Abstract
Objective
To evaluate the efficacy of combined oral medroxyprogesterone acetate (MPA)/levonorgestrel-intrauterine system (LNG-IUS) treatment and to compare the diagnostic accuracy of endometrial aspiration biopsy with dilatation & curettage (D&C) in young women with early-stage endometrial cancer (EC) who wished to preserve their fertility.
Methods
A prospective phase II multicenter study was conducted from January 2012 to January 2017. Patients with grade 1 endometrioid adenocarcinoma confined to the endometrium were treated with combined oral MPA (500 mg/day)/LNG-IUS. At 3 and 6 months of treatment, the histologic change of the endometrial tissue was assessed. The regression rate at 6 months treatment and the consistency of the histologic results between the aspiration biopsy and the D&C were evaluated.
Results
Forty-four patients were enrolled. Nine voluntarily withdrew and 35 patients completed the protocol treatment. The complete regression (CR) rate at 6 months was 37.1% (13/35). Partial response was shown in 25.7% of cases (9/35). There were no cases of progressive disease and no treatment-related complications. A comparison of the pathologic results from aspiration biopsy and D&C was carried out for 33 cases. Fifteen cases were diagnosed as “EC” by D&C. Among these, only 8 were diagnosed with EC from aspiration biopsy, yielding a diagnostic concordance of 53.3% (ĸ=0.55).
Conclusion
Combined oral MPA/LNG-IUS treatment for EC showed 37.1% of CR rate at 6 months. Considering the short treatment periods, CR rate may be much higher if the treatment continued to 9 or 12 months. So, this treatment is still a viable treatment option for young women of early-stage EC. Endometrial aspiration biopsy with the LNG-IUS in place is less accurate than D&C for follow-up evaluation of patients undergoing this treatment.
Trial Registration
ClinicalTrials.gov Identifier: NCT01594879
Keywords: Endometrial Neoplasms, Fertility Preservation, Mirena, Progestin
INTRODUCTION
Endometrial cancer (EC) is the most common gynecologic malignancy in developed countries. Although it is generally diagnosed in postmenopausal women, 3%–14% of cases occur in those younger than 40 years of age. Recently in Korea, the overall incidence of EC increased by 6.9% per year. Especially, EC in the young age group (age <30 years), who are frequently nulliparous and have a strong desire to preserve fertility at the time of their diagnosis, has increased by 11.2% per year [1,2,3].
EC in the young age group is usually well-differentiated endometrioid adenocarcinoma with infrequent myometrial invasion or lymph node metastasis. Therefore, it tends to show a favorable prognosis [4,5]. When EC is diagnosed in young patients wishing to preserve their fertility, the standard surgical treatment proceeding by hysterectomy and bilateral salpingo-oophorectomy may not be ideal. Moreover, given the increasing incidence of EC in younger patients, conservative management that preserves fertility has drawn attention and increasingly been investigated.
Current fertility-sparing treatment modalities are based on hormonal therapy, mainly with oral progestin. A number of studies have suggested that in selected patients with early-stage disease, EC can be effectively managed with oral progestin [6,7,8,9,10,11,12,13,14,15,16]. Additionally, other options have been investigated, including the use of the levonorgestrel-intrauterine system (LNG-IUS) or gonadotropin-releasing hormone [17,18,19,20,21,22].
In recent pilot and observational studies, combined medroxyprogesterone acetate (MPA) and LNG-IUS treatment in patients with stage IA (confined to endometrium), grade 1 EC showed promising results [19,22]. However, there has been no prospective trial on the effectiveness of combined oral MPA/LNG-IUS treatment. Furthermore, there is still no reliable data on the proper surveillance method during hormonal treatment of EC, especially when using the LNG-IUS. When using the LNG-IUS, the endometrial response could be evaluated by endometrial aspiration biopsy with the LNG-IUS in the uterus or by dilatation and curettage (D&C) after removal of the LNG-IUS. Notwithstanding, there has been no report on any comparison of these methods' accuracies.
Therefore, we designed a large phase II multicenter prospective study to evaluate the efficacy of combined oral MPA/LNG-IUS treatment in young women with early-stage EC who wish to preserve their fertility and to compare the diagnostic accuracy of endometrial aspiration biopsy with that of D&C in those patients.
MATERIALS AND METHODS
1. Study design
A prospective phase II multicenter study was conducted from January 2012 to January 2017. Thirteen institutions belonging to the Korean Gynecologic-Oncology Group were registered. Eligible subjects were women with histologically confirmed grade 1 endometrioid adenocarcinoma assumed to be FIGO Stage IA without myometrial invasion, who desired to preserve fertility and who were aged ≤40 years. The initial histologic diagnosis was made by D&C with the patient anesthetized. The clinical stage was assessed by transvaginal ultrasonography (TVS), abdomen-pelvic computed tomography (CT) and magnetic resonance imaging (MRI). No radiologic evidence of myometrial invasion, lymph node involvement or any extrauterine lesions were confirmed. All of the patients were fully informed of the study purposes and procedures, and their voluntary informed consent to participate (as approved by the institutional review board of each clinical trial institution) was obtained. This trial was registered at ClinicalTrials.gov (NCT01594879) and published in October 2012 [23].
2. Treatment and follow-up
Patients were treated with combined oral MPA (500 mg/day)/LNG-IUS. The follow-up schedule consisted of regular clinic review, transvaginal ultrasonography, and endometrial histological surveillance at 3-month intervals.
3. Evaluation of response
At 3 and 6 months of treatment, the histologic change of endometrial tissue was assessed via 2 methods: endometrial aspiration biopsy using a pipelle with the LNG-IUS remaining in the uterus, followed by D&C after removal of the LNG-IUS. The histologic diagnoses of the specimens were made by central pathologic review.
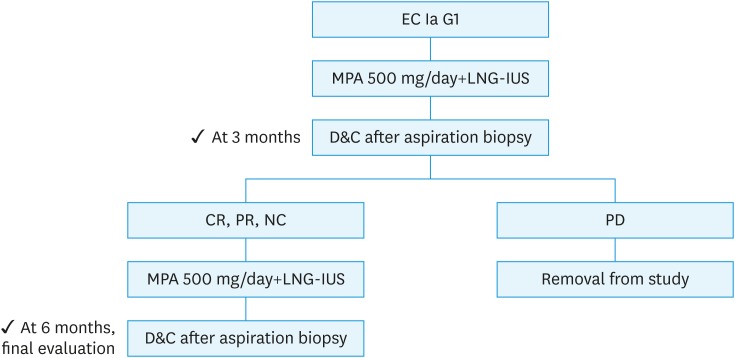
The treatment response was classified as follows: complete regression (CR), absence of any hyperplastic or cancerous lesion; partial response (PR), presence of residual hyperplasia or carcinoma with degeneration or atrophy of endometrial glands; no change (NC), presence of residual lesion without degeneration or atrophy of endometrial glands; progressive disease (PD), progression to higher-grade lesion or clinically progressive disease. At the 3-month treatment evaluation, patients with CR, PR or NC continued with the MPA (500 mg/day)/LNG-IUS treatment for an additional 3 months. If there was histological evidence of PD, the protocol treatment was stopped, and surgical treatment was suggested (Fig. 1).
Fig. 1. Study design.
CR, complete response (defined as absence of any hyperplastic or cancerous lesion); D&C, dilation and curettage; EC, endometrial carcinoma; G1, grade 1; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate; NC, no change (defined as residual lesion without degeneration or atrophy of endometrial glands); PD, progressive disease (defined as appearance of grade 2 or 3 endometrial carcinoma); PR, partial response (defined as residual lesion with degeneration and atrophy of endometrial glands).
4. Outcome measure
The primary outcome was the response rate at 6 months of treatment. This was determined by comparing the diagnosis of the follow-up D&C 6 months after MPA/LNG-IUS treatment with the initial histologic diagnosis. The secondary outcome was the consistency of the results between the endometrial aspiration biopsy and the D&C. At 3 and 6 months of treatment, we obtained endometrial tissues via the two methods noted above. To evaluate the diagnostic accuracy of the endometrial aspiration biopsy (with the LNG-IUS in the uterus) as compared with the D&C (after removal of the LNG-IUS), the histological results of the two methods were compared.
5. Sample size calculation and statistical considerations
This study was a single-arm phase II study with historical comparison. The primary endpoint was CR rate at 6 months of treatment. The historical control benchmark was a study by Ushijima et al. [12]. They had administered 600 mg of MPA and found CR in 55% of patients. With a 5% one-sided Type I error, we calculated that a total of 35 patients would provide 80% power in detecting of a 20% increase in CR rate. Assuming a 20% of dropouts or withdrawals, a total of 44 patients were accrued to the trial.
The secondary endpoint was the consistency of the results between the endometrial aspiration biopsy and the D&C. Kappa statistics were used to assess the agreement of the 2 methods. κ values <0 indicated no agreement, 0 to 0.20 slight, 0.21 to 0.40 fair, 0.41 to 0.60 moderate, 0.61 to 0.80 substantial, and 0.81 to 1 almost perfect agreement.
RESULTS
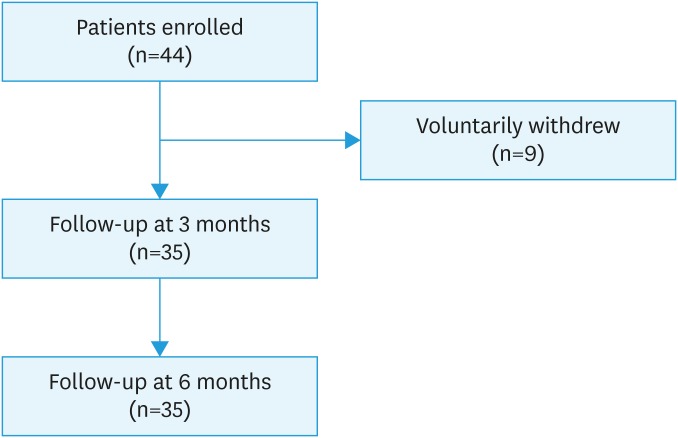
A total of 44 patients meeting the inclusion criteria were enrolled. Nine patients voluntarily withdrew and 35 patients completed the protocol treatment (Fig. 2).
Fig. 2. Flowchart of study population.
The patients' baseline characteristics are provided in Table 1. The mean age of the patients was 32.9±3.9 years (range, 27–40 years) and the mean body weight index (BMI) was 24.5±5.9 kg/m2 (range, 15.1–37.5 kg/m2). Thirty-three (94.3%) patients were nulliparous.
Table 1. Patients' characteristics (n=35).
| Characteristics | Values | |
|---|---|---|
| Age (yr) | 32.9±3.9 (27–40) | |
| Body mass index (kg/m2) | 24.5±5.9 (15.1–37.5) | |
| Parity | ||
| 0 | 33 (94.3%) | |
| 1 | 2 (5.7%) | |
Data are expressed as means±standard deviation or number.
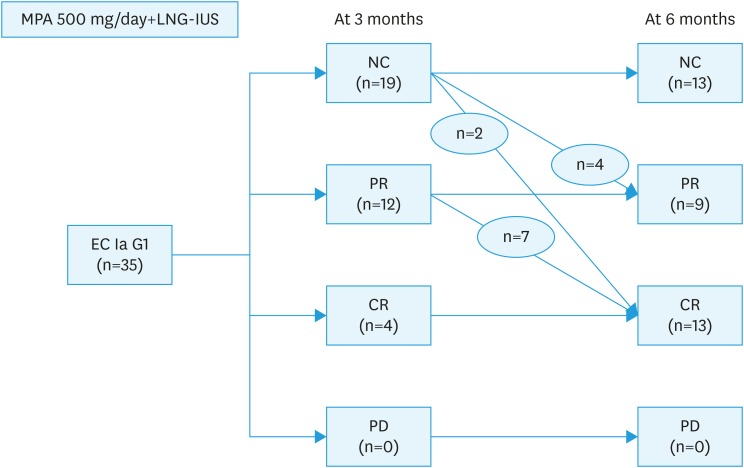
At 6 months of treatment, the CR rate was 37.1% (13/35). PR was shown in 25.7% (9/35) and NC in 37.1% (13/35) of cases. There were no cases of PD (Table 2).
Table 2. Response to combined MPA (500 mg/day)/LNG-IUS treatment (n=35).
| Response | 3 months | 6 months |
|---|---|---|
| CR | 11.4% (4/35) | 37.1% (13/35) |
| PR | 34.3% (12/35) | 25.7% (9/35) |
| NC | 54.3% (19/35) | 37.1% (13/35) |
| PD | 0% (0/35) | 0% (0/35) |
CR, complete response; LNG-IUS, levonorgestrel-intrauterine system; MPA, medroxyprogesterone acetate; NC, no change; PD, progressive disease; PR, partial response.
Of the 13 patients with CR at 6 months, 4 had already presented CR at 3 months. Seven patients with PR and 2 with NC at 3 months achieved CR after an additional 3 months of treatment (Fig. 3). Treatment generally was well tolerated, and there were no cases of treatment-related complication. There were no correlations between treatment response and clinical characteristics including age and BMI.
Fig. 3. Flowchart of treatment outcome.
CR, complete response (defined as absence of any hyperplastic or cancerous lesion); EC, endometrial carcinoma; G1, grade 1; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate; NC, no change (defined as residual lesion without degeneration or atrophy of endometrial glands); PD, progressive disease (defined as appearance of grade 2 or 3 endometrial carcinoma); PR, partial response (defined as residual lesion with degeneration and atrophy of endometrial glands).
Comparison of the pathologic results from aspiration biopsy and D&C was carried out for 33 cases. The histologic results by D&C were 15 (45.4%) with EC, 12 (36.4%) with EH and 6 (18.2%) with normal endometrium. Generally, 20 of 33 cases (60.6%) had diagnostic concordance, consisting of 4 cases potentially with normal endometrium, 8 cases with EC and 8 cases with EH. Among the 15 cases of EC on D&C, only 8 were diagnosed with EC from aspiration biopsy, yielding a diagnostic concordance of 53.3% (ĸ=0.55). Among the 9 cases of normal endometrium by aspiration biopsy, 3 were diagnosed as “EC,” and 2 as “EH” by D&C. There were 4 cases of “insufficient tissue for pathologic evaluation” histological results by aspiration biopsy. Among these 4 cases, 2 were diagnosed as “normal endometrium,” and the 2 others were diagnosed as “EH” by D&C (Table 3).
Table 3. Comparison of pathologic results from endometrial aspiration biopsy and D&C.
| D&C | No. (%) | Aspiration biopsy | No. | Concordance to D&C (%) |
|---|---|---|---|---|
| Normal | 6 (18.2) | Normal | 4 | 66.7 |
| Material insufficiency | 2 | |||
| EC | 15 (45.4) | EC | 8 | 53.3 |
| EH | 4 | |||
| Normal | 3 | |||
| EH | 12 (36.4) | EH | 8 | 66.6 |
| Normal | 2 | |||
| Material insufficiency | 2 | |||
| Total | 33 (100.0) | 60.6 |
D&C: dilatation and curettage; EC, endometrial adenocarcinoma; EH, endometrial hyperplasia.
DISCUSSION
Considering the recent rising incidence of EC in reproductive age women and their relatively good prognosis, it is imperative to provide them with an effective fertility-sparing treatment option. To date, there have been several promising results about progestin-based conservative treatment for early-stage EC, the mainstay option being treatment with oral progestin [6,7,8,9,10,11,12,13,14,15,16]. In previous retrospective studies and case series, the response rate varied widely, ranging from 57% to 78%. These large variations were due to differences in treatment protocol, including differences in drugs used, dosage, and treatment duration. In 2007, Ushijima et al. [12] reported the first results of a prospective multicenter study. They had administered 600 mg of MPA and low-dose aspirin daily for 26 weeks to 28 patients with stage I, grade 1 EC, and found CR in 55% of patients. This is relatively unsatisfactory, especially compared with the high cure rate (>93%) of surgical treatment. Therefore, a more effective treatment method with less adverse systemic effects was, and is still, sought.
In early-stage EC confined to the endometrium, LNG-IUS, which delivers a high concentration of progestin locally, can be an acceptable treatment option. According to a recent review, 17 of 37 patients with stage IA, grade 1 EC achieved CR with a LNG-IUS; the pooled complete response rate was 46% (95% confidence interval, 29%–63%) [21]. Moreover, some studies have investigated the combined use of LNG-IUS with oral progestin or gonadotropin-releasing hormone agonist to improve the treatment effect [19,20,21,22,23,24,25]. Based on a previous observational study, Kim et al. [22] reported promising results for 16 patients with stage IA, grade 1 EC who had been treated with combined oral MPA/LNG-IUS. The overall CR rate was 87.5% (14/16), and only 2 patients (14.3%) suffered recurrence.
Motivated by this result, we set out to conduct a multicenter prospective study to evaluate the efficacy of combined oral MPA/LNG-IUS treatment and a proper surveillance method. However, at 6 months follow-up, the CR rate was 37.1% (13/35), which is a somewhat disappointing outcome compared with those of previous studies. We considered that the relatively short treatment and follow-up periods could have been the culprits.
To date, the median treatment duration to CR and the total treatment duration have varied among studies, and there is no consensus on the optimal duration of treatment or when treatment failure should be determined. According to previous studies, most investigators recognize the need for a minimum of 3 months of treatment before assessment for a response [11,26]. Randall et al. reported that the median length of progestin treatment required for regression is 9 months; other studies have reported that patients achieved CR after 9–12 months of treatment [6,7,15,16]. According to a previous report of combined oral MPA/LNG-IUS treatment for early-stage EC, the median time to CR was 9.8±8.9 months (range, 3–35 months) [22]. Further, a study that investigated the efficacy of combined oral MPA/LNG-IUS treatment for patients with stage IA, grade 2 EC reported that CR was shown in 3 of the 5 patients, and that the median time to CR was 11.0±6.2 months (range, 6–18 months) [24]. In this regard, further investigation with longer treatment and follow-up periods is warranted. To the best of our knowledge, ours is the first multicenter prospective study to show the efficacy of combined oral MPA/LNG-IUS treatment in young women with early-stage EC who wish to preserve their fertility.
Another important issue that we wanted to investigate in this study is the proper surveillance method for EC patients treated with combined oral MPA/LNG-IUS: Can aspiration biopsy be as accurate as D&C as a follow-up evaluation method? The pathologic outcome at follow-up provides a basis for determining whether to continue conservative treatment of EC or not. Thus, the accuracy of the follow-up evaluation method is essential.
Although D&C is considered to be the most accurate method for diagnosis of EC, some studies have reported that aspiration biopsy seems to be as accurate as D&C [27]. However, these results were obtained for cases where there were no progestin effects on the endometrium and where the LNG-IUS was not in the uterus. Moreover, the evaluation of diagnostic accuracy focused on the initial diagnosis, not the follow-up evaluation, which can possibly be affected by hormonal treatment or LNG-IUS mechanical interference.
To date, there have been only 2 observational studies on the response of the endometrium in follow-up, particularly for EC patients treated with combined oral MPA/LNG-IUS [28,29]. In one study comparing the accuracy of aspiration biopsy (with the LNG-IUS in the uterus) with that of D&C (after LNG-IUS removal), the diagnostic concordance between the 2 examinations was only 32.1% [28]. Another study compared the accuracy of aspiration biopsy with that of D&C after LNG-IUS removal to avoid mechanical interference of LNG-IUS, and the diagnostic concordance was 39.3% [29]. That is, the diagnostic accuracy of endometrial aspiration biopsy with or without the LNG-IUS in place has been found to be very poor. Furthermore, a high prevalence of insufficient tissue for pathologic evaluation was noted (46.7%–60.7%) with endometrial aspiration biopsy.
We compared the diagnostic accuracy of endometrial aspiration biopsy with the LNG-IUS in the uterus versus D&C after removal of the LNG-IUS. Among the 15 cases of EC on D&C, only 8 were diagnosed with EC from aspiration biopsy, yielding a diagnostic concordance of 53.3% (ĸ=0.55). These findings, significantly, are consistent with those of earlier studies.
These results can be considered to indicate that aspiration biopsy failed to obtain adequate amounts of tissue for diagnosis, due to endometrial atrophy induced by high-dose oral progestin and LNG-IUS, not to mention mechanical interference from the LNG-IUS. The clinical significance of this study is that it is the first prospective multicenter study to show that D&C is more accurate than aspiration biopsy in the evaluation of treatment response for combined oral MPA/LNG-IUS treatment.
In conclusion, the combined oral MPA/LNG-IUS treatment for EC showed 37.1% of CR rate at 6 months of follow-up. Considering the present study's short treatment and follow-up periods, CR rate may be much higher if the treatment continued to 9 or 12 months. So, this treatment is still a viable treatment option for young women of early-stage EC and a long-term follow-up study will be valuable. It should also be noted that endometrial aspiration biopsy with LNG-IUS in place is less accurate than D&C after removal of LNG-IUS for follow-up evaluation in patients with combined oral MPA/LNG-IUS treatment for EC. As accurate diagnosis and response assessment are essential to successful conservative treatment of EC, D&C after removal of LNG-IUS should be implemented as a surveillance method.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.S.J.
- Data curation: S.S.J., K.S.B., B.D.S., K.J.W., N.J.H., L.M.C., L.T.S., K.S., P.J.
- Writing - original draft: K.M.K.
- Writing - review & editing: S.S.J., K.S.B., B.D.S., K.J.W., N.J.H., L.M.C., L.T.S., K.S., P.J.
References
- 1.Bogani G, Dowdy SC, Cliby WA, Ghezzi F, Rossetti D, Frigerio L, et al. Management of endometrial cancer: issues and controversies. Eur J Gynaecol Oncol. 2016;37:6–12. [PubMed] [Google Scholar]
- 2.Lim MC, Moon EK, Shin A, Jung KW, Won YJ, Seo SS, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea, 1999–2010. J Gynecol Oncol. 2013;24:298–302. doi: 10.3802/jgo.2013.24.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417–420. [PubMed] [Google Scholar]
- 4.Soliman PT, Oh JC, Schmeler KM, Sun CC, Slomovitz BM, Gershenson DM, et al. Risk factors for young premenopausal women with endometrial cancer. Obstet Gynecol. 2005;105:575–580. doi: 10.1097/01.AOG.0000154151.14516.f7. [DOI] [PubMed] [Google Scholar]
- 5.Duska LR, Garrett A, Rueda BR, Haas J, Chang Y, Fuller AF. Endometrial cancer in women 40 years old or younger. Gynecol Oncol. 2001;83:388–393. doi: 10.1006/gyno.2001.6434. [DOI] [PubMed] [Google Scholar]
- 6.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–440. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 7.Kaku T, Yoshikawa H, Tsuda H, Sakamoto A, Fukunaga M, Kuwabara Y, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- 8.Imai M, Jobo T, Sato R, Kawaguchi M, Kuramoto H. Medroxyprogesterone acetate therapy for patients with adenocarcinoma of the endometrium who wish to preserve the uterus-usefulness and limitations. Eur J Gynaecol Oncol. 2001;22:217–220. [PubMed] [Google Scholar]
- 9.Jadoul P, Donnez J. Conservative treatment may be beneficial for young women with atypical endometrial hyperplasia or endometrial adenocarcinoma. Fertil Steril. 2003;80:1315–1324. doi: 10.1016/s0015-0282(03)01183-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang CB, Wang CJ, Huang HJ, Hsueh S, Chou HH, Soong YK, et al. Fertility-preserving treatment in young patients with endometrial adenocarcinoma. Cancer. 2002;94:2192–2198. doi: 10.1002/cncr.10435. [DOI] [PubMed] [Google Scholar]
- 11.Ramirez PT, Frumovitz M, Bodurka DC, Sun CC, Levenback C. Hormonal therapy for the management of grade 1 endometrial adenocarcinoma: a literature review. Gynecol Oncol. 2004;95:133–138. doi: 10.1016/j.ygyno.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 12.Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, Saito T, et al. Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. J Clin Oncol. 2007;25:2798–2803. doi: 10.1200/JCO.2006.08.8344. [DOI] [PubMed] [Google Scholar]
- 13.Gunderson CC, Fader AN, Carson KA, Bristow RE. Oncologic and reproductive outcomes with progestin therapy in women with endometrial hyperplasia and grade 1 adenocarcinoma: a systematic review. Gynecol Oncol. 2012;125:477–482. doi: 10.1016/j.ygyno.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207:266.e1–266.12. doi: 10.1016/j.ajog.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 15.Park JY, Kim DY, Kim JH, Kim YM, Kim KR, Kim YT, et al. Long-term oncologic outcomes after fertility-sparing management using oral progestin for young women with endometrial cancer (KGOG 2002) Eur J Cancer. 2013;49:868–874. doi: 10.1016/j.ejca.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Park JY, Nam JH. Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist. 2015;20:270–278. doi: 10.1634/theoncologist.2013-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651–657. doi: 10.1067/mob.2002.122130. [DOI] [PubMed] [Google Scholar]
- 18.Dhar KK, NeedhiRajan T, Koslowski M, Woolas RP. Is levonorgestrel intrauterine system effective for treatment of early endometrial cancer? Report of four cases and review of the literature. Gynecol Oncol. 2005;97:924–927. doi: 10.1016/j.ygyno.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Kim MK, Yoon BS, Park H, Seong SJ, Chung HH, Kim JW, et al. Conservative treatment with medroxyprogesterone acetate plus levonorgestrel intrauterine system for early-stage endometrial cancer in young women: pilot study. Int J Gynecol Cancer. 2011;21:673–677. doi: 10.1111/IGC.0b013e3181fd9a06. [DOI] [PubMed] [Google Scholar]
- 20.Minig L, Franchi D, Boveri S, Casadio C, Bocciolone L, Sideri M. Progestin intrauterine device and GnRH analogue for uterus-sparing treatment of endometrial precancers and well-differentiated early endometrial carcinoma in young women. Ann Oncol. 2011;22:643–649. doi: 10.1093/annonc/mdq463. [DOI] [PubMed] [Google Scholar]
- 21.Baker J, Obermair A, Gebski V, Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125:263–270. doi: 10.1016/j.ygyno.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 22.Kim MK, Seong SJ, Kim YS, Song T, Kim ML, Yoon BS, et al. Combined medroxyprogesterone acetate/levonorgestrel-intrauterine system treatment in young women with early-stage endometrial cancer. Am J Obstet Gynecol. 2013;209:358.e1–358.e4. doi: 10.1016/j.ajog.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Kim MK, Seong SJ, Lee TS, Kim JW, Nam BH, Hong SR, et al. Treatment with medroxyprogesterone acetate plus levonorgestrel-releasing intrauterine system for early-stage endometrial cancer in young women: single-arm, prospective multicenter study: Korean Gynecologic Oncology Group Study (KGOG2009) Jpn J Clin Oncol. 2012;42:1215–1218. doi: 10.1093/jjco/hys171. [DOI] [PubMed] [Google Scholar]
- 24.Hwang JY, Kim DH, Bae HS, Kim ML, Jung YW, Yun BS, et al. Combined oral medroxyprogesterone/levonorgestrel-intrauterine system treatment for women with grade 2 stage IA endometrial cancer. Int J Gynecol Cancer. 2017;27:738–742. doi: 10.1097/IGC.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 25.Zhou H, Cao D, Yang J, Shen K, Lang J. Gonadotropin-releasing hormone agonist combined with a levonorgestrel-releasing intrauterine system or letrozole for fertility-preserving treatment of endometrial carcinoma and complex atypical hyperplasia in young women. Int J Gynecol Cancer. 2017;27:1178–1182. doi: 10.1097/IGC.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 26.Mao Y, Wan X, Chen Y, Lv W, Xie X. Outcomes of conservative therapy for young women with early endometrial adenocarcinoma. Fertil Steril. 2010;93:283–285. doi: 10.1016/j.fertnstert.2009.07.999. [DOI] [PubMed] [Google Scholar]
- 27.Dijkhuizen FP, Mol BW, Brölmann HA, Heintz AP. The accuracy of endometrial sampling in the diagnosis of patients with endometrial carcinoma and hyperplasia: a meta-analysis. Cancer. 2000;89:1765–1772. [PubMed] [Google Scholar]
- 28.Kim MK, Seong SJ, Song T, Kim ML, Yoon BS, Jun HS, et al. Comparison of dilatation & curettage and endometrial aspiration biopsy accuracy in patients treated with high-dose oral progestin plus levonorgestrel intrauterine system for early-stage endometrial cancer. Gynecol Oncol. 2013;130:470–473. doi: 10.1016/j.ygyno.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Seong SJ, Kim MK, Bae HS, Kim M, Yun BS, et al. Dilatation and curettage is more accurate than endometrial aspiration biopsy in early-stage endometrial cancer patients treated with high dose oral progestin and levonorgestrel intrauterine system. J Gynecol Oncol. 2017;28:e1. doi: 10.3802/jgo.2017.28.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]