Abstract
A green and an aqueous-mediated sonochemical synthesis of 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones from the multi-component reaction of Meldrum's acid, 3,4-methylenedioxy aniline and various aromatic aldehydes is described in the presence of catalytic amount of TiO2 NPs for the first time using high power sonicator. Initially, TiO2 NPs has also been synthesized by the biochemical method using leaf extract of Origanum majorana plant as a reducing and capping agent under sonication. Under the sonication, the catalytic activity of synthesized TiO2 NPs found to be excellent for synthesis of a series of 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones with operational simplicity, high yield under green reaction conditions without any environmental issue. The structure of TiO2 NPs was characterized by FT-IR, SEM, TEM, XRD and EDX studies.
Keyword: Organic chemistry
1. Introduction
Present drug innovation is faced with the challenges of scheming chemical reactions that are extremely able of providing target molecules with structural diversity and molecular complexity in one step by avoiding the use of volatile organic solvents and toxic catalyst. Recently, the development of well-organized and greener multi-component domino reactions has fascinated growing interest in organic synthesis in view of increasing environmental safety [1, 2, 3].
Growing awareness towards green chemistry has led to developing an eco-compatible process for the synthesis of chemical products without using hazardous reagents and solvents. The use of inexpensively and reusable catalyst plays a notable role in the synthesis of biologically active heterocyclic molecules to makes the synthetic protocol eco-friendly as well as economically-convenient. Nowadays, the use of nanoparticles (NPs) in synthetic organic chemistry seems to be an advance approach due to their large reactive surface area with good reactivity and selectivity, which allow the reactions to occurs in mild reaction conditions [4]. In addition, in nano catalyzed multi-component reactions the product isolation is very effortless and the catalyst can be reused for further reactions [5]. Recently, the utilization of TiO2 nanoparticles as proficient and greener heterogeneous catalyst to catalyze various organic reactions [6] has attracted the great attention of researchers because of their unique physical and chemical catalytic properties like high activity, non-toxicity strong oxidizing power [7] and has the closed approximately to an ideal catalysis because of its sustainability and environmental concerns [8].
Many methods such as sol-gel route [9], hydrothermal [10], polyol synthesis [11], and precipitation [12] had been reported for the synthesis of TiO2 nanoparticles (NPs) but all of these methods requires high pressure, energy, temperature, and toxic chemicals. Hence, to avoid these disadvantages, plant-mediated synthesis of NPs regarded as safe, cost-effective, biocompatible, non-toxic and environmentally friendly processes [13]. The literature survey reveals that there are few reports are available on the synthesis of TiO2 nanoparticles using plant extracts e.g. Catharanthus roseus [14a], Eclipta prostrata [14b], Annona squamosa [14c], Morinda citrifolia [14d] Jatropha curcas [14e]. These entire synthetic routes require a time in hours conventionally, for the process to reach completion. Hence, to reduce the synthetic time the use of ultrasonic waves is a remarkable and unique option in the field of nanocrystalline synthesis [15].
Origanum majorana (Lamiaceae) commonly known as "sweet majoram” is a pleasant smelling perennial and herbaceous plant growing up to height 30cm-60cm native to Cyperus, Anatolia, (Turkey) and naturalized in parts of Mediterranean region especially Egypt [16]. It is cultivated all over the world in different part of India, France, Hungry and United States for its flavor and fragrance. The well-dried majorana leaves are commonly used in food seasoning but are also medicinally valuable due to its antioxidant, antiviral, bactericidal, antiseptic and antifungal properties [17, 18]. Earlier it is used as traditional medicines against asthma, headache, and rheumatism. It has various biomolecules such as hydroquinone, sitosterol, flavonoids, tannins, cis-sabinene hydrate, phenolic terpenoids, triacontane and phenolic glycosides and these biomolecules are responsible for synthesis of nanoparticles [19]. The literature survey reveals some reports on the synthesis of silver [20] and ZnO NPs [21] using leaf extract of Origanum majorana, but no report is available on the synthesis of TiO2 NPs using Origanum majorana plant. Hence, due to the remarkable catalytic properties of TiO2 NPs in organic synthesis and well documented antioxidant properties of Origanum majorana [22] in the present paper, we have developed a green method for synthesis of TiO2 NPs using leaf extract of Origanum majorana for the first time.
Further, consumption of organic solvents at industrial scale has led not only to the environmental pollution but also cause many severe diseases and problems such as high cost, toxicity, need of high purity, and biodegradability [23]. In recent years, water as a reaction medium or an additive has emerged as a hot topic in green organic chemistry [24]. Water act as an endowment for the synthetic strategy as it exhibits unique reactivity and selectivity that not only accelerates the reaction rate but also enhances the selectivity even when the reactants are sparingly soluble or insoluble in this medium [25]. The use of sonication is a hub of scientific and industrial researches due to their lot of beneficial advantages. It is a more efficient, green technique and recently widely used in the synthesis of various important heterocyclic compounds and various organic transformations [26]. The use of sonication in organic synthesis has superiorities such as expediency, fewer reaction time, cleanness and controllability over the traditional heating methods which generally require longer reaction times, high temperature and typical reaction conditions. Therefore, nowadays it is a very challenging approach in organic synthesis and full-fill various aspects of green chemistry principles. A good reason to the rate enrichment of organic reactions under sonication can be explained by the acoustic cavitation theory and according to this theory during the irradiation under sonication there is a generation of high microscopic pressure and temperature within a few seconds and due to this rate of reaction increases and reaction occurred in short reaction time [27].
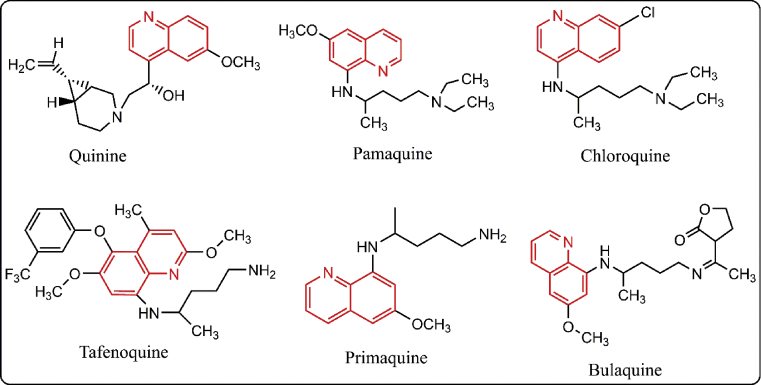
Nitrogen-containing heterocyclic system quinoline is of utmost significance in the field of pharmaceuticals and medicinal chemistry. It is prevalent in a large number of pharmacologically active natural and synthetic compounds such as chloroquine, quinine, amodiaquine, piperaquine, primaquine, mefloquine [28] and many more (Fig. 1). Furthermore, other quinoline derivatives are also possessing various other important biological activities such as antimicrobial, antileishmanial, antioxidant, antiproliferative, antitumor, anticancer and anti-inflammatory [29].
Fig. 1.
Selected quinoline ring containing drugs.
In the recent era, the concept of molecular hybridization or hybrid molecule has emerged great attention to the construction of new molecules by combining two or more pharmacophoric units in one structure [30]. The literature survey reveals that the slight modification or introduction of new group or entities in quinoline molecular frame enhances the remarkable biological profile.
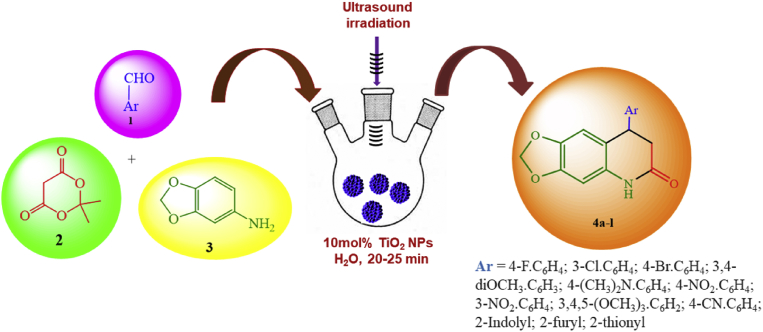
Encouraged, by the promising biological activities of the quinoline substructures and our continued research programme on developing a sustainable synthetic methodology for synthesis of medicinally interesting heterocyclic compounds using ultrasound irradiations [31], we wish to report here an effective synthesis of 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones (4a-l) from three-component reaction of Meldrum's acid 1, 3,4-(methylenedioxy)aniline 2 and various aromatic aldehydes 3 in the presence of catalytic amount of TiO2 NPs in aqueous medium using high intensity probe sonicator (HIU) in excellent yield in shorter reaction time for the first time (Fig. 2). Previously, Azarifar et. al reported the synthesis of other derivatives of the same framework under solvent-free conditions using low-intensity ultrasonic laboratory (LIU) cleaning bath [32]. However, the reported method has own merits but it is well known that the waves generated from an ultrasonic cleaner has significantly less power and energy and is not uniformly available when compared to generated from a direct immersion horn in sonicator [33]. This can lead to reproducibility problem due to the lower power involved for LIU [34]. In the present study, the synthesis of TiO2 NPs has been attempted using leaf extract of Origanum majorana under sonication in order to make whole the process more eco-friendly and convenient. Due to the cavitation effect of ultrasound the synthesis of non-aggregated TiO2 nanoparticles was achieved in reduces time with compared to other methods.
Fig. 2.
Schematic representation for synthesis of desired quinolone hybrids (4a-l).
2. Experimental
2.1. Chemicals and instruments
Melting points of all synthesized compounds were taken on a digital melting point apparatus. FT-IR spectra were recorded on a Perkin-Elmer spectrum version 10.4.00 and using a spectral range of 4000–400 cm−1 with KBr pellets. 1H NMR and 13C NMR spectra of all compounds have been carried out on Jeol Resonance (400MHz) using TMS as an internal reference. Xevo G2-S Q Tof (water, USA) mass spectrometer has been used for a mass spectrum of synthesized compounds. The purity of compounds was checked by TLC using benzene: ethyl acetate (8:2) as the solvent system. XRD measurements were carried out on X-ray diffractometer (Panalytical X Pert Pro) equipped with a CuKα radiation (λ = 1.54060 Å) operated at a voltage of 45 kV and current 40 mA. Surface morphology and Shape of the nanoparticles were estimated by Scanning Electron Microscope [Nova Nano FE-SEM 450 (FEI)]. The structural morphology of the nanoparticles was observed by Transmission Electron Microscope (Tecnai G2 20 (FEI) S-Twin 200 kV). Energy Dispersive Spectroscopy (EDS) was used to determine for identification of elements present in nanoparticles and to analyze its chemical composition. The ultrasound assisted reactions were carried out using an ultrasonic processor probe system (Qsonica700) operating at 20 kHz, 700W with 12 mm tip diameter probes attached with a sensor for temperature measurement. All chemicals used in the present study were purchased from Merck Chemical Company (Darmstadt, Germany) and used as such.
2.2. Synthesis
2.2.1. Preparation of leaf extract of Origanum majorana
Leaves of Origanum majorana are collected and thoroughly washed with water. Take 20 gm of leaves into 100 ml of distilled water at 80 °C for 2 hrs. After cooling, the aqueous extract was filtered using n° Whatman filter paper. The residue was removed and the filtrate was used for the synthesis of nanoparticles.
2.2.2. Synthesis of Titania nanoparticles using Origanum majorana leaf extract
0.5 mol L-1 solution of titanium (IV) isopropoxide was prepared. The 50 mL of freshly prepared leaf extract was mixed to 50 mL of 0.5 mol L-1 titanium (IV) isopropoxide solution and stirred at 500 rpm at 50 °C. After vigorous stirring for 5 h, the color of the mixture gradually changed to white yellowish gel, indicating the formation of TiO2 nanoparticles. The same mixture was immersed using ultrasonic processor probe system (Qsonica700) operating at 20 kHz, 700W with 12 mm tip. The operating conditions were a 30-sec pulse on and 30-sec pulse off time with an amplitude of 50% for 10 min. After 10 min the yellowish gel was obtained. The nanoparticles solution was then centrifuged at 6000 rpm for 20 min in order to clean any residue remaining from the extract. The obtained precipitate was washed with water several times to remove by-products and dried at 100 °C in the oven for overnight.
2.2.3. General procedure for synthesis of 8-(4-fluorophenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-ones (4a-l)
A mixture of Meldrum's acid 1 (1 mmol), 3,4-(methylenedioxy)aniline 2 (1 mmol) aldehydes 3 (1 mmol) and TiO2 NPs (10 mol %) was taken in 20 ml of tap water in a flask. Then, the mixture was sonicated for an appropriate time an amplitude of 50% (power 50 W) with 12 mm probe. After completion of the reaction as indicated by TLC, the solid precipitate was filtered and washed with water (10 mL) and dried to the obtained crude product. Ethyl acetate was added to this crude product and mixture was centrifuged at 10000 rpm for 25 min to separate out the TiO2 NPs. Then separated TiO2 NPs were washed with ethanol and dried to reuse. The product in ethyl acetate was obtained after evaporation and then recrystallized to get pure products.
The synthesized hybrids were characterized by calculating their Melting Point, analyzing their 1H NMR, 13C NMR and mass spectral data (spectra of representative compounds are given in supplementary information). The spectroscopic data revealed the formation of the hybrids.
2.2.3.1. 8-(4-Fluoro-phenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4a)
M.P. 195–200 °C; 1H NMR (400 MHz, DMSO-d6): δ 2.56–2.61 (dd, 1H, CH2, J = 6.4Hz), 2.74–2.80 (dd, 1H, CH2, J = 5.6Hz), 4.17–4.20 (t, 1H, CH, J = 5.6Hz), 5.89 (m, 2H, OCH2), 6.50 (s, 1H, Ar-H), 6.55 (s, 1H, Ar-H), 7.11 (d, 2H, Ar-H, J = 8.4Hz), 7.32 (d, 2H, Ar-H, J = 8.4Hz), 10.00 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): 36.92, 38.28, 97.89, 101.56, 108.68, 118.44, 129.09, 129.69, 131.76, 132.82, 142.24, 142.96, 147.10, 169.08 ppm. MS (ESI) m/z: 286 [M++1]. Anal. calc. for C16H12FNO3: C, 67.36; H, 4.24; N, 4.91%. Found C, 67.48; H, 4.21; N, 4.89%.
2.2.3.2. 8-(4-Bromo-phenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4b)
M.P. 210–212 °C; 1H NMR (400 MHz, DMSO-d6): 2.55–2.60 (dd, 1H, CH2, J = 5.6Hz), 2.74–2.80 (dd, 1H, CH2, J = 6.4Hz), 4.15–4.18 (t, 1H, CH, J = 6Hz), 5.90 (m, 2H, OCH2), 6.50 (s, 1H, Ar-H), 6.54 (s, 1H, Ar-H), 7.05 (d, 2H, Ar-H, J = 8.4Hz), 7.45 (d, 2H, Ar-H, J = 8.4Hz), 10.00 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): 36.28, 38.23, 97.90, 101.57, 108.68, 118.36, 120.35, 130.07, 132.00, 132.83, 142.67, 142.97, 147.11, 169.06 ppm. MS (ESI) m/z: 347 [M++1]. Anal. calc. for C16H12BrNO3: C, 55.51; H, 3.49; N, 4.05%. Found C, 55.63; H, 3.51; N, 4.03%.
2.2.3.3. 8-(3,4-Dimethoxy-phenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4c)
M.P. 205–210 °C; 1H NMR (400 MHz, DMSO-d6):δ 2.68–2.72 (dd, 1H, CH2, J = 5.8 Hz), 2.80–2.87 (dd, 1H, CH2, J = 6.2 Hz), 4.12–4.21 (t, 1H, CH, J = 5.6 Hz), 3.76 (s, 3H, OCH3), 3.81 (s, 3H, OCH3), 5.91 (m, 2H, OCH2), 6.43 (s, 1H, Ar-H), 6.78 (s, 1H, Ar-H), 6.84 (s, 1H, Ar-H) 7.28 (d, 1H, Ar-H, J = 8.2 Hz), 7.49 (d, 1H, Ar-H, J = 8.2 Hz), 10.02 (s, 1H, NH) ppm .13C NMR (100 MHz, DMSO-d6):δ 36.52, 38.34, 55.41, 56.16, 98.89, 112.82, 114.24, 118.79, 122.68, 129.61, 130.65, 133.15, 137.14, 143.92, 144.70, 145.92, 147.41, 147.83, 172.24 ppm. MS (ESI) m/z: 328 [M++1]. Anal. calc. for C18H17NO5: C, 66.05; H, 5.23; N, 4.28%. Found C, 66.20; H, 5.26; N, 4.25%.
2.2.3.4. 8-(4-Dimethylamino-phenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4d)
M.P. 250–252 °C, 1H NMR (400 MHz, DMSO-d6): δ2.58–2.62 (dd, 1H, CH2, J = 6.3 Hz), 2.74–2.79 (dd, 1H, CH2, J = 5.9 Hz), 2.82 (s, 6H, N-CH3), 4.17–4.22 (t, 1H, CH, J = 5.6 Hz), 5.84 (m, 2H, OCH2), 6.52 (s, 1H, Ar-H), 6.66 (s, 1H, Ar-H), 7.23 (d, 2H, Ar-H, J = 8.3 Hz), 7.39 (d, 2H, Ar-H, J = 8.3 Hz), 10.04 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): δ 36.92, 38.16, 42.81, 98.85, 101.42, 107.88, 117.62, 120.15, 129.36, 131.40, 132.28, 141.62, 142.66, 146.71, 169.81 ppm. MS (ESI) m/z: 311 [M++1]. Anal. calc. for C18H18N2O3: C, 69.66; H, 5.85; N, 9.03%. Found C, 69.78; H, 5.83; N, 9.01%.
2.2.3.5. 8-(4-Nitro-phenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4e)
M.P. 248–250 °C; 1H NMR (400 MHz, DMSO-d6): δ 2.54–2.60 (dd, 1H, CH2, J = 6.4Hz), 2.74–2.80 (dd, 1H, CH2, J = 6.4Hz), 4.52–4.63 (t, 1H, CH, J = 5 Hz), 5.90 (m, 2H, OCH2), 6.34 (s, 1H, Ar-H), 6.54 (s, 1H, Ar-H), 7.13 (d, 2H, Ar-H, J = 8.4Hz), 7.35 (d, 2H, Ar-H, J = 8.4Hz), 10.02 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): δ 36.63, 39.79, 98.56, 108.42, 128.18, 128.35, 134.51, 134.67, 137.24, 138.67, 149.88, 151.75, 152.64, 172.32 ppm. MS (ESI) m/z: 313 [M++1]. Anal. calc. for C16H12N2O5: C, 61.54; H, 3.87; N, 8.97 %. Found C, 61.45; H, 3.84; N, 8.95%.
2.2.3.6. 8-(3,4,5-Trimethoxy-phenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4f)
M.P. 241–243 °C; 1H NMR (400 MHz, DMSO-d6):δ 2.64–2.69 (dd, 1H, CH2, J = 6.8Hz), 2.72–2.80 (dd, 1H, CH2, J = 6.4Hz), 4.37–4.42 (t, 1H, CH, J = 5.7Hz), 3.62 (s, 6H, 2-OCH3), 3.68 (s, 3H, OCH3), 5.98 (m, 2H, OCH2), 6.62 (s, 2H, Ar-H), 6.99 (s, 1H, Ar-H), 7.12 (s, 1H, Ar-H), 9.58 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): δ 36.49, 39.17, 55.78, 58.16, 97.43, 105.65, 108.22, 113.54, 131.21, 133.18, 137.24, 138.11, 140.20, 146.55, 152.53, 153.02, 170.89 ppm. MS (ESI) m/z: 358 [M++1]. Anal. calc. for C19H19NO6: C, 63.86; H, 5.36; N, 3.92%. Found C,63.98; H, 5.34; N, 3.90 %.
2.2.3.7. 8-(3-Nitro-phenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4g)
M.P. 176–178 °C; 1H NMR (400 MHz, DMSO-d6):δ 2.52–2.61 (dd, 1H, CH2, J = 6.2Hz), 2.73–2.88 (dd, 1H, CH2, J = 6.2Hz), 4.28–4.52 (t, 1H, CH, J = 5.6Hz), 5.96 (m, 2H, OCH2), 6.79 (s, 1H, Ar-H), 6.82 (s, 1H, Ar-H), 7.01–7.51 (m, 3H, Ar-H), 8.05 (s, 1H, Ar-H), 10.02 (s, 1H, NH) ppm.13C NMR (100 MHz, DMSO-d6):δ 36.86, 38.62, 92.41, 106.55, 121.56, 122.84, 127.91, 130.13, 133.68, 134.74, 143.42, 144.21, 145.73, 146.23, 149.36, 173.74 ppm. MS (ESI) m/z: 313 [M++1]. Anal. calc. for C16H12N2O5: C, 61.54; H, 3.87; N, 8.97%. Found C, 61.66; H, 3.85; N, 8.95%.
2.2.3.8. 4-(6-Oxo-5,6,7,8-tetrahydro-[1,3]dioxolo[4,5-g]quinolin-8-yl)-benzonitrile (4h)
M.P.181–183 °C; 1H NMR (400 MHz, DMSO-d6):δ 2.62–2.74 (dd, 1H, CH2, J = 6.2Hz), 2.82–2.87 (dd, 1H, CH2, J = 6.4Hz), 4.28–4.52 (t, 1H, CH, J = 5.4Hz), 5.92 (m, 2H, OCH2), 6.57 (s, 1H, Ar-H), 6.68 (s, 1H, Ar-H), 7.22 (d, 2H, Ar-H, J = 8.1Hz), 7.42 (d, 2H, Ar-H, J = 8.1Hz), 10.01 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): δ 36.94, 38.62, 98.31, 106.89, 110.58, 115.67, 117.56, 127.84, 128.63, 132.11, 134.47, 142.96, 145.25, 146.85, 173.41 ppm. MS (ESI) m/z: 293 [M++1]. Anal. calc. for C17H12N2O3: C, 69.86; H, 4.14; N, 9.58%. Found C, 69.98; H, 4.12; N, 9.56%.
2.2.3.9. 8-(3-Chloro-phenyl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4i)
M.P. 240–245 °C; 1H NMR (400 MHz, DMSO-d6):δ 2.56–2.64 (dd, 1H, CH2, J = 6.8Hz), 2.79–2.81 (dd, 1H, CH2, J = 6.4Hz), 4.28–4.36 (t, 1H, CH, J = 5.2Hz), 5.98 (m, 2H, OCH2), 6.58 (s, 1H, Ar-H), 6.62 (s, 1H, Ar-H), 7.18–7.31 (m, 3H, Ar-H), 7.56 (s, 1H, Ar-H), 9.97 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): δ 36.73, 38.84, 97.45, 102.20, 108.21, 115.67, 126.95, 127.42, 128.36, 129.21, 131.44, 134.95, 143.53, 144.92, 146.33, 173.62 ppm. MS (ESI) m/z: 302 [M++1]. Anal. calc. for C16H12ClNO3: C, 63.69; H, 4.01; N, 4.64%. Found C, 63.81; H, 4.03; N, 4.62%.
2.2.3.10. 8-Thiophen-2-yl-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4j)
M.P. 257–259 °C; 1H NMR (400 MHz, DMSO-d6): δ. 2.61–2.66 (dd, 1H, CH2, J = 5.6 Hz), 2.82–2.88 (dd, 1H, CH2, J = 6 Hz), 4.40–4.42 (t, 1H, CH, J = 5.6Hz), 5.92 (m, 2H, OCH2), 6.47 (s, 1H, Ar-H), 6.64 (s, 1H, Ar-H), 6.74–6.77 (m, 1H, Ar-H), 6.89 (d, 1H, Ar-H, J = 6.5Hz), 7.29 (d, 1H, Ar-H, J = 5Hz), 9.97 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): 36.93, 38.68, 97.95, 101.58, 108.64, 118.89, 124.81, 125.14, 127.44, 132.41, 142.81, 147.13, 147.23, 168.84 ppm. MS (ESI) m/z: 274 [M++1]. Anal. calc. for C14H11NO3S: C, 61.52; H, 4.06; N, 5.12 %. Found C,61.64; H, 4.04; N,5.10%.
2.2.3.11. 8-(2H-Isoindol-1-yl)-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4k)
M.P. 252–254 °C; 1H NMR (400 MHz, DMSO-d6): δ. 2.56–2.62 (dd, 1H, CH2, J = 5.7Hz), 2.79–2.81 (dd, 1H, CH2, J = 6.1Hz), 4.37–4.42 (t, 1H, CH, J = 5.6Hz), 5.98 (m, 2H, OCH2), 6.74 (s, 1H, Ar-H), 6.82 (s, 1H, Ar-H), 7.04–7.89 (m, 5H, Ar-H), 9.23 (s, 1H, NH), 10.03 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): δ 36.76, 38.21, 98.32, 102.22, 103.03, 111.71, 112.54, 117.52, 122.56, 123.45, 128.81, 136.10, 140.06, 146.97, 147.83, 169.62 ppm. MS (ESI) m/z: 307 [M++1]. Anal. calc. for. C18H14N2O3: C, 70.58; H, 4.61; N, 9.15%. Found C, 70.70; H, 4.59; N, 9.13%.
2.2.3.12. 8-Furan-2-yl-7,8-dihydro-5H-[1,3]dioxolo[4,5-g]quinolin-6-one (4l)
M.P. 235–237 °C; 1H NMR (400 MHz, DMSO-d6):δ 2.56–2.60 (dd, 1H, CH2, J = 5.8 Hz), 2.80–2.86 (dd, 1H, CH2, J = 6.4 Hz), 4.40–4.42 (t, 1H, CH, J = 5.4 Hz), 5.92 (m, 2H, OCH2), 6.57 (s, 1H, Ar-H), 6.70 (s, 1H, Ar-H), 6.71–6.79 (m, 1H, Ar-H), 6.88 (d, 1H, Ar-H, J = 6.4Hz), 7.21 (d, 1H, Ar-H, J = 5Hz), 9.98 (s, 1H, NH) ppm. 13C NMR (100 MHz, DMSO-d6): 36.71, 38.50, 98.93, 109.56, 116.25, 120.74, 128.89, 131.68, 132.57, 134.81, 141.94, 142.78, 146.23, 172.60 ppm. MS (ESI) m/z: 258 [M++1]. Anal. calc. for C14H11NO4: C, 65.37; H, 4.31; N, 5.44 %. Found C, 65.49; H, 4.29; N, 5.42%.
3. Results and discussion
3.1. Preparation and characterization of TiO2 NPs
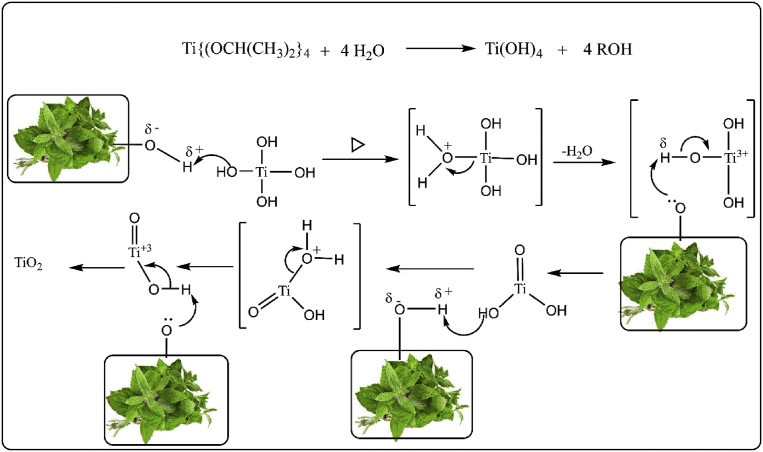
In the present paper, we report the fast synthesis of TiO2 NPs using Origanum majorana leaf extract using high-intensity sonicator in ambient conditions. Sonochemical synthesis of nanoparticles is based on the decomposition of precursor and its reduction and stabilization by appropriate agents present in the reaction mixture. The leaves extract of herb Origanum majorana used as the reducing and stabilizing agent. Origanum majorana is a herb of Lamiaceae family and this family known to contain a range of terpenoids and flavonoids. When a mixture of titanium (IV) isopropoxide and aqueous leaves extract of Origanum majorana was sonicated under high-intensity probe sonicator the TiO2 NPs was formed through the hydrolysis of precursor titanium (IV) isopropoxide to titanium hydroxide in presence of water and subsequently condensation of Ti(OH)4 to TiO2 NPs. The presences of various hydroxyl group compounds in extracts of Origanum majorana are responsible for the antioxidant capacity [19, 22] and catalyzed the condensation reaction and are also responsible for the stabilization of TiO2 NPs.
For the comparative study, the synthesis of TiO2 NPs has also been carried out conventionally but the ultrasound-assisted approach offered the advantage of reducing the reaction time to 20 min which was 5h in the case of the conventional approach. The reduction in the reaction time is due to the formation of non-aggregated nanoparticles within a short time and higher yield. Hence, this ultrasound assisted photosynthesis of TiO2 nanoparticle considered to be an effective method for their synthesis. The plausible mechanism for the synthesis of TiO2 in presence of leaf extract of Origanum majorana is presented in Fig. 3. Synthesized TiO2 NPs are characterized by FT-IR, SEM, TEM, XRD and EDX studies.
Fig. 3.
Possible reaction mechanism for the formation of TiO2 NPs in presence of hydroxyl group (-OH) of leaf extract of Origanum majorana as a capping agent.
3.1.1. Fourier transfer infrared spectroscopy
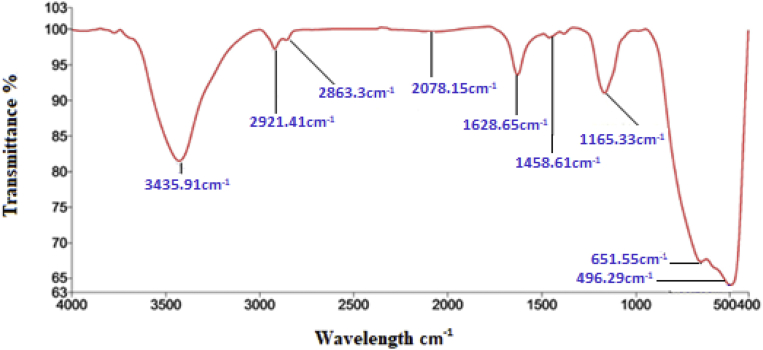
FT-IR spectrum of synthesized TiO2 NPs indicates the presence of various biomolecules localized on the surface of nanoparticles and responsible for the synthesis of TiO2 NPs. The absorption peaks at 3435.91, 2921.41, 2863.3 cm−1 confirm the presence of OH and CH3 & CH2 groups. Some peaks at 1628.65, 1458.61, 1165.33 cm−1 represents the stretching for C=C aromatic, CH3 bending, C-O stretching vibrations, respectively. The absorption peaks at 651.55 cm−1 and 496.29 cm−1 confirms the formation of TiO2 NPs (Fig. 4).
Fig. 4.
FT-IR of TiO2 nanoparticles synthesized using leaf extract.
3.1.2. X-ray diffraction
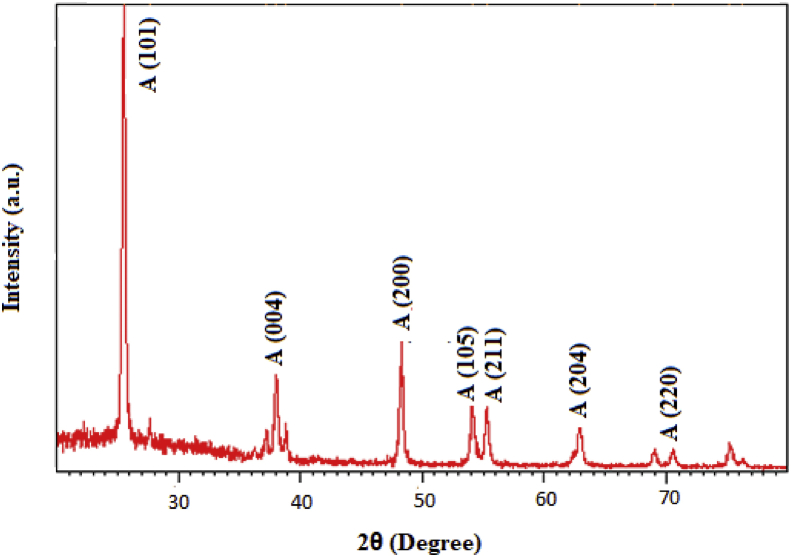
XRD pattern of Origanum majorana leaf broth reduced TiO2 NPs was carried out on X-ray diffractometer equipped with a CuKα radiation (λ = 1.54060Å) operated at voltage of 45 kV and current 40 mA (Fig. 5). The seven distinct peaks at 2θ values of 25.50, 38.80, 54.07, 55.28, 62.87, 69.07, 70.49 correspond to the diffraction of (101), (004), (112), (105), (211), (204), (220) crystal planes respectively that are matched with standard JCPDS (fill no 21–1272) clearly indicating the high purity anatase phase of TiO2 NPs. Furthermore, the size of the crystallite of TiO2 nanoparticles was estimated using the Debye-Scherrer equation.
Fig. 5.
XRD pattern of TiO2 nanoparticles.
Where D is crystallite diameter in Å, K is Scherer constant (average value 0.9), λ is X-ray wavelength, θ is diffraction angle, and β is full width at half maximum of XRD peak (in radian). The calculated average crystallite size of plant synthesized TiO2 nanoparticles was found to be in the range of 20 nm.
3.1.3. Scanning electron microscopy
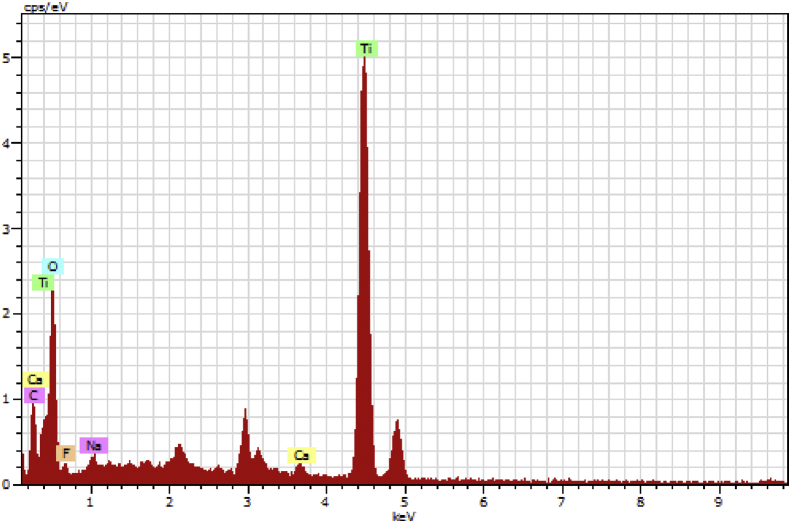
SEM analysis shows that nanoparticles formed clusters of aggregates, at scale bar 1μm and 200 nm (Fig. 6a and b) they appeared as a spherical shape with a particle size less than 20 nm as indicated in TEM (Fig. 7). The energy dispersive X-ray analysis study (EDX) shows the presence of titanium and oxygen (Fig. 8). The peaks due to carbon, oxygen, calcium, and sodium indicate the extracellular organic moieties are adsorbed on the surface of the metallic nanoparticles.
Fig. 6.
a) SEM image of TiO2 at inset bar: 1μm. b) SEM image of TiO2 at inset bar: 200 nm.
Fig. 7.
TEM image of TiO2 NPs.
Fig. 8.
EDX showing chemical composition in TiO2 NPs.
3.2. Optimization of reaction conditions for the synthesis of 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones
3.2.1. Effect of various catalyst and solvent on the synthesis of 4a
After the synthesis and characterization of TiO2 NPs as a catalyst, for optimizing the various conditions and parameters, we have chosen a model reaction of Meldrum's acid 1, 3,4-(methylenedioxy)aniline 2 and 4-fluorobenzeldehyde 3 for investigation. Initially, the model reaction has been carried out under ultrasonic irradiation in presence of a different catalyst to afford desired derivative 4a (Table 1, entries 1–6) in aqueous medium using various acid catalysts such as p-TSA, boric acid and sulfamic acid but only moderate yield of desired product was obtained (Table 1, entries 1–3).
Table 1.
Optimization of reaction conditions under ultrasound irradiation for the synthesis of 4aa.
| S.No. | Catalyst (mol%) | Solvent | Time (min) | ∗Yield (%) |
|---|---|---|---|---|
| 1 | p-TSA (10 mol %) | H2O | 35 | 50 |
| 2 | Boric acid (10 mol %) | H2O | 35 | 60 |
| 3. | Sulfamic acid (10 mol %) | H2O | 35 | 61 |
| 4 | ZnO NPs (10 mol %) | H2O | 35 | 72 |
| 5. | MgO NPs (10 mol %) | H2O | 35 | 74 |
| 6. | TiO2NPs (10 mol %) | H2O | 15 | 90 |
| 7. | Commercial TiO2 (10 mol %) | H2O | 15 | 79 |
| 8. | TiO2 NPs (10 mol %) | 1,4-dioxane | 30 | 70 |
| 9. | TiO2 NPs (10 mol %) | Acetonitrile | 30 | 72 |
| 10. | TiO2 NPs (10 mol %) | CH2Cl2 | 30 | 75 |
| 11. | Catalyst-free | H2O | 40 | traces |
| 12 | TiO2 NPs (5 mol %) | H2O | 15 | 70 |
| 13. | TiO2 NPs (15 mol %) | H2O | 15 | 90b |
Bold signifies the best reaction conditions for the present reaction.
Meldrum's acid (1 mmol), 3,4-(methylenedioxy)aniline (1 mmol) and 4-fluorobenzaldehyde (1 mmol), H2O (20 mL).
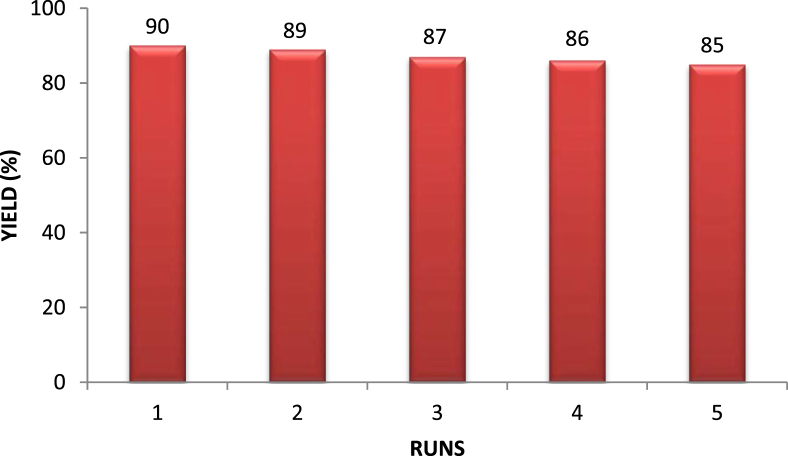
Yields of the five consequent runs by using the same TiO2 recovered NPs were 90, 89, 87, 86 and 85 %, respectively.
Isolated Yield.
Further, to enhances the yield the model reaction has also been checked in presence of various nanocatalysts of metal oxide such as ZnO, MgO, and TiO2 (Table 1, entries 4–6) and from the assessment of screening results, it is clearly apparent that out of other metal oxides TiO2 nanoparticles was found best catalyst for this reaction. To check the particle size of TiO2 the model reaction was also examined in the presence of commercial TiO2 (Table 1, entry 7) but the yield of synthesized compound 4a was comparatively low as obtained in case of TiO2 NPs. This may be due to its largest surface area and presence of the most reactive acidic sites due to its nano-sized nature.
After that, to check the effect of solvent the model reaction was also performed in various organic solvents such as 1,4-dioxane, CH3CN and CHCl2 (Table 1, entries 8–10) under sonication in presence of TiO2 NPs. The results obtained from Table 1 showed that using water as solvent best results were found that compared to other tested solvents which make this strategy further greener and suitable. The role of solvents under sonication is playing an important role as Imax (maximum cavitation intensity) and TImax (the temperature at Imax is reached) of any solvent have a profound effect on sonochemical reactivity for water Imax is 100, the Imax of water is responsible for the increase in the reaction rate compared to the other solvents for which Imax is lower [35].
Furthermore, an experiment was conducted in the absence of a catalyst (Table 1, entry 11). But only traces of product were obtained after 40 min under sonication. Therefore, TiO2 was important for this reaction. The effect of TiO2 NPs amount was also checked by changing the loading amount to 5%, 10%, and 15%, it was observed that 10 mol % of TiO2 NPs provided the upper limit yield (Table 1, entry12-13).
3.2.2. Effect of various power and amplitude of ultrasound sonicator on the synthesis of 4a
Further, to check the role of ultrasound and effect of various powers/amplitude of sonicator the model reaction in presence of optimizing catalyst and solvent has been performed by high stirring alone under a silent condition at room temperature (Table 2, entry -1). The results obtained from this experiment show that the yield of the desired product is poorer after continuing stirring for a longer time. This study shows the specific effect of ultrasound irradiation.
Table 2.
The effect of ultrasound irradiation on the synthesis of quinolone derivative 4a.
| Entry | Power (W)/Amplitude | Time (min.) | Yield∗ (%) |
|---|---|---|---|
| 1. | Silencec | 160 | 60 |
| 2 | 30 | 40 | 60 |
| 3. | 35 | 30 | 72 |
| 4. | 40 | 20 | 80 |
| 5. | 45 | 15 | 85 |
| 6. | 50 | 15 | 90 |
| 7 | 55 | 15 | 90 |
Without ultrasonic irradiation.
Isolated Yield.
To verify the effect of power/amplitude of sonicator the model reaction also performed at different power to obtained the target product in excellent yields (Table 2, entry 2–7). The results show that power of ultrasound sonicator play an important role on the rate of reaction as shown in (Table 2) the increasing power from 25 to 50 W enhances the yield of desired product with reducing the reaction time.
The possible explanation of this effect is due to the increasing number of active cavitation bubbles and resultant maximum collapse temperature. Hence, during this study, we employed various reaction conditions and it was found that best results have been obtained using 10 mol % of TiO2 NPs in an aqueous medium under sonication at power 50 watts. Furthermore, after the completion of the reaction, the catalyst was easily recovered and reused. Recovered TiO2 used for several times for the model reaction under optimize reaction conditions and it was observed that the catalytic activity of TiO2 NPs was not lost and after five runs there is no notable change observed in yields (Fig. 9).
Fig. 9.
Catalyst recyclability study on the synthesis of 4a.
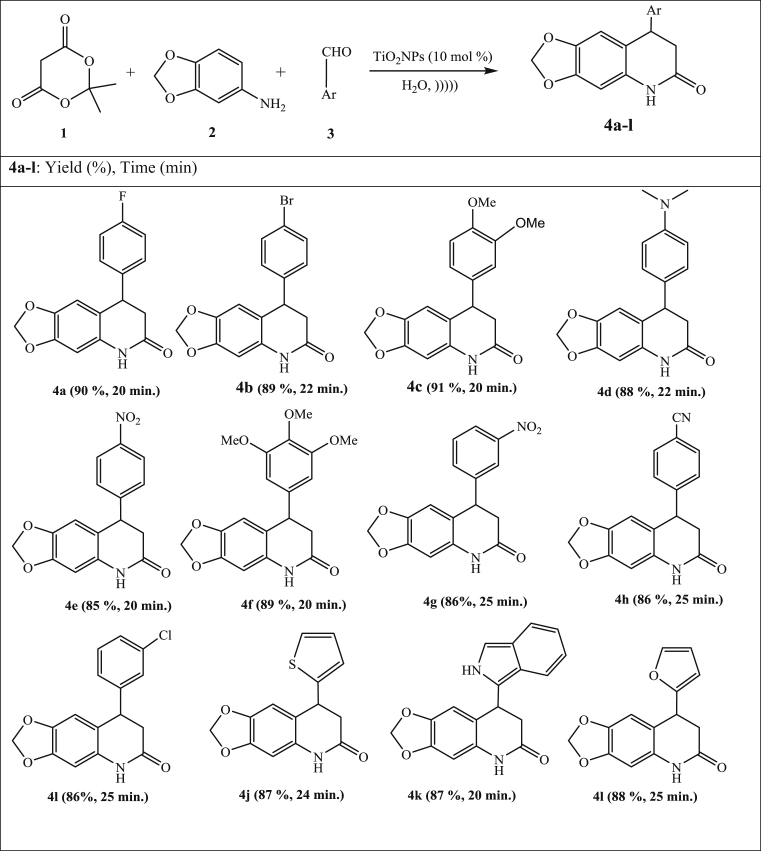
Under the optimized reaction conditions, this new Meldrum's acid-based multi-component reaction was extended for the synthesis of a series of 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones 4a-l using various aromatic aldehydes to explore the extent and generality of present reaction. There is no considerable effect has been observed due to nature and position of substituent's present on the aldehydes and reaction also proceeds smoothly in case of various heteroaldehydes such as thiophene-2-aldehyde, indole-2-carboxaldehyde, 2-furaldehyde leads to the formation of corresponding, 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones 4j-l in good yield under sonication using TiO2 NPs as catalyst and water as solvent. The results are summarized in Fig. 10.
Fig. 10.
Scope for the quinolone hybrids under optimized conditions.
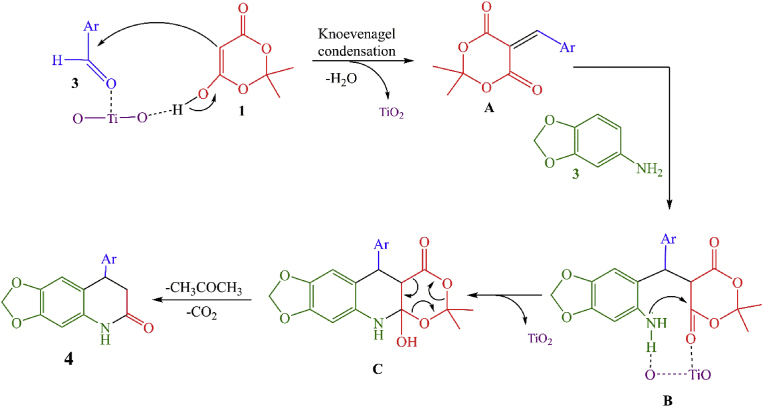
Due to the dual catalytic property of Lewis acid and base of TiO2 metal oxide the plausible mechanism suggested for the synthesis of 4a-l shown in Fig. 11. It is assumed that TiO2 NPs are coordinate with the carbonyl oxygen of aldehyde and it becomes activated. Then, the Knoevenagel condensation reaction between the activated aldehyde and Meldrum's acid 1 gives Knoevenagel adduct A. In next step Michael-type addition of 3,4-(methylenedioxy)aniline 3 take place with A to yielding intermediate B which subsequently undergoes intramolecular cyclization to form C, then after the desired quinoline hybrids, 4 was formed by releasing CO2 and acetone.
Fig. 11.
The proposed mechanism for synthesis of 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones (4a-l).
4. Conclusions
In summary, biologically, and pharmaceutically relevant new quinolone hybrids were synthesized by three-component reaction of Meldrum's acid, 3,4-(methylenedioxy)aniline and various aromatic aldehydes under ultrasonic irradiation in presence of a catalytic amount of TiO2 nanoparticles. In the presence of TiO2 NPs different substituted 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-ones were prepared by easy procedure with high atom economy, excellent yields, and mild reaction conditions. TiO2 NPs were also synthesized under ultrasound irradiation by a direct interaction of titanium (IV) isopropoxide and Origanum majorana leaves extract as reducing and capping agent. After the completion of the reaction, the prepared TiO2 NPs has been easily recovered and catalytic activity of catalyst remains unchanged after use.
Declarations
Author contribution statement
Diksha Bhardwaj, Aakash Singh: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ruby Singh: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Ruby Singh was supported by DST, New Delhi with a start-up grant (SERB) (YSS/2015/000972).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are thankful to the MNIT, Jaipur for the characterization of synthesized compounds and catalyst.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Shukla G., Verma R.K., Verma G.K., Singh M.S. Solvent-free sonochemical one-pot three-component synthesis of 2H-indazolo[2,1-b]phthalazine-1,6,11-triones and 1Hpyrazolo[1,2-b]phthalazine-5,10-diones. Tetrahedron Lett. 2011;52:7195. [Google Scholar]
- 2.(a) Staben S.T., Blaquiere N. Four-component synthesis of fully substituted 1,2,4-triazoles. Angew. Chem. Int. Ed. 2010;49:325. doi: 10.1002/anie.200905897. [DOI] [PubMed] [Google Scholar]; (b) Ma N., Jiang B., Zhang G., Tu S.-J., Wever W., Li G. New multicomponent domino reactions (MDRs) in water: highly chemo-, regio- and stereoselective synthesis of spiro{[1,3]dioxanopyridine}- 4,6-diones and pyrazolo[3,4-b]pyridines. Green Chem. 2010;12:1357. [Google Scholar]
- 3.(a) Haurena C., Gall E.L., Sengmany S., Martens T., Troupel M. A Straightforward Three-component synthesis of α-amino esters containing a phenylalanine or a phenylglycine scaffold. J. Org. Chem. 2010;75:2645. doi: 10.1021/jo1002328. [DOI] [PubMed] [Google Scholar]; (b) Chen W.-B., Wu Z.-J., Pei Q.-L., Cun L.-F., Zhang X.-M., Yuan W.-C. Highly enantioselective construction of spiro[4H-pyran-3,3′-oxindoles] through a domino Knoevenagel/Michael/cyclization sequence catalyzed by cupreine. Org. Lett. 2010;12:3132. doi: 10.1021/ol1009224. [DOI] [PubMed] [Google Scholar]; (c) Heravi M.M., Baghernejad B., Oskooie H.A., Hekmatshoar R. A novel and facile synthesis of 2-(cyclohexylamino)-6,7-dihydro-3-aryl-1H-indole-4(5H)-ones via a one-pot multi-component reaction. Tetrahedron Lett. 2008;49:6101. [Google Scholar]
- 4.(a) Herves P., Lorenzo M.P., Liz-Marzan L.M., Dzubiella J., Lu Y., Ballauff M. Catalysis by metallic nanoparticles in aqueous solution: model reactions. Chem. Soc. Rev. 2012;41:5577. doi: 10.1039/c2cs35029g. [DOI] [PubMed] [Google Scholar]; (b) Takale B.S., Bao M., Yamamoto Y. Gold nanoparticle (AuNPs) and gold nanopore (AuNPore) catalysts in organic synthesis. Org. Biomol. Chem. 2014;12:2005. doi: 10.1039/c3ob42207k. [DOI] [PubMed] [Google Scholar]
- 5.(a) Santra S., Bagdi A.K., Majee A., Hajra A. Metal nanoparticles in “on-water” organic synthesis: one-pot nano CuO catalyzed synthesis of isoindolo[2,1-a]quinazolines. RSC Adv. 2013;3:24931. [Google Scholar]; (b) Tailor Y., Khandelwal S., Kumara Y., Awasthi K. M. Kumar Gold nanoparticle (AuNPs) and gold nanopore (AuNPore) catalysts in organic synthesis. RSC Adv. 2015;15:46415. [Google Scholar]
- 6.Rahimizadeh M., Bakhtiarpoor Z., Eshghi H., Pordel M., Rajabzadeh G. TiO2 nanoparticles: an efficient heterogeneous catalyst for synthesis of bis(indolyl)methanes under solvent-free conditions. Monatsh. Chem. 2009;140:1465. [Google Scholar]
- 7.(a) Rana S., Brown M., Dutta A., Bhaumik A., Mukhopadhyay C. Site-selective multicomponent synthesis of densely substituted 2-oxo dihydropyrroles catalyzed by clean, reusable, and heterogeneous TiO2 nanopowder. Tetrahedron Lett. 2013;54:1371. [Google Scholar]; (b) Abdolmohammadi S. Simple route to indeno[1,2-b]quinoline derivatives via a coupling reaction catalyzed by TiO2 nanoparticles. Chin. Chem. Lett. 2013;24:318. [Google Scholar]; (c) Mondal A., Rana S., Mukhopadhyay C. One-pot, expeditious and chromatography-free synthesis of new chromeno[4,3- e][1,3]oxazine derivatives catalyzed by reusable TiO2 nanopowder at room temperature. Tetrahedron Lett. 2014;55:3498. [Google Scholar]
- 8.(a) Kuo C.H., Poyraz A.S., Jin L., Meng Y., Pahalagedara L., Chen S.Y., Kriz D.A., Guild C., Gudza A., Suib S.L. Heterogeneous acidic TiO2 nanoparticles for efficient conversion of biomass derived carbohydrates. Green Chem. 2014;16:785. [Google Scholar]; (b) Ayati A., Ahmadpour A., Bamoharram F.F., Tanhaei B., Manttari M., Sillanp M. A review on catalytic applications of Au/TiO2 nanoparticles in the removal of water pollutant. Chemosphere. 2014;107:163. doi: 10.1016/j.chemosphere.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Colleoni C., Massafra M.R., Rosace G. Photocatalytic properties and optical characterization of cotton fabric coated via sol–gel with non-crystalline TiO2 modified with poly(ethylene glycol) Surf. Coating. Technol. 2012;207:79. [Google Scholar]
- 10.Tan Z., Sato K., Ohara S. Synthesis of layered nanostructured TiO2 by hydrothermal method. Adv. Powder Technol. 2015;26:296. [Google Scholar]
- 11.Tripathy S.K., Sahoo T., Mohapatra M., Anand S., Yu Y.T. Polyol-assisted synthesis of TiO2 nanoparticles in a semi-aqueous solvent. J. Phys. Chem. Solid. 2009;70:147. [Google Scholar]
- 12.Morales J., Maldonado A., Olvera M.D.L. 10th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE), Mexico City, 30 September–4 October. 2013. Synthesis and characterization of nanostructured TiO2 anatase-phase powders obtained by the homogeneous precipitation method; p. 391. [Google Scholar]
- 13.Laura C., Ma Luisa B., Ángel M.J., Gonzalez G.F., Antonio B. Mechanism and applications of metal nanoparticles prepared by bio-mediated process. Rev. Adv. Sci. Eng. 2014;3:199. [Google Scholar]
- 14.(a) Velayutham K., Rahuman A.A., Rajakumar G., Santhoshkumar T., Marimuthu S., Jayaseelan C. Evaluation of Catharanthus roseus leaf extract-mediated biosynthesis of titanium dioxide nanoparticles against Hippobosca maculata and Bovicola ovis. Parasitol. Res. 2012;111:23. doi: 10.1007/s00436-011-2676-x. [DOI] [PubMed] [Google Scholar]; (b) Rajakumar G., Abdul Rahuman A., Priyamvada B., Gopiesh Khanna V., Kishore Kumar D., Sujin P.J., Rajakumar G., Abdul Rahuman A., Priyamvada B., Gopiesh Khanna V., Kishore Kumar D., Sujin P.J. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater. Lett. 2012;68:115. [Google Scholar]; (c) Roopan S.M., Bharathi A., Prabhakarn A., Rahuman A.A., Velayutham K., Rajakumar G. Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012;98:86. doi: 10.1016/j.saa.2012.08.055. [DOI] [PubMed] [Google Scholar]; (d) Sundrarajan M., Bama K., Bhavani M., Jegatheeswaran S., Ambika S., Sangili A., Nithya P., Sumathi R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photochem. Photobiol. B. 2017;171:117. doi: 10.1016/j.jphotobiol.2017.05.003. [DOI] [PubMed] [Google Scholar]; (e) Goutam S.P., Saxena G., Singh V., Yadav A.K., Bharagava R.N., Thapa K.B. Green synthesis of TiO2 nanoparticles using leaf extracts of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018;336:386. [Google Scholar]
- 15.(a) Suslick K.S., Price G.J. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999;29:295. [Google Scholar]; (b) Cubillana Aguilera L.M., Franco Romano M., Gil Montero M.L.A., Naranjo Rodriguez I., Hidalgo de Cisneros J.L., Palacios Santander J.M. New, fast and green procedure for the synthesis of gold nanoparticles based on sonocatalysis. Ultrason. Sonochem. 2011;18:789. doi: 10.1016/j.ultsonch.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Novak J., Langbehn J., Pank F., Franz C.M. Essential oil compounds in a historical sample of marjoram (Origanum majorana L., Lamiaceae) Flavour Frag. J. 2002;17:175. [Google Scholar]
- 17.el-Ashmawy I.M., el-Nahas A.F., Salama O.M. Protective effect of volatile oil, alcoholic and aqueous extracts of Origanum majorana on lead acetate toxicity in Mice. Basic Clin. Pharmacol. Toxicol. 2005;97:238. doi: 10.1111/j.1742-7843.2005.pto_136.x. [DOI] [PubMed] [Google Scholar]
- 18.Zaidi S.F.H., Yamada K., Kadowaki M., Usmanghani K., Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J. Ethnopharmacol. 2009;121:286. doi: 10.1016/j.jep.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Roby M.M.H., Sarhan M.A., Selim K.A.H., Khalel K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013;43:827. [Google Scholar]
- 20.Zahran M., El-Kemary M., Khalifa S., El-Seedi H. Spectral studies of silver nanoparticles biosynthesized by Origanum majorana. Green Process. Synth. 2018;7:100. [Google Scholar]
- 21.Mohammadian M., Eshaghi Z., Hooshmand S. Green and chemical synthesis of zinc oxide nanoparticles and size evaluation by UV–vis spectroscopy. J. Nanomed. Res. 2018;7:00175. [Google Scholar]
- 22.Shan B., Cai Y.Z., Sun M., Corke H. Antioxidant Capacity of 26 Spice extracts and characterization of their phenolic constituents. J. Agric. Food Chem. 2005;53:7749. doi: 10.1021/jf051513y. [DOI] [PubMed] [Google Scholar]
- 23.Clark J.H. Green chemistry: challenges and opportunities. Green Chem. 1999;1:1. [Google Scholar]
- 24.(a) LI C.J. Organic reactions in aqueous media with a focus on carbon−carbon bond formations: a decade update. Chem. Rev. 2005;105:3095. doi: 10.1021/cr030009u. [DOI] [PubMed] [Google Scholar]; (b) Butler R.N., Coyne A.G. Water: nature’s reaction enforcer-comparative effects for organic synthesis “in-water” and “on-water”. Chem. Rev. 2010;110:6302. doi: 10.1021/cr100162c. [DOI] [PubMed] [Google Scholar]
- 25.Chanda A., Fokin V.V. Organic synthesis “on water”. Chem. Rev. 2009;109:725. doi: 10.1021/cr800448q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(a) Banerjee B. Recent developments on ultrasound assisted catalyst-free organic synthesis. Ultrason. Sonochem. 2017;35:1. doi: 10.1016/j.ultsonch.2016.09.023. [DOI] [PubMed] [Google Scholar]; (b) Mosslemin M.H., Nateghi M.R. Rapid and efficient synthesis of fused heterocyclic pyrimidines under ultrasonic irradiation. Ultrason. Sonochem. 2010;17:162. doi: 10.1016/j.ultsonch.2009.07.002. [DOI] [PubMed] [Google Scholar]; (c) Fonseca S.F., Padilha N.B., Thurow S., Roehrs J.A., Savegnago de Souza L., Fronza M.G., Collares T., Buss J., Seixas F.K., Alves D., Lenardao E.J. Ultrasound-promoted copper-catalyzed synthesis of bis-arylselanyl chrysin derivatives with boosted antioxidant and anticancer activities. Ultrason. Sonochem. 2017;39:827. doi: 10.1016/j.ultsonch.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 27.(a) Cravotto G., Cintas P. Power ultrasound in organic synthesis: moving cavitational chemistry from academia to innovative and large-scale applications. Chem. Soc. Rev. 2006;35:180. doi: 10.1039/b503848k. [DOI] [PubMed] [Google Scholar]; (b) Mason T.J. Ultrasound in synthetic organic chemistry. Chem. Soc. Rev. 1997;26:443. [Google Scholar]
- 28.Marella A., Tanwar O.P., Saha R., Ali M.R., Srivastava S., Akhter M., Shaquiquzzaman M., Alam M.M. Quinoline : a versatile heterocyclic. Saudi Pharm J. 2013;21:1. doi: 10.1016/j.jsps.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(a) Daoud R., Desneves J., Deady L.W., Tilley L., Scheper R.J., Gros P., Georges E. The multidrug resistance protein is photoaffinity labeled by a quinoline-based drug at multiple sites. Biochemistry. 2000;39:6094. doi: 10.1021/bi9922188. [DOI] [PubMed] [Google Scholar]; (b) Suzuki T., Fukazawa N., San-nohe K., Sato W., Yano O., Tsuruo T. Structure−activity relationship of newly synthesized quinoline derivatives for reversal of multidrug resistance in cancer. J. Med. Chem. 1997;40:2047. doi: 10.1021/jm960869l. [DOI] [PubMed] [Google Scholar]; (c) Klingenstein R., Melnyk P., Leliveld S.R., Ryckebusch A., Korth C. Similar structure−activity relationships of quinoline derivatives for antiprion and antimalarial effects. J. Med. Chem. 2006;49:5300. doi: 10.1021/jm0602763. [DOI] [PubMed] [Google Scholar]; (d) Peng C.-C., Cape J.L., Rushmore T., Crouch G.J., Jones J.P. Cytochrome P450 2C9 Type II binding studies on quinoline-4-carboxamide analogues. J. Med. Chem. 2008;51:8000. doi: 10.1021/jm8011257. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lilienkampf A., Mao J., Wan B., Wang Y., Franzblauand S.G., Kozikowski A.P. Structure−activity relationships for a series of quinoline-based compounds active against replicating and non-replicating mycobacterium tuberculosis. J. Med. Chem. 2009;52:2109. doi: 10.1021/jm900003c. [DOI] [PubMed] [Google Scholar]; (f) Zishiri V.K., Joshi M.C., Hunter R., Chibale K., Smith P.J., Summers R.L., Martin R.E., Egan T.J. Quinoline antimalarials containing a dibemethin group are active against chloroquinone-resistant plasmodium falciparum and inhibit chloroquine transport via the p. falciparum chloroquine-resistance transporter (PfCRT) J. Med. Chem. 2011;54:6956. doi: 10.1021/jm2009698. [DOI] [PubMed] [Google Scholar]
- 30.Solomon V.R., Hu C., Lee H. Design and synthesis of anti-breast cancer agents from 4-piperazinylquinoline: a hybrid pharmacophore approach. Bioorg. Med. Chem. Lett. 2010;4:1563. doi: 10.1016/j.bmc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 31.(a) Dandia A., Singh R., Bhaskaran S., Samant S.D. Versatile three component procedure for combinatorial synthesis of biologically relevant scaffold spiro[indole-thiazolidinones] under aqueous conditions. Green Chem. 2011;13:1852. [Google Scholar]; (b) Dandia A., Singh R., Joshi J., Maheshwari S., Soni P. Ultrasound promoted catalyst-free and selective synthesis of spiro[indole-3,4′-pyrazolo[3,4-e][1,4]thiazepines] in aqueous media and evaluation of their anti-hyperglycemic activity. RSC Adv. 2013;3:18992. [Google Scholar]; (c) Dandia A., Singh R., Bhaskaran S. Ultrasound promoted greener synthesis of spiro[indole-3,5′-[1,3]oxathiolanes in water. Ultrason. Sonochem. 2010;17:399. doi: 10.1016/j.ultsonch.2009.08.003. [DOI] [PubMed] [Google Scholar]; (d) Dandia A., Singh R., Bhaskaran S. Facile stereoslective synthesis of spiro[indole-oxiranes] by combination of phase transfer catalyst and ultrasound irradiation and their bioassay. Ultrason. Sonochem. 2011;18:1114. doi: 10.1016/j.ultsonch.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 32.(a) Azarifar D., Sheikh D. Ultrasound-promoted one-pot synthesis of 8-aryl-7,8-dihydro-[1,3]-dioxolo[4,5-g]quinolin-6(5H)-one derivatives under catalyst-free and solvent-free conditions. Acta Chim. Slov. 2012;59:664. [PubMed] [Google Scholar]; (b) Azarifar D., Sheikh D. ZrOCl2·8H2O An efficient, ecofriendly, and recyclable catalyst for ultrasound- accelerated, one-pot, solvent-free synthesis of 8-aryl-7,8-dihydro-[1,3]dioxolo[4,5-g]quinolin-6-(5H)-one and 4-aryl-3,4-dihydroquinolin-2(1 h)- one derivatives. Synth. Commun. 2013;43:2516. [Google Scholar]
- 33.Cravotto G., Demetri A., Nano G.M., Palmisano G., Penoni A., Tagliapietra S. The aldol reaction under high-intensity ultrasound: a novel approach to an old reaction. Eur. J. Org. Chem. 2003;22:4438. [Google Scholar]
- 34.(a) Ross N.A., Bartsch R.A. High-intensity ultrasound-promoted reformatsky reactions. J. Org. Chem. 2003;68:360. doi: 10.1021/jo0261395. [DOI] [PubMed] [Google Scholar]; (b) Pugin B. Qualitative characterization of ultrasound reactors for heterogeneous sonochemistry. Ultrasonics. 1987;25:49. [Google Scholar]
- 35.(a) Mason T.J., Lorimer J.P. John Wiley and Sons; New York: 1988. Sonochemistry: Theory, Application and Uses of Ultrasound in Chemistry. [Google Scholar]; (b) Mason T.J. Sonochemistry and sonoprocessing: the link, the trends and (probably) the future. Ultrason. Sonochem. 2003;10:175. doi: 10.1016/S1350-4177(03)00086-5. [DOI] [PubMed] [Google Scholar]; (c) Mason T.J., Paniwnyk L., Lorimer J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996;3:253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.