Abstract
A study of prevalence of ruminant fascioliasis was undertaken from May 2017 to April 2018. A set of 7640 stool and 7640 bile samples were collected from slaughtered cattles, sheeps and goats in seven local abattoirs located within the seven Local Government Areas making up northern Bauchi state. The Sample collection was demarcated into four sections of three months each corresponding into four local seasons. 1910 samples were collected from the beginning to the end of each of the four local seasons. Direct postmortem investigation to detect adult Fasciola spp. was employed. Stool samples were analyzed using formol-etha concentration technique. ANOVA (Analysis of variance) was conducted to examine the prevalence of fascioliasis in six communities of northern Bauchi state. The prevalence of infection was statistically different on different localities. The highest infection rates from the seven sampling sites was Jama'are (48.5%) followed by Zaki (46.9%) p = 0.05. Specie specific prevalence of fascioliasis between the host species was statistically different. Cattles were more infected than sheep and goat. Prevalence of fascioliasis on gender was statistically different, with bulls showing a higher prevalence rate and female sheep and goat recorded higher prevalence. Prevalence of fascioliasis based on estimated ages of animals sampled was statistically significant, animals above 5 years had higher prevalence of 64.5% followed by animals below the age of 1 with 41.3% (p = 0.05). Prevalence of fascioliasis among sampled ruminants based on seasonal variations was statistically significant. Higher prevalence percentages were observed during the early and late rainy season (47.2% and 58.4%) compared to early and late dry seasons (36.2% and 20.1%) p = 0.05. The study, therefore, recommends regular meat inspection alongside public awareness campaigns.
Keywords: Prevalence, Fascioliasis, Ruminants, Infection
1. Introduction
Fascioliasis is a disease commonly found among cattle, buffalos, sheep, goats, horses, donkeys, rabbits, wild ruminants and humans. The disease is caused mainly by two species of parasitic Trematodes that affect the liver and other associated organs. Liver flukes belong to the group of food borne Trematodes infection and are zoonotic (World Health Organization, 2016). The two main species that cause fascioliasis (Fasciola hepatica and Fasciola gigantica) are similar and large enough to be visible to the naked eyes (Centres for Disease Control and Prevention, 2013). Farag reports that infection rates among animals may reach 90% in some regions (Farag, 1998). Fascioliasis has been classified as a neglected tropical disease (www.cdc.gov/parasites/fasciola/biology.html, 2016). Liver fluke development is evidently dependent on environmental characteristics, as infection of both definitive and intermediate host involves an association with external fresh water. Larval development occurs completely within species of freshwater snails which depend on environmental factors (Mas-Coma, 2005). Moreover, the transmission of Fasciola spp. is markedly influenced by human activities (Mas-Coma, 2005).
Nigeria is one of the four leading livestock producers in sub-Saharan Africa (FAO, 2009). Livestock contributes up to 12.7% of the total Nigerian agricultural gross domestic products (CBN, 1999). In Nigeria, cattle rearing is done under the transhumant husbandry system with little supplementary feeding resulting in low productivity (Ulayi et al., 2007). This may lead to weak body conditions, which in turn leads to increased susceptibility to trematode infection (Taylor, 1964).
The cattle, sheep and goat markets in northern Bauchi state have become hot selling points due to the ongoing Boko Haram crises in some of the neighbouring states of north-eastern Nigeria. Animals from neighbouring countries such as Cameroon, Chad and Niger Republic easily find their ways into these markets. The study area also have abundant grazing land and open fresh water bodies used for domestic and farming purposes; these are factors that contribute to the spread of fascioliasis. An attempt to establish prevalence rates of fascioliasis along other epidemiological variables has therefore become necessary against old and scanty information available.
Recently, many prevalence studies were conducted in different parts of Nigeria, although available data showed little or no record for the study area. Prevalence studies of liver flukes in cattle, and small ruminant at slaughter in Zaria, Nigeria revealed a total prevalence of 48.0% (Ieren et al., 2016). A ten year retrospective survey of bovine fascioliasis burdens in trade cattle slaughtered at abattoirs in north-central Nigeria conducted by Yatswako and Alhaji (Yatswako and Alhaji, 2017) also reported an overall prevalence of 32.34%, total economic loss from 47,931 condemned livers was put at 766,896.0 USD. The study also highlighted that all intrinsic factors of breed, sex and age significantly influenced occurrence of the disease at univariable analysis. Iboyi, Agada and Imande (Iboyi et al., 2017) recorded 42.0% prevalence out of the 400 sampled cattle slaughtered at the Minna modern abattoir of Niger state.
Elelu and Eisler (Elelu and Eisler, 2018) reviewed bovine fascioliasis and other trematode infections in Nigeria and suggested that Trematode infection poses widespread risk to livestock and possibly humans.
The objectives of this study are:
-
(i)
To establish prevalent rate of fascioliasis among cattle, sheep and goat at the seven abattoirs located within the study area.
-
(ii)
To study if differences exist in prevalence among the seven communities;
-
(iii)
To investigate the possible effects of sex, specie and seasonal variation on the prevalence of fascioliasis among sampled ruminants.
2. Materials and methods
2.1. The study area
Bauchi state, located in north eastern Nigeria was created in 1976, and currently consists of Twenty (20) local area councils. The state covers a total land Area of 49,119 km2 representing about 5.3% of Nigeria's total land mass. The total population of the state is 4,653,066 based on the 2016 population estimates and it is ranked 7th of the 36 states (Federal Republic of Nigeria Census Report, 2016). Bauchi state also ranks 5th among the 36 states in terms of land mass.
Rainfall in Bauchi state ranges between 1300 mm per annum in the South and only about 700 mm per annum in the extreme north. Mean maximum monthly temperature of the area is 37 °C occurring mostly between March and April. The state is watered by a number of rivers; they include the Gongola and Jama'are rivers.
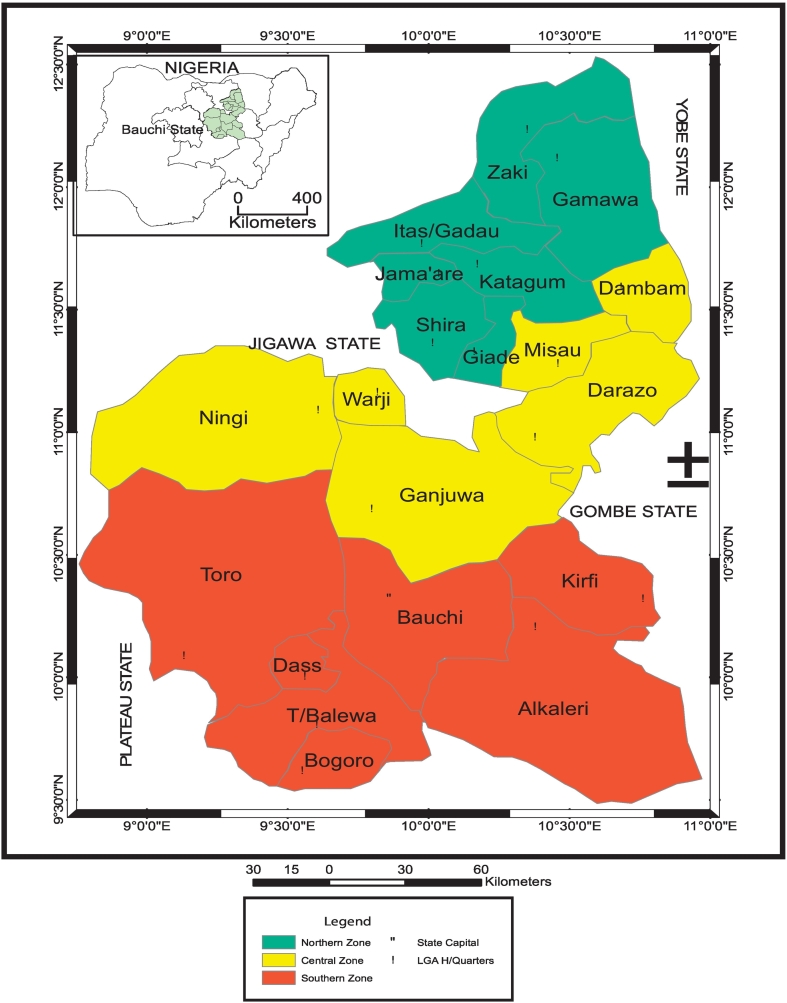
Bauchi north senatorial district is made up of seven (7) of the Twenty(20) local government areas of Bauchi state, comprising Gamawa, Giade, Itas-Gadau, Jama'are, Katagum, Shira and Zaki LGAs (Fig. 1). They make up a total land mass of 9, 717 km2 with a total population of 1, 512, 677 according to 2006 national population estimates.
Fig. 1.
Map of Bauchi state, Nigeria showing the position of northern Bauchi state in green.
2.2. Sampling procedure
Post mortem investigation was carried out in the seven main abattoirs; one located in each of the seven LGAs making up Bauchi north. One abattoir was visited each day of a week corresponding to market days on which majority of the animals were slaughtered. An Abattoir was only visited once in a week, and 52 times by the research assistants in one year. Systematic random sampling with an interval of two (2) was adopted. The collection of samples and analysis started from the month of May 2017 and ended in April 2018.
Early rainy season beginning May to July 2017
Late rainy season from August to October 2017
Early dry season from November 2017 to January 2018
Late dry season beginning February and ending April.
Sample size was determined according to the standard technique of sample size determination given by Thursfield (Thursfield, 1995). Expected prevalence was put at 50%, since there was no previous data from the study area.
A total of 7640 samples were collected, 3560 from cattle, 880 from sheep and 3200 from goat. The sample size was spread equally between 4 local seasons experienced within the study area (Table 1).
Table 1.
Number of sample examined per locality/specie for the seven local government areas of northern Bauchi state.
| Locality | Host species |
Total | ||
|---|---|---|---|---|
| Cattle | Sheep | Goat | ||
| Gamawa | 460 | 120 | 320 | 900 |
| Giade | 360 | 80 | 240 | 680 |
| Itas/Gadau | 360 | 80 | 240 | 680 |
| Jama'are | 460 | 120 | 240 | 820 |
| Katagum | 1200 | 240 | 1680 | 3120 |
| Shira | 360 | 120 | 240 | 720 |
| Zaki | 360 | 120 | 240 | 720 |
| Total | 3560 | 880 | 3200 | 7640 |
2.3. Sample analysis
The samples collected include fecal materials from the rectum and bile of slaughtered cattles, sheeps and goats. An approximately 4 g each of the sample was placed in a separate sample bottle and analyzed in the laboratory using the procedures outlined by Cheesbrough (Cheessbrough, 2005).
3. Results
From the 7640 set of samples examined, 3092 were found positive for Fasciola spp. eggs, adults and ova. A prevalence of 40.5% has therefore established from the study area (Table 2). Highest prevalence was recorded from samples collected in Jama'are (48.5%), followed by Zaki (46.9%) and lowest at Shira (27.8%). The prevalence of infection was statistically different on the seven localities (p = 0.0005).
Table 2.
Prevalence of infection for the seven community abattoirs in Bauchi north, north-eastern Nigeria.
| Communities | No. examined | No. positive | % prevalence | 95% confidence interval |
|---|---|---|---|---|
| Gamawa | 900 | 346 | 38.4 | 0.0742 |
| Giade | 680 | 211 | 31.0 | −0.0742 |
| Itas/Gadau | 680 | 281 | 41.3 | 0.0288 |
| Jama'are | 820 | 398 | 48.5 | 0.1009 |
| Katagum | 3120 | 1318 | 42.2 | 0.0380 |
| Shira | 720 | 200 | 27.8 | −0.3844 |
| Zaki | 720 | 338 | 46.9 | 0.0850 |
| Total | 7640 | 3092 | 40.5 |
p = 0.0005.
Specie specific prevalence occurred highest among cattle (45.7%). Prevalence of fascioliasis among sheep was 39.1% and 35.0% among goat. Differences between prevalence among cow, sheep and goats were also statistically significant (p = 0.0005) (Table 3).
Table 3.
Species specific prevalence of fascioliasis in northern Bauchi state, north-eastern Nigeria.
| Animal species | No. examined | No. positive | % prevalence | 95% confidence interval |
|---|---|---|---|---|
| Cattle | 3560 | 1628 | 45.7 | −0.2722 |
| Sheep | 880 | 341 | 39.1 | 0.2465 |
| Goat | 3200 | 1120 | 35.0 | −0.1525 |
| Total | 7640 | 3092 | 40.5 |
p = 0.0005.
Effect of sex on the prevalence of fascioliasis was also observed and recorded. While among cattle, males were more infected (55.3%) than females (41.3%); female sheep recorded higher prevalence (44.6%) than males (30.7%). Prevalence among goats also indicated higher value in females (42.6%) than among males (30.4%). Differences in respect of the animal gender were also significant at 5% level (Table 4).
Table 4.
Prevalence of fascioliasis based on sexes of the slaughtered animals in northern Bauchi state, north-eastern Nigeria.
| Sexes | No. examined | No. positive | % prevalence | 95% confidence interval |
|---|---|---|---|---|
| Cattle | 0.0742 | |||
| Female | 2438 | 1008 | 41.3 | −0.1091 - |
| Male | 1122 | 620 | 55.3 | −0.0793 |
| Sheep | ||||
| Female | 529 | 236 | 44.6 | 0.0236- |
| Male | 351 | 108 | 30.7 | 0.0026 |
| Goat | ||||
| Female | 1211 | 516 | 42.6 | 0.0793- |
| Male | 1989 | 604 | 30.4 | 0.0838 |
| Total | 7640 | 3092 | 40.5 |
p = 0.0005.
Prevalence of fascioliasis among the sampled animals seemed to respond to their estimated ages. Animals estimated to be <1 year had 41.3% prevalence. Highest prevalence was observed among the oldest animals (64.7%) and lowest among 1–2 year old (35.7%). It is interesting to note that, aged cattle recorded higher prevalence, as all animals sampled above five years were cattle. Older sheep and goats, on the other hand, between the ages of 3–4 years recorded lower prevalence of infection (Table 5).
Table 5.
Prevalence of fascioliasis based on estimated ages of sampled animals.
| Estimated age-group (months) | No. examined | No. positive | % prevalence | 95% confidence interval |
|---|---|---|---|---|
| <1 year | 1001 | 413 | 41.3 | −0.1041 |
| 1–2 years | 2850 | 1018 | 35.7 | 0.0067 |
| <3 years | 1711 | 679 | 39.7 | −0.0802 |
| 3–4 years | 1506 | 612 | 40.6 | −0.0914 |
| ≥5 years | 572 | 370 | 64.7 | −0.3504 |
| Total | 7640 | 3092 | 40.5 |
p = 0.0005.
Prevalence of fascioliasis among sampled ruminants within four local seasons corresponding to rainy and dry seasons were also statistically different. There are differences in the animals, in spite of the fact that an equal number of animals were examined in all the seasons. Out of the 1910 samples examined during the late rainy season, 1116 were positive, giving a prevalence of 58.4%. On the other hand, only 383 of the 1910 samples examined during the late dry season were positive (20.1%) (Table 6).
Table 6.
Prevalence of fascioliasis among sampled ruminants based on seasonal variations.
| Seasons | No. sampled | No. positive | % prevalence | 95% confidence interval |
|---|---|---|---|---|
| Early rainy season | 1910 | 902 | 47.2 | 0.0730 |
| Late rainy season | 1910 | 1116 | 58.4 | −0.1511 |
| Early dry season | 1910 | 691 | 36.2 | 0.1834 |
| Late dry season | 1910 | 383 | 20.1 | 0.2326 |
| Total | 7640 | 3092 | 40.5 |
p = 0.0005.
4. Discussion
A prevalence of 40.5% observed among ruminant animals within the sampled area indicates both presence of the disease and its contributing factors. The prevalence figures obtained from this study were higher than figures recorded from studies conducted Magaji et al., (Magaji et al., 2014), Aliyu, Ajogi, Ajanusi and Reuben (Aliyu et al., 2014), Yatswako and Alhaji (Yatswako and Alhaji, 2017). The result obtained however, appeared lower when compared with 80% prevalence rate reported from cows slaughtered at Maiduguri, north-eastern Nigeria. Ieren, Ajanusi and Mbaya (Ieren et al., 2016) also recorded a total prevalence of 48.0% from a study conducted at Zaria. Iboyi, Agada and Imande (Iboyi et al., 2017) reported a prevalence of 42.0% which nearly corresponds with the result of this present study. Based on reports available, it appeared that north-eastern Nigeria is prone to fascioliasis infection. The first incidence of fascioliasis in Nigeria was reported from this region (Burke, 1939). This may not be unconnected with the fact that, north-eastern Nigeria is a zone of rampant uncontrolled grazing, with no clean water sources for the animals except open ditches and ponds which are factors aiding transmission (World Health Organization, 2018). In addition to this, certain factors which were reported by Mas-Coma (Mas-Coma, 2005) to have great influence on liver fluke development are at play in this region (Greter et al., 2014).
A prevalence rate of 40.5% from the study area, which house ruminant animals that are transported to different parts of Nigeria due to the crisis in other parts of north-eastern Nigeria is important and requires public policy consideration.
In Nigeria, infection rates of fascioliasis at slaughter and among farm cattle have been documented with no significant variation. Aliyu, Ajogi, Ajanusi and Reuben (Aliyu et al., 2014) reported a 19.5% at slaughter and 14.5% prevalence from herds of cattle.
Infection rates recorded from the seven sampling communities occur highest at Jama'are (48.5%) followed by Zaki (46.9%). These are wetlands areas which river Jama'are passes through, with abundant grazing land favourable for the survival of the intermediary host. In addition, these areas serve as host communities to open grazing nomads, a reason advanced by various authors to be responsible for increased transmission (Smyth, 1996; Graycyzk and Freid, 1999; Bradleemaster et al., 2004).
Although more animals were sampled from Katagum due to high population of animals slaughtered, prevalence recorded was lower than Zaki and Jama'are.
In relation to specie specific prevalence, cattles were more infected than sheep and goats. Several authors have reported that cattle are more susceptible to Fasciola gigantica which is the prevalent specie in West Africa, especially Nigeria.
A number of authors have reported that, there is a relationship between host sex and the intensity of helminths infection (Noble and Noble, 1982; Rahman and Collins, 1992; Roberts and Janovy, 2010). Values from this study agreed with this assertion. Prevalence between sexes was statistically different. This does not preclude an interesting deviation shown by values of prevalence between female and male cattle, similar results were also reported by Magaji et al., (Magaji et al., 2014).
The age of the host is an important determinant of prevalence and intensity in most helminths infections (Rahman and Collins, 1992). Although differences between estimated ages of the sampled animals do not seem to indicate strong variation, differences in prevalence between estimated age groups were still significant.
Results from this study indicate that, prevalence rates among cattle stands higher than goats and sheep. The values from this study suggest that even among cattle, aged animals were more infected with fascioliasis. This may not be unconnected with the fact that female cattle slaughtered form a higher percentage of the sampled animals from the study area. Moreover, Rahman and Collins (Rahman and Collins, 1992) reported that females show an overall high infection than males.
Seasonal variations were also observed in this study. Higher prevalence (58.4%) was observed during the late rainy season from August to October followed by early rainy season (47.2%). Prevalence was lowest during the late dry season (20.1%). This is in agreement with reasons put forward by Graycyzk and Freid (Graycyzk and Freid, 1999) that rainfall determines the prevalence and intensity of fluke's infection more than any other factor. Liver fluke development has also been reported to be dependent on environmental characteristics such as rainfall (Mas-Coma, 2005).
5. Conclusion
It is important to note that, values obtained from this study were from abattoir studies, no intention is meant to generalize the results to include herd prevalence and its peculiarities.
This research has established a total prevalence of 40.5%, it can therefore be concluded that fascioliasis is prevalent in the study area, and its association with host characteristics were also reported. This calls for a policy plan targeted at prevention and control of this neglected tropical disease. In view of this, the following recommendations may assist.
There is urgent need for awareness campaign within the community on the danger of fascioliasis spread among ruminant animals. Since the area had become hot selling and buying spot of animals from crises zones, more effort should be geared towards meat supervision. The intermediary hosts of the parasite snails need to be reduced using environment friendly molluscides. Lack of sanitation facilities at the local abattoirs studied calls for an urgent concern.
Acknowledgments
Acknowledgements
The cooperation and understanding of the officials at the seven abattoirs is hereby acknowledged. Technical assistance of the principal laboratory technician of Federal University Dutse in person of Mr. Saidu Danwanka and the two technicians at undergraduate Biosciences laboratory of ASCOE Azare is greatly appreciated. To my seven research assistants who made large sample collections possible, I say thank you.
Conflict of opinion
None.
References
- Aliyu A.A., Ajogi I.A., Ajanusi O.J., Reuben Epidemiological studies of Fasciola gigantica in cattle in Zaria, Nigeria using coprology and serology. J. Public Health Epidemiol. 2014;6(2):85–91. [Google Scholar]
- Bradleemaster B., Wash A.W., Wayne T., Tritschler J. 2004. Analysis of Fasciola sp. Utilizing Abattoir Records in Hawaii. (retrieved August 2017) [Google Scholar]
- Burke J. In: The impact of Fascioliasis on Food Security in Nigeria. Mohammed B.R., editor. Vol. 2015. 1939. [Google Scholar]
- CBN . 1999. Central Bank of Nigeria Annual Report. [Google Scholar]
- Centres for Disease Control and Prevention Fasciola biology. 2013. www.cdc.gov/parasites/fasciola/biology.html retrieved from.
- Cheessbrough M. Cambridge University Press; Cambridge: 2005. District Laboratory Practice in Tropical Countries, Part 1, Cambridge Low Price Editions; pp. 224–226. [Google Scholar]
- Elelu N., Eisler M.C. A review of bovine fasciolosis and other trematode infections in Nigeria. J. Helminthol. 2018;92(2):128–141. doi: 10.1017/S0022149X17000402. [DOI] [PubMed] [Google Scholar]
- FAO Country profiles: Nigeria. 2009. www.fao.org/countryprofiles/index (retrieved October 2018)
- Farag H.F. Human fascioliasis in some countries of the Eastern Mediterranean Region. East Mediterr. Health J. 1998;4(1):156–160. [Google Scholar]
- Federal Republic of Nigeria Census Report. 2016. [Google Scholar]
- Graycyzk T.K., Freid B. Development of Fasciola hepatica in the intermediate host. In: Delton J.P., editor. Fasciolosis. CAB International publishing; Wallingford Oxon: 1999. pp. 31–46. [Google Scholar]
- Greter H., Jean-Richard V., Crump L., Bechir M., Alfaroukh I.O., Scheking E. The benefits of one health for pastoralist in Africa. Onderstepoort J. Vet. Res. 2014;81(2) doi: 10.4102/ojvr.v81i2.726. [DOI] [PubMed] [Google Scholar]
- Iboyi M.O., Agada P.A., Imandeh N.G. Study on the prevalence of fascioliasis on cattle slaughtered at Minna Modern abattoir, Niger state, Nigeria. J. Appl. Life Sci. Int. 2017;15(3):1–6. [Google Scholar]
- Ieren I.I., Ajanusi O.J., Mbaya P.Y. Prevalence of liver flukes in cattle, and small ruminants at slaughter in Zaria, Nigeria. Res. Zool. 2016;6(3):33–36. [Google Scholar]
- Magaji A.A., Ibrahim K., Salihu M.D., Saulawa M.A., Mohammed A.A., Musawa A.I. Advances in Epidemiology. vol. 2014. 2014. Prevalence of fascioliasis in cattle slaughtered in Sokoto Metropolitan Abattoir, Sokoto, Nigeria. (5 pages) [Google Scholar]
- Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J. Helminthol. 2005;79:207–216. doi: 10.1079/joh2005296. [DOI] [PubMed] [Google Scholar]
- Noble E.R., Noble G.A. 5th edition. Pitman Books Ltd.; 1982. Parasitology, the Biology of Animal Parasites. [Google Scholar]
- Rahman W.A., Collins G.A. An association of faecal egg counts and prolatic concentrations in sera of peri-parturient Angora goats. Vet. Parasitol. 1992;23:85–91. doi: 10.1016/0304-4017(92)90051-a. [DOI] [PubMed] [Google Scholar]
- Roberts L.S., Janovy J.J.R. Mc Graw Hill Int. Edtn.; Boston: 2010. Foundations of Parasitology (8th) [Google Scholar]
- Smyth J.D. The Cambridge University Press Ltd.; London: 1996. Introduction to Animal Parasitology. [Google Scholar]
- Taylor E.L. Food and Agricultural Organization of United Nations; 1964. Fasciolasis and Liver Fluke. [Google Scholar]
- Thursfield M. Second edition. Black Well Science Ltd; UK: 1995. Veterinary Epidemiology. [Google Scholar]
- Ulayi B.M., Umaru S.B., Adamu S. Prevalence of Dicrocoelom hopes and Fasciola gigantic infections in cattle at slaughter in Zaria. Niger. J. Anim. Vet. Adv. 2007;6:1112–1115. [Google Scholar]
- World Health Organization Food borne trematode infection – fascioliasis. 2016. www.who.int/foodborne_trematode_infections/fascioliasis/en/ Retrieved from:
- World Health Organization Fascioliasis epidemiology. 2018. http//www.who.int/foodborne_trematode_infections/fascioliasis Retrieved from.
- Centres for Disease Control and Prevention. 2016. www.cdc.gov/parasites/fasciola/biology.html
- Yatswako S., Alhaji N.B. Survey of bovine fasciolosis burdens in trade cattle slaughtered at abattoirs in North-central Nigeria: the associated predisposing factors and economic implication. Parasite Epidemiol. Control. 2017;2(2017):30–39. doi: 10.1016/j.parepi.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]