Abstract
Purpose
To describe the rare entity of concurrent herpetic acute retinal necrosis (ARN) and orbital inflammation.
Observations
Two cases of ARN with simultaneous orbital inflammation are described. A 40-year old male presented with a painful left eye and hand motion visual acuity (VA). Both panuveitis and orbital inflammation with involvement of the sclera and optic nerve were observed. He was initially treated with oral steroid therapy, after which the orbital inflammation improved but the panuveitis remained. A diagnostic anterior chamber paracentesis was positive for HSV-2 by PCR. He was started on systemic antivirals, but ultimately developed a retinal detachment. The second patient was an 18-year old female with hand motion VA in the left eye. Panuveitis and severe conjunctival chemosis were observed. MRI demonstrated dacryoadenitis with preseptal inflammation. The patient was initially started on oral steroid therapy, which alleviated the orbital inflammation but not the intraocular inflammation. An anterior chamber diagnostic paracentesis was positive for HSV-1, after which the patient underwent vitrectomy for a retinal detachment. The patient was started on systemic antiviral therapy.
Conclusions and Importance
Herpetic disease should remain on the differential for cases of concurrent intraocular and orbital inflammation. Early recognition of this process may help prevent severe vision loss. It is important to recognize that orbital inflammation secondary to herpetic disease may be diverse in its presentation.
Keywords: Acute retinal necrosis (ARN), Orbital inflammation
1. Introduction
First described in 1971, acute retinal necrosis (ARN) is a severe infection of the retina resulting in acute panuveitis,1 characterized by retinal whitening in areas of necrosis. The etiology of ARN is retinal infection with Herpesviridae, the most common of which is varicella zoster virus (VZV), followed by herpes simplex virus (HSV) types 1 and 2.2,3 Patients with ARN commonly develop retinal detachments, resulting in a poor visual prognosis. Bilaterality of this disease is also significant, estimated at 70%.4 The contralateral eye can be involved within months or even years later,4,5 especially in the absence of systemic antiviral treatment.
Diagnosis is largely based on clinical exam. Definitive diagnosis can be made with polymerase chain reaction (PCR) of aqueous obtained from the anterior chamber paracentesis. The sensitivity of anterior chamber PCR has been estimated as 88–100%.1 Treatment typically consists of systemic antiviral therapy with intravenous acyclovir or oral valacyclovir, augmented with intravitreal ganciclovir and/or foscarnet in severe cases and/or oral steroids.6 Rapid initiation of therapy is essential to limit the extent of damage and subsequent visual comorbidity. ARN usually presents solely with intraocular inflammation, namely panuveitis. We describe two cases of ARN that were accompanied by orbital inflammation.
2. Findings
2.1. Case 1
A 40-year old male presented to the Bascom Palmer Eye Institute (BPEI) Emergency Room with 1 week of painful, red eye and decreased vision in the left eye. A week prior, he was seen in a general emergency room, where he received a CT scan for headaches, which was negative. He was diagnosed with conjunctivitis and discharged. He returned to the same emergency room 5 days later with worsening vision. Repeat CT scan showed moderate pre-septal inflammation without extension into the post-septal space.
On examination at our institution, he was found to have a visual acuity (VA) of hand motion and intraocular pressure (IOP) of 25 mmHg OS with 3 mm of proptosis. Both left upper and lower eyelids were tense, with conjunctival injection and chemosis OS (Fig. 1A). No periorbital rash was noted. Extraocular movements were restricted (video available as Supplementary Material.) Intraocular examination displayed 2 + cell in the anterior chamber with 3 + keratic precipitates. There was no view of the fundus due to 3 + vitritis. The contralateral eye was normal.
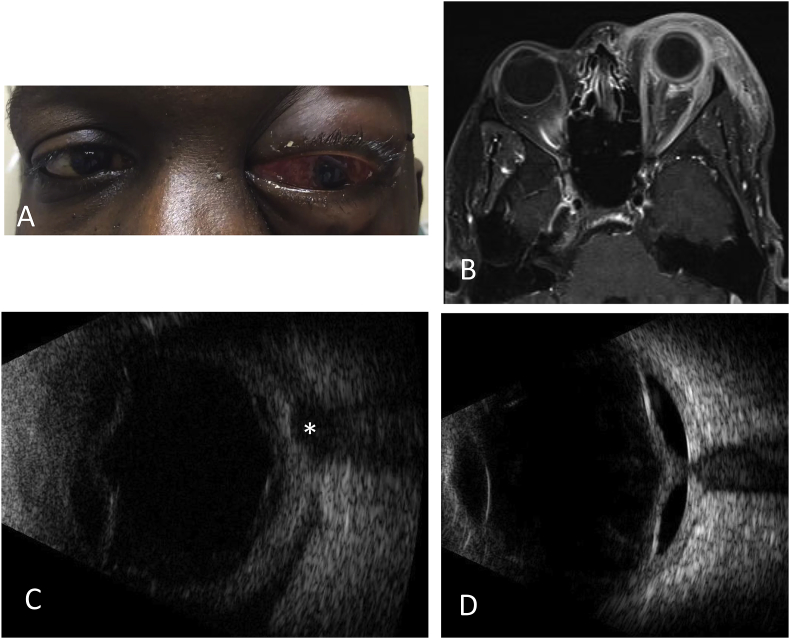
Fig. 1.
Images of Case 1. (A) External photograph demonstrating severe chemosis and proptosis of the left eye. (B) Transverse orbital MRI image demonstrating diffuse preseptal and postseptal orbital inflammation, including thickening of posterior sclera and optic nerve. (C) Ultrasound B-scan of left eye demonstrating posterior vitreous detachment and “T-sign” (asterisk) showing posterior Tenon infiltration, typically associated with posterior scleritis. (D) Two weeks later, ultrasound B-scan of left eye demonstrates retinal detachment.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajoc.2019.01.012.
The following is the supplementary data related to this article:
video
The patient was admitted to the general hospital for further workup and imaging. Subsequent MRI showed preseptal and postseptal enhancement with involvement of the sclera and optic nerve (Fig. 1B). Serum testing revealed negative HIV, Quantiferon, angiotensin converting enzyme (ACE), RPR and blood cultures. Due to the possibility of an orbital cellulitis, the patient received IV antibiotics for two days, but an ultrasound showed choroidal detachments throughout in addition to the classic “T-sign” of posterior scleritis (Fig. 1C). Severe extraocular movement restriction was also noted. At this point, the patient was diagnosed with posterior scleritis with concurrent orbital inflammation. The patient was started on 1 mg/kg IV methylprednisolone. After two days of treatment, the orbital inflammation improved significantly.
However, VA and intraocular inflammation remained unchanged. The patient then received two doses of sub-Tenon's Kenalog, as this presentation was thought to represent a non-infectious panuveitis. However, two weeks later, he developed a total retinal detachment as diagnosed on ultrasound (Fig. 1D). The patient underwent pars plana vitrectomy, at which time the retina was found to be necrotic with proliferative membrane formation. Intraoperative vitreous sample was sent for PCR, and the patient was started on valacyclovir 1 g three times daily. The vitreous sample was positive for HSV-2. At the last visit 20 months after initial presentation, the retina was attached under silicone oil with VA of light perception.
2.2. Case 2
An 18-year old African-American female presented to the BPEI Emergency Room with 1 week of eyelid swelling, pain, and headaches OS. She was on cephalexin at the initial visit for presumed preseptal cellulitis. VA in the left eye was hand motion and IOP was 26 mmHg. Eyelids were tense with edema. Temporal conjunctival chemosis with 2 + flare and 1 + cell was noted. No periorbital rash was noted. There was a poor view posteriorly, but grade II optic nerve edema was observed. Orbital MRI was limited by insufficient contrast, but preseptal inflammation and dacryoadenitis were observed (Fig. 2A). There was initial suspicion of sarcoidosis versus idiopathic orbital inflammation given these findings and the patient's ethnicity. Ultrasound B-scan was also performed, which showed dense vitreous opacities with membrane formation, fundus thickening, and a shallow retinal detachment, initially interpreted to be serous in nature given the lack of a retinal break (Fig. 2B). The patient was started on topical prednisolone and PO prednisone 60 mg with laboratory testing ordered.
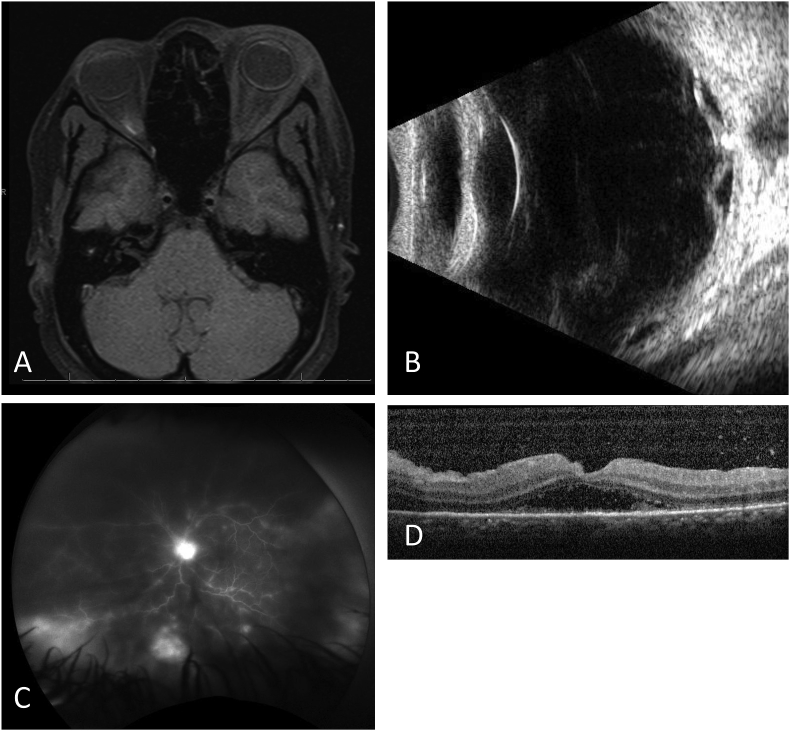
Fig. 2.
Images of Case 2. (A) MRI image demonstrating preseptal inflammation and dacryoadenitis. This study was limited by insufficient contrast. (B) Ultrasound B-scan demonstrating optic nerve drusen, dense vitreous opacities with membrane formation, fundus thickening and a shallow retinal detachment. (C) Late image of fluorescein angiography of the left eye demonstrating disc edema, peripheral staining of retinal lesions, and vascular leakage consistent with vasculitis. (D) Spectral domain optical coherent tomography (SD-OCT) demonstrating posterior vitreous debris, subretinal fluid, and subretinal inflammatory debris with loss of outer retinal segments.
Four days later, the patient felt some mild improvement but her vision remained the same. Repeat ultrasound showed a shallow posterior retinal detachment without shifting subretinal fluid. At this time, a herpetic etiology was entertained. A diagnostic anterior chamber paracentesis was completed and sent for herpesviridae. In addition to systemic prednisone, the patient was started on valacyclovir to cover for potential herpetic disease. ACE, RPR, and Quantiferon were all negative.
Fluorescein angiography was completed, and showed disc edema, vasculitis and peripheral retinal lesions (Fig. 2C), while indocyanine green angiography showed multiple hypofluorescent spots suggestive of choroidal dropout (not shown). OCT showed outer retinal loss and subretinal fluid parafoveally in addition to posterior vitritis (Fig. 2D).
Two days later, the patient's vitritis was thought to significantly worsen. Given the concurrent ultrasound findings concerning for retinal detachment, she underwent a pars plana vitrectomy with barricade endolaser. Intraoperatively, the retina was found to be attached but significantly necrotic. Aqueous humor PCR returned positive for HSV-1 at this time. The patient continued treatment with high dose valacyclovir. At the last visit 6 months after initial presentation, her VA was 20/80 with an early cataract also present.
Of note, Institutional Review Board (IRB) approval was not required due to the small size of this case series. Informed Consent was obtained from both patients, and the study was completed in accordance to HIPAA regulations.
3. Discussion & conclusions
Concurrent ARN and orbital inflammation is rare. Table 1 reviews our two cases with similar cases published in the literature.7, 8, 9, 10, 11 Notably, all cases with one exception were in female patients. Certainly the small sample size of this case series limits the extent of conclusions that can be drawn, but this combined intraocular and extraocular presentation may be more common in females. Previous cases often had poor VA outcome, which one might consider to be due to delayed recognition and treatment. Antiviral therapy was started 1 month after initial presentation in Case 1 when the patient had developed a retinal detachment, while antiviral therapy was initiated 1 week after initial presentation in Case 2. However, a review of the cases in Table 1 demonstrates that time to correct diagnosis and start of antiviral therapy was not necessarily correlated with overall outcome. The patient described in Badilla et al. had been previously treated for herpes zoster ophthalmicus,11 and this prior course of antiviral therapy might have facilitated her excellent final VA of 20/25. However, in Rozenbaum et al.,10 the patient progressed to develop a retinal detachment even while on antiviral therapy.
Table 1.
Clinical characteristics of case series.
| Case | Age (years) | Gender | Initial VA | Time to correct diagnosis (weeks) | Aqueous PCR result | Presence of RD | Orbital findings | Final VA | Interval between presentation and final follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Yaman et al.7 | 25 | F | CF | 1 | N/A | + | Diffuse inflammation | 20/400 | 6 |
| Foo et al.8 | 30 | F | 20/80 | 3 | HSV-1 | – | Diffuse inflammation, EOM restriction | NLP | 3 |
| Tornerup et al.9 | 34 | F | 20/25 | 1 | HSV-1 | + | Proptosis, diffuse inflammation | NLP | 3 |
| Rozenbaum et al.10 | 49 | F | HM | 0 | HSV-2 | + | Myositis | NLP | 2 |
| Badilla et al.11 | 81 | F | HM | 0 | N/A | – | Proptosis, myositis | 20/25 | 1 |
| Case 1 | 73 | M | HM | 4 | HSV-2 | + | Diffuse inflammation, posterior scleritis | LP | 20 |
| Case 2 | 18 | F | HM | 1 | HSV-1 | – | Diffuse inflammation, dacryoadenitis | 20/80 | 6 |
PCR = polymerase chain reaction; RD = retinal detachment; VA = visual acuity; CF = count fingers; HM = hand motion; NLP = no light perception; N/A = not applicable.
The two cases presented in this case series are unique in their presentation of specific orbital findings; Case 1 had findings suggestive of posterior scleritis, while Case 2 had findings consistent with dacryoadenitis. The latter finding led us to suspect an inflammatory process such as sarcoidosis before ARN was considered as a potential etiology. Sub-tenon Kenalog was administered in Case 1 due to presumed noninfectious panuveitis; a herpetic etiology was not suspected. In retrospect, this likely could have been delayed until all possible infectious etiologies were excluded.
One question that arises is whether the orbital inflammation in these presentations is solely a reaction to the viral infection of the eye, or an active viral infection within orbital tissues. Rozenbaum et al. completed a lacrimal gland biopsy, which showed a lymphocytic infiltrate.10 Immunohistochemistry was negative for HSV, suggesting that the orbital findings are part of a reactionary inflammatory response. There has been some discussion that ARN may represent a reactivation of a herpetic virus from the ciliary ganglion,12,13 which lies in close proximity to the globe; reactivation of the virus there could serve as the nidus for an orbital inflammatory reaction.
Ultimately, ARN should remain on the differential for any diffuse or focal orbital inflammation, especially in the presence of panuveitis. A similarity among almost all of these cases is that herpetic disease was not considered as a potential etiology until a significant amount of time had elapsed from initial presentation. Ophthalmologists tend to consider the combination of panuveitis and orbital inflammation to be caused by a purely inflammatory process such as sarcoidosis or smoldering infectious processes such as syphilis or tuberculosis. However, it is important to consider an acute infectious etiology, specifically a herpetic process, which requires rapid recognition and initiation of treatment.
Patient consent
The patients consented to publication of the case in writing/orally.
Conflicts of interest
The following authors have no financial disclosures: SSS, NAY, AJR, AMC, TAA.
All authors attest that they meet the current ICMJE criteria for authorship.
Funding sources
No funding or grant support.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.01.012.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Schoenberger S.D., Kim S.J., Thorne J.E. Diagnosis and treatment of acute retinal necrosis: a report by the American academy of ophthalmology. Ophthalmology. 2017;124(3):382–392. doi: 10.1016/j.ophtha.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Hillenkamp J., Nolle B., Bruns C. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology. 2009;116(10):1971–1975. doi: 10.1016/j.ophtha.2009.03.029. e2. [DOI] [PubMed] [Google Scholar]
- 3.Lau C.H., Missotten T., Salzmann J., Lightman S.L. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114(4):756–762. doi: 10.1016/j.ophtha.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 4.Tibbetts M.D., Shah C.P., Young L.H. Treatment of acute retinal necrosis. Ophthalmology. 2010;117(4):818–824. doi: 10.1016/j.ophtha.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Palay D.A., Sternberg P., Jr., Davis J. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112(3):250–255. doi: 10.1016/s0002-9394(14)76725-x. [DOI] [PubMed] [Google Scholar]
- 6.Wong R.W., Jumper J.M., McDonald H.R. Emerging concepts in the management of acute retinal necrosis. Br J Ophthalmol. 2013;97(5):545–552. doi: 10.1136/bjophthalmol-2012-301983. [DOI] [PubMed] [Google Scholar]
- 7.Yaman A., Ozbek Z., Saatci A.O. Unilateral acute retinal necrosis initially presenting with painful orbitopathy. Ann Ophthalmol (Skokie) 2008;40(3-4):180–182. [PubMed] [Google Scholar]
- 8.Foo K., Small K., Alexander D., Wellings P. Acute retinal necrosis associated with painful orbitopathy. Clin Exp Ophthalmol. 2003;31(3):270–272. doi: 10.1046/j.1442-9071.2003.00651.x. [DOI] [PubMed] [Google Scholar]
- 9.Tornerup N.R., Fomsgaard A., Nielsen N.V. HSV-1--induced acute retinal necrosis syndrome presenting with severe inflammatory orbitopathy, proptosis, and optic nerve involvement. Ophthalmology. 2000;107(2):397–401. doi: 10.1016/s0161-6420(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 10.Rozenbaum O., Rozenberg F., Charlotte F., Bodaghi B. Catastrophic acute retinal necrosis syndrome associated with diffuse orbital cellulitis: a case report. Graefes Arch Clin Exp Ophthalmol. 2007;245(1):161–163. doi: 10.1007/s00417-006-0265-y. [DOI] [PubMed] [Google Scholar]
- 11.Badilla J., Dolman P.J. Orbital myositis involving the oblique muscles associated with herpes zoster ophthalmicus. Ophthalmic Plast Reconstr Surg. 2007;23(5):411–413. doi: 10.1097/IOP.0b013e318137a373. [DOI] [PubMed] [Google Scholar]
- 12.Ansari W.H., Pichi F., Pecen P.E. Herpes zoster keratitis development after acute retinal necrosis. Int Ophthalmol. 2018;38(2):829–832. doi: 10.1007/s10792-017-0521-7. [DOI] [PubMed] [Google Scholar]
- 13.Bustos D.E., Atherton S.S. Detection of herpes simplex virus type 1 in human ciliary ganglia. Invest Ophthalmol Vis Sci. 2002;43(7):2244–2249. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
video