Abstract
Zinc is an essential trace element that serves as a cofactor for enzymes in critical biochemical processes and also plays a structural role in numerous proteins. Zinc transporter ZIP4 (ZIP4) is a zinc importer required for dietary zinc uptake in the intestine and other cell types. Studies in cultured cells have reported that zinc stimulates the endocytosis of plasma membrane–localized ZIP4 protein, resulting in reduced cellular zinc uptake. Thus, zinc-regulated trafficking of ZIP4 is a key means for regulating cellular zinc homeostasis, but the underlying mechanisms are not well understood. In this study, we used mutational analysis, immunoblotting, HEK293 cells, and immunofluorescence microscopy to identify a histidine-containing motif (398HTH) in the first extracellular loop that is required for high sensitivity to low zinc concentrations in a zinc-induced endocytic response of mouse ZIP4 (mZIP4). Moreover, using synthetic peptides with selective substitutions and truncated mZIP4 variants, we provide evidence that histidine residues in this motif coordinate a zinc ion in mZIP4 homodimers at the plasma membrane. These findings suggest that 398HTH is an important zinc-sensing motif for eliciting high-affinity zinc-stimulated endocytosis of mZIP4 and provide insight into cellular mechanisms for regulating cellular zinc homeostasis in mammalian cells.
Keywords: metal homeostasis, endocytosis, zinc, transport, trafficking, cellular import, extracellular sensing, metal toxicity, protein trafficking, ZIP4
Introduction
Zinc (Zn) is an essential nutrient required for a variety of enzymes as a catalytic cofactor or as a critical structural determinant. Zinc deficiency is associated with pathologies that include immune system dysfunction, abnormal morphogenesis, and growth retardation (1, 2). Similarly, an imbalance of zinc homeostasis can result in disease states. For example, excess zinc can result in cytotoxicity due to protein misfolding and aggregation, and may alter the homeostasis of other metals such as copper (3, 4). Accordingly, intracellular levels of zinc must be finely regulated, and this is accomplished by the coordinated actions of the membrane-bound ZIP (SLC39A) zinc importer and ZnT (SLC30A) zinc exporter families (5–8). Among 14 ZIP proteins, ZIP4 is the primary zinc importer for the absorption of dietary zinc by enterocytes, although it is expressed in other tissues such as kidney (9, 10). Mutations in ZIP4 cause acrodermatitis enteropathica (AE),4 an autosomal recessive genetic disorder of childhood zinc deficiency, which can be lethal without parenteral zinc administration (10–15). The essential role of ZIP4 in zinc acquisition was further demonstrated using an intestine-specific deletion of Zip4 in mice, which was found to phenocopy AE in humans (16).
Expression of ZIP4 is regulated by both transcriptional and post-translational mechanisms in response to varying zinc concentrations. Studies with mice have shown that mRNA levels of mouse ZIP4 (mZIP4) are elevated in enterocytes and in the yolk sac under dietary zinc limitation and lowered when mice are fed a zinc-replete diet (10, 17). Moreover, the abundance of mZIP4 at the apical membrane in enterocytes is regulated by zinc availability (17). Subsequent in vitro studies in HEK293 cells expressing epitope-tagged ZIP4 proteins have shown that low micromolar concentrations of zinc could promote endocytosis of mouse and human ZIP4 from the plasma membrane (18), and that potentially toxic levels of zinc concentrations lead to ubiquitin-mediated degradation of ZIP4 (19), suggesting that multiple levels of homeostatic regulation have evolved to precisely regulate the ZIP4-dependent transport of zinc across the plasma membrane. Mutational analysis has shown that the histidine-rich cluster found in the cytoplasmic loop between the third and fourth transmembrane domains (TMDs) is required for the zinc-induced ubiquitination and degradation of ZIP4 in response to excess zinc concentrations (19). However, the mechanism by which ZIP4 undergoes endocytosis in response to low zinc concentrations has not been determined.
All ZIP family members including ZIP4 share a similar predicted topology: the N-terminal extracellular domain (NED) and eight TMDs (20). Approximately half of known missense mutations in human ZIP4 (hZIP4) in AE patients are in the NED and these NED mutants exhibit reduced zinc uptake activity (21–23), thus indicating that the N-terminal extracellular domain of ZIP4 is a functionally important region. Additionally, biochemical studies have shown that ZIP13 forms homodimers on the Golgi membrane (24), and that several ZnT zinc efflux transporters require dimerization for zinc transport, including ZnT5–ZnT6 heterodimers and ZnT7 homodimers (25–27). Moreover, recent studies suggest the possibility of ZIP4 dimerization based on in silico structure homology modeling of a human ZIP4 that lacks the NED (28) and crystal structures of the N-terminal extracellular domain from Pteropus alecto (black fruit bat) ZIP4 (29). However, the functional significance of dimerization of full-length ZIP4 and its subcellular localization are not understood.
Here, we demonstrate that the conserved 398HTH motif in the external loop between the second and third TMD is required for endocytosis of mZIP4 in response to low micromolar concentrations of zinc ions. Site-directed mutations in the 398HTH motif resulted in a considerable decrease in zinc-sensitive endocytosis of the transporter. Using an in vitro assay, we provide evidence that two 398HTH motifs within adjacent mZIP4 proteins coordinate one zinc ion. Furthermore, we found that mZIP4 exists in homodimer form at the plasma membrane based on co-immunoprecipitation and chemical cross-linking experiments. These findings identify a key extracellular motif required for high sensitivity zinc-induced endocytosis of mZIP4 necessary for fine-tuned cellular zinc homeostasis.
Results
Isolation of HEK293 cell lines expressing mutant mZIP4 proteins
Previous studies have demonstrated that low micromolar zinc concentrations were sufficient to stimulate rapid endocytosis of the mouse ZIP4 protein from the plasma membrane, but the molecular mechanisms underlying this process are unknown (10, 18, 19, 30–32). A simple hypothesis to explain this phenomenon is that zinc may bind to extracellular region(s) of the mZIP4 protein, which then triggers conformational changes of mZIP4 resulting in increased endocytosis, possibly by stimulating interactions between an endocytic targeting motif of mZIP4 and the cellular trafficking machinery.
In addition to the cytoplasmic histidine-rich loop domain connecting TMD 3 and 4, which is required for zinc-stimulated mZIP4 degradation (19), there are two potential zinc-binding sites in extracellular regions of mZIP4. The N-terminal extracellular domains of both hZIP4 and mZIP4 are rich in clustered histidine residues that may bind to zinc (20, 29). Another histidine-containing extracellular region of mZIP4 is the loop region between TMD 2 and 3, which includes the sequence 398HTHGGEGHTHEEE410 (Fig. 1A). This extracellular loop may also be important for substrate binding because of the high frequency of histidines and glutamates, which are potential zinc-binding amino acids. Furthermore, mutations in potential metal-binding residues in this region of the mZIP4-related Irt1 transporter of Arabidopsis were found to either disrupt transport function altogether or change the metal selectivity (33). These results suggest the first extracellular loop between TMD 2 and 3 in mZIP4 proteins may play a general role in binding and/or sensing zinc.
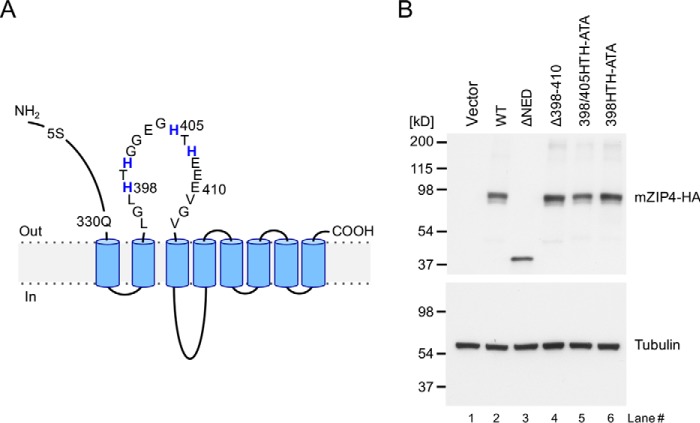
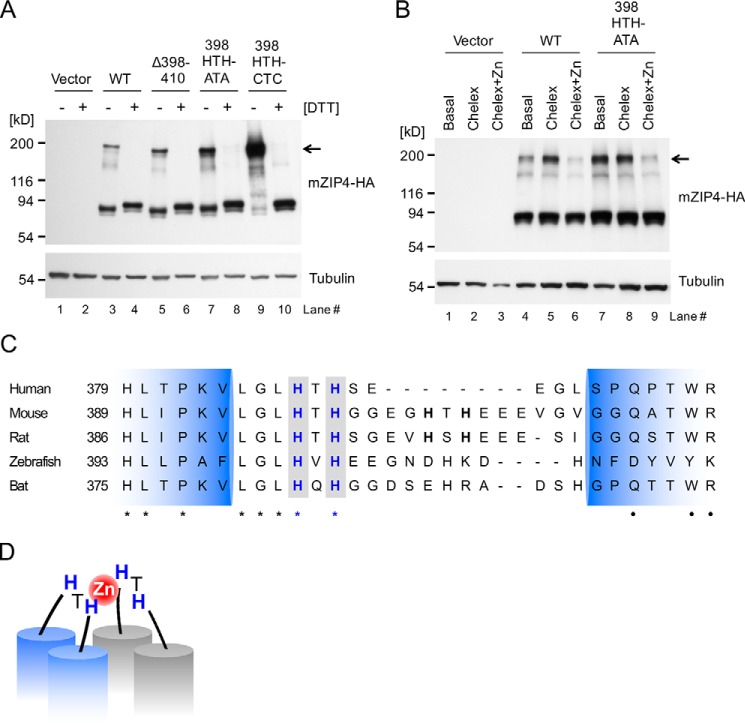
Figure 1.
Isolation of HEK293 cell lines expressing mZIP4 mutant proteins. A, schematic diagram of the mZIP4 protein and mutations investigated in this study. Most of the extracellular N-terminal region (residues 5 to 330) of mZIP4 is deleted in ΔNED-mZIP4-HA. Another deletion mutation incorporating residues 398 to 410 is shown, as well as two mutations involving alanine substitutions introduced into two HTH sequences in the first extracellular loop. B, Western blot analysis of HEK293 cells stably expressing mZIP4 mutant proteins shown in A. Total cell lysates were immunoblotted with anti-HA antibodies to detect mZIP4-HA protein, or anti-tubulin antibodies as a control for protein loading. As a negative control, HEK293 cells were transfected with empty vector and grown in basal medium (lane 1).
To investigate the role of potential metal-binding residues in regulating zinc-responsive mZIP4 trafficking, we used a site-directed mutagenesis approach targeting the loop domain as well as the NED (Fig. 1). Two deletion mutants were created, including ΔNED (which lacks the N-terminal extracellular domain) and Δ398–410 (which completely lacks the two HTH motifs). Alone (398HTH-ATA) or in combination (398HTH-ATA and 405HTH-ATA, hereafter 398/405HTH-ATA), we replaced individual histidine residues with alanines in the two HTH sequences in the first extracellular loop to disrupt potential metal binding while maintaining the general structure of the region (Fig. 1A). After confirming these mutations by DNA sequencing, HEK293 cells were stably transfected with the hemagglutinin (HA)-tagged mZIP4 constructs (mZIP4-HA) bearing these mutations and individual cell clones were selected for puromycin resistance. Immunoblot analysis was used to identify clones in which the expression of each mZIP4-HA variant was similar (Fig. 1B). Each mZIP4-HA variant migrated as a 90-kDa protein, with the exception of the truncated ΔNED mutant. Although the calculated molecular mass of mZIP-HA is ∼73 kDa, glycosylation is known to increase its apparent molecular mass to 90 kDa in HEK293 cells (31). As expected, there was no signal in HEK293 cells transfected with vector alone (Fig. 1B). Additional immunoblot analyses using available antibodies confirmed that HEK293 cells express little, if any, endogenous human ZIP4 protein (Fig. S1).
Deletion of the N-terminal extracellular domain in mZIP4 results in an increased rate of zinc-induced endocytosis
To characterize the function of the N-terminal extracellular domain in the zinc-responsive endocytosis of mZIP4-HA, we tested the zinc sensitivity of this response using an antibody uptake assay (Fig. 2A). The extracellular orientation of the HA-epitope tag on the C terminus of mZIP4-HA allowed mZIP4-HA endocytosis to be indirectly determined by measuring the levels of internalized anti-HA antibody added to the growth medium of living cells (18, 19). This was achieved by incubating live cells with anti-HA antibody, allowing sufficient time for internalization via endocytosis (5 min), and removal of surface-bound antibodies using acidic buffer followed by immunoblotting for the anti-HA antibody. This procedure was performed using cells grown in medium made zinc-deficient using Chelex 100; zinc was added back in the form of ZnCl2.
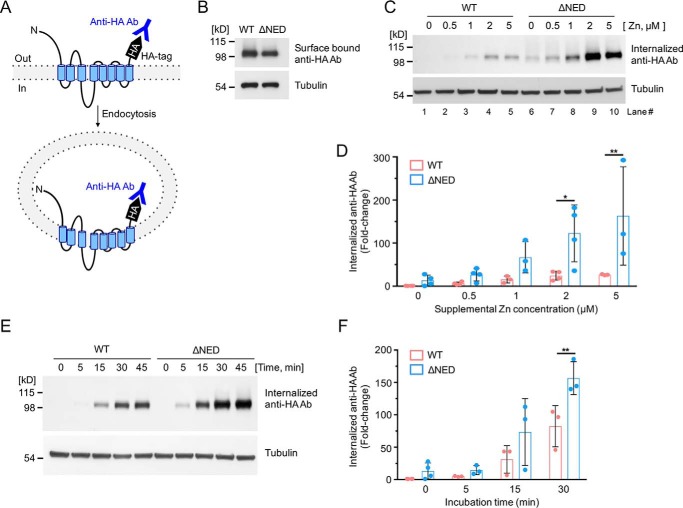
Figure 2.
Sensitivity of the mZIP4 N-terminal extracellular domain mutant to zinc-stimulated endocytosis. A, schematic illustration of mZIP4 showing the extracellular orientation of the HA tag, which permits labeling of mZIP4 by anti-HA antibodies at the plasma membrane, and measuring mZIP4 endocytosis. Zinc-stimulated endocytosis of WT and ΔNED-mZIP4-HA proteins was analyzed by measuring internalized anti-HA antibodies. B, WT-mZIP4-HA and ΔNED-mZIP4-HA levels at the cell surface were determined by incubating HEK293 cells in Chelex 100-treated medium. C, cells were preincubated with Chelex-treated medium for 3 h to increase surface levels of mZIP4-HA. Immunoblots were used to detect levels of internalized anti-HA antibodies endocytosed in HEK/WT-mZIP4-HA or HEK/ΔNED-mZIP4-HA cells for 5 min in either Chelex-treated medium (lanes 1 and 6) or Chelex-treated medium containing the indicated zinc concentrations. Tubulin levels shown in the lower panel demonstrate equal protein loading. D, quantification of zinc dose-dependent internalization of anti-HA antibodies in HEK293 cells expressing WT or ΔNED-mZIP4-HA (C). E, uptake of anti-HA antibodies by HEK/WT-mZIP4-HA or HEK/ΔNED-mZIP4-HA cells was assessed for the indicated time courses. Internalized anti-HA antibodies were detected by Western blot analysis as described under “Experimental procedures.” Tubulin is shown as a loading control. F, quantification of the internalization of anti-HA antibodies over time (Fig. 2E). Error bars in D and F indicate relative fold-changes compared with basal conditions of WT mZIP4-HA (mean ± S.D., n = 3–4). Statistics: two-way ANOVA, Sidak's post hoc test (*, p < 0.05, and **, p < 0.01).
The level of surface-bound anti-HA antibodies was similar in cells expressing the ΔNED-mZIP4-HA and wildtype (WT)-mZIP4-HA and incubated in Chelex-treated medium (Fig. 2B). This observation confirmed that both proteins accumulated at the plasma membrane at similar levels. As expected, the addition of zinc to the medium resulted in a dose-dependent increase in the endocytosis of WT-mZIP4-HA, as measured by the uptake of anti-HA antibodies (Fig. 2, C and D). Interestingly, endocytosis of the ΔNED-mZIP4-HA protein in response to zinc was significantly greater than WT-mZIP4-HA (Fig. 2, C and D). These finding suggest that loss of the N-terminal domain of mZIP4 increases the sensitivity to zinc-stimulated endocytosis. In additional experiments, WT-mZIP4-HA and ΔNED-mZIP4-HA were expressed in HEK293 cells pre-grown in Chelex-treated medium for 3 h to maximize protein levels at the plasma membrane and then exposed to zinc-containing medium containing anti-HA antibodies to permit internalization of these antibodies over time. The internalization of anti-HA antibodies by the ΔNED-mZIP4-HA mutant was markedly greater relative to the WT mZIP4-HA protein (Fig. 2, E and F). These results indicate that the ΔNED-mZIP4-HA mutant is internalized from the plasma membrane at a faster rate than the WT-mZIP4-HA protein.
Zinc-dependent trafficking of mZIP4 mutant proteins
Immunofluorescence microscopy was used to investigate the steady-state localization of each mZIP4-HA mutant protein in cells cultured in zinc-replete basal medium, and the ability of each protein to accumulate at the plasma membrane in response to zinc limitation. The WT-mZIP4 protein (WT-mZIP4-HA) was located in cytoplasmic vesicles when cells were cultured in basal medium (Fig. 3A, first column). Consistent with our previous observations (18), the exposure of the cells for 1 h to 10 μm TPEN, a membrane-permeable zinc chelator, resulted in the dispersal of WT-mZIP4-HA to the plasma membrane (Fig. 3A, first column). In contrast to WT-mZIP4, the NED deletion mutant protein (ΔNED-mZIP4-HA) was distributed in intracellular vesicles scattered throughout the cytoplasm in cells cultured in basal medium (Fig. 3A, second column). This distribution was quite distinct from that of the WT-mZIP4, which was more concentrated in the perinuclear region (Fig. 3A, first column). When zinc availability was limited by TPEN treatment, there was increased plasma membrane localization of the ΔNED-mZIP4-HA mutant, suggesting that the loss of the N-terminal extracellular domain did not grossly interfere with mZIP4-HA trafficking under zinc-deficient conditions. Compared with WT mZIP4, the steady-state localization of the TMD 2 and 3 loop region mutants (Δ398–410, 398/405HTH-ATA, and 398HTH-ATA) was shifted toward the cell periphery in both basal- and TPEN-treated medium, suggesting that these mutants may interfere with zinc-induced endocytosis (Fig. 3A, third to fifth columns).
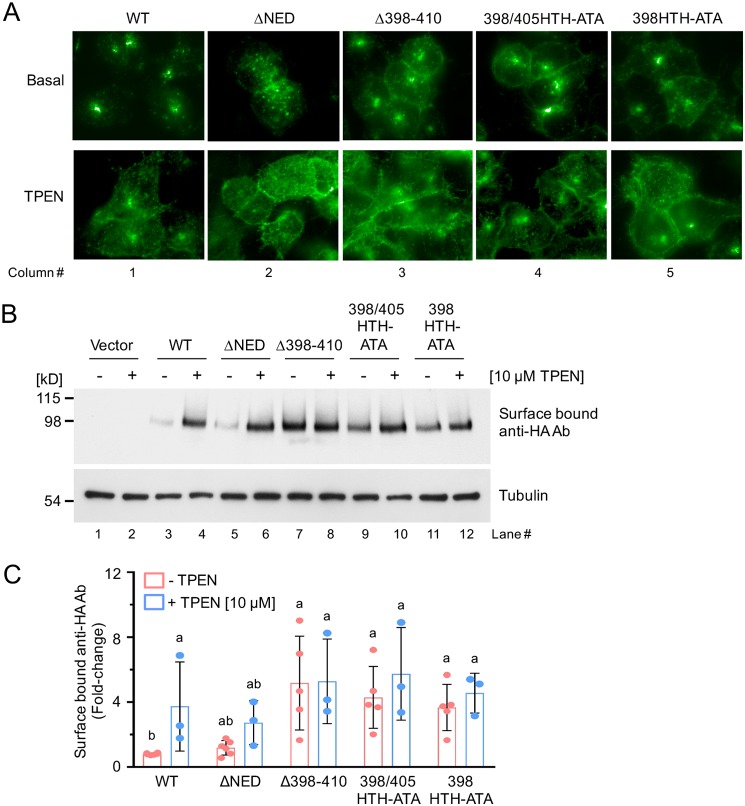
Figure 3.
Zinc-dependent relocalization of the mZIP4 mutant proteins. A, immunofluorescence microscopy analysis of HEK293 cells expressing each of the mZIP4-HA mutant proteins, which are exposed to either basal medium (∼2 μm zinc) or medium containing 10 μm of the zinc chelator TPEN for 1 h. Cells were fixed, permeabilized, and blocked prior to detection of WT and mutant mZIP4-HA proteins using anti-HA antibodies followed by anti-mouse Alexa 488 secondary antibodies. B, analysis of plasma membrane levels of WT and mutant mZIP4 proteins following TPEN treatment. Immunoblots were used to detect anti-HA antibodies bound to the surface of HEK293 cells expressing WT or mutant mZIP4 proteins after exposure to either basal medium (−) or medium containing 10 μm TPEN (+) for 1 h. Levels of anti-HA antibodies bound to mZIP4-HA at the plasma membrane were determined using Western blots as described under “Experimental procedures.” Tubulin protein was detected in each sample to indicate equal protein loading. C, quantification of surface bound anti-HA antibodies in HEK293 cells expressing mZIP4 WT and mutant proteins (Fig. 3B). Relative fold-changes compared with basal condition of WT mZIP4-HA are presented as mean ± S.D. (n = 3–6). Values with one different letter are significantly different from each other (p < 0.05). Statistics: two-way ANOVA, Sidak's post hoc test.
To more quantitatively assess the surface levels of mZIP4-HA mutant proteins under varying zinc concentrations, we used immunoblotting to detect levels of the extracellular HA-epitope tag at the plasma membrane of live cells (Fig. 2A) (18, 19). Live cells were placed on ice to inhibit endocytosis, incubated with anti-HA antibody, washed, and then cell lysates were prepared to detect anti-HA antibodies by immunoblotting. Using this approach, a low abundance of mZIP4-HA at the plasma membrane was observed for cells expressing WT-mZIP4-HA and ΔNED-mZIP4-HA grown in basal medium, as evident from low levels of surface-bound anti-HA antibodies (Fig. 3B, lanes 3 and 5). Consistent with the earlier immunofluorescence analysis (Fig. 3A, first and second columns), pre-treatment of cells for 1 h with 10 μm TPEN resulted in an increase in the WT-mZIP4-HA protein (Fig. 3B, lane 4) and the ΔNED-mZIP4-HA mutant protein at the plasma membrane (Fig. 3B, lane 6). However, all three mutants in the first external loop region showed higher surface level amounts in zinc-replete basal medium compared with the WT protein (Fig. 3, B, lanes 7, 9, and 11, and C), and zinc depletion by TPEN treatment did not further increase surface levels of the mZIP4-HA loop deletion mutant (Fig. 3, B, lane 8, and C), despite the equivalent amounts of total mZIP4 protein as shown by Western blotting controls (Fig. 1B). Note that the overall abundance of these mZIP4 mutant proteins was not significantly altered by these zinc- or TPEN-treated conditions (Fig. S2). These observations raise the possibility that mutations in the extracellular loop of mZIP4 may interfere with zinc responsive endocytosis.
The 398HTH motif of mZIP4 is required for high-sensitivity zinc-induced endocytosis
The first extracellular loop of mZIP4 contains two HTH motifs within the sequence 398HTHGGEGHTHEEE410, which may provide high-affinity zinc-binding sites to stimulate mZIP4 endocytosis in response to extracellular zinc. To test this hypothesis, we investigated whether these motifs are essential for mZIP4 endocytosis in response to a range of low zinc concentrations. As shown in Fig. 4, endocytosis of the WT-mZIP4-HA protein was stimulated by ∼0.5 μm zinc and saturated between 1 and 2 μm zinc. However, the first extracellular loop region mutants, Δ398–410, 398/405HTH-ATA, and 398HTH-ATA required ∼10 μm zinc to achieve an endocytic response that was similar to 1 μm zinc for the WT mZIP4 protein (Fig. 4). Importantly, the 405HTH-ATA mutant showed no reduction in endocytosis when compared with the WT-mZIP4-HA (Fig. S3). These findings indicate that the 398HTH motif determines the sensitivity of mZIP4 endocytosis to zinc.
Figure 4.
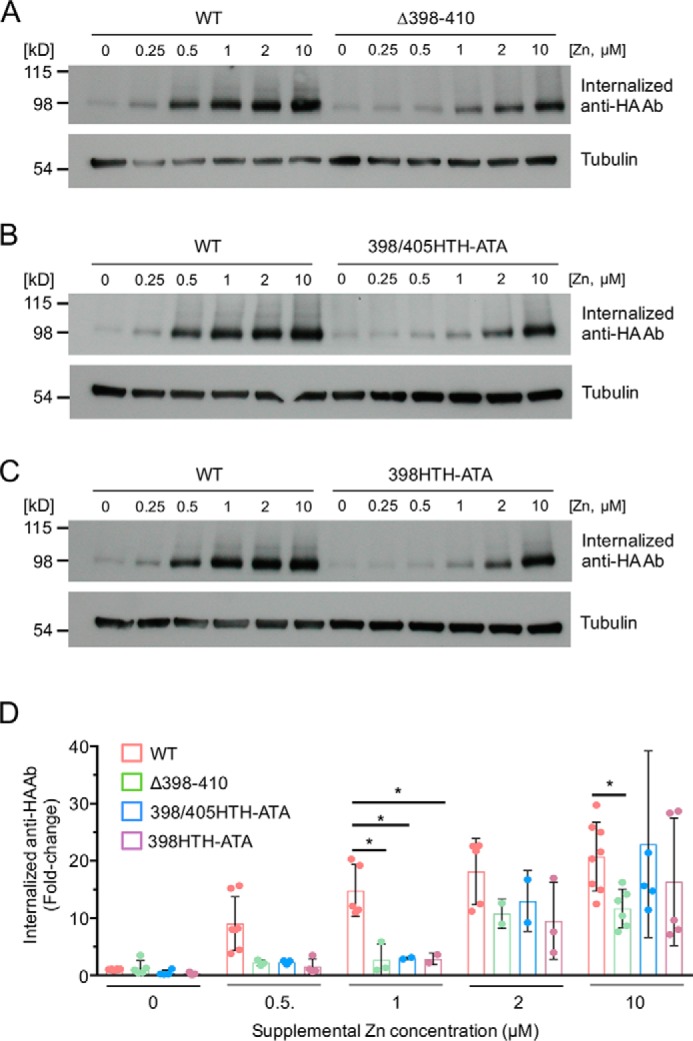
The first external loop region of mZIP4 is required for high-affinity zinc-stimulated endocytosis. A–C, HEK293 cells stably expressing WT and mutant mZIP4 proteins were preincubated with Chelex-treated medium for 3 h to increase surface levels of the proteins. Cells were then allowed to internalize anti-HA antibodies from Chelex-treated medium or the same medium containing the indicated zinc concentrations for 5 min. Immunoblots were then used to detect levels of internalized anti-HA antibodies endocytosed by WT and mutant mZIP4-HA proteins. Tubulin levels shown in the lower panel demonstrate equal protein loading. D, quantification of internalized anti-HA antibodies in HEK293 cells expressing WT and mutant mZIP4 proteins. Data are shown as relative fold-changes compared with basal condition of WT mZIP4-HA (mean ± S.D.) from multiple replicates per condition. Statistics: two-way ANOVA, Dunnett's post hoc test (*, p < 0.05).
Characterization of zinc binding to the first extracellular loop of mZIP4
To explore whether the HTH motifs in the first extracellular loop of mZIP4 are directly involved in zinc-binding, we generated a synthetic peptide corresponding to the first extracellular loop (peptide 1, 391IPKVLGLHTHGGEGHTHEEEVGVGGQATWR420) as well as a mutated version containing alanine substitutions for histidines in the 398HTH and 405HTH motif (peptide 2, 391IPKVLGLATAGGEGATAEEEVGVGGQATWR420) (Fig. 5A). The synthesized peptides were incubated with zinc ions and then subjected to gel filtration chromatography (PD-10 Sephadex G-25 column). Peptide–zinc interactions were then assessed by using atomic absorption spectroscopy to measure the concentration of zinc co-eluting with the peptides. Elution of the synthetic peptides from the column was monitored by UV spectrophotometry at 280 nm and quantified using the theoretical extinction coefficient. In the absence of peptide, a large peak of free zinc ions was observed at an elution position of fraction 9 (Fig. 5B). However, when peptide 1 was incubated with 1.0 molar eq of zinc, a significant amount of zinc ions from the original input was co-eluted with peptide 1 in fraction 4 (Fig. 5C). In contrast, the incubation of peptide 2 with 1.0 molar eq of zinc did not alter the elution of zinc ions, which peaked in fraction 9 (Fig. 5D). Together, these results suggest that the histidine residues in the external loop between the second and third TMD specifically interacts with zinc ions, and the bound zinc ions are likely coordinated by the histidines in the loop.
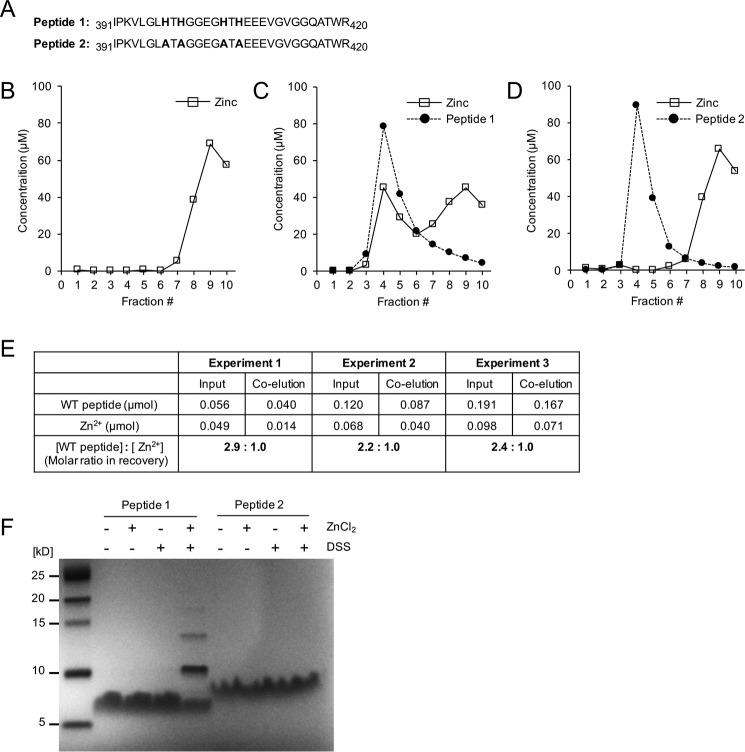
Figure 5.
Characterization of Zn2+ binding to the first extracellular loop of mZIP4. A, WT (peptide 1) and mutant (peptide 2) peptide sequences corresponding to the first extracellular loop region used in the zinc-binding assay. B–D, zinc binding analysis of peptides 1 and 2. The elution profiles are shown from a PD-10 column of 100 nmol of total zinc ions alone (B), 100 nmol of total zinc ions with 1.0 molar eq of peptide 1 (C), 100 nmol of zinc with 1.0 molar eq of peptide 2 (D). Concentrations of peptide are shown as filled circles with dashed lines, and eluted zinc levels are shown as open squares with a solid line. E, the stoichiometry of zinc binding under varied concentrations of the peptide 1 and zinc ions. F, zinc facilitates cross-linking of peptide 1 but not peptide 2. Peptides 1 and 2 (0.1 mm) were treated with 0.5 mm ZnCl2 and/or 2.5 mm of chemical cross-linker DSS and resolved on a Tris Tricine gel after quenching with 50 mm Tris (pH 7.5).
It was notable that the zinc ions co-eluted with peptide 1 were approximately one-half molar eq of the co-eluted peptide 1, suggesting a 2:1 binding ratio of the peptide 1 to zinc (Fig. 5C). To further examine the stoichiometry of the first extracellular loop in zinc binding at a higher resolution, we repeated the gel filtration assay using a Sephadex G-75 column (300 × 10 mm) for the WT peptide–zinc complex sample after incubation with varying concentrations of the components and measured concentrations of the co-eluted zinc and peptide 1 (Fig. 5E). Consistent with the previous result, the mean ± S.D. for the ratio of zinc to WT peptide was calculated as 2.46 ± 0.35, indicating that more than 2 molar eq of the peptide were co-eluted with zinc ions in all the tested conditions.
To investigate whether binding of zinc ions can promote the dimerization of peptide, the synthesized peptides were incubated with zinc ions and/or a chemical cross-linker, disuccinimidyl suberate (DSS), and assessed by Tris Tricine gel. Neither zinc ions nor DSS treatment promoted the multimerization of peptide 1 (Fig. 5F). However, incubation of peptide 1 with both zinc ions and DSS resulted in the formation of multimeric species of peptide 1, whereas peptide 2 lacking histidines remained unchanged under the same conditions. The predominant form of this multimer was 11 kDa, which presumably reflects the dimeric species (Fig. 5F). Interestingly, there were also larger species corresponding to the expected trimeric and tetrameric forms. However, these species were of lower abundance than the dimeric form, and may reflect zinc-mediated bridging between multiple peptides. Taken together, these results suggest that peptide 1 can form multimers, and that this depends on the histidine residues.
The mZIP4 protein forms homodimers
Next, we tested whether the mZIP4 protein forms an oligomer, as some ZIP and ZnT family members are reported to form homo- or hetero-oligomers, and in some cases multimerization is required for their function (24–27). Additionally, the crystal structure of the NED of black fruit bat ZIP4 suggests a dimeric structure of the N-terminal extracellular domain (29). As shown in Fig. 6A (lanes 4 and 6), both WT-mZIP4-HA and ΔNED-mZIP4-HA are monomeric and run at the predicted molecular weight under reducing conditions with dithiothreitol (DTT) followed by immunoblotting analysis. Under nonreducing conditions, both full-length mZIP4-HA and ΔNED-mZIP4-HA form oligomers with bands twice the apparent molecular weight of the each monomer, consistent with formation of a homodimer (Fig. 6A, lanes 3 and 5), in addition to the expected sizes of the monomeric proteins. These results indicated that the dimerized mZIP4 protein might contain a disulfide bond(s) or possess an oxidation-sensitive site between the monomers. To further evaluate the oligomeric state of the mZIP4 protein, we cross-linked HEK/WT-mZIP4-HA and HEK/ΔNED-mZIP4-HA cell extracts using the cross-linking agent ethylene glycol bis(succinimidyl succinate) (EGS) (34). As expected, EGS resulted in the expected migration of a glycosylated homodimeric form of WT mZIP4. As with the WT protein, EGS treatment resulted in reduced migration of the ΔNED mZIP4-HA protein corresponding to the expected sizes of both homodimeric as well as monomeric forms (Fig. 6B, lanes 2 and 4). These observations suggest that the NED is not essential for mZIP4 multimerization.
Figure 6.
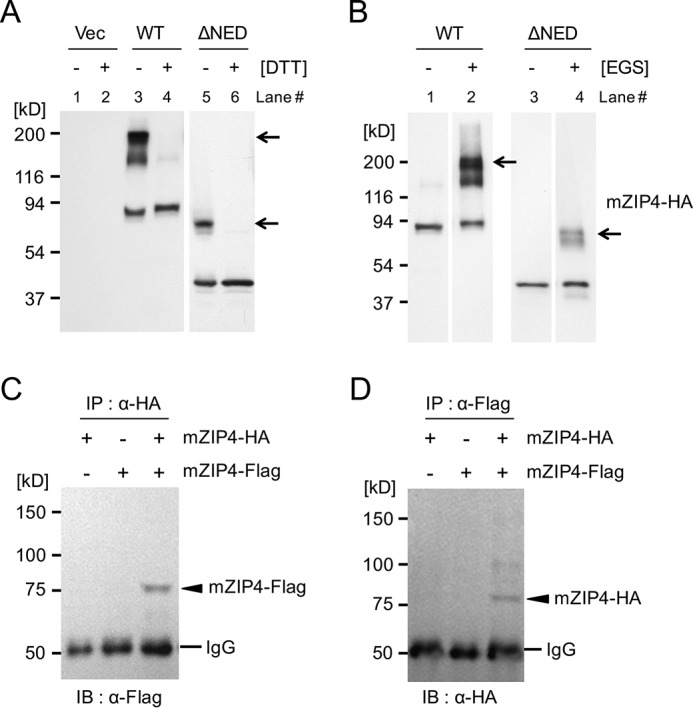
The mZIP4 protein forms homodimers. A, HEK293 cells were transfected with the pcDNA3.1 empty vector or the same vector harboring the WT or ΔNED mutant mZIP4 tagged with HA, and the lysates were analyzed by immunoblotting with (+) or without (−) DTT. Arrows indicate dimeric species of WT and ΔNED mutant mZIP4-HA, respectively. B, Western blot analysis of WT and ΔNED mutant mZIP4-HA in the presence and absence of the cross-linking agent EGS. Lysates of HEK/WT-mZIP4-HA and HEK/ΔNED-mZIP4-HA cells were incubated for 30 min at room temperature with EGS. Reactions were quenched with 45 mm Tris-HCl (pH 7.5), followed by incubation at room temperature for an additional 30 min. The cross-linked products were analyzed by SDS-PAGE and immunoblotting with anti-HA antibody. Polypeptide species are consistent with the expected sizes of monomeric and dimeric complexes of WT and ΔNED mutant mZIP4-HA. Arrows indicate the dimeric species of WT and ΔNED mutant mZIP4-HA, respectively. C and D, HEK293 cells were transiently transfected with expression plasmids for mZIP4-HA and/or mZIP4-FLAG followed by immunoprecipitation with the indicated antibodies. Immunoblotting was performed with either anti-FLAG or anti-HA antibodies. Arrowheads indicate monomeric mouse ZIP4. The IgG chain is indicated.
We next performed co-immunoprecipitation experiments to test whether indeed mZIP4 multimerization involves self-interactions. HEK293 cells were co-transfected with mZIP4-HA and/or mZIP4-FLAG, and whole cell lysates were immunoprecipitated with either anti-HA antibody or anti-FLAG antibody, followed by Western blot analysis using the indicated antibodies. These co-IP experiments showed that the mZIP4-FLAG and mZIP4-HA proteins were detected in immune complexes that precipitated with either the anti-HA or anti-FLAG antibodies, indicating that the complexes included self-interacting mZIP4 proteins (Fig. 6, C, lane 3, and D, lane 3). Taken together, these results suggest that the mZIP4 proteins are capable of forming homomultimeric complexes.
mZIP4 exists as homodimers at the cell surface
Because the histidine residues in the first extracellular loop of mZIP4 were able to bind zinc (Fig. 5F), we hypothesized that this motif may also be essential for the dimerization of mZIP4 via zinc binding. To test this possibility, loop region mutants of mZIP4 were analyzed to measure dimeric species of mZIP4 under reducing and nonreducing conditions. Loss of the HTH motifs did not result in decreased dimeric forms of either the 398HTH-ATA mutant or the loop deletion mutant (Δ398–410) mZIP4-HA (Fig. 7A, lanes 5 and 7), suggesting that zinc coordination through the loop is not necessary for mZIP4-HA dimer formation.
Figure 7.
mZIP4 exists as homodimers at the cell surface. A, HEK293 cells stably expressing WT and mutant mZIP4 proteins were analyzed by immunoblotting with (+) or without (−) DTT. Arrow indicates dimeric species of mZIP4 proteins. B, HEK293 cells stably expressing WT and mutant mZIP4-HA proteins were preincubated with basal medium, Chelex-treated medium, or Chelex-treated medium containing 10 μm zinc for 3 h to differentiate surface levels of the proteins, and then the cells were incubated with the membrane impermeable chemical cross-linker Sulfo-EGS at 37 °C to cross-link cell-surface proteins followed by quenching with Tris-HCl buffer, resolution via SDS-PAGE, and immunoblotting with anti-HA antibody. The expected molecular weight of the dimeric species of mZIP4 is indicated by the arrow. C, multiple sequence alignment of ZIP4 orthologs in mammals in the region of the first extracellular loop between the predicted TMD 2 and 3. NCBI reference sequence codes are: NP_570901.2 for human, NP_082340.1 for mouse, A0JPN2.1 for rat, XP_017207743.1 for zebrafish, and ELK11751.1 for bat. Conserved HXH motifs are shown in bold blue font with a gray background. Identical amino acids are indicated by asterisks (*), and high similarity amino acids are indicated by dots (●). Extracellular loop regions (389–420 amino acid residues) between TMD 2 and 3 of mZIP4 were predicted using TMHMM. D, a proposed model of zinc binding to the first extracellular loop of the ZIP4 homodimer. Histidine residues in the 398HTH motif in the first extracellular loops may coordinate a zinc ion and provide a high zinc sensitivity in zinc-stimulated endocytosis of ZIP4 proteins.
To clarify whether the 398HTH motif in the first external loop of mZIP4 contacts the adjacent 398HTH motif in the mZIP4 homodimer, we replaced both histidines in the 398HTH motif with cysteines to promote disulfide bridging if these residues come into close contact. Under nonreducing conditions, mZIP4 harboring 398HTH to CTC substitutions exhibited a substantially increased apparent molecular weight corresponding to the dimeric form (Fig. 7A, lane 9), suggesting that the first extracellular loops in the mZIP4 dimer are close enough to form a spontaneous intermolecular disulfide bond. Together with our earlier zinc binding studies, these data imply that two 398HTH motifs on the dimeric interface of mZIP4 may coordinate one zinc ion.
To investigate whether mZIP4 forms a dimer at the cell surface, a membrane impermeable chemical cross-linker, ethylene glycol bis(sulfosuccinimidyl succinate) (Sulfo-EGS) was added to HEK293 cells expressing WT-mZIP4-HA precultured with basal medium, Chelex-treated medium, or Chelex-treated medium containing 10 μm zinc for 3 h. Sulfo-EGS was used to trap dimeric forms of mZIP4-HA proteins strictly at the cell surface. In basal conditions, only a minor portion of total WT-mZIP4-HA was cross-linked by Sulfo-EGS (Fig. 7B, lane 4). However, the depletion of zinc by Chelex treatment resulted in significantly increased abundance of the mZIP4-HA dimer, which was suppressed by zinc treatments (Fig. 7B, lanes 5 and 6). Notably, levels of the dimeric form are well correlated with the mZIP4 abundance at the cell surface under Chelex-treated conditions (Fig. 7B, lanes 4 and 5; and Fig. 3B, lanes 3 and 4), indicating that the mZIP4-HA protein is likely present at the plasma membrane in its dimeric form. To verify that Sulfo-EGS only mediates mZIP4-HA cross-linking at the cell surface, cells expressing mZIP4-HA were preincubated with basal medium, with Chelex-treated medium, or with Chelex-treated medium plus zinc. The cells were then placed on ice to inhibit cellular endocytosis, treated with Sulfo-EGS, and then analyzed by immunoblotting. Changes in cross-linked dimeric mZIP4-HA abundance under endocytosis-free conditions (4 °C) behaved similarly to those under 37 °C conditions (Fig. S4), suggesting that cell surface pools of mZIP4 were dimerized.
Although HEK293 cells expressing 398HTH-ATA mutant mZIP4-HA showed elevated levels of dimeric proteins as compared with WT-mZIP4-HA proteins under basal conditions, zinc removal by Chelex treatment did not further increase the levels of 398HTH-ATA dimers (Fig. 7B, lanes 7 and 8), which was similar in magnitude to the increase in WT-mZIP4-HA abundance at the cell surface (Fig. 3B, lanes 11 and 12). These results suggest that zinc-induced endocytosis of the 398HTH-ATA mutant is impaired even in basal medium and this likely accounts for the elevated accumulation of this mutant protein at the cell surface under these conditions (Fig. 3).
To investigate whether the 398HTH motif is conserved in mammals, we scanned multiple sequences for the motif in ZIP4 from five mammalian species. Overall, there is relatively poor amino acid sequence conservation in the first external loop of mammalian ZIP4, whereas the second and third TMD regions are well conserved. Although the 405HTH motif is conserved only in rodents, the 398HTH motif is highly conserved in all mammal species examined (Fig. 7C), suggesting that the high sensitivity to zinc-responsive endocytosis of ZIP4 conferred by the 398HTH motif is functionally conserved in mammals. Taken together with earlier data, mZIP4 forms a homodimer at the plasma membrane, and the 398HTH motif in the first extracellular loop is required as a zinc sensor for the endocytosis of mZIP4 in response to low zinc concentrations but is dispensable for the dimerization of mZIP4.
Discussion
Dietary zinc availability can fluctuate widely depending upon an organism's immediate environment. ZIP4 zinc transporter proteins localize to the apical membrane of enterocytes and import dietary zinc ions from the lumen of the intestine (16). The mouse ZIP4 protein is regulated at multiple levels in response to varying zinc levels to maintain optimal organismal zinc status. Dietary zinc deficiency elevates mZIP4 mRNA levels in enterocytes and the embryonic visceral yolk sac, an effect that is suppressed upon zinc repletion by either zinc supplementation or parenteral injection (10, 17, 35). Moreover, ZIP4 proteins cycle between vesicular compartments and the plasma membrane, and the abundance of the ZIP4 protein at the plasma membrane is regulated by zinc availability.
Endocytosis of ZIP4 is stimulated by submicromolar concentrations of zinc ions, but the underlying molecular mechanism that regulates this process remained unknown. High concentrations of zinc promote the ubiquitin-mediated degradation of ZIP4. This process requires histidine-rich putative zinc-binding domains located in the second cytoplasmic loop between TMDs 3 and 4, which are not required for zinc-induced endocytosis (19). Thus, zinc-stimulated endocytosis and degradation are regulated by distinct zinc sensing motifs in ZIP4.
Our studies demonstrate that the 398HTH motif of mZIP4 is an important determinant of endocytosis for high zinc sensitivity. Histidine to alanine substitution of residues in this motif abolished sensitivity to low zinc in the metal-stimulated endocytosis of mZIP4 proteins (Figs. 3 and 4). Because the 398HTH motif faces the extracellular milieu, zinc occupancy of the motif may function as a sensor of extracellular zinc levels that promotes ZIP4 clearance from the plasma membrane when zinc levels increase. Zinc binding to the 398HTH motif may induce a conformational change in the cytoplasmic loop allowing binding of an adaptor protein to sorting signals required for endocytosis. Zinc-induced endocytosis of the closely related mZIP1 protein requires a dileucine sorting signal (144ETRALL) in the large cytoplasmic loop domain (36). A similar sequence (490ETPELL) found in the corresponding loop region of mZIP4 may be needed for endocytosis. It will be interesting to determine how zinc ion binding to the extracellular region of mZIP4 is coordinated with the endocytic machinery. Interestingly, the 398HTH motif is well conserved in the analogous loop region of ZIP6, ZIP7, and ZIP10 (Fig. S5), suggesting that this extracellular zinc sensing mechanism may also be conserved in other ZIP family members.
The accumulated data support the possibility that some ZIP and ZnT proteins exist as dimers (25–27). Here we showed that mZIP4 proteins form a homodimer at the cell surface (Fig. 7A), but the role and the mechanisms of the mZIP4 homodimer are still not completely understood. Some metal transporters require oligomerization for metal transport function. For example, the CTR1 copper transporter has three TMDs and forms a homotrimer that is required to create a copper-permeable pore (37, 38); however, it is not yet known whether the pore for zinc ion import is formed by the monomeric mZIP4 alone or requires additional proteins. Results from the zinc-binding assay with the synthetic peptide containing the first extracellular loop sequence (Fig. 5) and elevated accumulation of dimeric 398HTH-ATA mutant mZIP4-HA proteins at the plasma membrane (Fig. 7B, lanes 4 and 7) suggest that the HTH motif is not required for dimer formation. This dimerization may facilitate zinc binding mediated by the histidines in the 398HTH motif. We speculate that the dimerization of mZIP4 in response to elevated zinc concentrations is a homeostatic mechanism to avoid potential zinc toxicity by triggering endocytosis of the transporter. The 398HTH-ATA and 398HTH-CTC mutants generated in this study will be useful for determining the potential relationships between mZIP4 zinc sensing, dimerization, and transport activities. As mZIP4 is known to be glycosylated, it will also be important to determine the role of glycosylation in these homeostatic processes.
The sensitivity of mZIP4 dimers to reducing conditions (Fig. 6A) suggests that the formation of dimeric mZIP4 is mediated by inter-molecular disulfide bond(s). A recent crystallographic structure of the truncated NED of black fruit bat ZIP4 suggests that the PAL motif containing domain mediates the dimerization of NED (29). Consistent with this model, the ΔNED-mZIP4-HA dimeric forms were less abundant as compared with WT-mZIP4-HA dimers (Fig. 6, A and B) suggesting that the N-terminal extracellular domain may play a role in stabilizing mZIP4 dimers. Furthermore, our finding that deletion of this domain increased the sensitivity of mZIP4 endocytosis in response to zinc (Fig. 2) implies that this extracellular domain may function as a negative regulator of endocytosis. It will be interesting in future studies to determine whether the NED regulates zinc sensing by the 398HTH motif through cross-talk between the domains.
In the present work, we show that the 398HTH motif in the first extracellular loop of mouse ZIP4 functions as sensor of low zinc concentrations for the triggering of endocytosis. We propose that histidine residues in this motif coordinate a zinc ion in mZIP4 homodimers at the plasma membrane (Fig. 7D). Our observations in cultured cells suggest that other mammalian ZIP proteins may also have similar extracellular zinc sensing mechanisms and exist as dimers. These studies pave the way for future investigations such as whether dimerization is required for zinc uptake activity, and how and where ZIP4 dimer formation occurs within cells.
Experimental procedures
Plasmids, reagents, cell lines, and antibodies
The mZIP4 cDNA was cloned into the puromycin vector, pcDNA3.1, and the HA or FLAG antigen epitope was added using PCR at the C terminus, as described previously (18). The mZIP4 mutations in this study were generated via PCR mutagenesis and subcloned into the pcDNA3.1 plasmid vector or via a QuikChange II XL site-directed mutagenesis kit (Stratagene). All chemicals, unless stated otherwise, were purchased from Sigma. The human embryonic kidney 293 (HEK293) cell lines were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 units/ml of penicillin and streptomycin in 5% CO2 at 37 °C in an incubator. This medium contains ∼2 μm zinc. HEK293 cells lines were transfected with the empty pcDNA3.1 vector or the same vector harboring the WT or mutant mZIP4 tagged with HA or FLAG using the Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Stably transfected cells were established by the selection of transfected cells with 2 μg/ml of puromycin in the growth medium (Sigma). All horseradish peroxidase (HRP)-conjugated antibodies were from Roche Applied Science, and anti-rabbit IgG antibody was purchased from Sigma. Alexa 488-conjugated antibodies were purchased from Invitrogen. Zinc depletion of 10% fetal bovine serum, Dulbecco's modified Eagle's medium was performed using Chelex 100 as described previously (18, 19).
Immunoblot analysis of mZIP4 protein
Cells cultured in 6-well trays were scraped into ice-cold phosphate-buffered saline (PBS) and pelleted by centrifugation. After several washes in ice-cold PBS, the cells were lysed by sonication in lysis buffer containing 62 mm Tris-HCl, pH 6.8, 2% SDS, 100 mm DTT, and protease inhibitor mix (Roche Applied Science). Samples were centrifuged for 10 min at 16,000 × g, and the protein concentration of the lysates was determined using a DC protein assay kit (Bio-Rad). 20 μg of protein lysates was separated using 4–20% gradient SDS-PAGE, transferred to nitrocellulose membranes, and detected by chemiluminescence (Pierce) using anti-HA or -FLAG antibodies (1:1000) followed by HRP-conjugated secondary antibodies (1:5,000). As a loading control, the same membranes were stripped and re-probed with an anti-tubulin antibody (1:1,000).
Immunofluorescence microscopy
Cells were grown in 24-well trays for 24 h on sterile glass coverslips, washed twice with 1 ml of ice-cold PBS, and then fixed for 10 min at 25 °C using 4% paraformaldehyde. The cells were then permeabilized with 0.1% Triton X-100 in PBS for 5 min, blocked for 1 h with 1% bovine serum albumin (BSA) and 3% skim milk in PBS, and then probed with anti-HA antibodies (1:1000) followed by Alexa 488 anti-mouse antibodies (1:1000). Samples were viewed with a ×60 objective using a Leica DMRE microscope fitted with a Retiga Ex digital camera.
Detection of mZIP4-HA protein levels at the plasma membrane and intracellular pools
The pool of mZIP4-HA at the plasma membrane was assessed by measuring levels of anti-HA antibodies bound to the surface of HEK/mZIP4-HA cells as we have previously described (18, 19). Briefly, HEK/mZIP4-HA cells in 6-well trays were washed twice with PBS on ice, blocked with ice-cold 3% skim milk in PBS for 20 min, then incubated with 5 μg/ml of anti-HA antibody for 1 h on ice. Cells were washed five times in PBS to remove unbound antibodies, and then lysed by sonication in SDS lysis buffer as described above. Cell lysates containing the solubilized anti-HA antibodies that were bound to the mZIP4-HA protein at the plasma membrane were separated using 4–20% gradient SDS-PAGE, and transferred to nitrocellulose membranes. The anti-HA antibodies were then detected using HRP-conjugated antibodies (1:5000) by chemiluminescence. Tubulin protein levels were detected on parallel immunoblots using anti-tubulin antibodies.
The endocytosis of mZIP4-HA was indirectly determined by measuring the uptake of anti-HA antibodies added to the culture medium of living cells as described previously (18, 19). Cells were incubated at 37 °C in the indicated medium supplemented with 5 μg/ml of anti-HA antibodies for 5 min to allow internalization of antibody–mZIP4-HA complexes by endocytosis. Cells were then transferred to ice to prevent further endocytosis and washed three times with ice-cold PBS. Surface-bound antibodies were removed by five washes with ice-cold acidic buffer (100 mm glycine, 20 mm magnesium acetate, 50 mm potassium chloride, pH 2.2). After two additional washes with ice-cold PBS, the cells were harvested, lysed, and analyzed for anti-HA antibodies by immunoblotting as described above.
Zinc-binding assay
The 30-amino acid peptide mimicking the first extracellular loop (peptide 1) and the mutated version containing four alanine substitutions for the 398HTH and 405HTH motifs (peptide 2) were synthesized using solid-phase peptide synthesis (GenScript). The crude peptide was purified to homogeneity with HPLC, and the identity of the purified peptide was confirmed with MS. The sequence is given with the mutated residues highlighted in bold: peptide 1, 391IPKVLGLHTHGGEGHTHEEEVGVGGQATWR420; peptide 2, 391IPKVLGLATAGGEGATAEEEVGVGGQATWR420. The synthetic peptides were then solubilized using 0.01 n HCl and were incubated with 1 molar ratio of ZnCl2 for 1 h at room temperature. The pH of the peptide/zinc mixture was adjusted to 7.0 with neutralization buffer (100 mm Tris-HCl, pH 8.2) and was then loaded onto a PD-10 (Sephadex G-25) column equilibrated with the neutralization buffer. For each of the 10 collected fractions, UV-visible spectra were recorded on a Varian Cary 300 spectrophotometer using anaerobic quartz cells of 1.0-cm path length. For the stoichiometric determination of the zinc–peptide 1 complex, peptide 1 was prepared as described above and then incubated with various concentrations of ZnCl2. After neutralization with 100 mm Tris-HCl buffer (pH 8.2), the mixture was loaded onto a Sephadex G-75 column (300 × 10 mm) equilibrated with buffer containing 100 mm Tris-HCl (pH 7.5) and 150 mm NaCl. As described above, 1-ml fractions were collected and analyzed for A280 and zinc content by atomic absorption spectroscopy.
Cross-linking of peptide 1 with Zn2+
0.1 mm synthesized peptides dissolved in 20 mm HEPES (pH 7.5) were incubated with 0.5 mm ZnCl2 and/or 2.5 mm reversible cross-linker DSS (Pierce) in a total reaction volume of 20 μl for 30 min at 25 °C. Reactions were quenched using a final concentration of 50 mm Tris (pH 7.5) and incubated for 15 min at 25 °C. Samples were solubilized in Tricine sample buffer and analyzed by Tris Tricine gel.
Co-immunoprecipitations (co-IPs)
24 h after transfection with plasmids expressing mZIP4-HA and/or mZIP4-FLAG, HEK293 cells were collected from 10-cm plates in ice-cold PBS buffer. After lysis, whole cell lysates were centrifuged at 16,000 × g at 4 °C for 15 min. For the pre-clearing of the lysate, Protein G-Sepharose 4 Fast Flow beads (GE Healthcare) were first washed 3 times in ice-cold lysis buffer. 450 μl of the supernatants were then mixed with the pre-washed beads and incubated on a rotator for 1 h at 4 °C, after which the mixture was centrifuged at 12,000 × g at 4 °C for 10 min. 0.5 μl of anti-HA or anti-FLAG antibody was added to the pre-cleared supernatant and incubated on a rotator for 12 h at 4 °C. The mixture was additionally incubated with 50 μl of pre-cleared Protein G slurry on a rotator overnight at 4 °C. After centrifugation at 12,000 × g at 4 °C for 30 s, the supernatant was carefully discarded, and the pellets were washed with ice-cold lysis buffer three times. The pellet was boiled for 5 min in 50 μl of 1× SDS-PAGE sample buffer (62.5 mm Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 1% β-mercaptoethanol, and 0.02% bromphenol blue), and supernatants were collected after centrifugation at 12,000 × g at 4 °C for 5 min, followed by SDS-PAGE and Western blot analysis.
Statistics
For quantification of surface-bound or internalized anti-HA antibodies, band intensities on Western blots were determined by Image Studio Lite software (LI-COR). Tubulin band intensities were used to normalize surface-bound or internalized anti-HA antibodies for each sample. The relative fold-change was calculated by dividing the normalized expression from each lane by the normalized expression of the control sample (basal condition of WT mZIP4-HA). Statistical significance was determined using a two-way ANOVA followed by Sidak's or Dunnett's post hoc test in GraphPad Prism, version 7.04 (GraphPad Software). All data are presented as mean ± S.D., and p values less than 0.05 were considered statistically significant.
Sequence alignments
Clustal Omega was used to generate a multiple sequence alignment of ZIP4 orthologs in mammals and murine ZIP family members (39). TMDs and loop regions of mZIP4 were predicted using Trans-Membrane prediction using Hidden Markov Models (TMHMM) and the Conserved Domain Database (CDD) (40).
Author contributions
H. C., D. R. W., B.-E. K., and M. J. P. data curation; H. C., C.-J. L., H. J. C., D. R. W., and B.-E. K. formal analysis; H. C., T. K., and B.-E. K. validation; H. C., T. K., C.-J. L., H. J. C., D. R. W., B.-E. K., and M. J. P. investigation; H. C., T. K., and B.-E. K. visualization; H. C., T. K., H. J. C., and B.-E. K. methodology; H. C., T. K., C.-J. L., and B.-E. K. writing-original draft; T. K., B.-E. K., and M. J. P. writing-review and editing; D. R. W., B.-E. K., and M. J. P. supervision; B.-E. K. and M. J. P. conceptualization; B.-E. K. and M. J. P. funding acquisition; B.-E. K. and M. J. P. project administration.
Supplementary Material
Acknowledgments
We thank members of the Kim laboratory and Dr. David Eide for valuable comments on this project.
This work was supported by National Institutes of Health Grants DK110195 (to B.-E. K.) and DK66333 (to M. J. P.). The authors declare that they have no conflicts of interest with the contents of this article.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S5.
- AE
- acrodermatitis enteropathica
- HA
- hemagglutinin
- TPEN
- N,N,N′,N′-tetrakis(2-pyridyl-methyl)ethylenediamine
- HEK
- human embryonic kidney
- Sulfo-EGS
- ethylene glycol bis(sulfosuccinimidyl succinate)
- TMD
- transmembrane domain
- NED
- N-terminal extracellular domain
- IP
- immunoprecipitation
- HRP
- horseradish peroxidase
- ANOVA
- analysis of variance
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
References
- 1. Kambe T., Weaver B. P., and Andrews G. K. (2008) The genetics of essential metal homeostasis during development. Genesis 46, 214–228 10.1002/dvg.20382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prasad A. S. (1995) Zinc: an overview. Nutrition 11, 93–99 [PubMed] [Google Scholar]
- 3. Pawa S., Khalifa A. J., Ehrinpreis M. N., Schiffer C. A., and Siddiqui F. A. (2008) Zinc toxicity from massive and prolonged coin ingestion in an adult. Am. J. Med. Sci. 336, 430–433 10.1097/MAJ.0b013e31815f2c05 [DOI] [PubMed] [Google Scholar]
- 4. Maret W., and Li Y. (2009) Coordination dynamics of zinc in proteins. Chem. Rev. 109, 4682–4707 10.1021/cr800556u [DOI] [PubMed] [Google Scholar]
- 5. Fukada T., and Kambe T. (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3, 662–674 10.1039/c1mt00011j [DOI] [PubMed] [Google Scholar]
- 6. Taylor K. M., and Nicholson R. I. (2003) The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys. Acta 1611, 16–30 10.1016/S0005-2736(03)00048-8 [DOI] [PubMed] [Google Scholar]
- 7. Kambe T., Yamaguchi-Iwai Y., Sasaki R., and Nagao M. (2004) Overview of mammalian zinc transporters. Cell. Mol. Life Sci. 61, 49–68 10.1007/s00018-003-3148-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimura T., and Kambe T. (2016) The functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int. J. Mol. Sci. 17, 336 10.3390/ijms17030336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrews G. K. (2008) Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochem. Soc. Trans. 36, 1242–1246 10.1042/BST0361242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dufner-Beattie J., Wang F., Kuo Y. M., Gitschier J., Eide D., and Andrews G. K. (2003) The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J. Biol. Chem. 278, 33474–33481 10.1074/jbc.M305000200 [DOI] [PubMed] [Google Scholar]
- 11. Nakano A., Nakano H., Nomura K., Toyomaki Y., and Hanada K. (2003) Novel SLC39A4 mutations in acrodermatitis enteropathica. J. Investig. Dermatol. 120, 963–966 10.1046/j.1523-1747.2003.12243.x [DOI] [PubMed] [Google Scholar]
- 12. Kury S., Kharfi M., Kamoun R., Taieb A., Mallet E., Baudon J. J., Glastre C., Michel B., Sebag F., Brooks D., Schuster V., Scoul C., Dreno B., Bezieau S., and Moisan J. P. (2003) Mutation spectrum of human SLC39A4 in a panel of patients with acrodermatitis enteropathica. Hum. Mutat. 22, 337–338 10.1002/humu.9178 [DOI] [PubMed] [Google Scholar]
- 13. Wang K., Zhou B., Kuo Y. M., Zemansky J., and Gitschier J. (2002) A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am. J. Hum. Genet. 71, 66–73 10.1086/341125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Küry S., Dréno B., Bzieau S., Giraudet S., Kharfi M., Kamoun R., and Moisan J. P. (2002) Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat. Genet. 31, 239–240 10.1038/ng913 [DOI] [PubMed] [Google Scholar]
- 15. Liuzzi J. P., Bobo J. A., Lichten L. A., Samuelson D. A., and Cousins R. J. (2004) Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc. Natl. Acad. Sci. U.S.A. 101, 14355–14360 10.1073/pnas.0406216101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geiser J., Venken K. J., De Lisle R. C., and Andrews G. K. (2012) A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet. 8, e1002766 10.1371/journal.pgen.1002766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dufner-Beattie J., Kuo Y. M., Gitschier J., and Andrews G. K. (2004) The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J. Biol. Chem. 279, 49082–49090 10.1074/jbc.M409962200 [DOI] [PubMed] [Google Scholar]
- 18. Kim B. E., Wang F., Dufner-Beattie J., Andrews G. K., Eide D. J., and Petris M. J. (2004) Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J. Biol. Chem. 279, 4523–4530 10.1074/jbc.M310799200 [DOI] [PubMed] [Google Scholar]
- 19. Mao X., Kim B. E., Wang F., Eide D. J., and Petris M. J. (2007) A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J. Biol. Chem. 282, 6992–7000 10.1074/jbc.M610552200 [DOI] [PubMed] [Google Scholar]
- 20. Jeong J., and Eide D. J. (2013) The SLC39 family of zinc transporters. Mol. Aspects Med. 34, 612–619 10.1016/j.mam.2012.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schmitt S., Küry S., Giraud M., Dréno B., Kharfi M., and Bézieau S. (2009) An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum. Mutat. 30, 926–933 10.1002/humu.20988 [DOI] [PubMed] [Google Scholar]
- 22. Kury S., Kharfi M., Blouin E., Schmitt S., and Bezieau S. (2016) Clinical utility gene card for acrodermatitis enteropathica: update 2015. Eur. J. Hum. Genet. 24, 10.1038/ejhg.2015.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kambe T., and Andrews G. K. (2009) Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol. Cell. Biol. 29, 129–139 10.1128/MCB.00963-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bin B. H., Fukada T., Hosaka T., Yamasaki S., Ohashi W., Hojyo S., Miyai T., Nishida K., Yokoyama S., and Hirano T. (2011) Biochemical characterization of human ZIP13 protein: a homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers-Danlos syndrome. J. Biol. Chem. 286, 40255–40265 10.1074/jbc.M111.256784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ishihara K., Yamazaki T., Ishida Y., Suzuki T., Oda K., Nagao M., Yamaguchi-Iwai Y., and Kambe T. (2006) Zinc transport complexes contribute to the homeostatic maintenance of secretory pathway function in vertebrate cells. J. Biol. Chem. 281, 17743–17750 10.1074/jbc.M602470200 [DOI] [PubMed] [Google Scholar]
- 26. Fukunaka A., Suzuki T., Kurokawa Y., Yamazaki T., Fujiwara N., Ishihara K., Migaki H., Okumura K., Masuda S., Yamaguchi-Iwai Y., Nagao M., and Kambe T. (2009) Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J. Biol. Chem. 284, 30798–30806 10.1074/jbc.M109.026435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fukunaka A., Kurokawa Y., Teranishi F., Sekler I., Oda K., Ackland M. L., Faundez V., Hiromura M., Masuda S., Nagao M., Enomoto S., and Kambe T. (2011) Tissue nonspecific alkaline phosphatase is activated via a two-step mechanism by zinc transport complexes in the early secretory pathway. J. Biol. Chem. 286, 16363–16373 10.1074/jbc.M111.227173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antala S., Ovchinnikov S., Kamisetty H., Baker D., and Dempski R. E. (2015) Computation and functional studies provide a model for the structure of the zinc transporter hZIP4. J. Biol. Chem. 290, 17796–17805 10.1074/jbc.M114.617613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang T., Sui D., and Hu J. (2016) Structural insights of ZIP4 extracellular domain critical for optimal zinc transport. Nat. Commun. 7, 11979 10.1038/ncomms11979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang F., Kim B. E., Petris M. J., and Eide D. J. (2004) The mammalian Zip5 protein is a zinc transporter that localizes to the basolateral surface of polarized cells. J. Biol. Chem. 279, 51433–51441 10.1074/jbc.M408361200 [DOI] [PubMed] [Google Scholar]
- 31. Wang F., Kim B. E., Dufner-Beattie J., Petris M. J., Andrews G., and Eide D. J. (2004) Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum. Mol. Genet. 13, 563–571 10.1093/hmg/ddh049 [DOI] [PubMed] [Google Scholar]
- 32. Wang F., Dufner-Beattie J., Kim B. E., Petris M. J., Andrews G., and Eide D. J. (2004) Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J. Biol. Chem. 279, 24631–24639 10.1074/jbc.M400680200 [DOI] [PubMed] [Google Scholar]
- 33. Rogers E. E., Eide D. J., and Guerinot M. L. (2000) Altered selectivity in an Arabidopsis metal transporter. Proc. Natl. Acad. Sci. U.S.A. 97, 12356–12360 10.1073/pnas.210214197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gogada R., Prabhu V., Amadori M., Scott R., Hashmi S., and Chandra D. (2011) Resveratrol induces p53-independent, X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein oligomerization on mitochondria to initiate cytochrome c release and caspase activation. J. Biol. Chem. 286, 28749–28760 10.1074/jbc.M110.202440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liuzzi J. P., Guo L., Chang S. M., and Cousins R. J. (2009) Kruppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G517–523 10.1152/ajpgi.90568.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang L., and Kirschke C. P. (2007) A di-leucine sorting signal in ZIP1 (SLC39A1) mediates endocytosis of the protein. FEBS J. 274, 3986–3997 10.1111/j.1742-4658.2007.05933.x [DOI] [PubMed] [Google Scholar]
- 37. Lee J., Peña M. M., Nose Y., and Thiele D. J. (2002) Biochemical characterization of the human copper transporter Ctr1. J. Biol. Chem. 277, 4380–4387 10.1074/jbc.M104728200 [DOI] [PubMed] [Google Scholar]
- 38. Puig S., Lee J., Lau M., and Thiele D. J. (2002) Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J. Biol. Chem. 277, 26021–26030 10.1074/jbc.M202547200 [DOI] [PubMed] [Google Scholar]
- 39. McWilliam H., Li W., Uludag M., Squizzato S., Park Y. M., Buso N., Cowley A. P., and Lopez R. (2013) Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 41, W597–W600 10.1093/nar/gkt376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krogh A., Larsson B., von Heijne G., and Sonnhammer E. L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.