Abstract
Objectives
To assess the clinical and cost-effectiveness of human papillomavirus (HPV) primary screening triage with p16/Ki-67 dual stain cytology compared to cytology.
Methods
We conducted an Excel®-based budget impact model to estimate the preinvasive and invasive cervical cancer cases identified, mortality rate, direct medical costs, quality-adjusted life years (QALYs) and the incremental cost-effectiveness analysis of two strategies from the healthcare payer perspective. The study population is a cohort of women 30–65 years of age presenting for cervical screening.
Results
HPV primary screening triage with p16/Ki-67 dual stain showed higher sensitivity without losing specificity compared to conventional Pap smear. The improving the screening performance leads to decrease the prevalence of precancerous lesion, annual incidence and mortality of cervical cancer. The incidence of cervical cancer case detected by new algorithm compared with conventional method were 31,607 and 38,927, respectively. In addition, the new algorithm was more effective and more costly (average QALY 24.03, annual cost $13,262,693) than conventional cytology (average QALY 23.98, annual cost $7,713,251). The incremental cost-effective ratio (ICER) per QALY gained was $1,395. The sensitivity analysis showed if the cost of cytology and HPV test increased three times, the ICER would fall to $303/QALY gained and increased to $4,970/QALY gained, respectively.
Conclusion
Our model results suggest that screening by use of HPV genotyping test as a primary screening test combined with dual stain cytology as the triage of HPV positive women in Thai population 30–65 years old is expected to be more cost-effective than conventional Pap cytology.
Keywords: Cervical Cancer, Cost-Effectiveness Analysis, Cytology, Biomarkers, Cancer Screening, Human Papillomavirus DNA Tests
INTRODUCTION
Cervical cancer remains one of the most common cancers among Thai women. Even the age-standardized incidence rate (ASR) continue decreasing, it is still high (17.8/100,000) [1]. Nowadays, a comprehensive cervical cancer screening in Thailand includes two types; a cytology-based screening, and co-testing or combination of cytology plus human papillomavirus (HPV) testing. Thai government aims to reduce the ASR through HPV vaccination in combination with more effective screening strategy with HPV DNA testing. The latest update data regarding the cervical cancer screening in Thailand reported that the most cost-effectiveness strategy is high-risk human papillomavirus (hrHPV) testing. Compare with screening by cytology strategy, screening with hrHPV testing strategy could not only decrease the cost, but also detect more cases of cervical intraepithelial neoplasia 2 or higher (CIN2+) [2]. The results of this study support the full-scale implementation of HPV testing as primary cervical cancer screening in Thailand. Regarding the HPV positive women in HPV genotype algorithm form that study, the women positive for oncogenic HPV16/18 direct to colposcopy, with reflex cytological triage for women with other oncogenic types and direct referral for those in this group with high-grade cytological findings. The result of the Primary ASC-US LSIL Marker Study (PALMS) revealed that the p16/Ki-67 dual stain cytology combines superior sensitivity and non-inferior specificity over Pap cytology for detecting CIN2+ [3]. Different triage strategies involving p16/Ki-67 dual staining were evaluated in several studies [4,5,6,7]. Thomas et al assessed the performance of p16/Ki-67 dual stain cytology for triaging HPV-positive women undergoing primary HPV screening. The sensitivity, positive predictive value (PPV), and negative predictive value (NPV) were higher for dual staining than Pap cytology triaging HPV positive women with p16/Ki-67 dual stain cytology [4]. We assumed that using dual stain cytology instead of conventional pap smear for triaging other oncologic type HPV positive women would increase the detection rate of the precancerous lesion (CIN2/3). The objective of this study is to evaluate the clinical and cost-effectiveness of HPV primary screening triage with p16/Ki-67 dual stain cytology compared to primary conventional cytology algorithms from a payer perspective (Thai government).
MATERIALS AND METHODS
1. Modeling approach
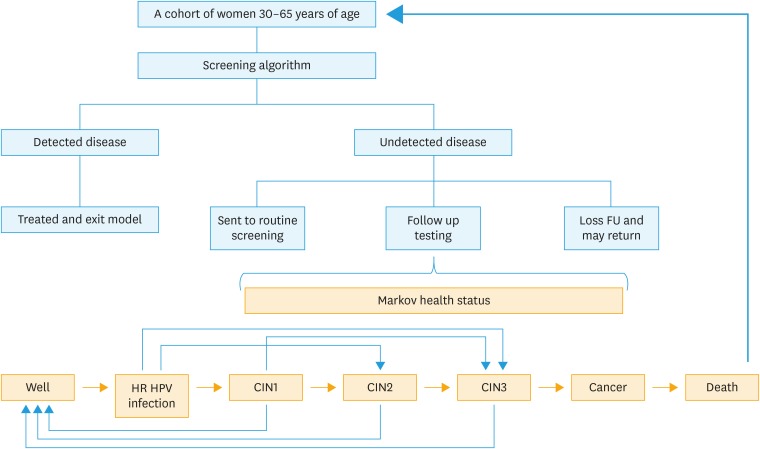
Excel-based budget impact model was constructed based on a Markov decision-analytic model in order to estimate the number of accumulated cases of CIN2, CIN3, cervical cancer and budget impact of each screening program. The Markov model was created based on the natural history of disease, including persistent high-risk HPV infection, CIN (low grade, high grade), invasive cervical cancer (ICC) and death. All women enter to screening model at the same time and exit from the model when they are diagnosed with CIN2, CIN3 or ICC. For women with undetected disease (either CIN or ICC) in the first round, they will be sent to routine screening or follow up testing. Some women may be lost to follow up and return unscheduled. The disease may regress, persist or progress spontaneously over time. Natural history and epidemiological data of HPV infection and cancer are based on published literature referenced in the model (Table 1). A model of the patient flow through a cervical cancer screening was showed in Fig. 1. The model considers 2 hypothetical cohorts, comparing costs and outcomes of a cohort screened with a new proposed algorithm (HPV primary screening triage with p16/Ki-67 dual stain cytology) with a control cohort of a conventional pap smear. Screening with the proposed algorithm is assumed to increase the detection rate of the precancerous lesion (CIN2/3) which leads to decrease the prevalence of cervical cancer, improve life expectancy and quality of life (QoL). The primary endpoint for the analysis was the incremental cost-effective ratio (ICER) per quality-adjusted life year (QALY) gained. ICER was calculated by additional annual cost divided by average QALY gained between two strategies.
Table 1. Data of HPV infection and cancer based on published reference.
| Clinical parameters | Input value | |||
|---|---|---|---|---|
| The performance of screening test [24,25] | ||||
| Cytology (threshold = ASCUS) | ||||
| Sensitivity of cytology for CIN2 | 53.20% | |||
| Sensitivity of cytology for CIN3 | 57.70% | |||
| Sensitivity of cytology for ICC | 57.70% | |||
| Specificity of cytology | 73.40% | |||
| HPV testing | ||||
| Sensitivity of pooled hrHPV testing for CIN2 | 86.40% | |||
| Sensitivity of pooled hrHPV testing for CIN3 | 89.90% | |||
| Sensitivity of pooled hrHPV testing for ICC | 89.90% | |||
| Specificity of pooled hrHPV testing | 62.70% | |||
| Sensitivity of genotyping 16/18 for CIN2 | 43.60% | |||
| Sensitivity of genotyping 16/18 for CIN3 | 53.40% | |||
| Sensitivity of genotyping 16/18 for ICC | 59.20% | |||
| Specificity of genotyping 16/18 | 91.90% | |||
| Dual staining (pooled HPV triage) | ||||
| Sensitivity for CIN2 | 86.80% | |||
| Sensitivity for CIN3 | 89.80% | |||
| Specificity for CIN2+ | 71.40% | |||
| Sensitivity for ICC | 93.80% | |||
| Epidemiology data [11,24,26,27,28,29,30,31] | ||||
| Prevalence of hrHPV | 5.6% | |||
| Prevalence of HPV16 and 18 | 1.7% | |||
| Prevalence of CIN1 | 0.6% | |||
| Prevalence of CIN2 | 0.3% | |||
| Prevalence of CIN3 | 0.8% | |||
| Prevalence of invasive cervical cancer | 0.075% | |||
| % of HSIL+ population that is HPV+ | 88.4% | |||
| % of LSIL population that is HPV+ | 61.5% | |||
| % of ASCUS population that is HPV+ | 21.4% | |||
| % CIN1 that are hrHPV 16/18 | 13.6% | |||
| % CIN2 that are hrHPV 16/18 | 23.1% | |||
| % CIN3 that are hrHPV 16/18 | 50.3% | |||
| % of ICC that are hrHPV 16/18 | 75.0% | |||
| General population annual death rate | 0.800% | |||
| Natural history parameters | ||||
| Progression [17,26,32,33,34,35,36,37,38,39] | ||||
| Well to hrHPV infection | 3.20% | |||
| Transformation from hrHPV (12 types) | ||||
| to CIN1 | 9.10% | |||
| to CIN2 | 0.10% | |||
| to CIN3 | 0.10% | |||
| Transformation from hrHPV 16/18 | ||||
| to CIN1 | 7.30% | |||
| to CIN2 | 2.20% | |||
| to CIN3 | 2.00% | |||
| Progression from CIN1 | ||||
| to CIN2 | 3.10% | |||
| to CIN3 | 0.90% | |||
| Progression from CIN2 (base case assumes CIN2 does not progress directly to ICC) | ||||
| to CIN3 | 4.20% | |||
| to ICC | 0.00% | |||
| CIN3 to ICC | 4.50% | |||
| Annual mortality rate for cervical cancer | 8.30% | |||
| Regression [32,35,39,40] | ||||
| Regression from hrHPV (12 types) to.. | ||||
| with NORMAL smear to well | 58.60% | |||
| with BORDERLINE/MILD smear to well | 45.60% | |||
| Regression from hrHPV 16/18 to.. | ||||
| with NORMAL smear to well | 43.80% | |||
| with BORDERLINE/MILD smear to well | 21.80% | |||
| Regression from CIN1 | ||||
| to well | 21.20% | |||
| to hrHPV | 2.40% | |||
| Regression from CIN2 | ||||
| to well | 9.40% | |||
| to CIN1 | 9.40% | |||
| Regression from CIN3 | ||||
| to well | 3.80% | |||
| to CIN1 | 1.60% | |||
ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; hrHPV, high-risk human papillomavirus; ICC, invasive cervical cancer.
Fig. 1. A model of the patient flow through a cervical cancer screening.
CIN, cervical intraepithelial neoplasia; FU, follow up; HPV, human papillomavirus.
2. Study population and time horizon
The study population is a cohort of women 30–65 years of age presenting for cervical screening. According to our estimations, the total population in Thailand (in 2015) is 67,959,360 [8]. Approximately 26.6% of those are women aged 30–65 years and only 50% attend to screening program [9,10]. After excluding ineligible individuals, 10.2% pregnant women and 12% women with HIV or hysterectomized women, the total number of the population eligible for screening is 7,953,963 [11]. The age of screening population and interval to rescreening were based on the current screening program of National Health Security Office and Ministry of Public Health of Thailand and also aligned with our previous studies [2,12].
3. Screening scenarios
The current practice in Thailand, conventional cytology method, provided the baseline reference for comparison with a new comparative practice, HPV DNA test with genotyping 16&18 triage with p16/Ki-67.
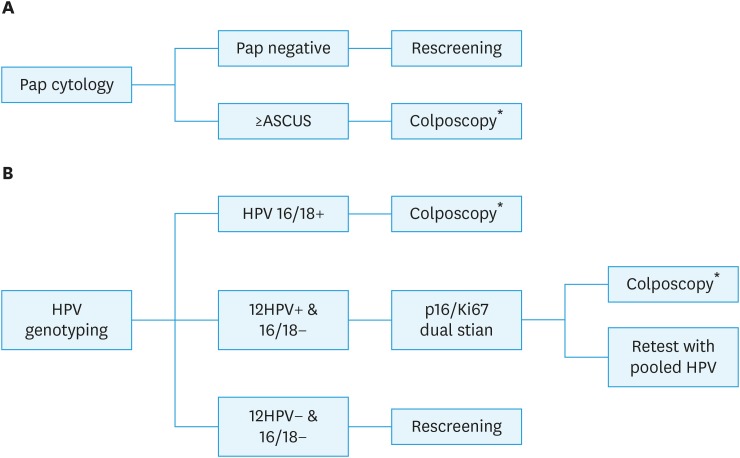
1. Cytology: Screening by a cytology-based program using Papanicolaou standard cytology followed by colposcopy of the result is atypical squamous cells of undetermined significance (ASCUS) or worse. The women with negative cytology return to screening in 5 years (Fig. 2A).
2. HPV/dual stain: HPV 16/18 genotyping is used as primary screening then refer to colposcopy if the result is positive for HPV 16 or 18. The dual staining was performed in cases of other 12 high risks HPV positive and those with positive result undergo colposcopy. Women with negative result of HPV genotyping return to screening in 5 years (Fig. 2B).
Fig. 2. Screening model. (A) Cytology: screening with conventional cytology. (B) HPV/dual stain: HPV DNA test with genotyping 16&18 plus triage with p16/Ki-67 dual stain.
ASCUS, atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ICC, invasive cervical cancer.
*Women with negative colposcopy return to routine screening, women with CIN or ICC were referred to treatment.
Compliance, treatment, and follow up protocol
The proportions of attendance at re-testing for management of both algorithms was 61.9%. We used the same compliance rate for management of both algorithms, thus it did not affect the result. The model assumed that all CIN1 cases had followed up visits without treatment, all CIN3 cases and 50% of CIN2 cases received treatment. Women with CIN1 or posttreatment CIN2+ returned to follow up every 6 months. A number of negative follow up visits before returning to routine screening population was 2, 4 times for CIN1 and CIN2-ICC woman respectively [13]. The recurrent rate after treatment for CIN2, CIN3 was assumed to be 4% and for ICC was assumed to be 20% [14]. For women treated with no recurrence within one routine screening cycle, they would re-enter to next screening interval or may loss follow up and return unscheduled. For women treated with recurrence, they would stay in the current status or progress to more severe states in the model.
4. Model parameters and assumptions
Clinical input
All model parameters were shown in Table 1. Demographic data were taken from national data for Thailand [15]. The screening performance inputs were taken from the Addressing THE Need for Advanced HPV Diagnostics HPV: high-risk Human Papillomavirus (ATHENA) trial and PALMS trial [3,16]. This model did not take into account of HPV vaccination. Colposcopy was considered to be a gold standard for diagnosis of CIN or ICC with 100% sensitivity and specificity. Disease progression and the proportion of fast-growing cancers were assumed not to be age-specific. The risk of progression and regression was assumed to be constant over time. Average probabilities of progression and regression of CIN were stratified by HPV status and showed in Table 1. The QoL or burden of disease was presented by the quality-adjusted life year or QALY. The QALY method could estimate the number of years lived and the QoL during those years that can be attributed to an intervention. Each year in perfect health was assigned the value of 1.0 down to a value of 0.0 for being dead. The QoL weight at the stage of ICC was the weighted average of cervical cancer at different stages (localized cervical cancer was 0.76; regional cervical cancer was 0.67, and distant cervical cancer is 0.48) (Supplementary Table 1) [17,18].
Cost input
The total direct medical costs were calculated from the cost of screening, diagnosis and treatment cost in provider perspective (Table 2). Screening costs of cytology including lab fee, profession fee, diagnostic costs and treatment cost for CIN and ICC were derived from our previous studies [2,19] which based on the cost of Center of Health Assurance at King Chulalongkorn Memorial Hospital. Cost of HPV DNA test and p16/Ki-67 were obtained from Roche Diagnostic (Thailand). As the treatment options for ICC depend on the International Federation of Gynecology and Obstetrics (FIGO) staging system, the cost of treatment was the weighted average of cervical cancer at different stages. Primary surgery is the treatment option for FIGO stage I to IIA cervical cancer. Concurrent chemotherapy and radiotherapy is the treatment of stage IIB to IVA cervical cancer. All cost and clinical input were discounted at an annual rate 3.5% [20].
Table 2. Details of total direct medical costs.
| Cost parameters | Input value (USD*) | ||
|---|---|---|---|
| Screening costs [41] | |||
| Office visit (routine/repeat screening) | 2.00 | ||
| Cytology test (lab fee) | 5.30 | ||
| Cytology test (professional fee) | 3.00 | ||
| HPV DNA test | 17.00 | ||
| P16/Ki-67 Dual staining | 35.00 | ||
| Diagnosis costs [41] | |||
| Office visit (diagnostic follow-up) | 12.86 | ||
| Colposcopy plus biopsy | 21.42 | ||
| CINtec® p16 Histology | 25.37 | ||
| Treatment costs [41,42,43] | |||
| Treatment for CIN2/CIN3 | 1,292.00 | ||
| Treatment for ICC† | 7,403.00 | ||
| • Stage IA1 | 1,206.29 | ||
| • Stage IA2–IIA | 2,904.94 | ||
| • Stage IIB–IVA | 163,334.63 | ||
| • Stage IVB | 9,168.74 | ||
| End of life cancer treatment cost | 10,019.00 | ||
| Discounting rate [20] | |||
| Discount rate for cost | 0.035 | ||
| Discount rate for health outcomes | 0.035 | ||
CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ICC, invasive cervical cancer.
*The currency used was US dollar (US Dollar exchange rate on May 3,2018; 1 USD = 35 THB); †The treatment for invasive cervical cancer cost was the weighted average of cervical cancer at different stages (stage I, 0.37; stage II, 0.19; stage III,0.33; and stage IV, 0.11) [43].
5. Sensitivity analysis
We performed a one-way sensitivity analysis to estimate the impact of uncertainty in different parameters of cost inputs. Because of the skewness in cost distribution, the ranges of costs were varied 10% below and 3 times above the base-case estimation.
RESULTS
1. Base case analysis
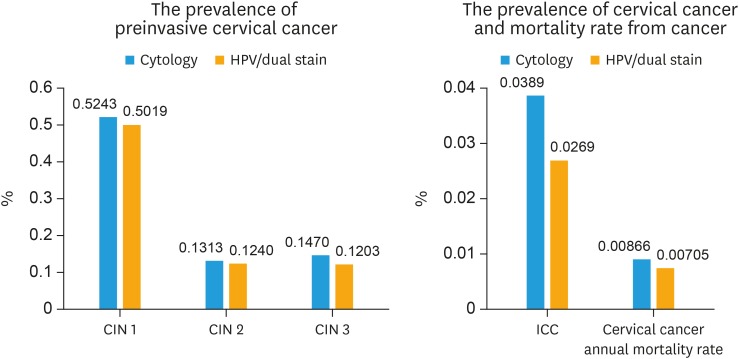
The screening performance, costs, average QALY and cost-effectiveness results based on a time horizon of 10 years were showed in Table 3. A proposed algorithm with HPV test as primary screening combined with p16/Ki-67 dual stain cytology showed better performance compared to Pap cytology by increasing the sensitivity by 48% (82.65% vs. 55.85%, respectively) without losing specificity (96.47 vs. 95.48%, respectively). This strategy was also lower the false positive by 22% results in less number of patients referred to colposcopy. Due to the gain in accuracy, the higher detection rate of precancerous cases leads to higher number of treated cases and reduce the number of cancer cases detected by 18.8%. Considering only the cost of treatment for invasive cervical cancer, the avoided treatment cost of the benefit of programmed as a consequence of reduced use of healthcare services is 54,189,960 USD (7320 cases × 7,403 USD). Moreover, the long-term outcome of the model showed that using HPV testing with dual stain cytology could decrease the prevalence of precancerous lesion (CIN2, CIN3), annual incidence and mortality of cervical cancer (Fig. 3). Under base case assumptions, the total and annual cost of the HPV testing with dual stain cytology model costed up to 71% more than cytology method. (total cost: $1,326,269,261 vs. $771,325,070, annual cost: $13,262,693 vs $7,713,251). The most influential direct cost component was the cost of screening (Supplementary Fig. 1). Using HPV testing with dual stain cytology for primary screening cervical cancer provided a more effective option at an ICER of $1,395 per QALY.
Table 3. Screening performance, cost, average QALY and ICER per QALY gained.
| Variables | Cytology | HPV/dual stain | |||
|---|---|---|---|---|---|
| Screening performance based on colposcopy population | |||||
| Number of screening cycles | 20 | 20 | |||
| Total colposcopy population (per screening cycle) | 270,487 | 220,535 | −18.5% | ||
| False positive (per screening cycle) | 249,800 | 194,562 | −22.1% | ||
| False negative (per screening cycle) | 16,350 | 5,452 | −66.7% | ||
| Sensitivity (≥CIN2) | 55.85% | 82.65% | 48.0% | ||
| Specificity (≥CIN2) | 95.48% | 96.47% | 1.0% | ||
| Screening performance and total number of cancer/precancer cases detected | |||||
| Screening performance (%) | |||||
| Cervical cancer detected | 57.7% | 88.9% | 54.1% | ||
| CIN3 detected | 57.7% | 85.2% | 47.7% | ||
| CIN2 detected | 53.2% | 79.2% | 49.0% | ||
| Total number of cancer/precancer cases detected | |||||
| Cervical cancer detected | 38,927 | 31,607 | −18.81% | ||
| CIN3 Detected | 213,218 | 257,188 | 20.62% | ||
| CIN2 Detected | 161,582 | 230,669 | 42.76% | ||
| Total annual cost, average QALY and ICER per QALY gained | |||||
| Total cost | $771,325,070 | $1,326,269,261 | |||
| Annual cost | $7,713,251 | $13,262,693 | |||
| Per person per year (over total screening population) | $1 | $2 | |||
| Per member per year (over total population) | $0 | $0 | |||
| Average QALY | 23.98 | 24.03 | |||
| ICER per QALY gained | - | $1,395 | |||
CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Fig. 3. The prevalence of preinvasive cervical cancer, cervical cancer, and mortality rate from cancer.
CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus.
2. Sensitivity analysis
One-way sensitivity analysis for ICER was performed by changing the parameter of cost inputs. The values for sensitivity analysis and results were presented as tornado diagram (Supplementary Table 2, Supplementary Fig. 2). The analysis showed that the costs of HPV and cytology had the greatest impact on ICER. The ICER fell to $303/QALY gained at a cytology cost of $15.9 and increased to $4,970/QALY gained at a HPV testing cost of $51. No other parameters changed the ICER by more than 25%. All of the variables had no impact on model conclusions.
DISCUSSION
The superior accuracy of HPV primary testing over Pap screening is well established [21]. Our earlier cost-effectiveness study also supported full scale implementation of HPV testing as a primary cervical cancer screening in Thailand [2]. In this study, we found that using dual-stained cytology as triage part of HPV algorithm could improve screening performance. The gaining in sensitivity without decreasing specificity is consistent with the results of Uijterwaal confirming that dual stain cytology is suitable for triage women with HPV positive with normal cytology to colposcopy [5]. Wentzensen et al. [6] compared the detection rate of CIN2+ cases in HPV positive women with different triage strategies; between PAP smear and dual stain cytology. The result revealed that the dual stain had similar sensitivity and statistically higher specificity compared with cytology. As a result of improvement in diagnostic accuracy for CIN2+ of HPV screening with dual stain triage algorithm, the number of women with precancerous detected and treated in the earlier round was higher. The prevalence and mortality rate of cancer case is lower. The number of women who return at next screening interval in this strategy might be more than the conventional cytology strategy. Subsequently, the cost of screening, treatment and follow up might be increased. The diagnostic cost calculated from the cost of colposcopy plus biopsy was slightly fewer than cytology method because of less patients referred to colposcopy. In addition, the new algorithm consisted of multiple steps and used more expensive tests. Therefore, the screening cost was much higher than other costs and had the most impact on the total cost.
The HPV and dual stain model was more effective and more costly than conventional cytology method with ICERs was 1,395 per QALY. All of the results including clinical outcome, QoL and budget impact are in line with cost-effectiveness analysis study in Belgium, conducted by Tjalma et al. [22]. The author compared the 2 strategies, primary cytology (the standard of care in Belgium) and the HPV primary screening and triage with dual stain cytology, same as our study. The result showed the latter strategy could decrease the prevalence of CIN and cervical cancer significantly and reduce the screening budget by 21%, resulting in saving 5.3 million euro a year in Belgium. The ICERs per QALY gained for the groups age 25–65 years and 30–65 years were -260 and -302 euro, respectively. However, the population cohort, time horizon, demographic data, cost data, and gross domestic product (GDP), are different. The population in model of Tjalma's study [22] has three different age groups for cervical cancer screening: women between ages 20–24, women between ages 25–65 and women between ages 30–65. The screening intervals were 3 years for primary cytology strategy and 5 years for HPV primary with reflex dual stain cytology.
Due to uncertain cost data, the sensitivity analysis was performed and found that the cost of cytology and HPV testing had an impact to ICER result. If HPV test cost was lower than base case 10%, it would decrease ICER by 12.7%. On the other hand, the ICER might rise to $4,970 per QALY gained if the cost of HPV test increased at least three times. Nevertheless, it was still more cost-effective than cytology method. The effect of cost variation in the HPV testing was similar to the previous study compared between cytology and HPV testing with cytology triage in Thailand [2]. In this study, the cost of HPV primary screening and dual stain does not play as the major parameter, even the cost of HPV test increase 3 times. HPV screening and dual stain triage still showed benefit over the cytology. We assumed that an ICER of less than three times the per capita GDP would be considered cost effective (current per capita GDP of Thailand $5,901) [23].
As far as we know, this is the first study using p16/Ki-67 dual stain cytology as triage part of HPV primary screening in Asia. We reported both intermediate and long-term clinical outcome, budget impact and cost-effectiveness data. Most of the data input derived from studies conducted in our country or in the setting that were similar to our practice. The results of study may differ by nations. However, the sensitivity analysis was conducted to display the effect of result due to variation in the cost of the screening tests. There are some limitations that need to be discussed further. This model did not take into account the impact of HPV vaccination which could affect the accuracy of HPV testing and prevalence of patients with precancerous lesions. According to the appropriate cervical cancer screening among vaccinated women, HPV test revealed more accurate than cytology [19]. Then the ICER would probably be decrease. We assumed that the rate of cervical lesion regression or progression were the same for all age groups and the treatment costs of cervical cancer in this model were the average costs across all stage. In the real practice, patients with advanced stage cervical cancer received concurrent chemoradiation which its cost is more expensive than early stage cancer. It is reasonable to assume that using difference cost would not change the result or may reduce ICER due to early detection cancer case and decreasing advance stage in the arm of new strategy (HPV/dual stain triage).
To find the best strategy, the future studies include new marker or triage with different tests are needed and the impact of HPV vaccination should be considered in the model. Moreover, our study was designed to focus on only direct medical cost this time. The effect of non-direct medical cost of social viewpoint is an important and should be included for future research.
This result is in line with our earlier study on health economic model with Thai context, primary screening with HPV-DNA (with partial genotyping 16&18 and 12 other high-risk HPV) demonstrated cost-effective compare to cytology screening. The p16/Ki-67 dual stain cytology could improve screening performance as a part of HPV algorithm. The new proposed HPV primary strategy allows early detection of cervical precancerous and cancer cases, reduction in mortality and lower treatment costs. We presented new information that should be considered to future national screening policy.
ACKNOWLEDGMENTS
The authors acknowledge support from Roche Diagnostics (Thailand) for the development of this article.
Footnotes
Presentation: This study has been presented in European Research Organisation on Genital Infection and Neoplasia (EUROGIN 2017) Congress during Oct 8–11, 2017 at Amsterdam RAI Exhibition and Convention Centre, Amsterdam, Noord Holland, Netherlands.
Funding: Funding for development of this article was partially provided by Roche Diagnostics (Thailand). The views expressed in this article are those of the authors and are not endorsed by the sponsor.
Conflict of Interest: A grant for this study was partially provided by Roche Diagnostics (Thailand). The views expressed in this article are those of the authors and are not endorsed by the sponsor.
- Conceptualization: T.W., H.P.
- Data curation: T.W., K.N., T.T., H.P.
- Formal analysis: T.W., T.T., H.P.
- Funding acquisition: T.W.
- Investigation: T.W., K.N., T.T.
- Methodology: T.W., H.P.
- Project administration: T.W.
- Resources: T.W., K.N.
- Software: T.W., T.T., H.P.
- Supervision: T.W., K.N., H.P.
- Validation: T.W., T.T., H.P.
- Visualization: T.W., H.P.
- Writing - original draft: T.W.
- Writing - review & editing: T.W., K.N., T.T., H.P.
SUPPLEMENTARY MATERIALS
Sensitivity analysis
Comparison of total cervical cancer screening program cost (including screening, diagnosis, treatment, and follow-up costs.
Tornado diagram: ICER comparator practice vs. current practice.
References
- 1.Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, et al. Human papillomavirus and related diseases in the world. Summary report [Internet] Barcelona: ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre); 2017. Jul 27, [cited 2018 Jan 20]. Available from: http://www.hpvcentre.net/statistics/reports/XWX.pdf. [Google Scholar]
- 2.Termrungruanglert W, Khemapech N, Tantitamit T, Sangrajrang S, Havanond P, Laowahutanont P. Cost-effectiveness analysis study of HPV testing as a primary cervical cancer screening in Thailand. Gynecol Oncol Rep. 2017;22:58–63. doi: 10.1016/j.gore.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikenberg H, Bergeron C, Schmidt D, Griesser H, Alameda F, Angeloni C, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105:1550–1557. doi: 10.1093/jnci/djt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright TC, Jr, Behrens CM, Ranger-Moore J, Rehm S, Sharma A, Stoler MH, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144:51–56. doi: 10.1016/j.ygyno.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Uijterwaal MH, Polman NJ, Witte BI, van Kemenade FJ, Rijkaart D, Berkhof J, et al. Triaging HPV-positive women with normal cytology by p16/Ki-67 dual-stained cytology testing: baseline and longitudinal data. Int J Cancer. 2015;136:2361–2368. doi: 10.1002/ijc.29290. [DOI] [PubMed] [Google Scholar]
- 6.Wentzensen N, Fetterman B, Castle PE, Schiffman M, Wood SN, Stiemerling E, et al. p16/Ki-67 dual stain cytology for detection of cervical precancer in HPV-positive women. J Natl Cancer Inst. 2015;107:djv257. doi: 10.1093/jnci/djv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovestad IT, Dalen I, Hansen E, Loge JL, Dybdahl BM, Dirdal MB, et al. Clinical value of fully automated p16/Ki-67 dual staining in the triage of HPV-positive women in the Norwegian Cervical Cancer Screening Program. Cancer Cytopathol. 2017;125:283–291. doi: 10.1002/cncy.21807. [DOI] [PubMed] [Google Scholar]
- 8.World Bank Group. Population, total 2015 [Internet] Washington, D.C.: World Bank Group; c2018. [cited 2018 Jan 8]. Available from: http://data.worldbank.org/indicator/SP.POP.TOTL?view=chart. [Google Scholar]
- 9.Ministry of Public Health. Cervical cancer screening rate in women aged 30–60 years [Internet] Muang Nonthaburi: Ministry of Public Health; c2018. [cited 2018 Jan 20]. Available from: https://hdcservice.moph.go.th/hdc/reports/report.php?source=pformated/format1.php&cat_id=6966b0664b89805a484d7ac96c6edc48&id=4eab25b045dc0a9453d85c98dc2fdef0. [Google Scholar]
- 10.National Statistical Office; United Nations Children's Fund; Ministry of Public Health; National Health Security Office; Thai Health Promotion Foundation; International Health Policy Program. Thailand monitoring the situation of children and women: multiple indicator cluster survey 2012 [Internet] Laksi Bangkok: National Statistical Office; c2013. [cited 2018 Jan 20]. Available from: http://web.nso.go.th/en/survey/monitoring/data/monitoring_full_report_2012.pdf. [Google Scholar]
- 11.Schneider A, Hoyer H, Lotz B, Leistritza S, Kühne-Heid R, Nindl I, et al. Screening for high-grade cervical intra-epithelial neoplasia and cancer by testing for high-risk HPV, routine cytology or colposcopy. Int J Cancer. 2000;89:529–534. [PubMed] [Google Scholar]
- 12.Tantitamit T, Termrungruanglert W, Khemapech N, Havanond P. A model approach for assessing the benefits of HPV testing against cytology in screening for cervical cancer precursors in Thailand. Asian Pac J Cancer Prev. 2017;18:1271–1275. doi: 10.22034/APJCP.2017.18.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedlander M, Grogan M U.S. Preventative Services Task Force. Guidelines for the treatment of recurrent and metastatic cervical cancer. Oncologist. 2002;7:342–347. [PubMed] [Google Scholar]
- 14.Mandelblatt JS, Lawrence WF, Womack SM, Jacobson D, Yi B, Hwang YT, et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA. 2002;287:2372–2381. doi: 10.1001/jama.287.18.2372. [DOI] [PubMed] [Google Scholar]
- 15.United Nations, Department of Economic and Social Affairs, Population Division (UNPD) World population prospects: the 2015 revision, key findings and advance tables. New York, NY: United Nations; 2015. [Google Scholar]
- 16.Wright TC, Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL, et al. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol. 2011;136:578–586. doi: 10.1309/AJCPTUS5EXAS6DKZ. [DOI] [PubMed] [Google Scholar]
- 17.Kulasingam SL, Benard S, Barnabas RV, Largeron N, Myers ER. Adding a quadrivalent human papillomavirus vaccine to the UK cervical cancer screening programme: a cost-effectiveness analysis. Cost Eff Resour Alloc. 2008;6:4. doi: 10.1186/1478-7547-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myers ER, Green S, Lipkus I. Patient preferences for health states related to HPV infection: visual analog scales vs time trade-off elicitation; Proceedings of 21st International Papillomavirus Conference; 2004 Feb 20–27; Mexico City. International Papillomavirus Society; 2004. Abstract 542 [Google Scholar]
- 19.Termrungruanglert W, Khemapech N, Havanond P, Pillsbury M, Shcheprov A, Numuang K, et al. Impact of vaccination: Health impact and cost-effectiveness to make informed policy decision on the introduction of human papillomavirus (HPV) vaccine to the national immunization Program (Nip) in Thailand. Value Health. 2014;17:A737. doi: 10.1016/j.jval.2014.08.117. [DOI] [PubMed] [Google Scholar]
- 20.National Institute for Health and Care Excellence. NICE process and methods guides. Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence; 2013. [Google Scholar]
- 21.Ogilvie GS, van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: the HPV FOCAL randomized clinical trial. JAMA. 2018;320:43–52. doi: 10.1001/jama.2018.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tjalma WA, Kim E, Vandeweyer K. The impact on women's health and the cervical cancer screening budget of primary HPV screening with dual-stain cytology triage in Belgium. Eur J Obstet Gynecol Reprod Biol. 2017;212:171–181. doi: 10.1016/j.ejogrb.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Macroeconomic and health: investing in health for economic development: report for the commission on macroeconomics and health. Geneva: World Health Organization; 2001. [Google Scholar]
- 24.Wright TC, Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206:46.e1–46.11. doi: 10.1016/j.ajog.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Cox JT, Castle PE, Behrens CM, Sharma A, Wright TC, Jr, Cuzick J Athena HPV Study Group. Comparison of cervical cancer screening strategies incorporating different combinations of cytology, HPV testing, and genotyping for HPV 16/18: results from the ATHENA HPV study. Am J Obstet Gynecol. 2013;208:184.e1–184.11. doi: 10.1016/j.ajog.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 26.Luyten A, Scherbring S, Reinecke-Lüthge A, Braun BE, Pietralla M, Theiler K, et al. Risk-adapted primary HPV cervical cancer screening project in Wolfsburg, Germany--experience over 3 years. J Clin Virol. 2009;46(Suppl 3):S5–S10. doi: 10.1016/S1386-6532(09)70294-X. [DOI] [PubMed] [Google Scholar]
- 27.Petry KU, Menton S, Menton M, van Loenen-Frosch F, de Carvalho Gomes H, Holz B, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer. 2003;88:1570–1577. doi: 10.1038/sj.bjc.6600918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klug SJ, Hukelmann M, Hollwitz B, Düzenli N, Schopp B, Petry KU, et al. Prevalence of human papillomavirus types in women screened by cytology in Germany. J Med Virol. 2007;79:616–625. doi: 10.1002/jmv.20863. [DOI] [PubMed] [Google Scholar]
- 29.Cuzick J, Myers O, Hunt WC, Saslow D, Castle PE, Kinney W, et al. Human papillomavirus testing 2007–2012: co-testing and triage utilization and impact on subsequent clinical management. Int J Cancer. 2015;136:2854–2863. doi: 10.1002/ijc.29337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 31.World Bank Group. Death rate, crude (per 1,000 people) 2014 [Internet] Washington, D.C.: World Bank Group; c2018. [cited 2018 Jan 20]. Available from: http://data.worldbank.org/indicator/SP.DYN.CDRT.IN. [Google Scholar]
- 32.Chen T, Jansen L, Gondos A, Emrich K, Holleczek B, Luttmann S, et al. Survival of cervical cancer patients in Germany in the early 21st century: a period analysis by age, histology, and stage. Acta Oncol. 2012;51:915–921. doi: 10.3109/0284186X.2012.708105. [DOI] [PubMed] [Google Scholar]
- 33.Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91:252–258. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 34.Insinga RP, Dasbach EJ, Elbasha EH. Epidemiologic natural history and clinical management of human papillomavirus (HPV) disease: a critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC Infect Dis. 2009;9:119. doi: 10.1186/1471-2334-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Progression and regression of incident cervical HPV 6, 11, 16 and 18 infections in young women. Infect Agent Cancer. 2007;2:15. doi: 10.1186/1750-9378-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Insinga RP, Perez G, Wheeler CM, Koutsky LA, Garland SM, Leodolter S, et al. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiol Biomarkers Prev. 2011;20:287–296. doi: 10.1158/1055-9965.EPI-10-0791. [DOI] [PubMed] [Google Scholar]
- 37.Kataja V, Syrjänen K, Mäntyjärvi R, Väyrynen M, Syrjänen S, Saarikoski S, et al. Prospective follow-up of cervical HPV infections: life table analysis of histopathological, cytological and colposcopic data. Eur J Epidemiol. 1989;5:1–7. doi: 10.1007/BF00145037. [DOI] [PubMed] [Google Scholar]
- 38.Khan MJ, Castle PE, Lorincz AT, Wacholder S, Sherman M, Scott DR, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst. 2005;97:1072–1079. doi: 10.1093/jnci/dji187. [DOI] [PubMed] [Google Scholar]
- 39.Matsumoto K, Yasugi T, Oki A, Fujii T, Nagata C, Sekiya S, et al. IgG antibodies to HPV16, 52, 58 and 6 L1-capsids and spontaneous regression of cervical intraepithelial neoplasia. Cancer Lett. 2006;231:309–313. doi: 10.1016/j.canlet.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Bulkmans NW, Berkhof J, Bulk S, Bleeker MC, van Kemenade FJ, Rozendaal L, et al. High-risk HPV type-specific clearance rates in cervical screening. Br J Cancer. 2007;96:1419–1424. doi: 10.1038/sj.bjc.6603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Termrungruanglert W, Havanond P, Khemapech N, Lertmaharit S, Pongpanich S, Khorprasert C, et al. Cost and effectiveness evaluation of prophylactic HPV vaccine in developing countries. Value Health. 2012;15:S29–34. doi: 10.1016/j.jval.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Lier D, Jacobs P. An economic analysis of the introduction of liquid-based cytology (LBC) and human papillomavirus (HPV) testing in Alberta [Internet] Calgary: Alberta Cervical Cancer Screening Program; c2018. [cited 2018 Jan 20]. Available from: https://www.albertahealthservices.ca/findhealth/service.aspx?Id=1016155. [Google Scholar]
- 43.Termrungruanglert W, Havanond P, Khemapech N, Lertmaharit S, Pongpanich S, Jirakorbchaipong P, et al. Model for predicting the burden and cost of treatment in cervical cancer and HPV-related diseases in Thailand. Eur J Gynaecol Oncol. 2012;33:391–394. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity analysis
Comparison of total cervical cancer screening program cost (including screening, diagnosis, treatment, and follow-up costs.
Tornado diagram: ICER comparator practice vs. current practice.