further demonstrated the molecular mechanisms underlying RAE1-mediated tumor progression. Our data revealing RAE1-mediated ZEB1-transcription regulation suggest that ZEB1, an EMT inducing transcription activator, contributes to the development of RAE1-driven EMT.
RAE1 is a component of the NPC. NPCs are large multi-protein structures that are present in the double membrane of the nuclear envelope mediating trafficking between the nucleoplasm and cytoplasm27–29. Alterations in nucleoporins, which compose each NPC, are frequently associated with particular defects in development and disease, and the resulting phenotypes are typically thought to be consequences of disturbed activity of nuclear transport24,29. Particularly, primary human specimens derived from different forms of cancer revealed dysregulation of mRNA export. Various components of mRNA export and its related factors contribute to the preferential export of transcripts encoding proteins involved in proliferation, survival, metastases, and invasion30.
Alternatively, NPCs can regulate the transcription levels of particular genes in a transport-independent manner24,26. Several studies have suggested that interactions between genes and the NPC modulate both the definition of hetero- and euchromatin boundaries and transcription25. Thus, NPCs are essential for controlling not only transport between the nucleoplasm and cytoplasm, but also organization of the genome and specific gene transcription31. The potential function of RAE1 as a transcription activator has not been reported before. However, some previous studies demonstrated the role of nuclear pore proteins (Nups), which interact with RAE1, as potential regulator of gene transcription. For example, Nup210 positions its target genes at the nuclear periphery, an environment where gene transcription can be controlled32,33. Here, we provide evidence that RAE1 regulates gene transcription by binding to the promoter region of a particular gene, ZEB1. Although we have realized the analysis of RAE1 expression in the Cancer Cell Line Encyclopedia (CCLE) databank did not find any correlation between RAE1 and ZEB1 mRNA expression nor with the breast cancer subtypes (Fig. S7), it may seem quite plausible to say that the regulation of ZEB1 expression by RAE1 is cell context-specific and/or may depend on the threshold of RAE1 expression. Taking this into account, our data showing the possibility of modulating ZEB1 expression by RAE1 suggest that the association of these two molecules may be important under certain conditions.
Apart from its function as a transcription factor, it is possible that RAE1 interacts with cytoplasmic components such as the cytoskeleton and contributes to genome organization and gene expression. RAE1, as a nuclear exporter, is expected to only be present in nuclear pores. However, according to public available databases (e.g. The Human Protein Atlas), RAE1 is present in the nucleoli fibrillar center and in the nucleus (Fig. S8). In addition, our ICC results show that RAE1 can be detected in the cytoplasm. (Fig. S2). The cytoskeleton is a well-organized dynamic cellular architecture that is typically involved in signal transduction in the cytoplasm34. In this regard, NPCs can act as docking sites for chromatin, ultimately contributing to the organization of the global topology of chromosomes in close association with other elements of the nuclear envelope25.
Based on the facts that the dysregulation of many NPC components is found in the different types of cancer and that various nuclear factors interacting with them, such as chromosomal maintenance 1 (CRM1, also known as exportin1), karyopherin family (KPNA2 and KPNB1), and chromosome segregation gene, contribute to the progression of cancer29,35–37, more attention is being focused on developing therapeutic drugs using transport factors35,38. In particular, CRM1/XPO1 is proposed as a promising drug candidate39,40, and is likely to bind to RAE1, either directly or indirectly (Fig. S9). Therefore, understanding the RAE1 function and mechanisms of action will be valuable enough to consider the value of therapeutic use. In conclusion, our study demonstrated that RAE1 promotes progression of breast cancer cells by activating ZEB1 at the transcription level and revealed the positive correlation between RAE1 and ZEB1 in breast cancer metastasis.
Methods
Cell lines
MCF7 and MDA-MB-231 breast cancer cell lines stably overexpressing RAE1 were generated and cultured as previously described18. For ZEB1 knockdown experiment, transient siRNA-mediated knockdown was carried out with HiPerFect transfection reagent (Qiagen, Hilden, Germany) according to the manufacturer’s protocol for 48 hrs. ZEB1 siRNA (Genolution, Seoul, Korea) or control siRNA were used to a final concentration of 40 nM.
Three-dimensional (3D) in vitro cell culture
To analyze cellular growth in 3D culture, an eight-well chamber slide was pre-coated with 50 μL of MatrigelTM (BD, San Jose, CA, USA) and incubated at 37 °C for 30 min to allow for gel formation. While the Matrigel was solidifying, 5 × 103 cells were diluted in cell culture medium (final concentration: 2.5 × 104 cells/mL) and mixed with 4% Matrigel-containing medium in a 1:1 ratio. The cell mixture was placed on top of the solidified Matrigel. Medium containing 2% Matrigel was changed every 3–4 days. Confocal images were acquired on the 10th day of culture after staining with anti-phalloidin (Invitrogen, Carlsbad, CA, USA) and fluorochrome 4′,6-diamidino-2-phenylindole (DAPI). Images were captured with a 40 × C-Apochromat water immersion lens on a Zeiss LSM 700 Confocal, using Zen 2011 software (Carl Zeiss, Oberkochen, Germany). For 3D images, z-stack scans were collected by incremental stepping through the 3D sample using a focal drive. The step size was automatically calculated with Zen 2011 software.
Tumor xenograft experiment
For xenograft experiments, male BALB/c nude mice were purchased from The Orient Bio, Inc. (Sungnam, Gyungki–do, Korea). RAE1-overexpressing MDA-MB-231 cells, and the same amount of MDA-MB-231 stable cells overexpressing empty vector and parent MDA-MB-231 cells, were used. For each mouse, 5 × 105 cells were stained with XenoLight Dir Fluorescence dye (PerkinElmer, Waltham, MA, USA), washed twice with cold sterile PBS without calcium chloride and magnesium chloride (Sigma-Aldrich, St. Louis, MO, USA), and mixed 50:50 (v/v) with Matrigel. The cells were injected into the right fat pad of 7 week-old male BALB/c nude mice to establish primary tumors. Each mouse was analyzed using IVIS (Caliper Life Sciences, Hopkinton, MA, USA) at 710 nm for excitation and 760 nm for emission from 6 hrs after injection up to 11 weeks. Mice were sacrificed in a CO2 chamber at 11 weeks after injection, and the liver, pancreas, spleen, kidney, lung, and heart tissues were collected to analyze metastasis. All animal procedures were approved by the Institutional Animal Care Committee at Yonsei University, and were carried out in accordance with the guidelines and regulations set by the ethics committee. Animals were then euthanized and selected tissues were processed for histology.
Immunohistochemistry (IHC) analysis
IHC analyses were performed as previously described with minor modification41. Briefly, slides were deparaffinized and rehydrated through series of graded ethanol. Antigen retrieval was performed using a pressure cooker. Endogenous peroxidase activity was blocked by incubation with 3% H2O2 for 30 min then incubated with protein blocking solution (Dako, Glostrup, Denmark) for 1 hour at room temperature. Primary antibody was incubated in a humid chamber at 4 °C overnight, and then slides were incubated with secondary rabbit IgG (Dako) for 15 min at room temperature, and developed with Dako Envision + System-HRP DAB (Dako). Anti-ZEB1 (Abcam, Cambridge, UK; dilution ratio 1:2000) was purchased. After counterstaining with Meyer’s Hematoxylin (Sigma), slides were mounted with mounting solution (Electron Microscopy Sciences, Hatfield, PA, USA). Quantitation of ZEB1-positive cells was done and then statistical analyses were performed with JMP software. Student’s t-tests were used to analyze differences between means. Data are represented as mean ± SEM.
Immunocytochemistry (ICC) analysis
ICC analyses were performed, with Abcam’s ICC protocol. An anti-RAE1 antibody (Abcam) was used to detect RAE1 protein. The nucleus was counterstained with DAPI. Images were captured with a 40 × C-Apochromat water immersion lens on a Zeiss LSM 700 Confocal, using Zen 2011 software.
Real-time PCR
Real-time PCR analysis was performed as previously described42. For quantitative PCR analysis, the StepOnePlusTM Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and Power SYBR Green PCR Master Mix (Applied Biosystems) kits were used. All samples were run in triplicate, and RAE1 and ZEB1 expression levels were normalized relative to that of β-Actin, which was used as an internal loading control. Primers for PCR are listed in Table 1.
Table 1.
Primer sequences used for real-time PCR.
| Genes | Sequence (5′ → 3′) |
|---|---|
| RAE1 | F- CAA CCT CAG GTT TTG GAA CC |
| R- CGA TGC CGT AAA CAC TTT GC | |
| ZEB1 | F- TCC TCT CGA ATG AGC ACG |
| R- CTT GCT CAC TAC TCT CG | |
| β-Actin | F- CATGTTTGAGACCTTCAACACCCC |
| R- GCCATCTCCTGCTCGAAGTCTAG |
Western blotting and antibodies
Western blot analyses were performed as previously described18. Anti-RAE1 (Abcam), anti-DDK-tag mouse monoclonal antibody (Origene, Rockville, MD, USA), anti-ZEB1 (Abcam), anti-E-cadherin (Abcam), anti-Integrinβ4 (Abcam), anti-β-catenin (BD, Franklin Lakes, USA), anti-N-cadherin (Abcam), anti-Vimentin (Sigma, St. Louis, MO, USA), and anti-β-Actin (Sigma) antibodies were used to detect each protein.
Matrigel invasion and migration assays
The MatrigelTM (BD) invasion and migration assays were performed previously described18.
Chromatin immunoprecipitation (ChIP) assay
ChIP analysis was performed as previously described42 with minor modifications. Chromatin was prepared from stable RAE1-overexpressing and control breast cancer cell lines. Briefly, 1 × 106 cells were cross-linked with 1% formaldehyde for 15 min, followed by the addition of glycine at 125 mM. Chromatin was sheared by sonication to fragments averaging between 0.5 and 1 kb in buffer containing 1% SDS, 1% Triton X-100, 0.1% sodium deoxycholate, 10 mM EDTA, 50 mM Tris-HCl (pH 8.0), and protease inhibitor cocktail (Roche Applied Science, Basel, Switzerland). Chromatin was pre-cleared with protein A/G beads containing 50% slurry (Santa Cruz Biotechnology, Dallas, TX, USA) and salmon sperm DNA, followed by immunoprecipitation with anti-RAE1 antibody (Abcam) coupled to protein A/G beads under each experimental condition. Nonimmune mouse IgG (Santa Cruz Biotechnology) was used as a control. ChIP-PCR data are shown as the percentage of input after normalization with IgG. Primers for ChIP-PCR are listed in Table 2.
Table 2.
Primer sequences used for ChIP-PCR assay.
| Amplicon sites | Sequence (5′ → 3′) |
|---|---|
| pZEB1 #1 | F- GGA TCC CAC GGT TCT ACG C |
| R- GCG ACC GGA GAG AGG CTA | |
| pZEB1 #2 | F- CTC ATC AAG GGA ACT CCC CG |
| R- GAA TTG AGG GGC GAG GGA AA | |
| pZEB1 #3 | F- CCC ACC ACA CCT GAG GAA AA |
| R- CAT GAT CCT CTC GCT TGT GTC | |
| Gene desert | F- TGG TGG TCT GCC TTC TGC CAG T |
| R- TCA CGT GGG AGG AAG AAG TAG GGC |
Dual luciferase assay
Dual luciferase assay was performed as described previously43. Genomic DNA fragment of the ZEB1 promoter region was cloned into the pGL3-Basic vector (Promega, Madison, WI, USA) using KpnI and HindIII sites. Control pGL3-Basic vector or the pGL3-ZEB1 constructs were transfected into HEK293T cells with the Renilla luciferase vector.
In silico analysis
The STRING web-accessible database version 10.5 (https://string-db.org) was used to evaluate RAE1 interaction partners in various human tissues. The Human Protein Atlas (https://www.proteinatlas.org) was used to determine the localization of RAE1 in several cell lines.
Statistical analysis
Data are expressed as the mean values with the standard error of the mean. Statistical differences were determined by Student’s t-test. A P-value of < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
This work was supported by the Brain Korea 21 PLUS Project for Medical Science, Yonsei University; the Basic Science Research Program through the National Research Foundation (NRF) funded by the Ministry of Education, Science, and Technology [NRF-2016R1A2B2011821, NRF-2016R1D1A1B03930822 and NRF-2017R1A2B2009850].
Author Contributions
J.H.O. and J.-Y.L. designed the experiments, analyzed data and wrote the manuscript. J.H.O. performed the in vitro studies. J.H.O., S.Y. and Y.C. performed the in vivo studies. K.T.N. and M.H.K. managed and supervised the study and finalized the manuscript. All authors discussed the results and commented on the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ki Taek Nam, Email: kitaek@yuhs.ac.
Myoung Hee Kim, Email: mhkim1@yuhs.ac.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-019-39574-8.
References
- 1.Wu, Y., Sarkissyan, M. & Vadgama, J. V. Epithelial-Mesenchymal Transition and Breast Cancer. J Clin Med5, 10.3390/jcm5020013 (2016). [DOI] [PMC free article] [PubMed]
- 2.Weigelt B, Peterse JL, Van ‘t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 3.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–2206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartis D, Mise N, Mahida RY, Eickelberg O, Thickett DR. Epithelial-mesenchymal transition in lung development and disease: does it exist and is it important? Thorax. 2014;69:760–765. doi: 10.1136/thoraxjnl-2013-204608. [DOI] [PubMed] [Google Scholar]
- 5.Gheldof A, Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog Mol Biol Transl Sci. 2013;116:317–336. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu PF, et al. Vimentin is a potential prognostic factor for tongue squamous cell carcinoma among five epithelial-mesenchymal transition-related proteins. PLoS One. 2017;12:e0178581. doi: 10.1371/journal.pone.0178581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al Moustafa AE, Achkhar A, Yasmeen A. Front Biosci (Schol Ed) 2012. EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas; pp. 671–684. [DOI] [PubMed] [Google Scholar]
- 8.Lien HC, et al. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: identification of genes potentially related to epithelial-mesenchymal transition. Oncogene. 2007;26:7859–7871. doi: 10.1038/sj.onc.1210593. [DOI] [PubMed] [Google Scholar]
- 9.Iseri OD, et al. Drug resistant MCF-7 cells exhibit epithelial-mesenchymal transition gene expression pattern. Biomed Pharmacother. 2011;65:40–45. doi: 10.1016/j.biopha.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, et al. Chemoresistance to 5-fluorouracil induces epithelial-mesenchymal transition via up-regulation of Snail in MCF7 human breast cancer cells. Biochem Biophys Res Commun. 2012;417:679–685. doi: 10.1016/j.bbrc.2011.11.142. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, et al. Acquisition of epithelial-mesenchymal transition is associated with Skp2 expression in paclitaxel-resistant breast cancer cells. Br J Cancer. 2014;110:1958–1967. doi: 10.1038/bjc.2014.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JA, et al. A mutation in the Schizosaccharomyces pombe rae1 gene causes defects in poly(A) + RNA export and in the cytoskeleton. J Biol Chem. 1995;270:7411–7419. doi: 10.1074/jbc.270.13.7411. [DOI] [PubMed] [Google Scholar]
- 13.Bharathi A, et al. The human RAE1 gene is a functional homologue of Schizosaccharomyces pombe rae1 gene involved in nuclear export of Poly(A) + RNA. Gene. 1997;198:251–258. doi: 10.1016/S0378-1119(97)00322-3. [DOI] [PubMed] [Google Scholar]
- 14.Pritchard CE, Fornerod M, Kasper LH, van Deursen JM. RAE1 is a shuttling mRNA export factor that binds to a GLEBS-like NUP98 motif at the nuclear pore complex through multiple domains. J Cell Biol. 1999;145:237–254. doi: 10.1083/jcb.145.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, et al. The mitotic checkpoint protein hBUB3 and the mRNA export factor hRAE1 interact with GLE2p-binding sequence (GLEBS)-containing proteins. J Biol Chem. 2001;276:26559–26567. doi: 10.1074/jbc.M101083200. [DOI] [PubMed] [Google Scholar]
- 16.Babu JR, et al. Rae1 is an essential mitotic checkpoint regulator that cooperates with Bub3 to prevent chromosome missegregation. J Cell Biol. 2003;160:341–353. doi: 10.1083/jcb.200211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker DJ, et al. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J Cell Biol. 2006;172:529–540. doi: 10.1083/jcb.200507081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh JH, et al. The mitotic checkpoint regulator RAE1 induces aggressive breast cancer cell phenotypes by mediating epithelial-mesenchymal transition. Sci Rep. 2017;7:42256. doi: 10.1038/srep42256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan Y, Curtis C, Caldas C, Markowetz F. A sparse regulatory network of copy-number driven gene expression reveals putative breast cancer oncogenes. IEEE/ACM Trans Comput Biol Bioinform. 2012;9:947–954. doi: 10.1109/TCBB.2011.105. [DOI] [PubMed] [Google Scholar]
- 20.Chin K, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Kao J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 23.Kim JB, Stein R, O’Hare MJ. Three-dimensional in vitro tissue culture models of breast cancer–a review. Breast Cancer Res Treat. 2004;85:281–291. doi: 10.1023/B:BREA.0000025418.88785.2b. [DOI] [PubMed] [Google Scholar]
- 24.Capelson M, Hetzer MW. The role of nuclear pores in gene regulation, development and disease. EMBO Rep. 2009;10:697–705. doi: 10.1038/embor.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohler A, Hurt E. Gene regulation by nucleoporins and links to cancer. Mol Cell. 2010;38:6–15. doi: 10.1016/j.molcel.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 26.Ibarra A, Hetzer MW. Nuclear pore proteins and the control of genome functions. Genes Dev. 2015;29:337–349. doi: 10.1101/gad.256495.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’Angelo MA, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–466. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fahrenkrog B, Koser J, Aebi U. The nuclear pore complex: a jack of all trades? Trends Biochem Sci. 2004;29:175–182. doi: 10.1016/j.tibs.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Xu S, Powers MA. Nuclear pore proteins and cancer. Semin Cell Dev Biol. 2009;20:620–630. doi: 10.1016/j.semcdb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Culjkovic-Kraljacic B, Borden KL. Aiding and abetting cancer: mRNA export and the nuclear pore. Trends Cell Biol. 2013;23:328–335. doi: 10.1016/j.tcb.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon DN, Rout MP. Cancer and the nuclear pore complex. Adv Exp Med Biol. 2014;773:285–307. doi: 10.1007/978-1-4899-8032-8_13. [DOI] [PubMed] [Google Scholar]
- 32.Satomura A, Brickner JH. Nuclear Pore Complexes: A Scaffold Regulating Developmental Transcription? Trends Cell Biol. 2017;27:621–622. doi: 10.1016/j.tcb.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akhtar A, Gasser SM. The nuclear envelope and transcriptional control. Nat Rev Genet. 2007;8:507–517. doi: 10.1038/nrg2122. [DOI] [PubMed] [Google Scholar]
- 34.Kumeta M, Yoshimura SH, Hejna J, Takeyasu K. Nucleocytoplasmic shuttling of cytoskeletal proteins: molecular mechanism and biological significance. Int J Cell Biol. 2012;2012:494902. doi: 10.1155/2012/494902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stelma T, et al. Targeting nuclear transporters in cancer: Diagnostic, prognostic and therapeutic potential. IUBMB Life. 2016;68:268–280. doi: 10.1002/iub.1484. [DOI] [PubMed] [Google Scholar]
- 36.Kuusisto HV, Wagstaff KM, Alvisi G, Roth DM, Jans DA. Global enhancement of nuclear localization-dependent nuclear transport in transformed cells. FASEB J. 2012;26:1181–1193. doi: 10.1096/fj.11-191585. [DOI] [PubMed] [Google Scholar]
- 37.van der Watt PJ, Ngarande E, Leaner VD. Overexpression of Kpnbeta1 and Kpnalpha2 importin proteins in cancer derives from deregulated E2F activity. PLoS One. 2011;6:e27723. doi: 10.1371/journal.pone.0027723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol. 2012;83:1021–1032. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J, et al. XPO1-dependent nuclear export is a druggable vulnerability in KRAS-mutant lung cancer. Nature. 2016;538:114–117. doi: 10.1038/nature19771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lapalombella R, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120:4621–4634. doi: 10.1182/blood-2012-05-429506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nam KT, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037 e2029. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh, J. H., Lee, J. Y., Kong, K. A., Kim, J. M. & Kim, M. H. The histone acetylation mediated by Gcn5 regulates the Hoxc11 gene expression in MEFs. Acta Biochim Biophys Sin (Shanghai), 1–6, 10.1093/abbs/gmx051 (2017). [DOI] [PubMed]
- 43.Lee JY, Kim JM, Jeong DS, Kim MH. Transcriptional activation of EGFR by HOXB5 and its role in breast cancer cell invasion. Biochem Biophys Res Commun. 2018;503:2924–2930. doi: 10.1016/j.bbrc.2018.08.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.