Abstract
α4 integrin plays a crucial role in retention and release of neutrophils from bone marrow. Although α4 integrin is known to be a potential target of reactive oxygen species (ROS)–induced cysteine glutathionylation, the physiological significance and underlying regulatory mechanism of this event remain elusive. Here, using in vitro and in vivo biochemical and cell biology approaches, we show that physiological ROS-induced glutathionylation of α4 integrin in neutrophils increases the binding of neutrophil-associated α4 integrin to vascular cell adhesion molecule 1 (VCAM-1) on human endothelial cells. This enhanced binding was reversed by extracellular glutaredoxin 1 (Grx1), a thiol disulfide oxidoreductase promoting protein deglutathionylation. Furthermore, in a murine inflammation model, Grx1 disruption dramatically elevated α4 glutathionylation and subsequently enhanced neutrophil egress from the bone marrow. Corroborating this observation, intravenous injection of recombinant Grx1 into mice inhibited α4 glutathionylation and thereby suppressed inflammation-induced neutrophil mobilization from the bone marrow. Taken together, our results establish ROS-elicited glutathionylation and its modulation by Grx1 as pivotal regulatory mechanisms controlling α4 integrin affinity and neutrophil mobilization from the bone marrow under physiological conditions.
Keywords: glutathionylation, reactive oxygen species (ROS), integrin, neutrophil, inflammation, α4 integrin, glutaredoxin, immune response, neutrophil mobilization, vascular cell adhesion molecular 1 (VCAM-1)
Introduction
GSH reaction with the free sulfhydryl (–SH) on certain cysteines of proteins induces protein glutathionylation and forms protein–GSH mixed disulfide adducts (Pr-SSG). Glutathionylation was previously considered to prevent proteins from irreversible oxidizing to sulfenic acid, sulfinic acid, or sulfonic acid (1, 2). However, it has become increasingly clear that glutathionylation, an important redox posttranslational modification, regulates cellular functions and signal transduction by affecting target proteins, including both intracellular molecules such as actin, titin, and NF-κB (3–5) and extracellular components such as some cytokines and HMGB1 (6).
Glutathionylation is mostly induced by ROS.9 ROS are a series of highly reactive chemicals mainly containing superoxide, hydrogen peroxide, hydroxyl radicals, etc. (7). ROS are largely produced by phagocytes to fight against microorganisms in inflammatory responses. Recently, there has been a growing body of evidence showing novel functions of ROS in physiological cellular signaling and functionality. Deglutathionylation is mainly regulated by oxidoreductases such as Grx (8, 9). Grx, a family of thioltransferases, removes GSH from Pr-SSG via a thiol–disulfide exchange reaction (10, 11). In mammalian cells, there are two main Grx isoforms, Grx1 and Grx2. Grx2 is primarily located at mitochondria, whereas Grx1 is found in many locations, including the cytosol, nucleus, and extracellular matrix (12, 13), suggesting that glutathionylation of extracellular proteins might be mediated by Grx1. A recent study has shown that Grx1 regulates interleukin-1β deglutathionylation and its bioactivity (14).
A previous studies suggested that glutathionylation might regulate the binding of α4 integrin on eosinophil to its ligand VCAM-1 (15). However, whether glutathionylation is essential for α4-mediated neutrophil adhesion and related physiological cellular processes is unknown. In this study, we demonstrated that ROS induced α4 integrin glutathionylation and subsequently regulated the adhesion of neutrophils to endothelial cells. We further showed that Grx1 reversed the glutathionylation of α4 integrin physiologically. Disruption of Grx1 increased α4 integrin glutathionylation and inhibited neutrophil binding to endothelial cells. On the contrary, Grx1 overexpression or Grx1 protein addition resulted in opposite effects.
Under rest conditions, most neutrophils exist in the bone marrow. During infection or inflammation, massive neutrophils are mobilized from the bone marrow to the circulation (16). To avoid improper infection or excessive inflammation, it is of great importance to appropriately govern bone marrow neutrophil retention and mobilization. Multiple lines of evidence have demonstrated that α4 integrin is the central integrin to regulate marrow neutrophil retention and mobilization (17–20). As stress conditions induce elevated level of ROS, we speculated that Grx1- and ROS-mediated α4 integrin glutathionylation might be involved in neutrophil mobilization from the bone marrow. Our results showed that Grx1 depletion resulted in increased neutrophil egress from the bone marrow and concomitantly increased α4 integrin glutathionylation and vice versa. This study reveals that Grx1-mediated and ROS-induced α4 integrin glutathionylation are important physiological regulatory mechanisms controlling α4 integrin functions in neutrophils and then participate in bone marrow neutrophil mobilization under stress conditions.
Results
ROS induced elevated α4 integrin glutathionylation in neutrophils
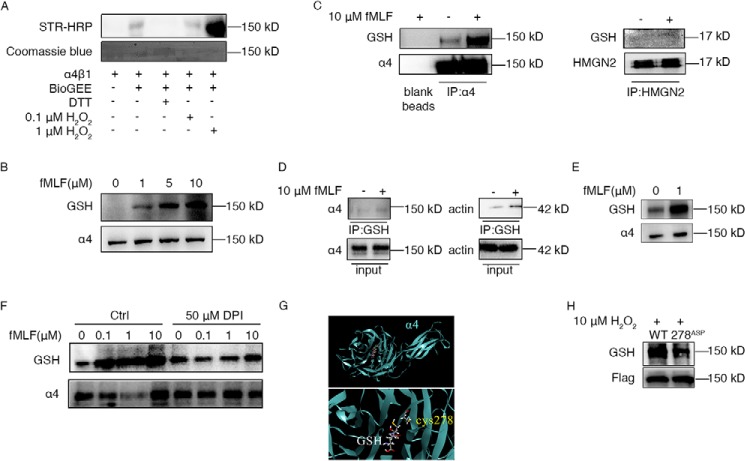
The bone marrow extracellular space is highly oxidizing, even stronger than the intracellular compartment (14). Thus, extracellular proteins of neutrophils might be regulated by ROS-induced glutathionylation. First, we performed in vitro glutathionylation assays with biotinylated GSH (BioGEE) to confirm the possible modifications of α4 integrin. As expected, recombinant α4 integrin was glutathionylated in vitro (Fig. 1A). H2O2 significantly increased the α4–BioGEE mixed disulfide adducts (BioGSS-α4) signal, whereas treatment with DTT eliminated glutathionylation of α4. To determine the modification of α4 integrin in neutrophils, we tested α4 glutathionylation with a GSH-specific antibody. Although no band was detected in neutrophil-like differentiated HL60 (dHL60) cell lysates, there was a band corresponding to the size of α4 integrin after formyl-methionyl-leucyl-phenylalanine (fMLF) stimulation (Fig. 1B). The glutathionylation signals elevated along with increasing concentrations of fMLF (1–10 μm) (Fig. 1B) and reached a peak at the 5-min time point, which then decreased along the time course (Fig. S1). To confirm whether the modified protein was α4 integrin, lysates of fMLF-treated dHL60 cells were pulled down with α4 antibody or GSH antibody and then were tested with α4 and GSH antibodies. Both assays showed that α4 integrin of neutrophils was indeed glutathionylated by ROS (Fig. 1, C and D). Glutathionylated α4 signals were detected after pulldown enrichment, even in nontreated dHL60 cells. Similarly, in murine neutrophils, fMLF triggered the glutathionylation of α4 integrin (Fig. 1E). Also, glutathionylated α4 was detected even without fMLF treatment. It is well known that ROS are mostly generated by NADPH oxidase in chemoattractant-stimulated neutrophils (13). To investigate whether glutathionylation of α4 integrin was directly induced by ROS, dHL60 cells were pretreated with diphenyleneiodonium chloride (DPI), which is an inhibitor of NADPH oxidase. After fMLF treatment, DPI-pretreated cells showed a significant decrease in α4 glutathionylation (Fig. 1F). These data indicate that α4 integrin is glutathionylated in neutrophils and that physiological ROS drastically augments the glutathionylation signal.
Figure 1.
ROS induced elevated α4 integrin glutathionylation in neutrophils. A, recombined integrin α4β1 protein was treated with biotinylated GSH (BioGEE), DTT, and H2O2 at the indicated concentrations for 30 min. Then the solutions were subjected to immunoblotting to detect BioGEE-modified α4 with streptavidin–horseradish peroxidase (STR-HRP). Total protein loading was evaluated with Coomassie blue dye. Every treatment used 0.5 μg protein. B, neutrophil-like dHL60 cells were treated with the indicated concentrations of fMLF for 5 min. Cells were harvested and subjected to immunoblotting for GSH and α4. C, dHL60 cells were left untreated or stimulated with 10 μm fMLF for 5 min. Whole-cell lysates were then subjected to α4 immunoprecipitation (IP) followed by immunoblotting for GSH-modified α4. Blank agarose was tested as a negative control. HMGN2 (without cysteine) antibody–induced pulldown was used as a control. D, dHL60 cells were left untreated or stimulated with 10 μm fMLF for 5 min. Whole-cell lysates were then subjected to GSH IP followed by immunoblotting for α4. Actin antibody was used as a control. E, murine neutrophils were left untreated or stimulated with 1 μm fMLF for 5 min. Whole-cell lysates were subjected to immunoblotting for GSH and α4. F, dHL60 cells were pretreated or not pretreated with 50 μm DPI for 30 min. Then both groups of dHL60 cells were stimulated with the indicated concentrations of fMLF for 5 min. Cells were harvested and subjected to immunoblotting for GSH and α4. G, GSH was docked into the α4 integrin crystal structure in complex with the α4 integrin β-propeller and thigh domain crystal structure (PDB code 4IRZ). The dominant pose of 10 docking runs is represented as sticks, and interacting cysteine is represented as sticks. H, dHL60 cells were transfected with constructs expressing either the WT or the 278Asp mutant form of FLAG–α4 integrin. These dHL60 cells were stimulated with 10 μm H2O2 for 5 min. Whole-cell lysates were then subjected to FLAG IP followed by immunoblotting for GSH-modified FLAG-α4. All data are representative of at least three separate experiments.
To predict the modified cysteines of α4 integrin by glutathionylation, molecular docking was performed by docking GSH into the α4 integrin β-propeller and thigh domain crystal structure (PDB code 4IRZ). It was shown that the S atom of Cys278 of α4 integrin was quite close to the S atom of GSH (4.9 Å) (Fig. 1G), indicating that these two S atoms might be able to form a disulfide bond. To verify the role of Cys-278 in α4 integrin glutathionylation, constructs expressing either mutation of Cys278 to Asp (278Asp) or WT FLAG-α4 were transfected in HL60 cells. dHL60 cells with 278Asp mutant α4 integrin displayed much lower H2O2-elicited glutathionylation of α4 integrin (Fig. 1H). The result suggests that Cys278 might be one of the glutathionylated sites of α4 integrin.
ROS regulated the binding of neutrophils to HUVECs by α4 integrin and VCAM-1
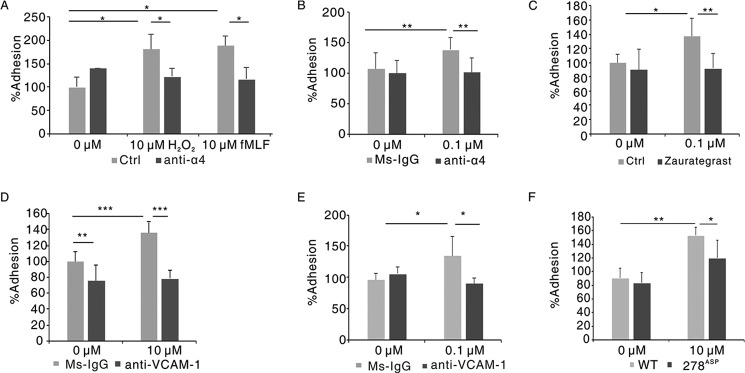
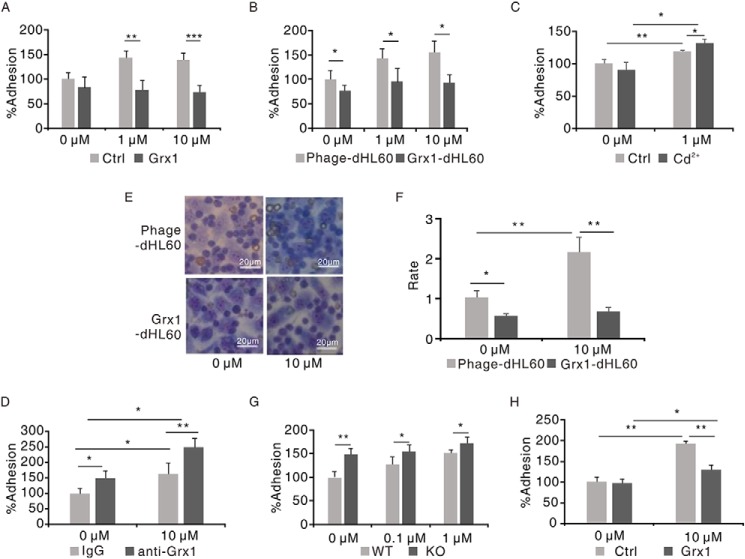
Next we tested the role of ROS-induced glutathionylation of α4 integrin in neutrophils adhering to endothelial cells using an in vitro adhesion assay of 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled neutrophils binding to a human umbilical vein endothelial cell (HUVEC) monolayer. As shown in Fig. 2A, either H2O2 or fMLF treatment elicited apparent increased adhesion of dHL60 cells to HUVECs, whereas α4 antibody treatment disrupted the effect. Consistently, fMLF-treated murine neutrophils showed elevated adhesion to HUVECs, and the increased adhesion was inhibited by the α4 antibody (Fig. 2B). Also, zaurategrast, an inhibitor of α4 integrin (21), displayed a similar effect (Fig. 2C). A membrane-impermeable sulfhydryl blocker, N-ethylmaleimide treatment largely decreased adhering neutrophils (Fig. S2). The adhesion assay was also performed with VCAM-1 antibody or IgG-pretreated HUVECs. VCAM-1 antibody, but not IgG, abolished the effect of ROS-triggered elevated adhesion (Fig. 2D). Similar results were obtained in the adhesion of murine neutrophils to HUVECs (Fig. 2E). We also examined the effect of mutation of Cys278 to Asp on ROS-induced increased adhesion of neutrophils to HUVECs. As shown in Fig. 2F, the adhesion rate of dHL60 cells with 278Asp mutant α4 integrin to HUVECs was significantly decreased compared with WT dHL60 cells after H2O2 stimulation. These results indicate that ROS-induced glutathionylation promotes the adhesion of neutrophils to endothelial cells through α4 integrin and VCAM-1.
Figure 2.
ROS regulated the binding of neutrophils to HUVECs by α4 integrin and VCAM-1. A, CFSE-labeled dHL60 cells were preincubated with 10 μg/ml α4 antibody or IgG for 30 min and then stimulated with 10 μm H2O2 or fMLF for 30 min, followed by adhesion to the HUVEC monolayer. The adhesion of IgG-pretreated cells without stimulation was defined as 100%. *, p < 0.05 versus IgG-treated dHL60 cells. Ctrl, control. B, CFSE-labeled murine neutrophils were preincubated with 10 μg/ml α4 antibody or IgG for 30 min and then stimulated with 0.1 μm fMLF for 30 min, followed by adhesion to the HUVEC monolayer. The adhesion of IgG-pretreated cells without stimulation was defined as 100%. **, p < 0.01 versus IgG-treated murine neutrophils. C, CFSE-labeled dHL60 cells were preincubated or not with 2 mm zaurategrast for 1 h and then stimulated with 10 μm fMLF for 30 min, followed by adhesion to the HUVEC monolayer. The adhesion of non-pretreated cells without stimulation was defined as 100%. *, p < 0.05; **, p < 0.01 versus non-treated dHL60 cells. D, HUVECs were preincubated with 10 μg/ml IgG or VCAM-1 antibody and then performed in vitro adhesion assay with CFSE-labeled dHL60 cells. The adhesion of IgG-pretreated cells without stimulation was defined as 100%. **, p < 0.01; ***, p < 0.001 versus IgG-treated dHL60 cells. E, HUVECs were preincubated with 10 μg/ml IgG or VCAM-1 antibody and then performed in vitro adhesion assay with CFSE-labeled murine neutrophils. The adhesion of IgG pretreated cells without stimulation was defined as 100%. *, p < 0.05 versus IgG-treated murine neutrophils. F, dHL60 cells were transfected with constructs expressing either the WT or 278Asp mutant form of FLAG–α4 integrin. GFP vector plasmids were co-transfected simultaneously in dHL60 cells to label transduced dHL60 cells. GFP-labeled dHL60 cells were stimulated with 10 μm H2O2 or not for 30 min, followed by adhesion to the HUVEC monolayer. The adhesion of dHL60 cells with WT FLAG-α4 without stimulation was defined as 100%. *, p < 0.05 versus WT FLAG-α4 dHL60 cells with 10 μm H2O2 treatment; **, p < 0.01 versus WT FLAG-α4 dHL60 cells without stimulation. Data are the means ± S.D. of three individual experiments.
Grx1 physiologically catalyzed α4 integrin deglutathionylation
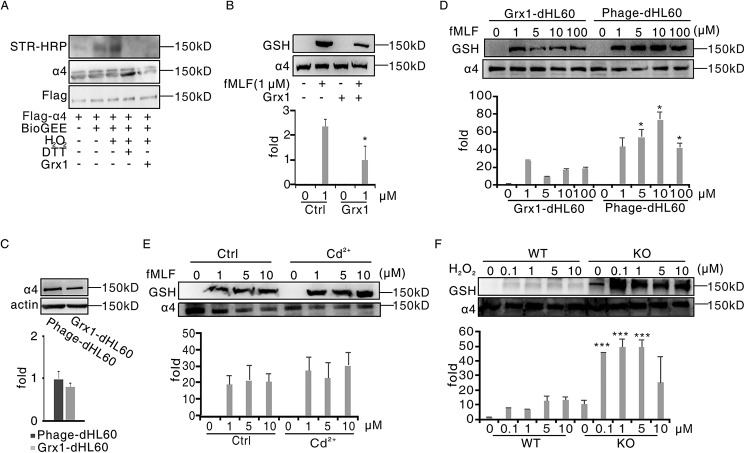
Grx is the main reductase of glutathionylation in mammals. In addition to the action in intracellular components, Grx1 has been reported to exist with catalytic activity in some extracellular environments, such as sputum, plasma, and bone marrow extracellular space (14,22,23). Therefore, we speculated that Grx1 might physiologically modulate the glutathionylation of α4 integrin. We primarily tested the effect of Grx1 on glutathionylation of α4 integrin in vitro. In a very similar fashion as DTT treatment, fusion Grx1 protein addition significantly weakened the glutathionylation signal of FLAG-α4 (Fig. 3A). The glutathionylated α4 integrin signal in dHL60 cells was also decreased when adding Grx1 protein to the culture medium (Fig. 3B).
Figure 3.
Grx1 physiologically catalyzed α4 integrin deglutathionylation. A, HEK293T cells were transfected with plasmids encoding FLAG-α4. After 48 h, cells were harvested and pulled down through anti-FLAG–agarose beads. Beads were treated with BioGEE and H2O2 at the indicated concentrations for 15 min. After adding 1 μg of Grx1 protein and being incubated for another 15 min, the solutions were subjected to immunoblot analysis for BioGEE-modified α4 with streptavidin–horseradish peroxidase (STR-HRP), and total α4 loading was evaluated with FLAG and α4 antibodies. B, dHL60 cells were preincubated with 1 μg of Grx1 protein or not for 30 min and then treated with 1 μm fMLF for 5 min. Cells were harvested and subjected to immunoblot analysis for GSH and α4. C, control dHL60 cells (Phage-dHL60) and Grx1-overexpressing dHL60 cells (Grx1-dHL60) were harvested and subjected to immunoblot analysis for α4 and actin detection. D, phage-dHL60 and Grx1-dHL60 cells were treated with the indicated concentrations of H2O2 for 5 min. Cells were harvested and subjected to SDS-PAGE followed by immunoblot analysis for GSH and α4. E, Grx1-dHL60 cells were pretreated with 2 mm Grx1 inhibitor Cd2+ or not for 30 min and then stimulated with the indicated concentration of H2O2 for 5 min. Cells were harvested and subjected to SDS-PAGE followed by immunoblot analysis for GSH and α4. F, WT and Grx1−/− mouse neutrophils were treated with the indicated concentrations of H2O2 for 5 min. Cells were harvested and subjected to SDS-PAGE followed by immunoblot analysis for GSH and α4. All data are representative of at least three separate experiments.
To further evaluate the role of Grx1 in glutathionylation of α4 integrin, we examined the glutathionylation of α4 integrin in Grx1-overexpressing dHL60 cells. Grx1 overexpression drastically decreased the glutathionylation of α4 in response to fMLF but barely affected the expression of α4 integrin (Fig. 3, C and D, and Fig. S3A). With increasing concentrations of fMLF stimulation, the α4-SSG signals of Grx1-overexpressing dHL60 cells also elevated but were always weaker than the control. By contrast, a Grx inhibitor, Cd2+, slightly increased α4 glutathionylation in neutrophils (Fig. 3E). To investigate whether Grx1 directly participated in α4 integrin glutathionylation, a Grx1 knockout mouse was used (Fig. S3B). Similar to Cd2+ treatment, murine Grx1-deficient neutrophils showed much stronger α4 glutathionylation than the WT cells (Fig. 3F). Together, our results demonstrate that α4 deglutathionylation is physiologically controlled by Grx1.
Grx1 inhibited the ligand binding activity of α4 integrin in neutrophils to VCAM-1
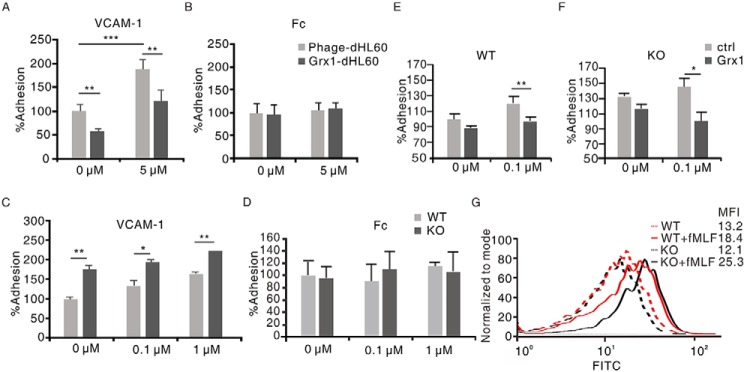
As our data show, glutathionylation modulated integrin α4/VCAM-1–dependent neutrophil adhesion by modifying α4. Grx1, the main regulator of α4 integrin deglutathionylation, might also be involved in this process. Therefore, the effect of Grx1 on α4-mediated neutrophil binding to immobilized VCAM-1 was tested first. According to a previous study (15) and our pretest, 1 μg/ml was chosen as the concentration of recombinant ligands. The effects of Grx1 on neutrophil binding to immobilized Fc were also examined as controls. Grx1 overexpression reduced dHL60 cell adhesion to VCAM-1 but not Fc (Fig. 4, A and B). Then the binding activity in murine Grx1−/− neutrophils was detected. In contrast, Grx1 deficiency activated murine neutrophils adhering to VCAM-1 but not Fc (Fig. 4, C and D). Interestingly, even without H2O2 treatment, Grx1 already displayed an impairing effect in ligand binding activity of α4 integrin in this assay (Fig. 4, A and C). Our results suggested that Grx1 might inhibit neutrophil binding to VCAM-1. To further evaluate the function of extracellular Grx1, murine WT and Grx1−/− neutrophils were pretreated with Grx1 protein. The result showed that Grx1 pretreatment suppressed the adhesion of WT neutrophils to VCAM-1 and restored the adhesion of Grx1−/− neutrophils to immobilized VCAM-1 (Fig. 4, E and F).
Figure 4.
Grx1 inhibited the ligand binding activity of α4 integrin in neutrophils to VCAM-1. A, CFSE-labeled Phage-dHL60 and Grx1-dHL60 cells were stimulated with 5 μm H2O2 or not and allowed to adhere to immobilized recombinant VCAM-1 proteins. The adhesion of cells without stimulation was defined as 100%. **, p < 0.01; ***, p < 0.001 versus Phage-dHL60 cells. B, CFSE-labeled Phage-dHL60 and Grx1-dHL60 cells were stimulated with 5 μm H2O2 or not and allowed to adhere to immobilized recombinant Fc proteins. The adhesion of cells without stimulation was defined as 100%. C, CFSE-labeled WT and Grx1−/− murine neutrophils were stimulated with the indicated concentrations of H2O2 and allowed to adhere to immobilized VCAM-1. The adhesion of WT murine neutrophils without stimulation was defined as 100%. *, p < 0.05; **, p < 0.01 versus WT murine neutrophils. D, CFSE-labeled WT and Grx1−/− murine neutrophils were stimulated with the indicated concentrations of H2O2 and allowed to adhere to immobilized Fc. The adhesion of WT murine neutrophils without stimulation was defined as 100%. E, CFSE-labeled WT murine neutrophils were preincubated with Grx1 protein (10 μg/ml) for 30 min and then stimulated with 0.1 μm H2O2 or not and allowed to adhere to immobilized VCAM-1. The adhesion of WT murine neutrophils without stimulation was defined as 100%. **, p < 0.01 versus control. F, CFSE-labeled Grx1−/− murine neutrophils were preincubated with Grx1 protein (10 μg/ml) for 30 min and then stimulated with 0.1 μm H2O2 or not and allowed to adhere to immobilized VCAM-1. The adhesion of WT murine neutrophils without stimulation was defined as 100%. *, p < 0.05 versus control. G, ligand binding assays for murine WT or Grx1−/− neutrophils with or without fMLF treatment were performed at the indicated concentrations of FITC–VCAM-1. The competitive assays were performed in the presences of 10 μg/ml FITC–VCAM-1 and subjected to flow cytometric analysis. MFI, mean fluorescence intensity. One representative of three experiments is shown. Data are the means ± S.D. of three individual experiments.
In addition, we performed a ligand-binding assay to soluble VCAM-1. Soluble VCAM-1 was prelabeled with FITC and then detected by flow cytometry. The results showed that more murine Grx1−/− neutrophils bound to FITC-labeled VCAM-1 than the WT after H2O2 treatment, whereas, under rest conditions, cells displayed a similar binding ability to VCAM-1 (Fig. 4G). These results also support the inhibiting effect of Grx1 on the ligand-binding activity of α4 integrin to VCAM-1.
Grx1 suppressed the adhesion of neutrophils to endothelial cells
Next, the role of Grx1 in neutrophils adhering to endothelial cells was investigated. Primary results showed that recombinant Grx1 pretreatment decreased the adhesion of dHL60 cells to the HUVEC monolayer in response to H2O2 (Fig. 5A), and fewer Grx1-overexpressing dHL60 cells adhered to HUVECs (Fig. 5B). Then Grx1-overexpressing dHL60 cells were pretreated with Cd2+, which rescued the impairing effect of Grx1 on dHL60 cells adhering to HUVECs after H2O2 stimulation (Fig. 5C). As Cd2+ is membrane-permeant, it may inhibit both intracellular and extracellular Grx1 activities (24). We thus performed the experiment pretreated with a Grx1-specific antibody to block the extracellular Grx1 activity. Similarly, Grx1 antibody–treated dHL60 cells displayed significantly increased adhesion to HUVECs (Fig. 5D). A Wright–Giemsa staining assay was also explored to directly observe the adhesion of neutrophils to HUVECs. As Fig. 5E shows, the morphology of HUVEC was much larger than that of dHL60 cell. Therefore, it is very clear to distinguish dHL60 cells from HUVECs. It can be observed that much fewer Grx1-overexpressing dHL60 cells attached to HUVECs than the control (Fig. 5, E and F).
Figure 5.
Grx1 suppressed the adhesion of neutrophils to endothelial cells. A, CFSE-labeled dHL60 cells were preincubated with Grx1 protein (10 μg/ml) or not for 30 min and then stimulated with the indicated concentrations of fMLF and allowed to adhere to HUVECs. The adhesion of cells without Grx1 incubation and stimulation was defined as 100%. **, p < 0.01; ***, p < 0.001 versus control (Ctrl). B, CFSE-labeled Phage-dHL60 or Grx1-dHL60 cells were stimulated with the indicated concentrations of H2O2 and allowed to adhere to HUVECs. The adhesion of Phage-dHL60 cells without stimulation was defined as 100%. *, p < 0.05 versus Phage-dHL60 cells. C, CFSE-labeled Grx1-dHL60 cells were preincubated with 2 mm Cd2+ or not for 30 min and then stimulated with 1 μm H2O2 and allowed to adhere to HUVECs. The adhesion of non-preincubated dHL60 cells without stimulation was defined as 100%. *, p < 0.05; **, p < 0.01 versus control. D, CFSE-labeled Grx1-dHL60 cells were preincubated with IgG or Grx1 antibody for 30 min and then stimulated with 10 μm H2O2 and allowed to adhere to HUVECs. The adhesion of IgG-pretreated cells without stimulation was defined as 100%. *, p < 0.05; **, p < 0.01 versus control. E, Phage-dHL60 or Grx1-dHL60 cells were stimulated with or without 10 μm fMLF and allowed to adhere to HUVECs. Cells were stained with Wright–Giemsa staining. Scale bars = 20 μm. F, quantification of the adhesion ratio of Phage-dHL60 or Grx1-dHL60 cells to HUVECs. *, p < 0.05; **, p < 0.01 versus Phage-dHL60 cells. Results are represented as mean ± S.D. of three individual experiments. G, CFSE-labeled murine WT and Grx1−/− murine neutrophils were stimulated with 1 μm H2O2 and allowed to adhere to HUVECs. The adhesion of WT neutrophils without stimulation was defined as 100%. *, p < 0.05; **, p < 0.01 versus murine WT neutrophils. H, CFSE-labeled murine Grx1−/− neutrophils were preincubated with Grx1 protein (10 μg/ml) or not for 30 min and then stimulated with or without 10 μm fMLF and allowed to adhere to HUVECs. The adhesion of non-incubated cells without stimulation was defined as 100%. *, p < 0.05; **, p < 0.01 versus control. Results are represented as mean ± S.D. of three individual experiments.
In murine neutrophils, Grx1 pretreatment also impaired the adhesion of neutrophils to HUVECs (Fig. S4). In contrast, Grx1 abolishment promoted murine neutrophils adhering to the HUVEC monolayer (Fig. 5G). Then murine Grx1−/− neutrophils were treated with recombinant Grx1 protein. As expected, the elevated adhesion after H2O2 stimulation was attenuated (Fig. 5H). It is noteworthy that sometimes Grx1 showed an impairing effect on adhesion of neutrophils to HUVECs under rest conditions, which might be due to the basic ROS level under rest conditions that has been detected in a previous study (25). Consistently, we observed a visible glutathionylated α4 signal in murine Grx1−/− neutrophils without stimulation (Fig. 3F). Collectively, these data show that Grx1 might negatively modulate the adhesion of neutrophils to endothelial cells through α4/VCAM-1 signaling in response to ROS.
Grx1 inhibited neutrophil mobilization from the bone marrow via α4 integrin
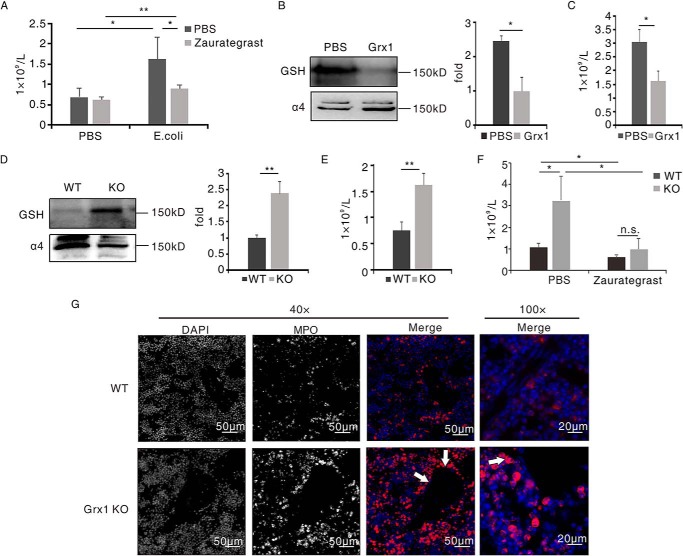
Increasing evidence has shown that integrin α4/VCAM-1 is critical adhesion signaling in the mobilization of neutrophils from the bone marrow. We explored neutrophil mobilization from the bone marrow during inflammation using an intraperitoneal Escherichia coli–induced acute peritonitis model in live mice. As expected, mice pretreated with zaurategrast displayed impaired neutrophil egress from the bone marrow (Fig. 6A).
Figure 6.
Grx1 inhibited neutrophil mobilization from the bone marrow via α4 integrin. A, mice were pretreated with zaurategrast (5 mg/kg) or PBS for 1 h. Mice were then challenged with E. coli (injected intraperitoneally, 1 × 107/mouse) or PBS. Neutrophils in peripheral blood were counted 1 h after challenge (n = 3/group). *, p < 0.05; **, p < 0.01 versus control. B and C, mice were pretreated with Grx1 (injected intravenously, 1 μg/mouse) for 1 h. Mice were then challenged with E. coli (injected intraperitoneally, 1 × 107/mouse). B, murine neutrophils purified at the 1-h time point and subjected to immunoblot analysis for GSH and α4 (n = 3/group). C, neutrophils in peripheral blood were counted 1 h after challenge (n = 3/group). *, p < 0.05 versus PBS pre-administrated mice. D and E, WT and Grx1−/− mice were challenged with E. coli (injected intraperitoneally, 1 × 107/mouse). D, murine neutrophils purified at the 1-h time point and subjected to immunoblot analysis for GSH and α4 (n = 3/group). E, neutrophils in peripheral blood were counted 1 h after challenge (n = 3/group). **, p < 0.01 versus WT mice. F, WT and Grx1−/− mice were pretreated with zaurategrast (5 mg/kg) or PBS for 1 h. Mice were then challenged with E. coli (injected intraperitoneally, 1 × 107/mouse). Neutrophils in peripheral blood were counted 1 h after challenge (n = 3/group). *, p < 0.05 versus PBS-pretreated WT mice; n.s., not significant. Data are the mean ± S.D. of three mice. One representative of three experiments is shown. G, the bone marrows of WT and Grx1−/− mice after treatment as in E was stained with myeloperoxidase antibody (MPO, red) and 4′,6-diamidino-2-phenylindole (DAPI, blue). Representative fluorescence images are shown. KO, knockout.
As our in vitro results showed that Grx1 modulated integrin α4/VCAM-1 adhesion signaling, we speculated that Grx1 might take part in neutrophil mobilization from the bone marrow by regulating α4 integrin. Preintravenous injection of recombinant Grx1 protein attenuated the glutathionylated α4 integrin of bone marrow neutrophils (Fig. 6B). Grx1-pretreated mice showed fewer neutrophils in peripheral blood, only half compared with control mice, at the 1-h time point after challenge (Fig. 6C), and even before peritonitis, administration of Grx1 impaired the glutathionylation of α4 integrin and decreased the number of neutrophils in peripheral blood (Fig. S5).
On the contrary, we compared bone marrow neutrophil mobilization between WT and Grx1−/− mice. We also checked the glutathionylation of α4 integrin and neutrophil number in peripheral blood 1 h after intraperitoneally injecting live E. coli. The Western blot result showed that Grx1 deficiency induced a dramatically elevated level of glutathionylated α4 integrin signal (Fig. 6D), and many more neutrophils in the circulation were detected in Grx1−/− mice (Fig. 6E). We also measured the alternation of neutrophil number in the bone marrow at the 1-h time point after the challenge by flow cytometry. The variation tendencies of neutrophil numbers in the bone marrow were contrary to those in peripheral blood (Fig. S6), but no significant differences were observed in zaurategrast pretreatment, Grx1 pretreatment, or Grx1−/− mice compared with the control. As noted previously, Grx1 knockout mice were similar to their WT littermates in differential leukocyte counts in the bone marrow and peripheral blood (13). Furthermore, α4 integrin inhibitor (zaurategrast) pretreatment lessened the effects of Grx1 depletion. Zaurategrast-treated Grx1−/− mice showed similar neutrophil number as WT mice in the circulation (Fig. 6F).
Immunohistochemistry was used to examine the location of neutrophils in the bone marrow after inflammation. The slides showed that more Grx1-attenuated neutrophils appeared near the marrow venous sinusoidal endothelium than WT neutrophils (Fig. 6G and Fig. S7, red). These results suggest that Grx1 might prevent neutrophil egress from the bone marrow via α4 integrin in response to stimuli.
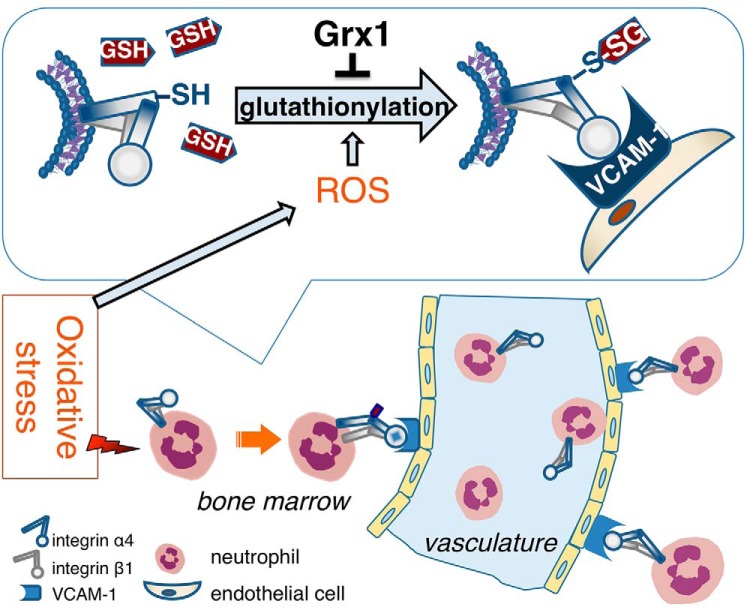
As reported previously (26–28), CXCR2 ligands (such as macrophage inflammatory protein 2 (MIP-2), chemokine (C-X-C motif) ligand 1 (CXCL1, also called KC)) and granulocyte-colony stimulating factor (G-CSF) signaling play crucial roles in neutrophil mobilization from the bone marrow. We examined the glutathionylation of MIP-2, KC, and G-CSF with an in vitro glutathionylation assay. None of them were glutathionylated (Fig. S8). These data suggest that Grx1- and ROS-mediated glutathionylation might have little direct effect on CXCR2 ligands/G-CSF mobilization signaling. Collectively, we found that ROS-induced α4 integrin glutathionylation and its reversal by Grx1 were crucial mechanisms controlling binding of VCAM-1 by α4 integrin in neutrophils and then promoted neutrophil mobilization from the bone marrow in inflammation (Fig. 7), providing some new clues for the treatment of exaggerated infections or inflammation-related diseases.
Figure 7.
Schematic of Grx1 and ROS involvement in neutrophil mobilization from the bone marrow via integrin α4/VCAM-1. Under stress conditions, the level of ROS is elevated in the bone marrow microenvironment. ROS induced the glutathionylation of α4 integrin in neutrophils, which increased the affinity of α4 of neutrophils to VCAM-1 on endothelial cells in the bone marrow. Furthermore, glutathionylation of α4 promoted neutrophil mobilization from the bone marrow. These effects were negatively mediated by Grx1.
Discussion
Integrins are a series of transmembrane α and β subunit–formed adhesion molecules mediating cell adhesion in many processes (29). In this study, we revealed that Grx- and ROS-mediated glutathionylation regulated α4 integrin affinity in neutrophils and further established a role for Grx1 in acute bone marrow neutrophil mobilization through suppressing integrin α4/VCAM-1 adhesion signaling.
ROS-induced glutathionylation, as a reversible redox modification, controls the activities of target proteins and further modulates cellular function and signaling. Accumulating studies have reported that some extracellular molecules might be regulated by glutathionylation (6). Our data showed that neutrophil surface α4 integrin was glutathionylated by ROS both in vitro and in vivo, and demonstrated that ROS-triggered glutathionylation promoted α4 integrin-derived neutrophils adhering to the endothelium. Other studies on α4 integrin in eosinophils, RAW264.7 cells, or melanoma B16 cells also supported our findings (15, 30, 31). They found that a high concentration of H2O2 (100 μm) suppressed the adhesion of eosinophil surface α4 integrin to VCAM-1. Similarly, in our study, 100 μm H2O2 also inhibited the adhesion of neutrophils to endothelial cells and decreased glutathionylation of α4 integrin (Fig. 3D). It is possible that overdose of ROS might induce irreversible oxidization, but not glutathionylation. However, recent studies showed that the level of ROS was less than 10 μm in the bone marrow extracellular space under stress conditions (14, 25). We also examined the ROS level of the bone marrow extracellular space under both rest and inflammatory conditions. The value was no more than 20 μm even after inducing inflammation. In short, ROS may rarely reach 100 μm under physiological conditions. Therefore, we chose no more than 10 μm of H2O2 to perform experiments to investigate the role of physiological ROS-triggered glutathionylation on α4 integrin.
Glutathionylation is reversed by some reducing enzymes, which is called deglutathionylation. There are three main groups of molecules acting for deglutathionylation, including Grx, thioredoxin (Trx), and sulfiredoxin (Srx) (32, 33). They oxidize their own free thiols and then reduce protein thiols. Classically they are found to mediate deglutathionylation of intracellular proteins. Some groups have reported activity of Grx1 in the extracellular area (6, 14, 22, 23, 34), implying that Grx1 might play reducing roles in the extracellular space. Structural analysis of α4 integrin shows that all 24 cysteines are located at its extracellular domains (35). Therefore, we assumed that deglutathionylation of α4 integrin might be modulated by extracellular Grx1. As expected, we found that recombinant Grx1 protein treatment or Grx1 overexpression attenuated the glutathionylation of α4 integrin and, thus, drastically inhibited α4-derived neutrophils adhering to VCAM-1 or endothelial cells. By contrast, an inhibitor of Grx (Cd2+), neutralizing Abs against Grx1, or Grx1 depletion significantly elevated the level of glutathionylated α4 integrin and thus promoted the adhesion of neutrophils to VCAM-1 or endothelial cells. Moreover, the increasing adhesion was prevented by preculture with Grx1 protein in murine Grx1−/− neutrophils. To the best of our knowledge, these results are the first to show the effect of extracellular Grx1 on membrane-anchored proteins via glutathionylation. However, the roles of Trx and Srx in glutathionylation of α4 integrin need to be studied in the future.
As reported previously (36), the α4 subunit participates in two integrins, α4β1 (very late antigen 4, VLA-4) and α4β7 (lymphocyte Peyer's patch adhesion molecule, LPAM). LPAM is expressed at very low levels on neutrophils and appears to have few effects on neutrophil mobilization from the bone marrow (19). Therefore, the effects of Grx1 on adhesion of neutrophils to endothelial cells might be contributed by regulating VLA-4 specifically. Although the β1 subunit has been proven not to be modified by glutathionylation (30), Grx1 and ROS might mainly target the α4 subunit. A previous study showed that Cys278 is important for α4 integrin ligand-binding (15, 37). A docking analysis of GSH and α4 integrin predicted that the sites of glutathionylation might include Cys278, and mutation of Cys278 to Asp showed reduced glutathionylation of α4 integrin and impaired ROS-induced elevated adhesion of neutrophils to HUVECs, suggesting that glutathionylation is crucial to increase α4 integrin affinity. α4 integrin contains some other unique cysteine residues at positions 717, 767, and 828 in non-analyzed domains (30, 38). Thus, there might be other modified cysteine residues in α4 integrin. The exact sites of glutathionylation still need to be determined in a future study.
Integrins are of importance in many immunological, physiological, and pathological processes, especially leukocyte extravasation from blood to tissue and progenitor cell/leukocyte release from the bone marrow (39). It is quite clear that β2 integrins are the key integrins driving the adhesion of neutrophil migration from blood to tissue (40, 41). α4 integrin seems to play few roles in this process, with controversial expression on peripheral blood neutrophils (42–44). A wide range of studies revealed that VLA-4/VCAM-1 adhesion signaling is essential in homeostatic stem cell/progenitor cell egress from the bone marrow (45, 46). Although the effect of VLA-4 on neutrophil release from the bone marrow under rest condition is debated, VLA-4 has been proven to mediate marrow neutrophil mobilization under stress conditions (17, 19). Bone marrow neutrophils have been shown to express VLA-4 (17), and a specific blocking antibody or antagonist of α4 inhibits MIP-2–induced bone marrow neutrophil mobilization (17). Our results also demonstrated that inhibition of α4 integrin decreased the number of neutrophils in the circulation after inflammation.
As we know, α4 integrin is also expressed in some other cells, such as monocytes, macrophages, and T cells, and in some tumor cells, such as melanoma cells. Several studies have shown that oxidative stress is involved in some α4-dependent cellular functions. Low-dose ionizing radiation–induced ROS promoted the avidity of VLA4 of RAW264.7 and VCAM-1 (30). Treatment with the ROS scavenger N-acetylcysteine elevates peripheral blood mononuclear cell (PBMC) surface thiol and regulates VLA4-dependent adhesion and Jurkat aggregation (47). Grx1 has been reported to participate in some related processes or diseases. Recent studies showed that overexpression of Grx1 inhibited monocyte chemotaxis induced by MCP-1 (48), and Grx1 KO mice are more susceptible to pulmonary fibrosis (49). Elevated Grx1 activity diminishes the development of Parkinson's disease (50). However, few studies have reported interactions of Grx1 and VLA4 in those processes, which need further investigation.
Given that Grx1 regulates the affinity of α4 integrin in neutrophils, Grx1 might be involved in stress-induced bone marrow neutrophil mobilization. Here we uncovered Grx1-modulated neutrophil mobilization from the bone marrow in inflammatory conditions. We examined the neutrophil number in peripheral blood at the 1-h time point after induction of peritonitis. Mice preadministered recombinant Grx1 protein showed significantly decreased neutrophil numbers in the circulation and suppressed glutathionylated α4 integrin in bone marrow neutrophils. By contrast, compared with WT mice, Grx1−/− mice displayed many more neutrophils in peripheral blood and enhanced glutathionylation of α4 integrin of neutrophils in the bone marrow. Similarly, we observed that Grx1−/− mice egressed more neutrophils from the bone marrow after MIP-2 stimulation.
In conclusion, our study identified Grx1-mediated and ROS-induced glutathionylation as a novel regulating mechanism of bone marrow neutrophil mobilization under stress conditions. By mediating α4 integrin glutathionylation, Grx1 suppressed the adhesion of neutrophils to the marrow venous sinusoidal endothelium and thus impaired neutrophil migration from marrow to blood in response to infection or stress.
Experimental procedures
Cell culture
HL60 cells were purchased from the ATCC (CCL-240TM) and cultured in RPMI 1640 medium (Gibco) supplemented with 2 mm l-glutamine, 15% heat-inactivated fetal bovine serum (Gibco), and 1% penicillin/streptomycin. dHL60 cells were treated with 1.3% DMSO for 6 days in Iscove's modified Dulbecco's medium to be differentiated to a neutrophil-like phenotype. HUVECs were purchased from the ATCC (CRL-1730TM) and maintained in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. HEK293T cells were obtained from the ATCC (CRL-11268TM) and cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin.
Mice
Grx1−/− mice were obtained from Cyagen (KOCMP-23992-Glrx). In all experiments, we used age-matched C57BL/6 mice as WT controls. 6- to 8-week-old C57BL/6 mice were obtained from DaShuo and housed in the animal facilities of the West China School of Basic Medical Sciences and Forensic Medicine at Sichuan University. All procedures that involved mice were approved by the ethics committee of the West China School of Basic Medical Sciences and Forensic Medicine at Sichuan University.
Reagents
Biotinylated GSH (GSH ethyl ester and biotin amide (BioGEE), G36000) were obtained from Invitrogen. 30% hydrogen peroxide solution (216763), fMLF (F3506), N-ethylmaleimide (E3876), and cadmium chloride (20899) were obtained from Sigma. DPI (HY-100965, 10 mg) was obtained from MCE. CFSE (C1031) was obtained from Beyotime. The cell line nucleofector kits (VCA-1003) were obtained from Lonza. Anti-FLAG tag agarose-conjugated beads (273887) were obtained from Abmart. Rat anti-mouse VCAM-1 (sc-18864L, Santa Gruz Biotechnology), anti-mouse/rabbit Grx1 (ab55059 and ab45953, respectively, Abcam), anti-glutathione (ab19534, Abcam), anti-rabbit integrin α4 (8440S, CST), and appropriate isotype control Abs were purchased. For flow cytometry, FITC-conjugated rat anti-mouse LY-6C (553126), phosphatidylethanolamine-Cy7–conjugated rat anti-mouse CD11b (552850) mAbs, and isotype control Abs were obtained from BD Pharmingen. Human recombinant VCAM-1 (ADP4-050) was obtained from R&D Systems. CD31 (GB11063-3) and MPO (GB11224-1) were obtained from Servicebio.
Cell adhesion assay
Here we performed an in vitro adhesion assay based on a previous study (15). For adhesion to immobilized proteins, soluble VCAM-1 or Fc at 10 μg/ml in PBS was cultured overnight at 4 °C in 96-well plates. We treated the plate wells with 0.5% BSA to block nonspecific binding. Cells were labeled with 5 μm CFSE and cultured for 20 min at room temperature. The CFSE-labeled dHL60 cells were pretreated with H2O2 or fMLF and then adhered to immobilized proteins for 60 min at 37 °C. For adhesion to the HUVEC monolayer, HUVECs were seeded at 5 × 103 cells/well into a 96-well plate and cultured overnight. CFSE-labeled cells were pretreated with H2O2 or fMLF and adhered to the HUVEC monolayer for 30 min at 37 °C before washing and detection of cell adhesion. Next, we removed nonadherent cells and detected the fluorescence. The adhesion ratio refers to the ratio of adherent cell fluorescence to input fluorescence.
Alternatively, we stained both attached dHL60 cells and HUVECs via Wright–Giemsa staining and then counted these two types of cells by light scope. The cell adhesion ratio was defined as the ratio of dHL60 cells to HUVECs.
FITC-labeled recombinant protein binding assay
The binding assay was modified from a previous study (15). Briefly, recombinant proteins were labeled with FITC using a FITC labeling kit (Gbiosciense). 1 × 106 cells/ml were pretreated with or without H2O2 in Hank's balanced salt solution (HBSS) for 10 min and then cultured with 1 μg of FITC–VCAM-1 in 0.1 ml HBSS at room temperature for 10 min. Finally, we tested the intensity of FITC by flow cytometry.
Molecular docking
Possible glutathionylated sites of α4 were predicted by docking GSH into the α4 integrin β-propeller and thigh domain crystal structure (extraction from the α4β7 crystal structure in complex with Fab natalizumab, PDB code 4IRZ) via CDOCKER, which was implemented in Accelrys Discovery Studio Client (DS) version 3.1 (Accelrys Software Inc., San Diego, CA) (51).
Immunohistochemistry
Marrow plugs were obtained from mouse femora after decalcification and embedded with paraffin before sectioning and mounting. For staining, slides were permeabilized with 0.1% Triton X-100 in PBS and blocked with 1% BSA followed by 10% goat serum, and then incubated with rabbit anti-CD31 (GB11063-3, 1:100) overnight at 4 °C, followed by Alexa phosphatidylethanolamine goat anti-rabbit IgG (P9795, 1:400) for 2 h. Slides were then washed, counterstained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) before scanning with Pannoramic MIDI (3D HISTECH) with the attached Quant center software (Optical Analysis) and associated CaseViewer software. Marrow for MPO staining was prepared as above, except for imaging with an Olympus BX50 inverted microscope (Olympus America) with an attached Optronics MagnaFire digital camera (Optical Analysis) and associated MagnaFire software (version 2.0) when the images were magnified about ×100.
Marrow neutrophil acute mobilization model, glutathionylation, and other neutrophil functional assays
Peritonitis was induced by intraperitoneal injection with 1 × 107 E. coli (strain 19138, ATCC) in WT and Grx1−/− mice. One hour after the challenge, peripheral blood was collected through the fundus after anesthetizing mice with isoflurane and tested using a hematology analyzer. Then mice were sacrificed, and bone marrow neutrophils were purified. Purified neutrophils were probed with GSH and α4 integrin antibodies. For recombinant Grx1 treatment, 1 μg of Grx1 protein was intravenously injected into the tail vein 1 h before inducing peritonitis. For α4 inhibitor treatment assay, zaurategrast was intraperitoneally injected at a concentration of 5 mm/kg 3 h in advance. Some other related assays, including neutrophil isolation, immunoprecipitation, and Wright–Giemsa staining, have been described in a previous publication (13).
Statistical analysis
All comparisons were performed with two-tailed Student's t test using Microsoft Excel. p < 0.05 was considered significant *, p < 0.05, **, p < 0.01, ***, p < 0.001. All experiments were performed at least three times.
Author contributions
Y. Y., J. C., X. G., N. H., and J. L. conceptualization; Y. Y., N. H., and J. L. resources; Y. Y., J. C., F. Z., Q. X., L. H., X. Z., J. M., L. G., and J. L. data curation; Y. Y., J. C., and F. Z. software; Y. Y., J. C., and J. L. formal analysis; Y. Y., X. G., and J. L. validation; Y. Y., J. C., F. Z., Q. X., L. H., X. Z., X. S., H. R., Y. D., L. G., X. W., and J. L. investigation; Y. Y. visualization; Y. Y., J. C., F. Z., Q. X., L. H., X. G., X. Z., J. M., X. S., H. R., Y. D., and J. L. methodology; Y. Y., N. H., and J. L. writing-original draft; J. C., X. S., X. W., Y. W., S. C., N. H., and J. L. funding acquisition; J. C., H. R. L., N. H., and J. L. project administration; J. C., H. R. L., Y. W., S. C., N. H., and J. L. writing-review and editing; Y. W., S. C., N. H., and J. L. supervision.
Supplementary Material
Acknowledgments
We thank Yuanfu Xu, Yan Teng, Xiaofei Shen, Fumei Zhang, Xiaojuan Guo, Peng Liu, Xuemei Xie, Xinle Li, Lin Lin, and Li Zhao for helpful discussions and Chao Li and Zihan Li for family support.
The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S8.
- ROS
- reactive oxygen species
- VCAM-1
- vascular cell adhesion molecule 1
- Grx1
- glutaredoxin 1
- -SSG
- -GSH mixed disulfide adducts
- BioGEE
- biotinylated GSH
- dHL60 cells
- differentiated HL60 cells
- fMLF
- formyl-methionyl-leucyl-phenylalanine
- CFSE
- 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester
- HUVEC
- human umbilical vein endothelial cell
- DPI
- diphenyleneiodonium chloride
- Ab
- antibody
- IP
- immunoprecipitation
- MPO
- myeloperoxidase
- MIP-2
- macrophage inflammatory protein 2
- G-CSF
- granulocyte-colony stimulating factor
- VLA-4
- very late antigen 4
- HBSS
- Hank's balanced salt solution.
References
- 1. Thomas J. A., Zhao W., Hendrich S., and Haddock P. (1995) Analysis of cells and tissues for S-thiolation of specific proteins. Methods Enzymol. 251, 423–429 10.1016/0076-6879(95)51145-8 [DOI] [PubMed] [Google Scholar]
- 2. Pastore A., and Piemonte F. (2012) S-glutathionylation signaling in cell biology: progress and prospects. Eur. J. Pharm. Sci. 46, 279–292 10.1016/j.ejps.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 3. Giganti D., Yan K., Badilla C. L., Fernandez J. M., and Alegre-Cebollada J. (2018) Disulfide isomerization reactions in titin immunoglobulin domains enable a mode of protein elasticity. Nat. Commun. 9, 185 10.1038/s41467-017-02528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen C. A., Wang T. Y., Varadharaj S., Reyes L. A., Hemann C., Talukder M. A., Chen Y. R., Druhan L. J., and Zweier J. L. (2010) S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature 468, 1115–1118 10.1038/nature09599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stojkov D., Amini P., Oberson K., Sokollik C., Duppenthaler A., Simon H. U., and Yousefi S. (2017) ROS and glutathionylation balance cytoskeletal dynamics in neutrophil extracellular trap formation. J. Cell Biol. 216, 4073–4090 10.1083/jcb.201611168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shelton M. D., and Mieyal J. J. (2008) Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol. Cells 25, 332–346 [PMC free article] [PubMed] [Google Scholar]
- 7. Tafani M., Sansone L., Limana F., Arcangeli T., De Santis E., Polese M., Fini M., and Russo M. A. (2016) The interplay of reactive oxygen species, hypoxia, inflammation, and sirtuins in cancer initiation and progression. Oxid. Med. Cell Longev. 2016, 3907147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dalle-Donne I., Rossi R., Colombo G., Giustarini D., and Milzani A. (2009) Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem. Sci. 34, 85–96 10.1016/j.tibs.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 9. Holmgren A. (1976) Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc. Natl. Acad. Sci. U.S.A. 73, 2275–2279 10.1073/pnas.73.7.2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuipers I., Bracke K. R., Brusselle G. G., Aesif S. W., Krijgsman R., Arts I. C., Wouters E. F., and Reynaert N. L. (2012) Altered cigarette smoke-induced lung inflammation due to ablation of Grx1. PLoS ONE 7, e38984 10.1371/journal.pone.0038984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lillig C. H., Berndt C., and Holmgren A. (2008) Glutaredoxin systems. Biochim. Biophys. Acta 1780, 1304–1317 10.1016/j.bbagen.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 12. Fernandes A. P., and Holmgren A. (2004) Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid. Redox Signal. 6, 63–74 10.1089/152308604771978354 [DOI] [PubMed] [Google Scholar]
- 13. Sakai J., Li J., Subramanian K. K., Mondal S., Bajrami B., Hattori H., Jia Y., Dickinson B. C., Zhong J., Ye K., Chang C. J., Ho Y. S., Zhou J., and Luo H. R. (2012) Reactive oxygen species-induced actin glutathionylation controls actin dynamics in neutrophils. Immunity 37, 1037–1049 10.1016/j.immuni.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang X., Liu P., Zhang C., Chiewchengchol D., Zhao F., Yu H., Li J., Kambara H., Luo K. Y., Venkataraman A., Zhou Z., Zhou W., Zhu H., Zhao L., Sakai J., et al. (2017) Positive regulation of interleukin-1β bioactivity by physiological ROS-mediated cysteine S-glutathionylation. Cell Rep. 20, 224–235 10.1016/j.celrep.2017.05.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu S. Y., Tsai M. Y., Chuang K. P., Huang Y. F., and Shieh C. C. (2008) Ligand binding of leukocyte integrin very late antigen-4 involves exposure of sulfhydryl groups and is subject to redox modulation. Eur. J. Immunol. 38, 410–423 10.1002/eji.200737556 [DOI] [PubMed] [Google Scholar]
- 16. Zhao E., Xu H., Wang L., Kryczek I., Wu K., Hu Y., Wang G., and Zou W. (2012) Bone marrow and the control of immunity. Cell Mol. Immunol. 9, 11–19 10.1038/cmi.2011.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burdon P. C., Martin C., and Rankin S. M. (2005) The CXC chemokine MIP-2 stimulates neutrophil mobilization from the rat bone marrow in a CD49d-dependent manner. Blood 105, 2543–2548 10.1182/blood-2004-08-3193 [DOI] [PubMed] [Google Scholar]
- 18. Sadik C. D., Kim N. D., and Luster A. D. (2011) Neutrophils cascading their way to inflammation. Trends Immunol. 32, 452–460 10.1016/j.it.2011.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petty J. M., Lenox C. C., Weiss D. J., Poynter M. E., and Suratt B. T. (2009) Crosstalk between CXCR4/stromal derived factor-1 and VLA-4/VCAM-1 pathways regulates neutrophil retention in the bone marrow. J. Immunol. 182, 604–612 10.4049/jimmunol.182.1.604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hyun Y. M., and Hong C. W. (2017) Deep insight into neutrophil trafficking in various organs. J. Leukoc. Biol. 102, 617–629 10.1189/jlb.1RU1216-521R [DOI] [PubMed] [Google Scholar]
- 21. Rettig M. P., Ansstas G., and DiPersio J. F. (2012) Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia 26, 34–53 10.1038/leu.2011.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peltoniemi M. J., Rytilä P. H., Harju T. H., Soini Y. M., Salmenkivi K. M., Ruddock L. W., and Kinnula V. L. (2006) Modulation of glutaredoxin in the lung and sputum of cigarette smokers and chronic obstructive pulmonary disease. Respir. Res. 7, 133 10.1186/1465-9921-7-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura H., Vaage J., Valen G., Padilla C. A., Björnstedt M., and Holmgren A. (1998) Measurements of plasma glutaredoxin and thioredoxin in healthy volunteers and during open-heart surgery. Free Radic. Biol. Med. 24, 1176–1186 10.1016/S0891-5849(97)00429-2 [DOI] [PubMed] [Google Scholar]
- 24. Chrestensen C. A., Starke D. W., and Mieyal J. J. (2000) Acute cadmium exposure inactivates thioltransferase (glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J. Biol. Chem. 275, 26556–26565 10.1074/jbc.M004097200 [DOI] [PubMed] [Google Scholar]
- 25. Zhu H., Kwak H. J., Liu P., Bajrami B., Xu Y., Park S. Y., Nombela-Arrieta C., Mondal S., Kambara H., Yu H., Chai L., Silberstein L. E., Cheng T., and Luo H. R. (2017) Reactive oxygen species-producing myeloid cells act as a bone marrow niche for sterile inflammation-induced reactive granulopoiesis. J. Immunol. 198, 2854–2864 10.4049/jimmunol.1602006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bajrami B., Zhu H., Kwak H. J., Mondal S., Hou Q., Geng G., Karatepe K., Zhang Y. C., Nombela-Arrieta C., Park S. Y., Loison F., Sakai J., Xu Y., Silberstein L. E., and Luo H. R. (2016) G-CSF maintains controlled neutrophil mobilization during acute inflammation by negatively regulating CXCR2 signaling. J. Exp. Med. 213, 1999–2018 10.1084/jem.20160393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eash K. J., Greenbaum A. M., Gopalan P. K., and Link D. C. (2010) CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. J. Clin. Invest. 120, 2423–2431 10.1172/JCI41649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Petit I., Szyper-Kravitz M., Nagler A., Lahav M., Peled A., Habler L., Ponomaryov T., Taichman R. S., Arenzana-Seisdedos F., Fujii N., Sandbank J., Zipori D., and Lapidot T. (2002) G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 3, 687–694 10.1038/ni813 [DOI] [PubMed] [Google Scholar]
- 29. Shimizu Y., Rose D. M., and Ginsberg M. H. (1999) Integrins in the immune system. Adv. Immunol. 72, 325–380 10.1016/S0065-2776(08)60024-3 [DOI] [PubMed] [Google Scholar]
- 30. Yuan Y., Lee S. H., and Wu S. (2013) The role of ROS in ionizing radiation-induced VLA-4 mediated adhesion of RAW264.7 cells to VCAM-1 under flow conditions. Radiat. Res. 179, 62–68 10.1667/RR3119.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valcárcel M., Carrascal T., Crende O., and Vidal-Vanaclocha F. (2014) IL-18 regulates melanoma VLA-4 integrin activation through a hierarchized sequence of inflammatory factors. J. Invest. Dermatol. 134, 470–480 10.1038/jid.2013.342 [DOI] [PubMed] [Google Scholar]
- 32. Popov D. (2014) Protein S-glutathionylation: from current basics to targeted modifications. Arch. Physiol. Biochem. 120, 123–130 10.3109/13813455.2014.944544 [DOI] [PubMed] [Google Scholar]
- 33. Sevilla F., Camejo D., Ortiz-Espín A., Calderón A., Lázaro J. J., and Jiménez A. (2015) The thioredoxin/peroxiredoxin/sulfiredoxin system: current overview on its redox function in plants and regulation by reactive oxygen and nitrogen species. J. Exp. Bot. 66, 2945–2955 10.1093/jxb/erv146 [DOI] [PubMed] [Google Scholar]
- 34. Du Y., Zhang H., Montano S., Hegestam J., Ekberg N. R., Holmgren A., Brismar K., and Ungerstedt J. S. (2014) Plasma glutaredoxin activity in healthy subjects and patients with abnormal glucose levels or overt type 2 diabetes. Acta Diabetol. 51, 225–232 10.1007/s00592-013-0498-2 [DOI] [PubMed] [Google Scholar]
- 35. Takada Y., Elices M. J., Crouse C., and Hemler M. E. (1989) The primary structure of the α4 subunit of VLA-4: homology to other integrins and a possible cell-cell adhesion function. EMBO J. 8, 1361–1368 10.1002/j.1460-2075.1989.tb03516.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Harp J. A., Waters T. E., and Goff J. P. (2005) Adhesion molecule and homing receptor expression on blood and milk polymorphonuclear leukocytes during the periparturient period of dairy cattle. Vet. Immunol. Immunopathol. 104, 99–103 10.1016/j.vetimm.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 37. Pujades C., Teixidó J., Bazzoni G., and Hemler M. E. (1996) Integrin α4 cysteines 278 and 717 modulate VLA-4 ligand binding and also contribute to α 4/180 formation. Biochem. J. 313, 899–908 10.1042/bj3130899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu Y., Schürpf T., and Springer T. A. (2013) How natalizumab binds and antagonizes α4 integrins. J. Biol. Chem. 288, 32314–32325 10.1074/jbc.M113.501668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rose D. M., Han J., and Ginsberg M. H. (2002) α4 integrins and the immune response. Immunol. Rev. 186, 118–124 10.1034/j.1600-065X.2002.18611.x [DOI] [PubMed] [Google Scholar]
- 40. Kolaczkowska E., and Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 41. Tseng A., Kim K., Li J., and Cho J. (2018) Myeloperoxidase negatively regulates neutrophil-endothelial cell interactions by impairing αMβ2 integrin function in sterile inflammation. Front. Med. 5, 134 10.3389/fmed.2018.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bochner B. S., Luscinskas F. W., Gimbrone M. A. Jr., Newman W., Sterbinsky S. A., Derse-Anthony C. P., Klunk D., and Schleimer R. P. (1991) Adhesion of human basophils, eosinophils, and neutrophils to interleukin 1-activated human vascular endothelial cells: contributions of endothelial cell adhesion molecules. J. Exp. Med. 173, 1553–1557 10.1084/jem.173.6.1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ibbotson G. C., Doig C., Kaur J., Gill V., Ostrovsky L., Fairhead T., and Kubes P. (2001) Functional α4-integrin: a newly identified pathway of neutrophil recruitment in critically ill septic patients. Nat. Med. 7, 465–470 10.1038/86539 [DOI] [PubMed] [Google Scholar]
- 44. Kirveskari J., Bono P., Granfors K., Leirisalo-Repo M., Jalkanen S., and Salmi M. (2000) Expression of α4-integrins on human neutrophils. J. Leukoc. Biol. 68, 243–250 [PubMed] [Google Scholar]
- 45. Papayannopoulou T., Priestley G. V., Nakamoto B., Zafiropoulos V., and Scott L. M. (2001) Molecular pathways in bone marrow homing: dominant role of α4β1 over β2-integrins and selectins. Blood 98, 2403–2411 10.1182/blood.V98.8.2403 [DOI] [PubMed] [Google Scholar]
- 46. Richter R., Forssmann W., and Henschler R. (2017) Current developments in mobilization of hematopoietic stem and progenitor cells and their interaction with niches in bone marrow. Transfus. Med. Hemother. 44, 151–164 10.1159/000477262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laragione T., Bonetto V., Casoni F., Massignan T., Bianchi G., Gianazza E., and Ghezzi P. (2003) Redox regulation of surface protein thiols: identification of integrin α-4 as a molecular target by using redox proteomics. Proc. Natl. Acad. Sci. U.S.A. 100, 14737–14741 10.1073/pnas.2434516100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ullevig S., Zhao Q., Lee C. F., Seok Kim H., Zamora D., and Asmis R. (2012) NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler. Thromb. Vasc. Biol. 32, 415–426 10.1161/ATVBAHA.111.238899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anathy V., Lahue K. G., Chapman D. G., Chia S. B., Casey D. T., Aboushousha R., van der Velden J. L. J., Elko E., Hoffman S. M., McMillan D. H., Jones J. T., Nolin J. D., Abdalla S., Schneider R., Seward D. J., et al. (2018) Reducing protein oxidation reverses lung fibrosis. Nat. Med. 24, 1128–1135 10.1038/s41591-018-0090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gorelenkova Miller O., and Mieyal J. J. (2018) Critical roles of glutaredoxin in brain cells-implications for Parkinson's disease. Antioxid. Redox Signal. 10.1089/ars.2017.7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang X., Sha K., Xu G., Tian H., Wang X., Chen S., Wang Y., Li J., Chen J., and Huang N. (2016) Subinhibitory concentrations of allicin decrease uropathogenic Escherichia coli (UPEC) biofilm formation, adhesion ability, and swimming motility. Int. J. Mol. Sci. 17, E979 10.3390/ijms17070979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.