Abstract
Estrogen hormones play an important role in controlling glucose homeostasis and pancreatic β-cell function. Despite the significance of estrogen hormones for regulation of glucose metabolism, little is known about the roles of endogenous estrogen metabolites in modulating pancreatic β-cell function. In this study, we evaluated the effects of major natural estrogen metabolites, catechol estrogens, on insulin secretion in pancreatic β-cells. We show that catechol estrogens, hydroxylated at positions C2 and C4 of the steroid A ring, rapidly potentiated glucose-induced insulin secretion via a nongenomic mechanism. 2-Hydroxyestrone, the most abundant endogenous estrogen metabolite, was more efficacious in stimulating insulin secretion than any other tested catechol estrogens. In insulin-secreting cells, catechol estrogens produced rapid activation of calcium influx and elevation in cytosolic free calcium. Catechol estrogens also generated sustained elevations in cytosolic free calcium and evoked inward ion current in HEK293 cells expressing the transient receptor potential A1 (TRPA1) cation channel. Calcium influx and insulin secretion stimulated by estrogen metabolites were dependent on the TRPA1 activity and inhibited with the channel-specific pharmacological antagonists or the siRNA. Our results suggest the role of estrogen metabolism in a direct regulation of TRPA1 activity with potential implications for metabolic diseases.
Keywords: estrogen, ion channel, transient receptor potential channels (TRP channels), insulin secretion, pancreatic islet
Introduction
Estrogen hormones play a critical role in the regulation of energy homeostasis. The classical nuclear hormone estrogen receptor α and β isoforms (ERα and ERβ)2 are believed to mediate the majority of the protective effects of estrogen hormones in metabolic diseases and diabetes (1). In pancreatic β-cells, treatment with estrogens increases insulin secretion and insulin biosynthesis, protects cells from an array of harmful diabetogenic stimuli, and stimulates β-cell proliferation and regeneration (2–6). During pregnancy, elevation of estrogen levels can contribute to improved islet insulin secretion and augmented plasma insulin responses (7). Activation of estrogen signaling can also be important for pancreatic β-cell adaptation to higher insulin demands at the states of insulin resistance and hyperglycemia (8). Classical ERα and ERβ receptors have been described in pancreatic β-cells and play somewhat different roles. Whereas ERα regulates insulin biosynthesis and improves β-cell survival, ERβ is involved in regulation of insulin secretion and pancreatic β-cell numbers (4, 6).
Action of estrogens and other steroids through the classical nuclear steroid receptors is relatively slow and requires RNA transcription, translation, and protein synthesis. However, rapid effects for steroid hormones were reported, and these effects did not require changes in gene expression (9). Similar to other steroids, estrogens can activate rapid signals, functional within seconds or minutes, that are mediated through binding to cell plasma membrane-associated receptors (10). The identity of receptors mediating the rapid nongenomic action of estrogens is not fully elucidated, and multiple putative plasma membrane estrogen binding sites were reported. Nevertheless, one such receptor that binds estrogen, G protein–coupled estrogen receptor 1 (GPER1), has been identified (11). In pancreatic β-islets, activation of GPER1 protects β-cells from apoptosis and induces mild elevation in glucose-stimulated insulin secretion (3, 12).
Rapid nongenomic effects of several steroids including estrogens involve modulation of calcium ion fluxes and ion channel activity. Steroids were reported to alter the activity of one particular ion channel family, transient receptor potential (TRP) channels (13). Pregnenolone sulfate and related neurosteroids directly stimulate ion fluxes through TRPM3 channels (14, 15), whereas progesterone inhibits TRPM3 ion currents (16). TRPC5 channels can be activated by glucocorticoids and inhibited by progesterone and dihydrotestosterone (17, 18). Estradiol was reported to activate TRPV5 and TRPV6 channels (19, 20). TRP channels are expressed in pancreatic β-cells and can regulate insulin secretion through modulation of membrane depolarization and calcium influx (21).
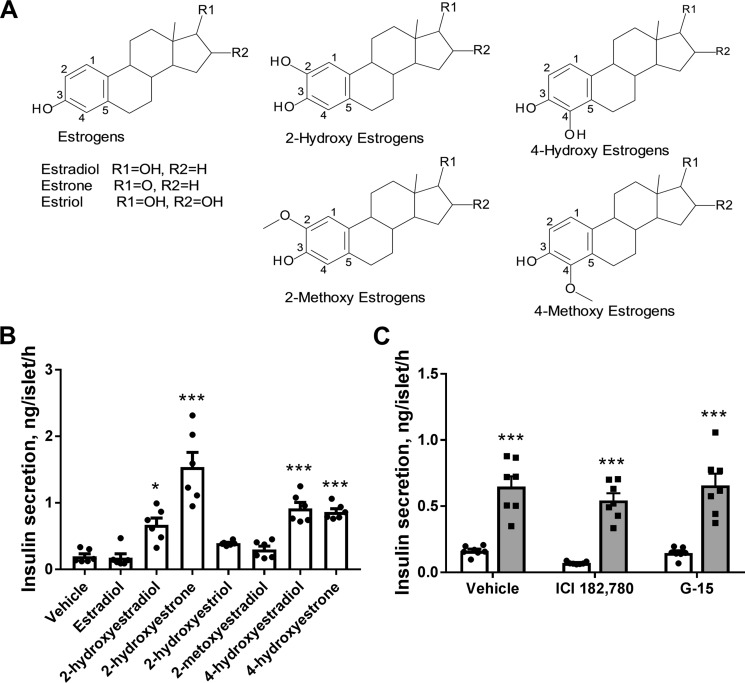
All three major endogenous estrogens: estrone, estradiol, and estriol, are metabolized via hydroxylation by cytochrome P450 enzymes (22). Catechol estrogens formed by the hydroxylation reaction are the major endogenous estrogen metabolites (see Fig. 1A). Hydroxylation at the position C2 of the steroid A ring is the prevalent metabolic pathway in the liver, whereas C4 hydroxylated estrogens are mostly formed in extrahepatic tissues (23). 2-Hydroxyestrone is the most abundant catechol estrogen, whereas 2-hydroxyestradiol and 2-hydroxyestriol are also detected at smaller amounts (23, 24). All 2-hydroxy catechol estrogens displayed low affinity to the classical nuclear estrogen receptors. However, catechol estrogen-specific membrane-binding sites that do not display any affinity to estrogen hormones were described (25, 26). The biological significance of catechol estrogens is not entirely clear.
Figure 1.
Catechol estrogens produce acute potentiation of glucose-induced insulin secretion in mouse pancreatic islets. A, chemical structures of estrogen hormone metabolites hydroxylated at positions C2 and C4 of the steroid A ring. B, effects of estradiol and hydroxylated estrogen metabolites on glucose-induced insulin secretion in isolated pancreatic islets. Accumulated insulin secretion during 1 h of incubation was measured in response to 17 mm glucose and 10 μm steroid or the vehicle (n = 6). C, antagonists of estrogen receptors ICI 182,780 (1 μm) and G-15 (10 μm) did not inhibit 2-hydroxyestrone-mediated potentiation of glucose-induced insulin secretion in isolated pancreatic islets. Accumulated 1 h of insulin secretion was measured in response to 17 mm glucose (open bars) and 17 mm glucose with 10 μm 2-hydroxyestrone (gray bars, n = 7). *, p < 0.05; and ***, p < 0.001 versus insulin secretion in the absence of steroid.
In the current study we demonstrated that endogenous metabolites of estrogen hormones, 2- and 4-hydroxylated catechol estrogens, can directly activate TRPA1 channels. Activation of the TRPA1 activity with catechol estrogens in pancreatic β-cells led to elevation in cytosolic free Ca2+ concentration ([Ca2+]i) and stimulation of glucose-induced insulin secretion. Thus, we propose a novel mechanism by which estrogen metabolism can regulate insulin secretion and glucose homeostasis.
Results
Catechol estrogens induce rapid stimulation of insulin secretion in pancreatic β-cells
Considering the important role of estrogen hormones in the regulation of pancreatic β-cell function and insulin secretion, we assessed the effects of acute administration of endogenous estrogen metabolites, catechol estrogens (Fig. 1A), on glucose-induced insulin secretion in isolated pancreatic islets. Insulin secretion was evaluated in mouse pancreatic islets in the presence of high glucose concentration (17 mm) during 1 h of incubation with 10 μm steroids or the vehicle (Fig. 1B). 2-Hydroxyestradiol, 2-hydroxyestrone, 4-hydroxyestradiol, and 4-hydroxyestrone, metabolites of estradiol and estrone hydroxylated at positions 2 and 4, all significantly increased insulin secretion, whereas 2-hydroxyestriol, the metabolite of another estrogen hormone estriol, did not modify insulin secretion. The most abundant endogenous estrogen metabolite, 2-hydroxyestrone, was the most efficacious catechol estrogen in inducing insulin secretion and generated a 7-fold increase in the insulin response. 2-Methoxyestradiol, which is formed by further methylation of 2-hydroxyestradiol, had no effect on insulin secretion. The precursor of metabolites active in the insulin secretion assay, estrogen hormone estradiol, did not stimulate insulin secretion under used experimental conditions.
To rule out the involvement of the classical estrogen receptors described in pancreatic β-cells (ERα, ERβ, and GPER1) in stimulating insulin secretion with catechol estrogens, we utilized selective antagonists of these receptors: ICI 182,780 and G-15 (27, 28). Treatment of mouse pancreatic islets with either of these two antagonists did not inhibit insulin secretion induced with 2-hydroxyestrone (Fig. 1C). This observation, as well as a lack of stimulation of insulin secretion with estrogen hormone estradiol under our experimental conditions (Fig. 1A), suggests that catechol estrogens induce insulin secretion by a novel mechanism different from activation of the known estrogen receptors.
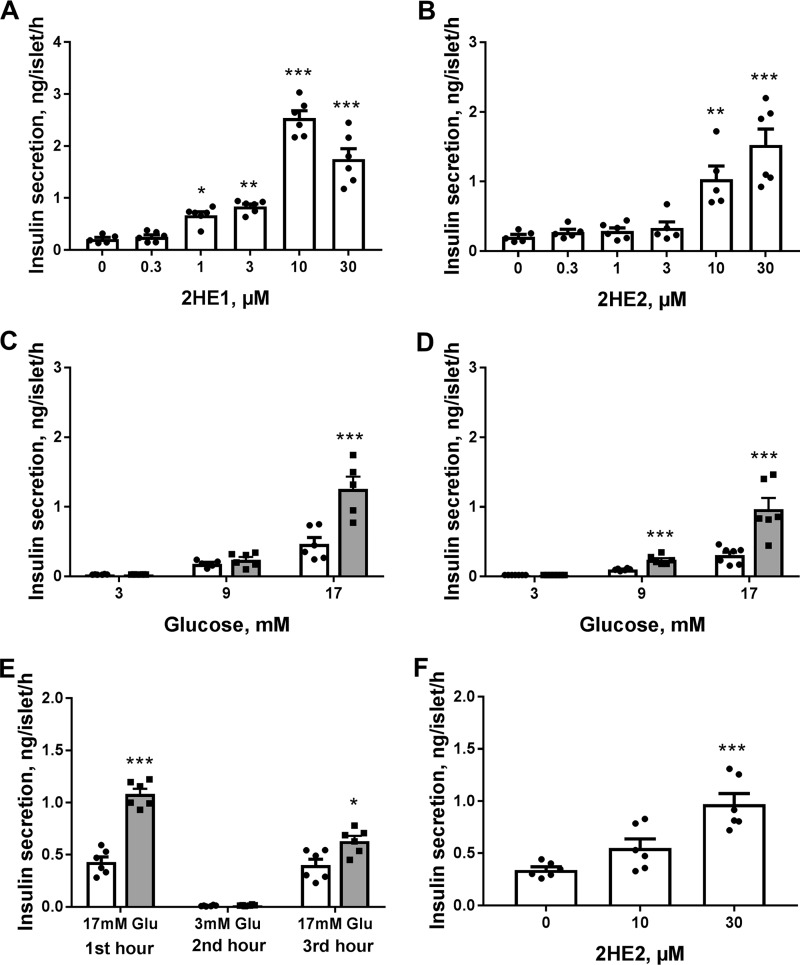
We selected two catechol estrogens, 2-hydroxyestrone and 2-hydroxyestradiol, which demonstrated the insulinotropic activity in pancreatic islets, to further explore effects of this class of compounds. Both 2-hydroxyestrone and 2-hydroxyestradiol produced concentration-dependent potentiation of glucose-induced insulin secretion in mouse islets (Fig. 2, A and B). 2-Hydroxyestrone displayed slightly higher potency and efficacy in enhancing insulin secretion as compared with that for 2-hydroxyestradiol. Effects of both compounds on insulin secretion were also studied at different glucose concentrations. Modulation of insulin secretion by these steroid metabolites was glucose concentration–dependent (Fig. 2, C and D). At a basal glucose concentration of 3 mm, neither of these two compounds modulated insulin secretion, whereas at intermediate glucose levels (9 mm), only 2-hydroxyestrone significantly elevated insulin secretion, and at high glucose levels (17 mm), both compounds produced strong stimulation of insulin secretion.
Figure 2.
2-Hydroxyestrone (2HE1) and 2-hydroxyestradiol (2HE2) induce concentration- and glucose-dependent increases in insulin secretion in pancreatic islets. A, effects of increasing concentrations of 2HE1 on insulin secretion in mouse islets at 17 mm glucose (n = 6). B, effects of increasing concentrations of 2HE2 on insulin secretion in mouse islets at 17 mm glucose (n = 6). C, effects of 10 μm 2HE1 on insulin secretion in mouse islets at different glucose concentrations (n = 6). Insulin secretion in the absence of steroid is depicted as open bars, and insulin secretion in the presence of 10 μm steroid is depicted as gray bars. D, effects of 10 μm 2HE2 on insulin secretion in mouse islets at different glucose concentrations (n = 6). Insulin secretion in the absence of steroid depicted as open bars and in the presence of 10 μm steroid as gray bars. E, preservation of glucose-induced insulin secretion in mouse islets pre-exposed to 2HE1. Islets were treated for the first hour of incubation with 17 mm glucose in the absence (open bars) or presence of 10 μm 2HE1 (gray bars). Then islets were washed and treated with 3 mm glucose (second hour of incubation) followed by treatment with 17 mm glucose (third hour of incubation). Insulin secretion was measured during each incubation (n = 6). F, effects of 2HE2 on insulin secretion in isolated human pancreatic islets measured in the presence of 11 mm glucose (n = 6). *, p < 0.05; **, p < 0.01; and ***, p < 0.001 versus insulin secretion in the vehicle group at appropriate glucose concentration.
To evaluate whether treatment of cells with catechol estrogens can perturb glucose-induced insulin secretion, we measured insulin secretion in response to low and high glucose concentrations in mouse islets that were pre-exposed to 10 μm 2-hydroxyestrone for 1 h and compared it with the vehicle control (Fig. 2E). Exposure of islets to 2-hydroxyestone did not significantly disturb the subsequent islet glucose response. Islets pretreated with 2-hydroxyestrone did not have elevated insulin secretion at 3 mm glucose, whereas only slightly higher insulin secretion was observed in these islets at 17 mm glucose (Fig. 2E).
Finally, the effects of 2-hydroxyestradiol on glucose-induced insulin secretion were examined in human pancreatic islets isolated from nondiabetic donors. 2-Hydroxyestradiol produced up to 3-fold elevation in secreted insulin in islets from a human nondiabetic donor (Fig. 2F). Similar stimulation of insulin secretion with 2-hydroxyestradiol was observed in islets from two additional human donors.
Catechol estrogens induce calcium influx and elevation of cytosolic free Ca2+ levels
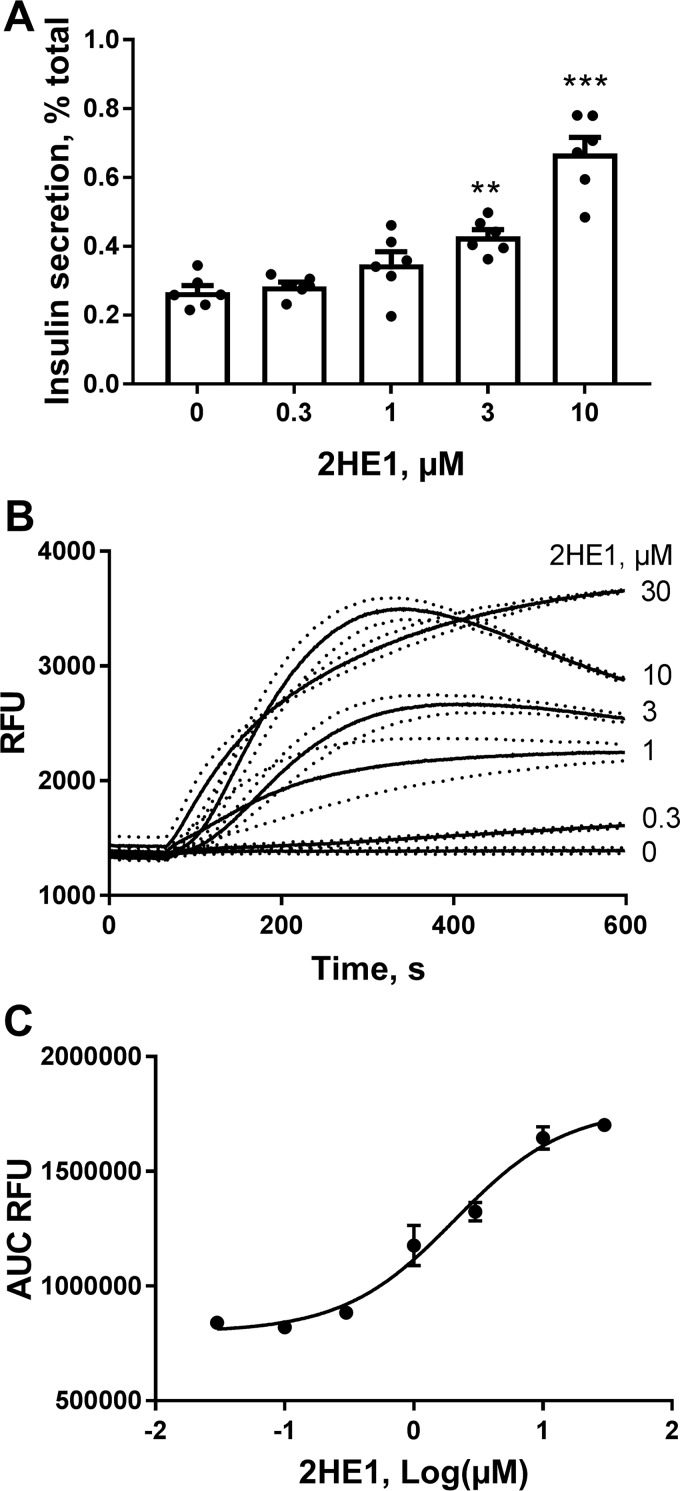
To study the molecular mechanisms of catechol estrogen-mediated potentiation of insulin secretion, we employed insulin-secreting INS-1 832/13 (INS-1) cells, which display robust responsiveness to glucose stimulation (29). Similar to isolated islets, in INS-1 cells 2-hydroxyestrone produce strong and concentration-dependent elevation of insulin secretion at high glucose concentration (Fig. 3A). Elevation of [Ca2+]i is a major triggering signal for stimulation of insulin secretion. We evaluated effects of 2-hydroxyestrone on [Ca2+]i in INS-1 cells using FLIPR technology (Fig. 3B). Treatment with 2-hydroxyestone led to rapid and concentration-dependent increases in [Ca2+]i. The steroid produced elevations in [Ca2+]i in INS-1 cells with half-maximal effective concentration (EC50) of 2.1 ± 1.2 μm (Fig. 3C). The increases in [Ca2+]i induced by 2-hydroxyestone were dependent on the presence of extracellular calcium and were not observed in cells incubated in the calcium-free buffer (data not shown), which suggest that the steroid raises [Ca2+]i through activation of calcium influx into the cell via a plasma membrane–located calcium channel.
Figure 3.
2-Hydroxyestrone (2HE1) increases insulin secretion and induces calcium influx in INS-1 cells. A, stimulation of insulin secretion with increasing concentrations of 2HE1 in INS-1 cells (n = 6). **, p < 0.01; and ***, p < 0.001 versus insulin secretion in the vehicle group. B, cytosolic free Ca2+ responses to increasing concentrations of 2HE1 in INS-1 cells (n = 3). Dotted lines depict S.E. values. C, concentration–response curve constructed from experiments shown in B for the average area-under-the-curve (AUC) values for Ca2+ responses to 2HE1 (n = 3).
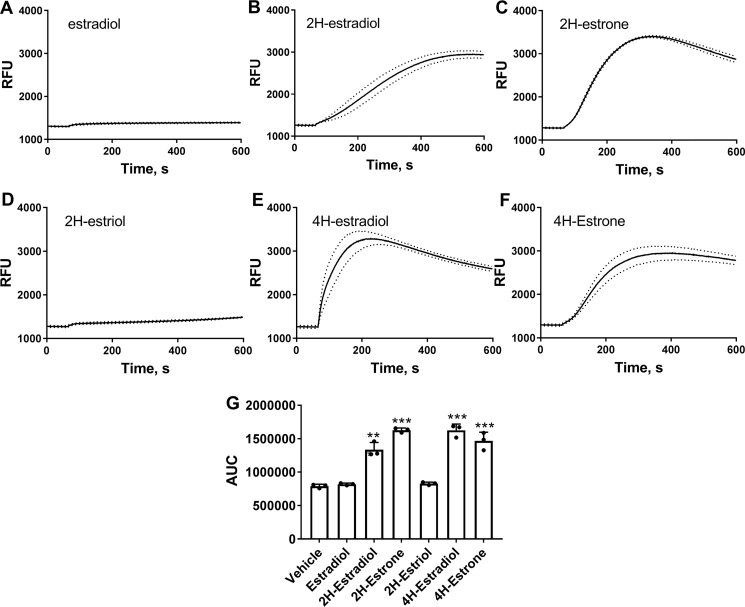
Effects of other catechol estrogens on [Ca2+]i were measured at single 10 μm steroid concentration. The parental hormone estradiol and 2-hydroxyestriol did not increase [Ca2+]i in INS-1 cells (Fig. 4, A and D). Other tested metabolites, 2-hydroxyestradiol, 2-hydroxyestrone, 4-hydroxyestradiol, and 4-hydroxyestrone, produced rapid elevations in [Ca2+]i (Fig. 4, B, C, E, and F). Overall, the ability of estrogen metabolites to increase [Ca2+]i correlated well with the insulinotropic activity of these compounds (Figs. 1B and 4G).
Figure 4.
Catechol estrogens induce increases in cytosolic free Ca2+ in INS-1 cells. A–F, changes in cytosolic free Ca2+ in response to 10 μm of indicated steroid in INS-1 cells (n = 3). Dotted lines depict S.E. values. G, average area-under-the-curve (AUC) values for Ca2+ responses to 10 μm steroid constructed from experiments shown in A–F (n = 3). ***, p < 0.001 versus Ca2+ area under the curve in the vehicle group.
Activation of calcium flux and insulin secretion by catechol estrogens depends on TRPA1 activity
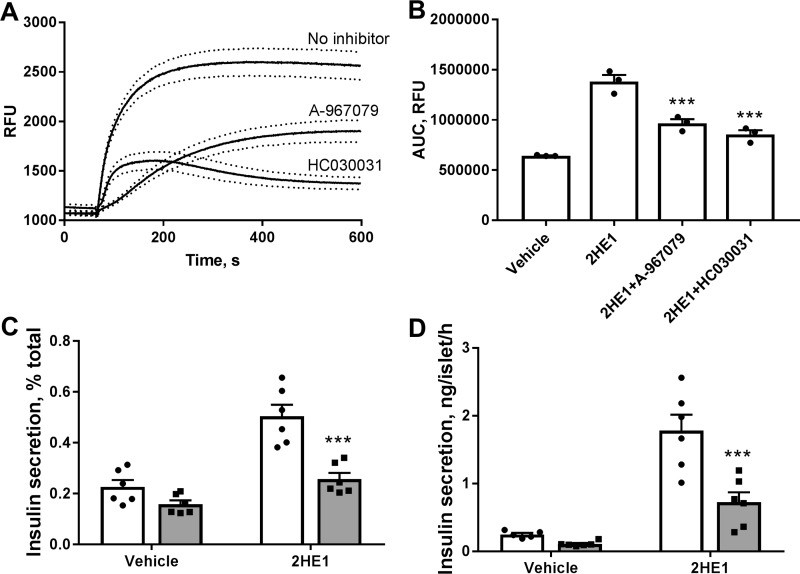
Steroids are known to modulate activities of multiple members of the TRP ion channel family. We hypothesized that elevations in [Ca2+]i with catechol estrogens can be mediated via activation of one of the TRP channels. To test this hypothesis, we evaluated the effects of known pharmacological inhibitors of TRP channels on 2-hydroxyestrone–induced [Ca2+]i increases. Of various TRP inhibitors tested (data not shown), two compounds, A-967079 and HC030031, specific inhibitors of TRPA1 channels (30, 31), produced significant blockade of [Ca2+]i increases generated by 2-hydroxyestrone (Fig. 5, A and B). We used one of the TRPA1 inhibitors, HC030031, to assess the role of TRPA1 channels in elevation of insulin secretion with catechol estrogens. Treatment of INS-1 cells (Fig. 5C) and pancreatic islets (Fig. 5D) with HC030031 blocked the stimulatory effect of 2-hydroxyestrone on insulin secretion.
Figure 5.
Pharmacological inhibitors of TRPA1 channels block 2-hydroxyestrone (2HE1) induced increases in calcium fluxes and insulin secretion. A, cytosolic free Ca2+ in INS-1 cells in response to 10 μm 2HE1 and in the presence of selective TRPA1 blockers, 3 μm A-967079 and 30 μm HC030031. Dotted lines depict S.E. values. B, average area-under-the-curve (AUC) values for Ca2+ responses constructed from experiments shown in A (n = 3). ***, p < 0.001 versus Ca2+ area under the curve in the 2HE1 group. C, effects of selective TRPA1 blocker 30 μm HC030031 on insulin secretion in INS-1 cells induced with 15 mm glucose and 10 μm 2HE1. Open bars, insulin secretion without the inhibitor; gray bars, secretion with HC030031 (n = 6). D, effects of selective TRPA1 blocker 30 μm HC030031 on insulin secretion in isolated mouse islets induced with 17 mm glucose and 10 μm 2HE1. Open bars, insulin secretion without the inhibitor; gray bars, secretion with HC030031 (n = 6). ***, p < 0.001 versus insulin secretion without the inhibitor.
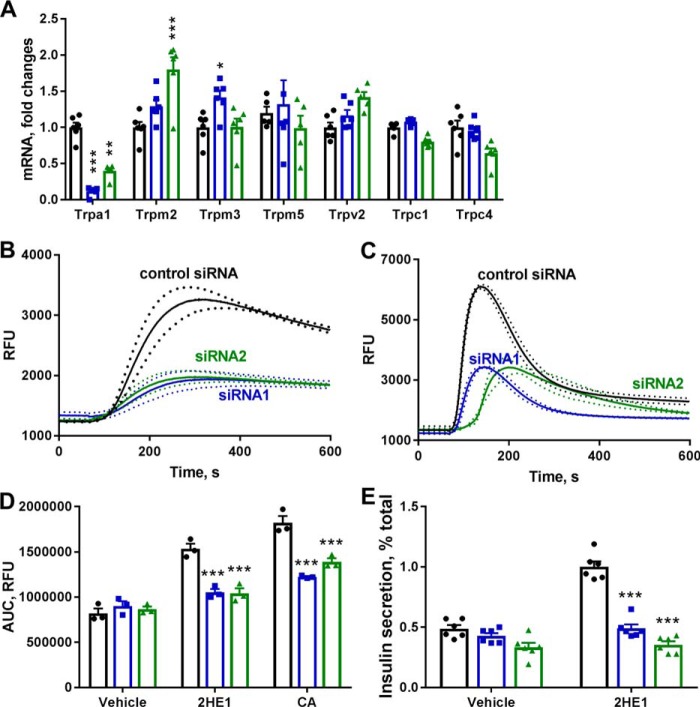
Furthermore, we utilized the TRPA1-specific siRNA probes to down-regulate expression of TRPA1 channels in INS-1 cells and to substantiate our hypothesis that catechol estrogens stimulate calcium influx and insulin secretion in pancreatic β-cells through activation of TRPA1 channels. Transfection of INS-1 cells with TRPA1-specific siRNAs led to strong decreases in TRPA1 mRNA levels as determined by quantitative RT-PCR, by 89% with siRNA1 and 60% with siRNA2 (Fig. 6A). Neither of two used TRPA1-specific siRNAs induce down-regulation of other TRP genes implicated in regulation of insulin secretion (32), and only some up-regulation of TRPM2 and TRPM3 mRNAs was seen with siRNA treatments (Fig. 6A).
Figure 6.
Treatment with the TRPA1-specific siRNA blocks calcium fluxes induced by TRPA1 agonist cinnamaldehyde (CA) and 2-hydroxyestrone (2HE1). A, mRNA expression of TRP channel mRNA in INS-1 cells treated with control nontargeted siRNA (black dots) and TRPA1-specific siRNA1 (blue dots) and siRNA2 (green dots, n = 6). B, cytosolic free Ca2+ in response to 10 μm 2HE1 in INS-1 cells treated with siRNA probes (n = 3). Dotted lines depict S.E. values. C, cytosolic free Ca2+ in response to 50 μm CA in INS-1 cells treated with siRNA probes (n = 3). Dotted lines depict S.E. values. D, average area-under-the-curve (AUC) values for Ca2+ responses constructed from experiments shown in B and C. Black dots, nontargeted siRNA; blue dots, TRPA1 siRNA1; green dots, TRPA1 siRNA2 (n = 3). E, insulin secretion in response 10 μm 2HE1 in INS-1 cells treated with nontargeted siRNA (black dots) and TRPA1-specific siRNA1 (blue dots) and siRNA2 (green dots, n = 6). *, p < 0.05; and ***, p < 0.001 versus signal in the nontargeted siRNA group.
In INS-1 cells transfected with the control nontargeted siRNA, the selective TRPA1 activator trans-cinnamaldehyde (33) and 2-hydroxyestrone generated strong increases in [Ca2+]i (Fig. 6, B and C). Interestingly, these two compounds showed differences in kinetics of calcium flux activation. If cinnamaldehyde generated a transient increase in [Ca2+]i that faded 300 s after compound addition, 2-hydroxyestrone induced more sustained elevation in [Ca2+]i. In INS-1 cells treated with either of the two TRPA1-specific siRNAs, [Ca2+]i responses to both cinnamaldehyde and 2-hydroxyestrone were significantly inhibited (Fig. 6, B and C). Down-regulation of TRPA1 expression with the specific siRNA probes blocked [Ca2+]i responses to cinnamaldehyde by 50–70% and to 2-hydroxyestrone by 75–80% (Fig. 6D). Knockdown of TRPA1 expression with either of two siRNA probes in INS-1 cells also produced strong inhibition of 2-hydroxyestone induced insulin secretion (Fig. 6E). Thus, data with TRPA1-specific siRNAs in INS-1 cells further supported our hypothesis on the key role of TRPA1 channels in the stimulation of calcium fluxes and insulin secretion with cathechol estrogens.
Catechol estrogens induce direct activation of TRPA1 channels
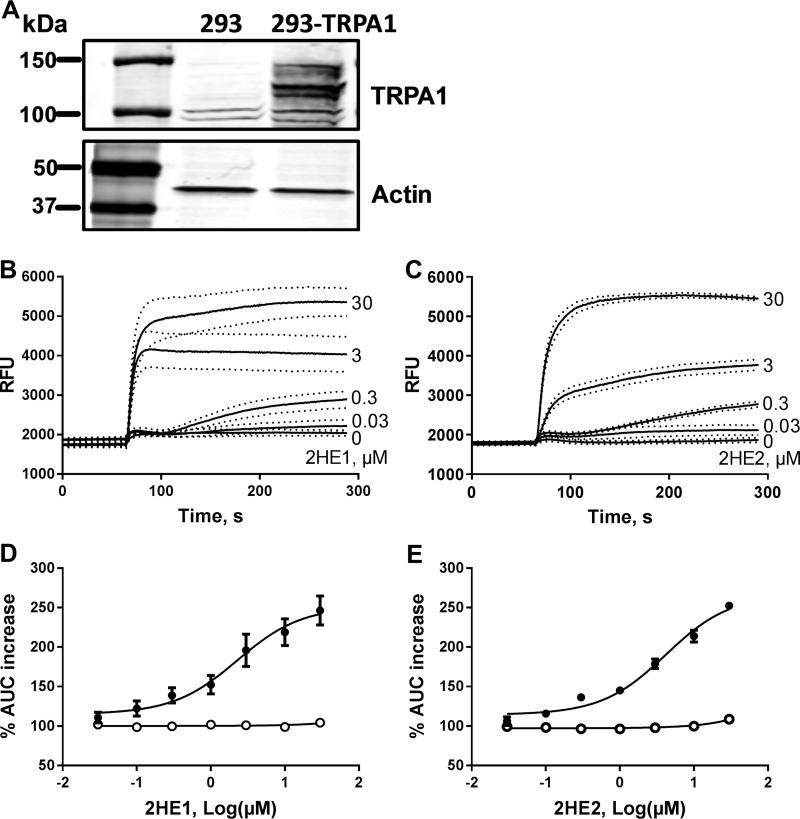
To demonstrate activation of TRPA1 channels with catechol estrogens, we examined [Ca2+]i responses to these compounds in HEK293 cells transfected with human TRPA1 cDNA (HEK293-TRPA1 cells). No expression of TRPA1 in the parental HEK293 cells and specific expression of TRPA1 protein in HEK293 cells transfected with TRPA1 cDNA were confirmed by Western blotting (Fig. 7A). HEK293-TRPA1 cells generated robust [Ca2+]i responses to increasing concentrations of 2-hydroxyestrone and 2-hydroxyestradiol (Fig. 7, B and C). Both catechol estrogens caused sustained elevation in [Ca2+]i in HEK293-TRPA1 cells with kinetics similar to that observed in INS-1 cells. The EC50 values for activation of TRPA1 channels in HEK293-TRPA1 cells were 2.3 ± 1.6 and 4.2 ± 1.3 μm for 2-hydroestrone and 2-hydroxyestradiol, respectively (Fig. 7, D and E). In parental HEK293 cells that do not express TRPA1, both compounds failed to induce any activation of calcium fluxes and elevations in [Ca2+]i (Fig. 7, D and E).
Figure 7.
Catechol estrogens stimulate Ca2+ influx in HEK293-TRPA1 cells. A, Western blotting analysis of TRPA1 protein expression in HEK293 cells that were either nontransfected or transfected with TRPA1 cDNA (HEK293-TRPA1). B, cytosolic free Ca2+ responses to increasing concentrations of 2-hydroxyestrone (2HE1) in HEK293-TRPA1 cells (n = 3). Dotted lines depict S.E. values. C, cytosolic free Ca2+ responses to increasing concentrations of 2-hydroxyestradiol (2HE2) in HEK293-TRPA1 cells. Dotted lines depict S.E. values. D, concentration–response curves for average Ca2+ responses to 2HE1 in HEK293-TRPA1 cells (black circles, constructed from experiments shown in B) and in parental HEK293 cells (white circles, n = 3). E, concentration–response curves for average Ca2+ responses to 2HE2 in HEK293-TRPA1 cells (black circles, constructed from experiments shown in C) and in nontransfected HEK293 cells (white circles, n = 3). AUC, area under the curve.
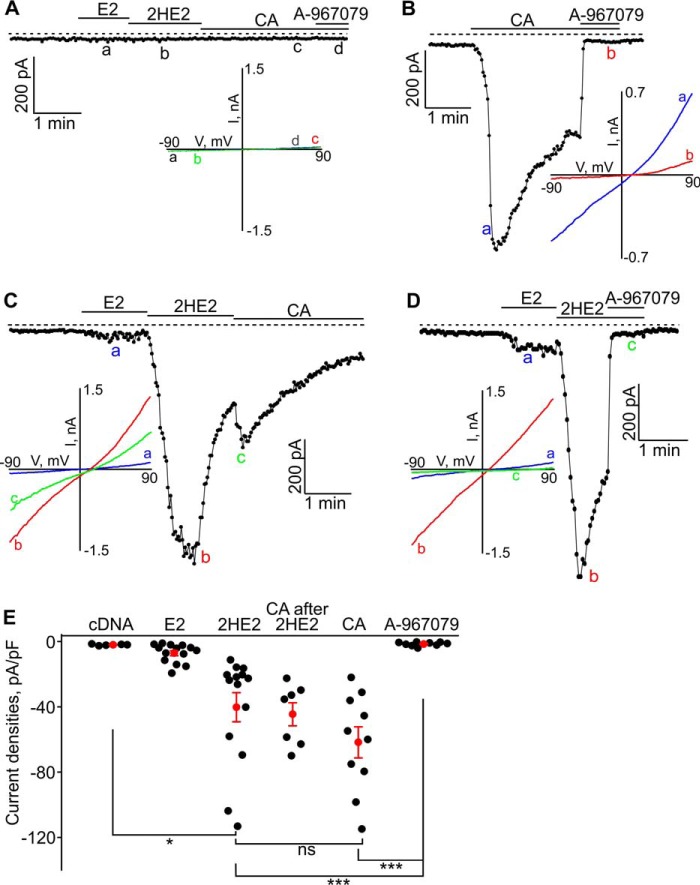
We next performed whole-cell patch-clamp experiments to confirm the ability of catechol estrogens to activate TRPA1 currents. In parental HEK293 cells, lacking the TRPA1 protein, estradiol, 2-hydroxyestradiol, or cinnamaldehyde evoked no inward currents (Fig. 8A). As expected, in HEK293 cells transfected with TRPA1 cDNA, the TRPA1 agonist cinnamaldehyde induced strong inward currents that were inhibited with the TRPA1 antagonist A-967079 (Fig. 8B). In HEK293-TRPA1 cells, administration of 10 μm estradiol did not generate any significant currents, whereas administration of 10 μm 2-hydroxyestradiol produced strong stimulation of ion currents (Fig. 8C). The inward current evoked by 2-hydroxyestradiol in HEK293-TRPA1 cells was blocked by A-967079 (Fig. 8D). To further characterize 2-hydroxyestradiol-activated TRPA1 current, we investigated the current–voltage relationships (I–V) of the evoked current. The I–V exhibited no rectification and a reversal potential between +10 and +20 mV (Fig. 8C), which was similar to the characteristics of the cinnamaldehyde-evoked TRPA1 current I–V. The inward currents induced by 2-hydroxyestradiol and cinnamaldehyde were of similar magnitude, and administration of cinnamaldehyde after 2-hydroxyestrdaiol did not generate further significant current increases (Fig. 8E). Together, these results show that 2-hydroxyestradiol is an agonist of the TRPA1 channel.
Figure 8.
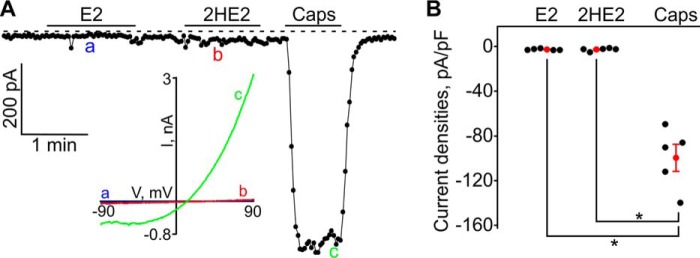
β-Estradiol (E2) and 2-hydroxyestradiol (HE2) evoked inward currents in HEK293-TRPA1 cells. A, neither E2 (10 μm), HE2 (10 μm), CA (cinnamaldehyde (CA); 50 μm), nor A-967079 (2 μm) affected the background currents in HEK293-pcDNA cells. A sample trace is shown. The inset shows the current–voltage relationships (I–V) acquired at the time points indicated with letters a, b, c, and d. B, cinnamaldehyde induced large inward currents in HEK-TRPA1 cells, which were inhibited by a specific TRPA1 blocker, A-967079 (2 μm). The inset shows the I–V acquired at the time points indicated with letters a and b. C, E2 (10 μm), HE2 (10 μm), and CA (50 μm)-induced inward currents in a HEK293-TRPA1 cell. The inset shows the I–V acquired at the time points indicated with letters a, b, and c. D, HE2-induced currents (10 μm) can be inhibited by A-967079 (2 μm), a specific inhibitor of TRPA1. The inset shows the I–V acquired at the time points indicated with letters a, b, and c. E, summary for the data shown in A–D. The black filled dots represent the individual data points, and the red dots and bars are the means ± S.E. (S.E.). *, p < 0.05; and ***, p < 0.001; ns, not significant. The horizontal bars indicate the times when the tested compounds were added to the bath. The broken line shows the zero current level.
Finally, we assessed the specificity of the TRPA1 activation with catechol estrogens by exploring effects of 2-hydroxyestradiol on the TRPV1 channel, a member of TRP family closely related to TRPA1. In HEK293 cells expressing TRPV1, neither estradiol nor 2-hydroxyestradiol stimulated ion currents, whereas the TRPV1 agonist capsaicin generated strong inward currents (Fig. 9, A and B), indicating that catechol estrogens are selective TRPA1 activators.
Figure 9.
β-Estradiol (E2) and 2-hydroxyestradiol (HE2) did not induce inward currents in HEK293-TRPV1 cells. A, E2 (10 μm) nor HE2 (10 μm) did not evoke any inward currents in a HEK293-TRPV1 cell, whereas capsaicin (Caps; 1 μm) activated the large inward current. The inset shows the I–V acquired at the time points indicated with letters a, b, and c. The horizontal bars indicate the times when the tested compounds were added to the bath. The broken line shows the zero current level. B, a summary for the data shown in A. The black filled dots represent the individual data points, and the red dots and bars are the means ± S.E. *, p < 0.05.
Discussion
Estrogen hormones are important physiological regulators of nutrient metabolism. In pancreatic islets, estrogens modulate insulin synthesis, insulin secretion, β-cell health, and numbers through multiple mechanisms that involve activation of classical nuclear ERα and ERβ receptors and the plasma membrane GPER1 receptor. Here we propose an additional mechanism for estrogens to regulate insulin secretion via production of endogenous metabolites, catechol estrogens, which in turn stimulate calcium influx and insulin secretion in pancreatic β-cells.
2- and 4-hydroxylated metabolites of estradiol and estrone potently potentiated glucose-induced insulin secretion in isolated pancreatic islets and insulin-secreting INS-1 cells. Stimulation of insulin secretion with these catechol estrogens was only observed at high glucose concentrations but not at basal glucose levels. Hydroxylated estrogens produced rapid increases in [Ca2+]i through stimulation of calcium influx into the cell. These calcium responses were blocked with selective TRPA1 inhibitors and by down-regulation of TRPA1 expression with the specific siRNA. The selective TRPA1 inhibitor and specific siRNAs blocked stimulation of insulin secretion induced by catechol estrogens. Altogether, these findings suggest that catechol estrogens stimulate insulin secretion in pancreatic β-cells via activation of TRPA1 channels and induction of calcium influx.
Furthermore, we provide direct evidence that catechol estrogens activate TRPA1 channels and demonstrate rapid inward current and calcium responses to these compounds in HEK293 cells expressing TRPA1, whereas no calcium response or ion current was observed in parental HEK293 cells and/or HEK293 cells expressing TRPV1. Notably, 2-hydroxyestrone evoked ion current in HEK293 cells expressing TRPA1 in the whole-cell patch-clamp configuration, supporting the direct effect of catechol estrogens on the TRPA1 channel.
Endogenous estrogen hormones undergo extensive hydroxylation at various positions of the steroid rings. Formation of hydroxylated estrogen metabolites can occur in liver, as well as in extrahepatic tissues (22, 23, 34). Enzymes, members of the cytochrome P450 family responsible for the oxidative metabolism of estrogen hormones, were well-characterized (35). Expression of cytochromes with the highest catalytic activities for estrogen 2-hydroxylation, CYP1A1 and 1A2 (35), was detected in both pancreatic exocrine acinar and endocrine islet cells (36), suggesting that endogenous synthesis of catechol estrogens can take place in pancreas. Unfortunately, only scarce data exist on catechol estrogen tissue levels. In the breast tissue, where hydroxylated estrogens were analyzed in several independent studies, catechol estrogens accounted for majority of total tissue estrogens, and their levels were significantly higher than levels of the classical estrogen hormones (37, 38). In plasma, catechol estrogen concentrations are fairly low and rarely exceed 500 pg/ml levels in premenopausal women (24, 39) because of high clearance of hydroxylated estrogens from the blood through further metabolism to metoxyestrogens (40). High clearance rates and local tissue synthesis of catechol estrogens are consistent with the idea that effects of these compounds are local and paracrine in nature.
TRPA1 is a nonselective cation channel that can conduct Ca2+ and is activated by elevated intracellular calcium. TRPA1 is also activated by multiple noxious, dietary, and endogenous compounds with the majority of these activators containing electrophilic carbons (41). Here, we report another class of endogenous compounds, novel TRPA1 activators. Recent data implicate TRPA1 in the control of glucose metabolism. TRPA1 agonists lower blood glucose, increase insulin secretion, stimulate glucose uptake, inhibit food intake, and increase secretion of intestinal incretin hormones (42–45). Previous studies demonstrated that TRPA1 is expressed in pancreatic β-cells and insulin-secreting cell lines (46, 47). TPRA1 agonists invoke inward ion current, membrane depolarization, and calcium influx in pancreatic β-cells, which leads to potentiation of glucose-dependent insulin secretion (46). Our results are consistent with the previously published reports on TRPA1 effects in pancreatic β-cells and confirm the role of TRPA1 in regulating insulin secretion. Moreover, this study may implicate TRPA1 in mediating processes of adaptation of maternal β-cells to secrete higher amounts of insulin during pregnancy.
TRPA1 is expressed in sensory neurons, and the role of TRPA1 in mediating different types of pain is well-established (41). Although modulation of pain with catechol estrogens was not the topic of this study, our results may have implications in the area of pain sensation and can provide potential explanation for modulation of pain sensitivity with estrogen and for differences in pain perception between men and women (48). In summary, we provide evidence of a novel mechanism through which metabolites of estrogen hormones may stimulate insulin secretion in pancreatic β-cells through direct activation of TRPA1.
Experimental procedures
Pancreatic islet isolation and insulin secretion
Mouse pancreatic islets were isolated from male C57BL/6 mice (Envigo, Indianapolis, IN) by collagenase digestion. Use of animals was approved by Eli Lilly and Company's Institutional Animal Care and Use Committee. Human pancreatic islets from listed cadaver organ donors that were refused for pancreas or islet transplantation were obtained from Prodo Labs (Irvine, CA) and InSphero AG (Schlieren, Switzerland) and were used in accordance with internal review board ethical guidelines for use of human tissue. Islets were cultured in RPMI 1640 medium (Invitrogen) supplemented with 11 mm glucose, 10% (v/v) heat-inactivated fetal bovine serum (Invitrogen), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). For insulin secretion studies, pancreatic islets were starved for 30 min in Earle's balanced salt solution (EBSS) containing 0.1% BSA and 3 mm glucose. For static insulin secretion studies, groups of three islets were selected and cultured with test compounds at appropriate glucose concentrations in 0.3 ml of EBSS for 60 min at 37 °C. All tested compounds were obtained from Sigma–Aldrich and Steraloids, Inc. (Newport, RI). At the end of incubation, the supernatant was collected and submitted for insulin analysis. Insulin levels were determined with the Meso Scale Discovery (Gaithersburg, MD) electrochemiluminescence insulin assay.
Insulinoma INS-1832/13 cell culture and insulin secretion
INS-1 832/13 cells were kindly provided by Dr. C. Newgard (Duke University, Durham, NC). INS-1 cells were cultured in RPMI 1640 medium supplemented with 11 mm glucose, 10% fetal bovine serum, 10 mm HEPES, 2 mm l-glutamine, 1 mm sodium pyruvate, and 50 μm mercaptoethanol. The cells were seeded in 96-well plates (30,000 cells/well) and cultured for 3 days. The cells were washed and then incubated in EBSS containing 0.1% BSA and no glucose for 30 min. The starvation buffer was removed, and 0.2 ml of EBSS containing 15 mm glucose and test compounds was added to cells, and plates were incubated at 37 °C for additional 60 min. At the end of incubation, supernatant was collected and used for insulin analysis. The cell were lysed, and the insulin content of the lysate was analyzed and used to normalize insulin secretion values.
FLIPR assays in INS-1 832/13
INS-1 cells were plated in 96-well plates at 50,000 cells/well and allowed 24 h for attachment. FLIPR Calcium 6 Assay (Molecular Devices, San Jose, CA) was used to monitor changes in [Ca2+]i. The cells were incubated for 30 min at 37 °C in EBSS with 0.1% BSA and then loaded with the calcium dye for 90 min at room temperature. Compounds were added to plates, and signal was quantified by FLIPR TETRA system (Molecular Devices).
siRNA transfection in INS-1 832/13
For siRNA studies, INS-1 were seeded into 96-well plates at a density of 20,000 cells/well, incubated for 24 h and then transfected with On-TargetPlus siRNAs (Dharmacon, Inc., Lafayette, CO) for the rat Trpa1 gene (siRNA1, catalog no. J-100647-05; and siRNA2, catalog no. J-100647-08) or nontargeting On-TargetPlus Control siRNA (catalog no. D-001810-02). The cells were transfected with 25 nm siRNA using Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific). After 24 h, the medium was replaced with regular culture medium, and the cells were incubated for another 72 h before calcium and insulin secretion measurements were made. To measure changes in the gene expression in transfected cells, RNA was isolated with the SV 96 RNA isolation system (Promega, Madison, WI). Gene expression was assessed with TaqMan real-time PCR assays (Thermo Fisher Scientific) for the following genes: Trpa1, Rn01473803_m1; Trpm2, Rn01429410_m1; Trpm3, Rn01479074_m1; Trpm5, Rn01479552_m1; Trpv2, Rn00567974_m1; Trpc1, Rn00585625_m1; and Trpc4, Rn00696282_m1.
FLIPR assays in HEK293 cells
HEK293 cells (ATCC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium/F-12 (3:1, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum, 2 mm glutamine, 20 mm HEPES. HEK293 cells were seeded on a 96-well plate at 15,000 cells/well, allowed 24 h for attachment, and then transfected with 75 ng/well human TRPA1-pcDNA3.1 plasmid using Lipofectamine LTX and PLUS reagent (Thermo Fisher Scientific). After 24 h the medium was replaced with regular culture medium, and the cells were incubated for another 24 h before TRPA1 protein and cytosolic calcium measurements were made. For TRPA1 Western blotting protein measurements, the cells were lysed with the radioimmune precipitation assay buffer (Sigma–Aldrich), and equal amounts of protein samples were separated by SDS-PAGE, transferred to the membrane, and probed with antibodies against TRPA1 (NB110-40763; Novus Biologicals, Centennial, CO) and β-actin (ab6276; Abcam, Cambridge, MA). FLIPR Calcium 6 Assay (Molecular Devices) was used to monitor changes in [Ca2+]i. The cells were incubated in Hanks' balanced salt solution with 0.1% BSA for 30 min and then loaded with calcium dye for 90 min at 37 °C. Compounds were added to plates, and signal was quantified by the FLIPR TETRA system (Molecular Devices).
Patch-clamp electrophysiology
The electrophysiological experiments were performed as described elsewhere (49–51). Briefly, HEK cell were plated at a low density on round 15-mm glass coverslips, and the experiments were performed 24–48 h after transfection of the cDNA constructs. An Axopatch 200B amplifier and Digidata 1550A digitizer were employed to record the currents in voltage-clamped HEK cells using the whole-cell patch-clamp mode. The sampling rate was set to 1 kHz. Series resistance compensation was set to 50–70%. The cells were voltage-clamped at a holding potential of −60 mV, and the voltage ramps from −90 to +90 mV were applied with 2-s intervals. The extracellular solution contained 145 mm NaCl, 2.5 mm KCl, 1.2 mm CaCl2, 1 mm MgCl2, 10 mm HEPES, and 5.5 mm glucose (pH 7.2 adjusted with NaOH); the pipette solution contained 125 mm CsMeSO3, 3.77 mm CaCl2, 2 mm MgCl2, 10 mm EGTA (100 nm free Ca2+), and 10 mm HEPES (pH 7.2 adjusted with Trizma base). The pCLAMP 10 software package was used for data analyses. The current densities were determined by dividing the current amplitude values by the cell capacitance. The experiments were performed at 22–25 °C. Kruskal–Wallis one-way analysis of variance on ranks followed by the Dunn's all pairwise multiple comparison post hoc test was used to determine the significance of changes in the tested groups.
Statistical analysis
The results are presented as the means ± S.E. for the indicated number of experiments. The data were analyzed with GraphPad or SigmaPlot 13 using a four-parameter nonlinear logistic algorithm and analysis of variance.
Author contributions
W. M., X. C., R. C., S. K. S., J. V. F., O. C., A. G. O., and A. M. E. data curation; W. M., X. C., R. C., S. K. S., J. V. F., O. C., A. G. O., and A. M. E. formal analysis; W. M., X. C., R. C., S. K. S., J. V. F., O. C., and A. M. E. investigation; W. M., R. C., S. K. S., and O. C. methodology; X. C., J. V. F., A. G. O., and A. M. E. visualization; J. V. F., A. G. O., and A. M. E. writing-review and editing; A. G. O. and A. M. E. conceptualization; A. G. O. and A. M. E. supervision; A. G. O. and A. M. E. validation; A. G. O. and A. M. E. writing-original draft; A. M. E. project administration.
W. M., R. C., S. K. S., J. V. F., O. C., and A. M. E. are employed by Eli Lilly and Company.
- ER
- estrogen receptor
- GPER
- G protein–coupled estrogen receptor
- TRP
- transient receptor potential
- EBSS
- Earle's balanced salt solution.
References
- 1. Mauvais-Jarvis F., Clegg D. J., and Hevener A. L. (2013) The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 34, 309–338 10.1210/er.2012-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Le May C., Chu K., Hu M., Ortega C. S., Simpson E. R., Korach K. S., Tsai M. J., and Mauvais-Jarvis F. (2006) Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. U.S.A. 103, 9232–9237 10.1073/pnas.0602956103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu S., Le May C., Wong W. P., Ward R. D., Clegg D. J., Marcelli M., Korach K. S., and Mauvais-Jarvis F. (2009) Importance of extranuclear estrogen receptor-α and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 58, 2292–2302 10.2337/db09-0257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alonso-Magdalena P., Ropero A. B., García-Arévalo M., Soriano S., Quesada I., Muhammed S. J., Salehi A., Gustafsson J. A., and Nadal A. (2013) Antidiabetic actions of an estrogen receptor β selective agonist. Diabetes 62, 2015–2025 10.2337/db12-1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuchi Y., Cai Y., Legein B., De Groef S., Leuckx G., Coppens V., Van Overmeire E., Staels W., De Leu N., Martens G., Van Ginderachter J. A., Heimberg H., and Van de Casteele M. (2015) Estrogen receptor α regulates β-cell formation during pancreas development and following injury. Diabetes 64, 3218–3228 10.2337/db14-1798 [DOI] [PubMed] [Google Scholar]
- 6. Zhou Z., Ribas V., Rajbhandari P., Drew B. G., Moore T. M., Fluitt A. H., Reddish B. R., Whitney K. A., Georgia S., Vergnes L., Reue K., Liesa M., Shirihai O., van der Bliek A. M., Chi N. W., et al. (2018) Estrogen receptor α protects pancreatic β-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J. Biol. Chem. 293, 4735–4751 10.1074/jbc.M117.805069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Costrini N. V., and Kalkhoff R. K. (1971) Relative effects of pregnancy, estradiol, and progesterone on plasma insulin and pancreatic islet insulin secretion. J. Clin. Invest. 50, 992–999 10.1172/JCI106593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kilic G., Alvarez-Mercado A. I., Zarrouki B., Opland D., Liew C. W., Alonso L. C., Myers M. G. Jr, Jonas J. C., Poitout V., Kulkarni R. N., and Mauvais-Jarvis F. (2014) The islet estrogen receptor-α is induced by hyperglycemia and protects against oxidative stress-induced insulin-deficient diabetes. PLoS One 9, e87941 10.1371/journal.pone.0087941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Losel R. M., Falkenstein E., Feuring M., Schultz A., Tillmann H. C., Rossol-Haseroth K., and Wehling M. (2003) Nongenomic steroid action: controversies, questions, and answers. Physiol. Rev. 83, 965–1016 10.1152/physrev.00003.2003 [DOI] [PubMed] [Google Scholar]
- 10. Kelly M. J., and Levin E. R. (2001) Rapid actions of plasma membrane estrogen receptors. Trends Endocrinol. Metab. 12, 152–156 10.1016/S1043-2760(01)00377-0 [DOI] [PubMed] [Google Scholar]
- 11. Filardo E. J., Quinn J. A., Bland K. I., and Frackelton A. R. (2000) Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 14, 1649–1660 10.1210/mend.14.10.0532 [DOI] [PubMed] [Google Scholar]
- 12. Mårtensson U. E., Salehi S. A., Windahl S., Gomez M. F., Swärd K., Daszkiewicz-Nilsson J., Wendt A., Andersson N., Hellstrand P., Grände P. O., Owman C., Rosen C. J., Adamo M. L., Lundquist I., Rorsman P., et al. (2009) Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150, 687–698 10.1210/en.2008-0623 [DOI] [PubMed] [Google Scholar]
- 13. Kumar A., Kumari S., Majhi R. K., Swain N., Yadav M., and Goswami C. (2015) Regulation of TRP channels by steroids: Implications in physiology and diseases. Gen. Comp. Endocrinol. 220, 23–32 10.1016/j.ygcen.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 14. Wagner T. F., Loch S., Lambert S., Straub I., Mannebach S., Mathar I., Düfer M., Lis A., Flockerzi V., Philipp S. E., and Oberwinkler J. (2008) Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic β cells. Nat. Cell Biol. 10, 1421–1430 10.1038/ncb1801 [DOI] [PubMed] [Google Scholar]
- 15. Majeed Y., Agarwal A. K., Naylor J., Seymour V. A., Jiang S., Muraki K., Fishwick C. W., and Beech D. J. (2010) cis-Isomerism and other chemical requirements of steroidal agonists and partial agonists acting at TRPM3 channels. Br. J. Pharmacol. 161, 430–441 10.1111/j.1476-5381.2010.00892.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majeed Y., Tumova S., Green B. L., Seymour V. A., Woods D. M., Agarwal A. K., Naylor J., Jiang S., Picton H. M., Porter K. E., O'Regan D. J., Muraki K., Fishwick C. W., and Beech D. J. (2012) Pregnenolone sulphate-independent inhibition of TRPM3 channels by progesterone. Cell Calcium 51, 1–11 10.1016/j.ceca.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beckmann H., Richter J., Hill K., Urban N., Lemoine H., and Schaefer M. (2017) A benzothiadiazine derivative and methylprednisolone are novel and selective activators of transient receptor potential canonical 5 (TRPC5) channels. Cell Calcium 66, 10–18 10.1016/j.ceca.2017.05.012 [DOI] [PubMed] [Google Scholar]
- 18. Majeed Y., Amer M. S., Agarwal A. K., McKeown L., Porter K. E., O'Regan D. J., Naylor J., Fishwick C. W., Muraki K., and Beech D. J. (2011) Stereo-selective inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids. Br. J. Pharmacol. 162, 1509–1520 10.1111/j.1476-5381.2010.01136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Irnaten M., Blanchard-Gutton N., and Harvey B. J. (2008) Rapid effects of 17β-estradiol on epithelial TRPV6 Ca2+ channel in human T84 colonic cells. Cell Calcium 44, 441–452 10.1016/j.ceca.2008.02.007 [DOI] [PubMed] [Google Scholar]
- 20. Irnaten M., Blanchard-Gutton N., Praetorius J., and Harvey B. J. (2009) Rapid effects of 17β-estradiol on TRPV5 epithelial Ca2+ channels in rat renal cells. Steroids 74, 642–649 10.1016/j.steroids.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 21. Colsoul B., Vennekens R., and Nilius B. (2011) Transient receptor potential cation channels in pancreatic β cells. Rev. Physiol. Biochem. Pharmacol. 161, 87–110 [DOI] [PubMed] [Google Scholar]
- 22. Zhu B. T., and Conney A. H. (1998) Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis 19, 1–27 10.1093/carcin/19.1.1 [DOI] [PubMed] [Google Scholar]
- 23. Fishman J. (1983) Aromatic hydroxylation of estrogens. Annu. Rev. Physiol. 45, 61–72 10.1146/annurev.ph.45.030183.000425 [DOI] [PubMed] [Google Scholar]
- 24. De Crée C., Ball P., Seidlitz B., Van Kranenburg G., Geurten P., and Keizer H. A. (1997) Plasma 2-hydroxycatecholestrogen responses to acute submaximal and maximal exercise in untrained women. J. Appl. Physiol. 82, 364–370 10.1152/jappl.1997.82.1.364 [DOI] [PubMed] [Google Scholar]
- 25. Schaeffer J. M., Stevens S., Smith R. G., and Hsueh A. J. (1980) Binding of 2-hydroxyestradiol to rat anterior pituitary cell membranes. J. Biol. Chem. 255, 9838–9843 [PubMed] [Google Scholar]
- 26. Markides C. S., and Liehr J. G. (2005) Specific binding of 4-hydroxyestradiol to mouse uterine protein: evidence of a physiological role for 4-hydroxyestradiol. J. Endocrinol. 185, 235–242 10.1677/joe.1.06014 [DOI] [PubMed] [Google Scholar]
- 27. de Cupis A., Noonan D., Pirani P., Ferrera A., Clerico L., and Favoni R. E. (1995) Comparison between novel steroid-like and conventional nonsteroidal antioestrogens in inhibiting oestradiol- and IGF-I-induced proliferation of human breast cancer-derived cells. Br. J. Pharmacol. 116, 2391–2400 10.1111/j.1476-5381.1995.tb15085.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dennis M. K., Burai R., Ramesh C., Petrie W. K., Alcon S. N., Nayak T. K., Bologa C. G., Leitao A., Brailoiu E., Deliu E., Dun N. J., Sklar L. A., Hathaway H. J., Arterburn J. B., Oprea T. I., et al. (2009) In vivo effects of a GPR30 antagonist. Nat. Chem. Biol. 5, 421–427 10.1038/nchembio.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., and Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 10.2337/diabetes.49.3.424 [DOI] [PubMed] [Google Scholar]
- 30. Chen J., Joshi S. K., DiDomenico S., Perner R. J., Mikusa J. P., Gauvin D. M., Segreti J. A., Han P., Zhang X. F., Niforatos W., Bianchi B. R., Baker S. J., Zhong C., Simler G. H., McDonald H. A., et al. (2011) Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 152, 1165–1172 10.1016/j.pain.2011.01.049 [DOI] [PubMed] [Google Scholar]
- 31. McNamara C. R., Mandel-Brehm J., Bautista D. M., Siemens J., Deranian K. L., Zhao M., Hayward N. J., Chong J. A., Julius D., Moran M. M., and Fanger C. M. (2007) TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. U.S.A. 104, 13525–13530 10.1073/pnas.0705924104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Islam M. S. (2011) TRP channels of islets. Adv. Exp. Med. Biol. 704, 811–830 10.1007/978-94-007-0265-3_42 [DOI] [PubMed] [Google Scholar]
- 33. Bandell M., Story G. M., Hwang S. W., Viswanath V., Eid S. R., Petrus M. J., Earley T. J., and Patapoutian A. (2004) Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 10.1016/S0896-6273(04)00150-3 [DOI] [PubMed] [Google Scholar]
- 34. Ball P., and Knuppen R. (1978) Formation of 2- and 4-hydroxyestrogens by brain, pituitary, and liver of the human fetus. J. Clin. Endocrinol. Metab. 47, 732–737 10.1210/jcem-47-4-732 [DOI] [PubMed] [Google Scholar]
- 35. Lee A. J., Cai M. X., Thomas P. E., Conney A. H., and Zhu B. T. (2003) Characterization of the oxidative metabolites of 17β-estradiol and estrone formed by 15 selectively expressed human cytochrome P450 isoforms. Endocrinology 144, 3382–3398 10.1210/en.2003-0192 [DOI] [PubMed] [Google Scholar]
- 36. Standop J., Schneider M., Ulrich A., Büchler M. W., and Pour P. M. (2003) Differences in immunohistochemical expression of xenobiotic-metabolizing enzymes between normal pancreas, chronic pancreatitis and pancreatic cancer. Toxicol. Pathol. 31, 506–513 10.1080/01926230309793,10.1080/01926230390226041 [DOI] [PubMed] [Google Scholar]
- 37. Castagnetta L. A., Granata O. M., Traina A., Ravazzolo B., Amoroso M., Miele M., Bellavia V., Agostara B., and Carruba G. (2002) Tissue content of hydroxyestrogens in relation to survival of breast cancer patients. Clin. Cancer Res. 8, 3146–3155 [PubMed] [Google Scholar]
- 38. Rogan E. G., Badawi A. F., Devanesan P. D., Meza J. L., Edney J. A., West W. W., Higginbotham S. M., and Cavalieri E. L. (2003) Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis 24, 697–702 10.1093/carcin/bgg004 [DOI] [PubMed] [Google Scholar]
- 39. Fuhrman B. J., Xu X., Falk R. T., Dallal C. M., Veenstra T. D., Keefer L. K., Graubard B. I., Brinton L. A., Ziegler R. G., and Gierach G. L. (2014) Assay reproducibility and interindividual variation for 15 serum estrogens and estrogen metabolites measured by liquid chromatography-tandem mass spectrometry. Cancer. Epidemiol. Biomarkers. Prev. 23, 2649–2657 10.1158/1055-9965.EPI-14-0438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Longcope C., Femino A., Flood C., and Williams K. I. (1982) Metabolic clearance rate and conversion ratios of [3H]2-hydroxyestrone in normal men. J. Clin. Endocrinol. Metab. 54, 374–380 10.1210/jcem-54-2-374 [DOI] [PubMed] [Google Scholar]
- 41. Bautista D. M., Pellegrino M., and Tsunozaki M. (2013) TRPA1: A gatekeeper for inflammation. Annu. Rev. Physiol. 75, 181–200 10.1146/annurev-physiol-030212-183811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Anand P., Murali K. Y., Tandon V., Murthy P. S., and Chandra R. (2010) Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chem. Biol. Interact. 186, 72–81 10.1016/j.cbi.2010.03.044 [DOI] [PubMed] [Google Scholar]
- 43. Kim M. J., Son H. J., Song S. H., Jung M., Kim Y., and Rhyu M. R. (2013) The TRPA1 agonist, methyl syringate suppresses food intake and gastric emptying. PLoS One 8, e71603 10.1371/journal.pone.0071603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Emery E. C., Diakogiannaki E., Gentry C., Psichas A., Habib A. M., Bevan S., Fischer M. J., Reimann F., and Gribble F. M. (2015) Stimulation of GLP-1 secretion downstream of the ligand-gated ion channel TRPA1. Diabetes 64, 1202–1210 10.2337/db14-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chepurny O. G., Leech C. A., Tomanik M., DiPoto M. C., Li H., Han X., Meng Q., Cooney R. N., Wu J., and Holz G. G. (2016) Synthetic small molecule GLP-1 secretagogues prepared by means of a three-component indole annulation strategy. Sci. Rep. 6, 28934 10.1038/srep28934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cao D. S., Zhong L., Hsieh T. H., Abooj M., Bishnoi M., Hughes L., and Premkumar L. S. (2012) Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic β cells. PLoS One 7, e38005 10.1371/journal.pone.0038005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Numazawa S., Takase M., Ahiko T., Ishii M., Shimizu S., and Yoshida T. (2012) Possible involvement of transient receptor potential channels in electrophile-induced insulin secretion from RINm5F cells. Biol. Pharm. Bull. 35, 346–354 10.1248/bpb.35.346 [DOI] [PubMed] [Google Scholar]
- 48. Fillingim R. B., King C. D., Ribeiro-Dasilva M. C., Rahim-Williams B., and Riley J. L. 3rd (2009) Sex, gender, and pain: a review of recent clinical and experimental findings. J. Pain 10, 447–485 10.1111/j.1526-4637.2009.00590.x,10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Obukhov A. G., Schultz G., and Lückhoff A. (1998) Regulation of heterologously expressed transient receptor potential-like channels by calcium ions. Neuroscience 85, 487–495 10.1016/S0306-4522(97)00616-7 [DOI] [PubMed] [Google Scholar]
- 50. Kumar S., Chakraborty S., Barbosa C., Brustovetsky T., Brustovetsky N., and Obukhov A. G. (2012) Mechanisms controlling neurite outgrowth in a pheochromocytoma cell line: the role of TRPC channels. J. Cell. Physiol. 227, 1408–1419 10.1002/jcp.22855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen X., Li W., Riley A. M., Soliman M., Chakraborty S., Stamatkin C. W., and Obukhov A. G. (2017) Molecular determinants of the sensitivity to Gq/11-phospholipase C-dependent gating, Gd3+ potentiation, and Ca2+ permeability in the transient receptor potential canonical type 5 (TRPC5) channel. J. Biol. Chem. 292, 898–911 10.1074/jbc.M116.755470 [DOI] [PMC free article] [PubMed] [Google Scholar]