Abstract
The goal of the present study was to determine the efficacy of osimertinib (AZD9291), a third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor for the treatment of aggressive EGFR-mutant non-small cell lung cancer (NSCLC), compared to cisplatinum (CDDP) + pemetrexed (PEM). The NSCLC cell line PC-9 expressing green fluorescence protein (PC-9-GFP) was implanted in the brain of nude mice and was treated with CDDP + PEM or AZD9291. Tumors were observed by non-invasive fluorescence imaging. AZD9291 treatment caused tumor regression in contrast to CDDP + PEM which had only a slight inhibitory effect. These results suggest that AZD9291 is a promising clinical option for NSCLC patients with brain metastasis.
Introduction
Non-small cell lung cancer (NSCLC), which accounts for approximately 85% of all lung cancers, is one of the most frequent cancers to metastasize to brain [1], [2], [3]. It is estimated that about 30% to 50% of patients with metastatic NSCLC will develop brain metastasis [4], [5]. Clinical studies have demonstrated that survival time is significantly reduced after the occurrence of brain metastases in NSCLC patients [6]. The current treatment options for NSCLC with brain metastases include surgery, radiotherapy and chemotherapy. The efficacy of conventional systemic chemotherapy of brain metastases of NSCLC patients is limited due in large part to the blood–brain barrier (BBB) [2], [7], [8]. Higher incidences of brain metastases for patients with epidermal growth factor receptor (EGFR)-mutant metastatic NSCLC were found compared to EGFR wild type [9], [10]. Several generations of EGFR tyrosine kinase inhibitors (TKIs) have been found to be highly effective compared to chemotherapy for NSCLC patients with brain metastases [11], [12], [13], [14]. Recent pre-clinical and clinical studies suggest that some third-generation inhibitors can cross the BBB and show anti-tumor activity [15], [16], [17], [18].
Osimertinib (AZD9291), a third-generation inhibitor of mutant EGFR, has been approved by the United States Food and Drug Administration (FDA) for EGFR T790 M–positive NSCLC [19], [20]. Osimertinib was highly active in patients with lung cancer with the EGFR T790 M mutation [21], [22], [23], [24], [25], and is more efficacious compared to standard first line therapies [21], [26], [27], [28]. Osimertinib showed higher concentrations in mouse brain tissue compared to plasma [29]. Osimertinib has improved BBB penetration ability and has potential for NSCLC patients with brain metastasis [1], [30], [31]. Koba et al. [32] reported that NSCLC patients containing an EGFR T790 M mutation with multiple brain metastases showed a strong response to osimertinib within 2 weeks without radiation therapy. Further, Xie et al. [33], in a retrospective study, showed that osimertinib is effective for patients with progressing brain metastases and that radiation therapy is not needed before osimertinib treatment. Osimertinib showed high efficacy in a leptomeningeal carcinomatosis (LMC) model with EGFR-mutant lung cancer [34], against lung cancer with multiple HER2 aberrations [35], induced apoptosis in oral epidermoid and colorectal cancer cells [36], [37], and showed good efficacy against breast cancer with L755P and L755S mutations [38].
In the present study, we established an imageable orthotopic xenograft mouse model of PC-9 expressing green fluorescence protein (PC-9-GFP) growing in the brain and determined the efficacy of osimertinib compared with conventional chemotherapy.
Materials and Methods
Cell lines and Cell Culture
The PC-9-GFP human EGFR-mutant NSCLC cell line with stable high-expression of GFP (AntiCancer, Inc., San Diego, CA) was maintained in RPMI-1640 (Mediatech, Inc. Manassas, VA) with 10% fetal bovine serum. All media were supplemented with penicillin and streptomycin. Cells were cultured at 37 °C with 95% air and 5% CO2.
Mice
Athymic nu/nu nude mice (AntiCancer Inc., San Diego, CA), 6–7 weeks old, were used in this study. The animals were fed an autoclaved laboratory rodent diet. All animal studies were conducted in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873–1. Animals were anesthetized by subcutaneous injection of a ketamine mixture (0.02 ml solution of 20 mg/kg ketamine, 15.2 mg/kg xylazine, and 0.48 mg/kg acepromazine maleate). The animals were observed on a daily basis and humanely sacrificed by CO2 inhalation if they met the following humane endpoint criteria: severe tumor burden (more than 20 mm in diameter), prostration, significant body weight loss, difficulty breathing, rotational motion and body temperature drop.
Subcutaneous Tumor Growth
PC-9-GFP cells growing in culture were harvested by trypzinization and washed two times with phosphate-buffered saline (PBS, Mediatech, Inc. Manassas, VA). Cells (2 × 106) were injected subcutaneously into the right flank of mice in a total volume of 100 μl PBS. The subcutaneous tumors were used as the source of tissue for orthotopic implantation into the brain.
Surgical Orthotopic Implantation (SOI) for Establishment of Brain Implantation Model
Tumor pieces (1 mm3) derived from PC-9-GFP subcutaneous tumors growing in the nude mouse were implanted by surgical orthotopic implantation (SOI) onto the left intracranial space of mice. Briefly, a small incision (0.4–0.5 cm) on the top of the head was made and osteotomy was performed with a sharp pointed scalpel to make a flap. A single tumor fragment (1 mm3) was inserted to the subcranial space from the flap to establish the brain tumor model. The wound was closed with 6–0 nylon suture (Ethilon, Ethicon, Inc., Bridgewater, NJ, USA). All procedures of the operation described above were performed with a 7× microscope.
Treatment Study Design
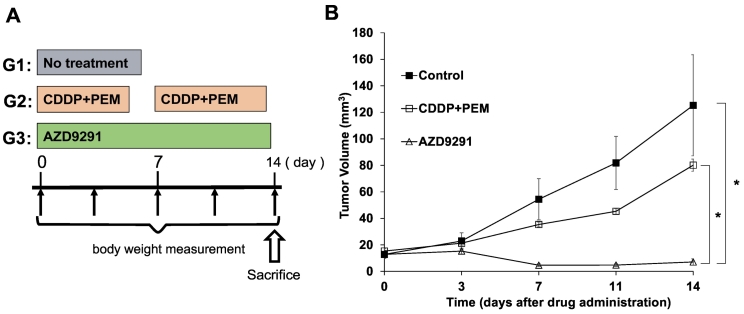
The mouse models were randomized into 3 groups of 8 mice each (Figure 1A): G1, untreated control; G2, cisplatinum (CDDP, 6 mg/kg, intraperitoneal injection [i.p.], once a week for 2 weeks) + pemetrexed (PEM, 100 mg/kg, i.p., once a week for 2 weeks); G3, AZD 9291 (25 mg/kg/day, oral gavage, 14 consecutive days). Treatment started when all tumors reached 10–20 mm3. Tumor length, width and mouse body weight were measured twice in a week using florescence imaging. Tumor volume was calculated with the following formula: Tumor volume (mm3) = length (mm) × width (mm) × width (mm) × 1/2. Data are presented as mean ± standard error of the mean (SEM).
Figure 1.
(A) Treatment regime. (B) Efficacy of the cisplatinum (CDDP) plus pemetrexed (PEM) combination compared to osimertinib (AZD9291) on non-small cell lung cancer (NSCLC) growing in the brain in nude mice. Line graphs indicate tumor volume at each time point after the onset of treatment N = 8 mice/group. * P < .01. Error bars: ± SEM.
Histological Analysis
Fresh tumor samples were fixed in 10% formalin and embedded in paraffin before sectioning and staining. Tissue sections were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (HE) staining was performed according to standard protocol. Ki-67 immunofluorescence staining with anti-Ki-67 antibody (Abcam Ltd., Cambridge, MA), in combination with diamino-benzidine (DAB, Dako Japan Inc., Kyoto, Japan) staining, and hematoxylin counterstaining was performed according to manufacturer's protocols.
Statistical Analysis
All statistical analyses were performed by statistical software EZR (Saitama Medical Center, Jichi Medical University), which is a graphical-user interface for R (The R Foundation for Statistical Computing, version 3.4.1). It is a modified version of R commander (version 2. 4–0) including statistical functions for biostatistics. A normal distribution was assessed with the Shapiro–Wilk test. The Bartlett's test was used to verify the homogeneity of variances across groups. One-way ANOVA with Tukey HSD for post hoc analysis was used for the parametric test for inter-group comparison. Kruskal-Wallis with Steel-Dwass for post hoc analysis was used as the non-parametric test for inter-group comparison. All P-values were two-sided and P-values of 0.05 or less were considered statistically significant.
Results
Efficacy of AZD9291 and CDDP + PEM on PC-9 Growing in the Brain
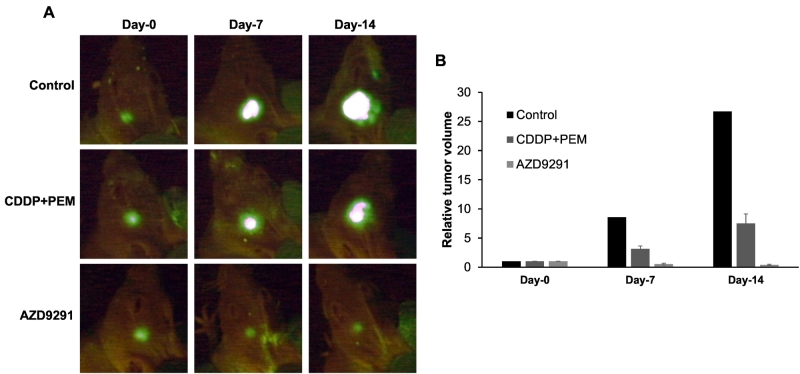
AZD9291 significantly regressed the NSCLC brain metastasis tumors compared to the untreated control and CDDP + PEM groups (AZD9291 vs. untreated control: P = .006, AZD9291 vs. CDDP+PEM: P = .004, Steel-Dwass test, Figure 1) on day 14 after initiation of treatment. The tumor had totally regressed at day 11 in one mouse of the AZD9291 group. CDDP + PEM slightly inhibited tumor growth, although there was no significant difference between the CDDP + PEM and the untreated control groups (P = .98, Figure 1B). Mean tumor volumes at day 14 were as follows: control: 125.3 ± 38.1; CDDP + PEM: 80.1 ± 7.13; and AZD9291: 7.1 ± 2.4 (Figure 2, A and B). The effect of treatment on relative tumor volume is presented in Figure 2B.
Figure 2.
(A) Fluorescence images of representative green fluorescent protein (GFP)-expressing NSCLC growing in the brain of nude mice from each treatment group at each time point after the onset of treatment. The tumors grew rapidly in the control group more than that in CDDP+PEM treated group. The tumors gradually regressed in the mice of the AZD9291 treated group. (B) The mean relative tumor volume capmared to day 0 of representative mice in each group in panel A.
Histology of the Treated and Untreated Tumors in the Brain
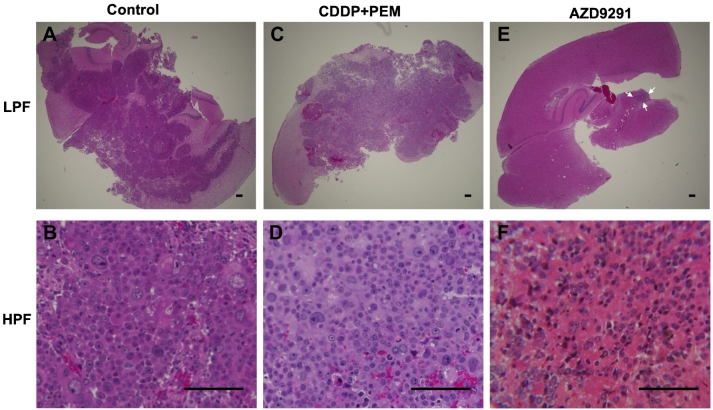
Figure 3 shows the tumor histology of each group. The tumor tissue of the control group mainly comprised viable highly-dense cancer cells with nuclear atypia and atypical mitosis which could be detected in high-power fields (HPF). Spreading cancer cells in the tumor section could be observed in low-power fields (LPF). Tumors treated with CDDP+PEM also comprised highly-dense cancer cells which could be detected by both HPF and LPF, although the cancer-cell density was lower than the control. Only a small area of cancer cells was detected from the LPF of AZD9291-treated tumors with cancer-cell density lower than the control and CDDP+PEM groups. In addition, more scar tissue and hemosiderin deposition in the stroma were detected in the tumors treated by AZD9291.
Figure 3.
Tumor histology. (A) Untreated control. Low-power field (LPF). (B) Untreated control. High-power field (HPF). (C) CDDP+PEM treated. (LPF) (D) CDDP+PEM treated. (HPF). (E) AZD9291 treated. (LPF). (F) AZD9291 treated. (HPF). Scale bars: 100 μm.
Ki-67 Immunofluorescence Staining
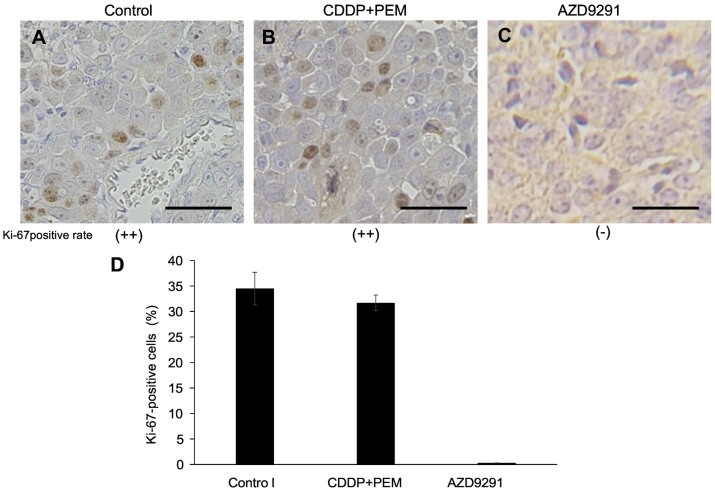
In order to evaluate the proliferative capacity of cancer cells after treatment, immunofluorescence staining with the Ki-67 proliferation marker, which is present during all active phases of the cell cycle (G1, S, G2 and mitosis) and is absent in resting cells (G0), was performed on the tumor sections (Figure 4, A–C). Semi-quantitative Ki-67 positivity was evaluated as either (++, moderate, 30–50% Ki-67-positive cells) in the tumor sections of control and CDDP+PEM treated groups and (−, none, 0%) in the AZD9291-treated group, suggesting of AZD9291 inhibited cancer-cell proliferation (Figure 4, A–D).
Figure 4.
Ki-67 immunohistochemistry. (A) Untreated control. Semi-quantitative Ki-67 positive frequency is evaluated as (++). (B) CDDP+PEM treated. (++). (C) AZD9291 treated. (− ~ +). (D) % of Ki-67 positive cells in each group. Scale bars: 100 μm. Error bars ± SEM.
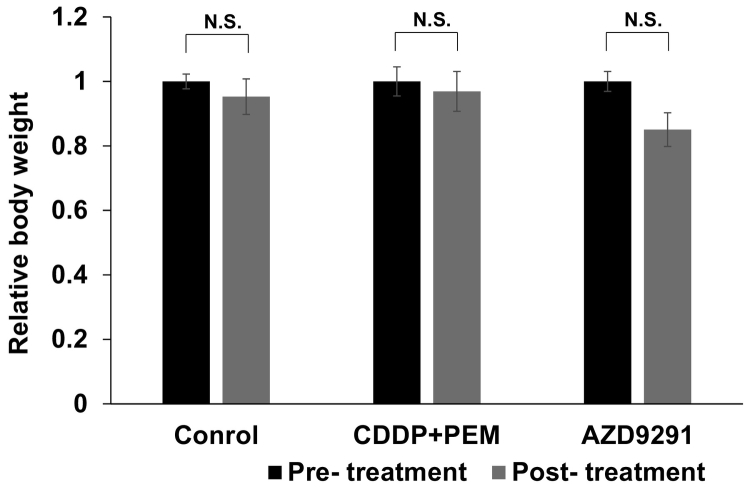
Effect of Treatment on Body Weight
Mouse body weight was measured pre-treatment and post-treatment, and body weight relative to the initial tumor volume was calculated. There was no significant difference in body weight between pre- and post-treatment in any group (Figure 5). There were no other observed side effects.
Figure 5.
Mouse body weight. Bar graphs show mouse body weight relative to the initial body weight for each group at pre- and post-treatment times. N.S., not significant. Error bars: ± SEM.
Discussion
Several first-line treatment options have been developed for patients with NSCLC containing an EGFR-TKI–sensitizing mutation [1], [39]. However, approximately one-third of patients with NSCLC undergo disease progression during treatment with these first-line therapies due to brain metastases [40], [41]. In the present study, evaluation of first-line treatment CDDP+PEM and a new targeted drug, osimertinib, for NSCLC brain metastasis were compared in a model of a GFP-labeled EGFR-mutant NSCLC growing in the brain of nude mice.
A combination of CDDP with PEM (PP) has been a standard treatment for patients with nonsquamous metastatic NSCLC [42], [43], [44], [45], [46]. However, PP therapy has limited efficacy if the disease metastasizes to the brain. Median progression-free survival and overall survival in the nonsquamous NSCLC patients with brain metastasis receiving PP therapy were reported to be 5 months and 11 months, respectively [45]. Chemotherapeutic agents for the treatment of lung-cancer brain metastasis have poor efficacy, which may be due to the BBB [2]. Both CDDP and PEM can cross the BBB to a limited extent. Cerebrospinal fluid penetration of CDDP and PEM are very low, reported to be 3.7% and 1.6%, respectively [47], [48]. In the present study, the efficacy of the CDDP and PEM treatment on the NSCLC brain tumor models was limited and it could not significantly inhibit tumor growth.
Osimertinib is a third-generation of EGFR-tyrosine kinase inhibitors for the treatment of advanced EGFR-mutant NSCLC with improved BBB permeability [1], [49]. A recent study suggests that osimertinib has CNS efficacy in patients with untreated EGFR-mutant NSCLC [50]. Rho et al. [51] showed the efficacy of two small-molecule EGFR-kinase inhibitors that are selective for T790 M-mutant isoforms of EGFR, in a preclinical model of lung cancer and found that both drugs were effective against intracranial metastasis of EGFR-mutant lung adenocarcinoma. Goss et al. [52] reported that osimertinib showed clinically-meaningful efficacy against patients with T790 M-positive advanced NSCLC and CNS metastases. Nishii et al. [53] demonstrated safety and efficacy of osimertinib for NSCLC patients with CNS lesions and poor performance status.
In the present study, we implanted GFP-labeled EGFR-mutant NSCLC tumors orthotopically to the mouse brain to track the intracranial tumors in real time without craniotomy and evaluate the drug's efficacy. The tumor regression caused by osimertinib suggests this drug has potential to achieve efficacy against NSCLC brain metastasis in the clinic.
Conflicts of Interest
The authors declare that they have no competing interests.
Contributor Information
Michael Bouvet, Email: mbouvvet@ucsd.edu.
Shree Ram Singh, Email: singhshr@mail.nih.gov.
Hiroyuki Tsuchiya, Email: tsuchi@med.kanazawa-u.ac.jp.
Robert M Hoffman, Email: all@anticancer.com.
References
- 1.Ballard P., Yates J.W., Yang Z., Kim D.W., Yang J.C., Cantarini M., Pickup K., Jordan A., Hickey M., Grist M. Preclinical comparison of osimertinib with Other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH. Role of chemotherapy on brain metastasis. Prog Neurol Surg. 2012;25:110–114. doi: 10.1159/000331183. [DOI] [PubMed] [Google Scholar]
- 3.Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol. 2014;9(2):195–199. doi: 10.1097/JTO.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 4.Arrieta O., Villarreal-Garza C., Zamora J., Blake-Cerda M., de la Mata M.D., Zavala D.G., Muñiz-Hernández S., de la Garza J. Long-term survival in patients with non-small cell lung cancer and synchronous brain metastasis treated with whole-brain radiotherapy and thoracic chemoradiation. Radiat Oncol. 2011;6:166. doi: 10.1186/1748-717X-6-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang T, Su C, Li X, Zhao C, Zhou F, Ren S, Zhou C, Zhang J. EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol. 2016;11(10):1718–1728. doi: 10.1016/j.jtho.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Noronha V, Joshi A, Gokarn A, Sharma V, Patil V, Janu A, Purandare N, Chougule A, Jambhekar N, Prabhash K. the importance of brain metastasis in EGFR mutation positive NSCLC patients. Chemother Res Pract. 2014;2014 doi: 10.1155/2014/856156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelman M.J., Belani C.P., Socinski M.A., Ansari R.H., Obasaju C.K., Chen R., Monberg M.J., Treat J., Alpha Oncology Research Network Outcomes associated with brain metastases in a three-arm phase III trial of gemcitabine-containing regimens versus paclitaxel plus carboplatin for advanced non-small cell lung cancer. J Thorac Oncol. 2010;5:110–116. doi: 10.1097/JTO.0b013e3181c59a3a. [DOI] [PubMed] [Google Scholar]
- 8.Barlesi F., Gervais R., Lena H., Hureaux J., Berard H., Paillotin D., Bota S., Monnet I., Chajara A., Robinet G. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01) Ann Oncol. 2011;22:2466–2470. doi: 10.1093/annonc/mdr003. [DOI] [PubMed] [Google Scholar]
- 9.Hsu F, De Caluwe A, Anderson D, Nichol A, Toriumi T, Ho C. EGFR mutation status on brain metastases from non-small cell lung cancer. Lung Cancer. 2016;96:101–107. doi: 10.1016/j.lungcan.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Luo S, Lin H, Yang H, Chen H, Liao Z, Lin W, Zheng W, Xie X. Correlation between EGFR mutation status and the incidence of brain metastases in patients with non-small cell lung cancer. J Thorac Dis. 2017;9(8):2510–2520. doi: 10.21037/jtd.2017.07.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosell R., Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., Palmero R., Garcia-Gomez R., Pallares C., Sanchez J.M. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 12.Togashi Y., Masago K., Masuda S., Mizuno T., Fukudo M., Ikemi Y., Sakamori Y., Nagai H., Kim Y.H., Katsura T., Mishima M. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405. doi: 10.1007/s00280-012-1929-4. [DOI] [PubMed] [Google Scholar]
- 13.Sequist L.V., Yang J.C., Yamamoto N., O'Byrne K., Hirsh V., Mok T., Geater S.L., Orlov S., Tsai C.M., Boyer M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 14.Baik CS, Chamberlain MC, Chow LQ. Targeted therapy for brain metastases in EGFR-mutated and ALK-rearranged non-small-cell lung cancer. J Thorac Oncol. 2015;10:1268–1278. doi: 10.1097/JTO.0000000000000615. [DOI] [PubMed] [Google Scholar]
- 15.Park SJ, Kim HT, Lee DH, Kim KP, Kim SW, Suh C, Lee JS. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77(3):556–560. doi: 10.1016/j.lungcan.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 16.Porta R, Sánchez-Torres JM, Paz-Ares L, Massutí B, Reguart N, Mayo C, Lianes P, Queralt C, Guillem V, Salinas P. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37(3):624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 17.Hoffknecht P., Tufman A., Wehler T., Pelzer T., Wiewrodt R., Schütz M., Serke M., Stöhlmacher-Williams J., Märten A., Maria Huber R. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol. 2015;10:156–163. doi: 10.1097/JTO.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuler M., Wu Y.L., Hirsh V., O'Byrne K., Yamamoto N., Mok T., Popat S., Sequist L.V., Massey D., Zazulina V., Yang J.C. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016;11:380–390. doi: 10.1016/j.jtho.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Greig SL. Osimertinib: First Global Approval. Drugs. 2016;76(2):263–273. doi: 10.1007/s40265-015-0533-4. [DOI] [PubMed] [Google Scholar]
- 20.Khozin S, Weinstock C, Blumenthal GM, Cheng J, He K, Zhuang L, Zhao H, Charlab R, Fan I, Keegan P. Osimertinib for the Treatment of Metastatic EGFR T790M Mutation-Positive Non-Small Cell Lung Cancer. Clin Cancer Res. 2017;23(9):2131–2135. doi: 10.1158/1078-0432.CCR-16-1773. [DOI] [PubMed] [Google Scholar]
- 21.Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372(18):1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 22.Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, Kim SW, Kim JH, Planchard D, Felip E. Osimertinib in pretreated T790M positive advanced non-small-cell lung cancer: AURA Study Phase II Extension Component. J Clin Oncol. 2017;35(12):1288–1296. doi: 10.1200/JCO.2016.70.3223. [DOI] [PubMed] [Google Scholar]
- 24.Ramalingam S.S., Yang J.C., Lee C.K., Kurata T., Kim D.W., John T., Nogami N., Ohe Y., Mann H., Rukazenkov Y. Osimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer. J. Clin. Oncol. 2018;36(9):841–849. doi: 10.1200/JCO.2017.74.7576. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Qiu T, Guo L, Ling Y, Gao Y, Ying J, He J. Primary and acquired EGFR T790M-mutant NSCLC patients identified by routine mutation testing show different characteristics but may both respond to osimertinib treatment. Cancer Lett. 2018;423:9–15. doi: 10.1016/j.canlet.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Vansteenkiste J, Reungwetwattana T, Nakagawa K, Cho BC, Dols MAC, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH. CNS response to osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFR-TKI sensitising mutation (EGFRm)-positive advanced non-small cell lung cancer (NSCLC): Data from the FLAURA study. Ann Oncol. 2017;28(Suppl._10) [Google Scholar]
- 27.Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, Hodge R, Kaur P, Brown AP, Ghiorghiu D. CNS Efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3) J Clin Oncol. 2018;36(26):2702–2709. doi: 10.1200/JCO.2018.77.9363. [DOI] [PubMed] [Google Scholar]
- 28.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 29.Kim D, Yang J, Cross D. Preclinical evidence and clinical cases of AZD9291 activity in EGFR-mutant non-small cell lung cancer (NSCLC) brain metastases (BM) Ann Oncol. 2014;25(Suppl._4):iv152. [Google Scholar]
- 30.Reichegger H, Jochum W, Förbs D, Hader C, Früh M. Rapid intracranial response to osimertinib in a patient with epidermal growth factor receptor T790M-positive adenocarcinoma of the lung. Oncol Res Treat. 2016;39(7–8):461–463. doi: 10.1159/000446759. [DOI] [PubMed] [Google Scholar]
- 31.Ricciuti B, Chiari R, Chiarini P, Crinò L, Maiettini D, Ludovini V, Metro G. Osimertinib (AZD9291) and CNS response in two radiotherapy-naïve patients with EGFR-mutant and T790M-positive advanced non-small cell lung cancer. Clin Drug Investig. 2016;36(8):683–686. doi: 10.1007/s40261-016-0411-1. [DOI] [PubMed] [Google Scholar]
- 32.Koba T, Kijima T, Takimoto T, Hirata H, Naito Y, Hamaguchi M, Otsuka T, Kuroyama M, Nagatomo I, Takeda Y. Rapid intracranial response to osimertinib, without radiotherapy, in nonsmall cell lung cancer patients harboring the EGFR T790M mutation: Two Case Reports. Medicine (Baltimore) 2017;96(6) doi: 10.1097/MD.0000000000006087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie L, Nagpal S, Wakelee HA, Li G, Soltys SG, Neal JW. Osimertinib for EGFR-mutant lung cancer with brain metastases: results from a single-center retrospective study. Oncologist. 2018 doi: 10.1634/theoncologist.2018-0264. [pii: theoncologist.2018-0264] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanjo S., Ebi H., Arai S., Takeuchi S., Yamada T., Mochizuki S., Okada Y., Nakada M., Murakami T., Yano S. High efficacy of third generation EGFR inhibitor AZD9291 in a leptomeningeal carcinomatosis model with EGFR-mutant lung cancer cells. Oncotarget. 2016;7:3847–3856. doi: 10.18632/oncotarget.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu S, Li S, Hai J, Wang X, Chen T, Quinn MM, Gao P, Zhang Y, Ji H, Cross DAE. Targeting HER2 aberrations in non-small cell lung cancer with osimertinib. Clin Cancer Res. 2018;24(11):2594–2604. doi: 10.1158/1078-0432.CCR-17-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z, Chen Y, Xu M, Chen L, Zhang X, To KK, Zhao H, Wang F, Xia Z, Chen X. Osimertinib (AZD9291) enhanced the efficacy of chemotherapeutic agents in ABCB1- and ABCG2-overexpressing cells in vitro, in vivo, and ex vivo. Mol Cancer Ther. 2016;15(8):1845–1858. doi: 10.1158/1535-7163.MCT-15-0939. [DOI] [PubMed] [Google Scholar]
- 37.Guo L, Huang S, Wang X. PUMA mediates the anti-cancer effect of osimertinib in colon cancer cells. Onco Targets Ther. 2017;10:5281–5288. doi: 10.2147/OTT.S139382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagano M, Kohsaka S, Ueno T, Kojima S, Saka K, Iwase H, Kawazu M, Mano H. High-throughput functional evaluation of variants of unknown significance in ERBB2. Clin Cancer Res. 2018;24(20):5112–5122. doi: 10.1158/1078-0432.CCR-18-0991. [DOI] [PubMed] [Google Scholar]
- 39.Masters G.A., Temin S., Azzoli C.G., Giaccone G., Baker S., Jr., Brahmer J.R., Ellis P.M., Gajra A., Rackear N., Schiller J.H. Systemic therapy for Stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2015;33:3488–3515. doi: 10.1200/JCO.2015.62.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mujoomdar A., Austin J.H., Malhotra R., Powell C.A., Pearson G.D., Shiau M.C., Raftopoulos H. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology. 2007;242:882–888. doi: 10.1148/radiol.2423051707. [DOI] [PubMed] [Google Scholar]
- 41.Heon S., Yeap B.Y., Britt G.J., Costa D.B., Rabin M.S., Jackman D.M., Johnson B.E. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013;20(4):e300–e306. doi: 10.3747/co.20.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23(25):6207–6219. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto N, Kenmotsu H, Yamanaka T, Nakamura S, Tsuboi M. Randomized phase III study of cisplatin with pemetrexed and cisplatin with vinorelbine for completely resected nonsquamous non-small-cell lung cancer: The JIPANG Study Protocol. Clin Lung Cancer. 2018;19(1):e1–e3. doi: 10.1016/j.cllc.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Zhu W., Røe O.D., Wu C., Li W., Guo R., Gu Y., Liu Y., Shu Y., Chen X. Activity of pemetrexed-based regimen as first-line chemotherapy for advanced non-small cell lung cancer with asymptomatic inoperable brain metastasis: a retrospective study. J Chemother. 2015;27(4):221–226. doi: 10.1179/1973947815Y.0000000005. [DOI] [PubMed] [Google Scholar]
- 46.Scagliotti G.V., Parikh P., von Pawel J., Biesma B., Vansteenkiste J., Manegold C., Serwatowski P., Gatzemeier U., Digumarti R., Zukin M. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Onc. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 47.Jacobs SS, Fox E, Dennie C, Morgan LB, McCully CL, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin Can Res. 2005;11(4):1669–1674. doi: 10.1158/1078-0432.CCR-04-1807. [DOI] [PubMed] [Google Scholar]
- 48.Kumthekar P., Grimm S.A., Avram M.J., Kaklamani V., Helenowski I., Rademaker A., Cianfrocca M., Gradishar W., Patel J., Mulcahy M., McCarthy K., Raizer J.J. Pharmacokinetics and efficacy of pemetrexed in patients with brain or leptomeningeal metastases. J Neurooncol. 2013;112(2):247–255. doi: 10.1007/s11060-013-1055-0. [DOI] [PubMed] [Google Scholar]
- 49.Cross D.A., Ashton S.E., Ghiorghiu S., Eberlein C., Nebhan C.A., Spitzler P.J., Orme J.P., Finlay M.R., Ward R.A., Mellor M.J. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, Bohnet S, Zhou C, Lee KH, Nogami N. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;28 doi: 10.1200/JCO.2018.78.3118. [JCO2018783118] [DOI] [PubMed] [Google Scholar]
- 51.Rho JK, Lee IY, Choi YJ, Choi CM, Hur JY, Koh JS, Lee J, Suh BC, Song HJ, Salgaonkar P. Superior efficacy and selectivity of novel small-molecule kinase inhibitors of T790M-mutant EGFR in preclinical models of lung cancer. Cancer Res. 2017;77(5):1200–1211. doi: 10.1158/0008-5472.CAN-16-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goss G, Tsai CM, Shepherd FA, Ahn MJ, Bazhenova L, Crinò L, de Marinis F, Felip E, Morabito A, Hodge R. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol. 2018;29(3):687–693. doi: 10.1093/annonc/mdx820. [DOI] [PubMed] [Google Scholar]
- 53.Nishii Y, Hataji O, Ito K, Watanabe F, Kobayashi T, D'Alessandro-Gabazza C, Toda M, Taguchi O, Yamamoto N, Gabazza EC. Efficacy of osimertinib in a patient with non-small cell lung cancer harboring epithelial growth factor receptor exon 19 deletion/T790M mutation, with poor performance status. Mol Clin Oncol. 2018;8(2):246–249. doi: 10.3892/mco.2017.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]