Abstract
BACKGROUND: Large cell neuroendocrine carcinoma (LCNEC) was categorized into pulmonary neuroendocrine tumors (NETs) according to the World Health Organization classification guideline. However, LCNEC patients often received the chemotherapy regimens similar to non-small cell lung carcinoma (NSCLC) in advanced stage and the therapeutic effect was unsatisfactory. Therefore, this study aimed to investigate the hidden clinical features, prognosis and immunoprofile of the LCNEC, compared with carcinoid and SCLC, to explore whether LCNEC shares similarity with SCLC and potential treatment approaches could be revealed. METHODS: One hundred seventeen pulmonary NETs cases were retrospectively retrieved in this study. The Kaplan–Meier estimator was employed to draw survival curves. Immunohistochemistry was applied to detect NET-related markers expression. RESULTS: In clinical features, compared with carcinoid, LCNEC patients were older, more commonly in male and advanced stage. The parallel phenomena were also found in the high-grade subgroup when compared with the low- to intermediate-grade one. In survival analysis, the 5-year overall survival of LCNECs was 59.1%, which was poorer than that of carcinoids, but better than that of SCLCs. Immunohistochemistry showed that p53 and PTEN functional inactivation, up-regulation of CD117 expression, down-regulation of SSR2A and SSR5 expression were commonly involved in LCNECs when compared with carcinoids, or in the high-grade subgroup when compared with the low- to intermediate-grade one. However, no significant difference was found in the comparison between LCNECs and SCLCs, or NSCLCs and SCLCs. CONCLUSION: In clinical features, survival and immunoprofile, LCNEC showed more similarity with SCLC rather than carcinoid, which might guide novel therapy for pulmonary NETs.
Introduction
Neuroendocrine tumors (NETs) occupy a distinctive group of tumors for their unique biological characteristics and various original sites [1]. Lung cancer is one kind of frequent malignant tumor that possesses of the top morbidity and mortality compared with other tumors in the world. Pulmonary neuroendocrine tumors (NETs) account for nearly 25% of all types of lung cancers [2]. According to the 2015 WHO classification guideline, pulmonary NETs were categorized into typical carcinoid (TC), atypical carcinoid (AC), large cell neuroendocrine carcinoma (LCNEC) and small cell lung carcinoma (SCLC). According to the differentiation of tumors, TC and AC were classified into the low- to intermediate-grade pulmonary NETs, while LCNEC and SCLC were classified into the high-grade pulmonary NETs [3].
Clinically, no specific chemotherapy regimen has been proved effective for LCNEC. Therefore, LCNEC patients generally receive chemotherapy similar to non-small cell lung carcinoma (NSCLC) in advanced stage. In contrast, SCLC patients receive specific chemotherapy regimens at any stage [4], [5]. However, in clinical practice, we discovered that LCNEC might share some similarity with SCLC, other than TC or AC, in morphology, immunoprofile and poor prognosis.
The p53 protein, as a tumor suppressor gene, predictive of sensitivity to platinum, was reported to be commonly functional inactivated in the high-grade NETs compared to the low- to intermediate-grade NETs [6], [7]. In addition, in gastrointestinal and pancreatic neuroendocrine tumors, p53 expression was also up-regulated in the high-grade NETs compared with the low- to intermediate-grade ones [8]. Besides, CD117 as a transmembrane tyrosine kinase receptor (TKR) was reported to be overexpressed in most of the high-grade NETs, which suggested a potential therapy of Imatinib [8], [9]. Moreover, somatostatin receptor 2A/5 (SSR2A/5) and phosphatase and tensin homolog (PTEN) expression levels, which were predictive of sensitivity to somatostatin analog and mammalian target of rapamycin (mTOR) inhibitor treatment, respectively, were reported significantly decreased with tumor grade progression, regardless of the site of tumors [10], [11]. Therefore, we wondered whether some similar phenomena in the expression of these markers could be discovered in pulmonary NETs.
In this study, we retrospectively retrieved 117 cases of pulmonary NETs in the Department of Thoracic Surgery, Zhongshan Hospital, which were subdivided into TC (10), AC (13), LCNEC (59) and SCLC (35) according to the 2015 WHO classification guideline. Survival curves were drawn and analyzed by the Kaplan–Meier product limit estimator. Immunohistochemical studies were applied to detect p53, CD117, SSR2A, SSR5 and PTEN expressional level in pulmonary NETs. Taking together the data above with statistical analysis, we tried to explore whether LCNEC and SCLC share some similarity in some aspects, which might guide novel therapy for pulmonary NETs.
Materials and Methods
Case Selection And Follow-Up
The flow chart of the case selection was shown in Figure 1. From 2005 to 2016, a total of 154 patients who received complete surgical removal of the primary lung tumor and were diagnosed with pulmonary NETs in the Department of Thoracic Surgery, Zhongshan Hospital, Fudan University, China, were selected for the study. Each pathological section was reviewed by three experienced pathologists, making the final diagnosis according to the 2015 WHO classification guideline. To eliminate the interference factors, we excluded those patients that had history of other malignancy, neoadjuvant therapy or were pathological diagnosed as “mixed carcinoma”. After exclusion, 117 patients were eligible for the study and subdivided as follows: 10 TCs, 13 ACs, 59 LCNECs and 35 SCLCs. It is worth mentioning that all the patients were selected only in the Department of Thoracic surgery, not including those patients that in extensive stage with distant metastasis, especially SCLC patients, which led to the little proportion of SCLC cases enrolled in this study. Each patient has been informed of the research purpose and procedure in details, and agreed with the subject. This study was approved by the ethics committee on human research of Zhongshan Hospital.
Figure 1.
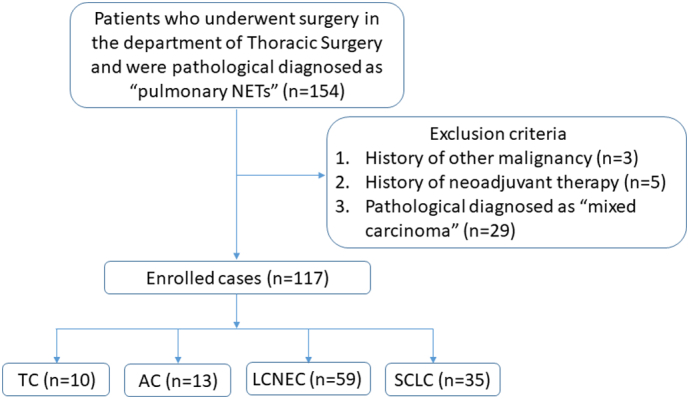
The flow chart of the case selection.
In the analysis of survival, 84 patients who had detailed 5-year follow-up records were selected for overall survival (OS). For the follow-up, each patient was required to take chest CT, abdominal ultrasonography and serum tumor markers every 3-month during the first year after surgery. Thereafter, patients were followed-up twice a year. Brain MRI and full body bone scan were applied at every anniversary follow-up of the surgery. Patients' data was collected in details, including age, sex, smoking status, tumor site, tumor size, tumor pathology, lymph node metastasis, TNM stage (according to American Joint Committee on Cancer (AJCC), eighth edition) [12], etc. Patients whose pathology were classified as SCLC or proved N1 lymph nodes metastasis received postoperative adjuvant chemotherapy. SCLC patients received EP regimen (etoposide and platinum), and carcinoid and LCNEC patients received TP regimen (paclitaxel and platinum). Those patients with N2 lymph nodes metastasis were received postoperative adjuvant radiotherapy.
Immunohistochemical Staining (IHC)
IHC was performed as previously described [13]. Three experienced pathologists, who were unknown of the content and purpose of the study, were invited to score the IHC results. The scoring standards were listed as follows. For SSR2A and SSR5 expression, we performed semi-quantitative scoring based on both subcellular localization and the extent of staining. The scoring system was as follow: 0 (absence of immunoreactivity); 1 (pure cytoplasmic immunoreactivity); 2 (≤50% membranous immunoreactive cells); or 3 (>50% membranous immunoreactive cells). Scores 0 and 1 were considered as negative, while scores 2 and 3 were considered as positive [14].
PTEN expression was evaluated on a semi-quantitative scale according to both the extent (score, 0–3 for positive cells, <5, 5–40, 40–70, and >70%, respectively) and the intensity (score, 0–3 for “-”, “+”, “++”, and “+++”, respectively) of staining. Multiply the score of the degree and intensity, cases with a score 0 were considered as negative, 1–4 were considered as low expression, and score of 6 and 9 were considered as high expression [15].
The p53 expression was considered as positive if the percentage of immunoreactions was observed in >20% of tumor cells, while the rest were considered as negative [16].
CD117 expression was classified as follows: positivity of CD117 was defined when 1% or more tumor cell membranes were reactive, and the negative group was defined as absent expression or cytoplasmic expression of CD117 [17].
Statistical Analysis
Statistical analysis was performed with the SPSS 17.0 software program. The Kaplan–Meier estimator was employed to draw OS curves. Correlation of variables were evaluated by the χ2 test, Fisher exact test, or and Spearman coefficients test, as appropriate, to analyze the difference between each subgroup. OS was defined as the time elapsed from the date of surgery to death. Two-tailed test results were considered significant at P < .05.
Results
LCNEC Shared Similarity With SCLC, Not Carcinoid, in Clinicopathology Features
As shown in Table 1, we gathered related clinicopathologic features, including sex, age, smoking status, tumor site, tumor size, lymph node metastasis, TNM stage. Through statistical analysis, we could conclude that, compared with carcinoid (TC and AC), the LCNEC patients were older (mean age, 59.6 ± 9.5 vs. 48.1 ± 13.1 y, P = .007), and more commonly happened in male (83.1% vs. 52.2%, P = .004) and in advanced stage (stage II and above: 57.6% vs. 34.8%, P = .063). Smoking status, tumor site, tumor size and lymph node metastasis showed no significant difference between two subgroups. Notably, lymph node metastasis happened most frequently in SCLCs (48.6%), followed by LCNECs (37.3%) and least in Carcinoids (26.1%).
Table 1.
The Analysis of the Associations Between Pulmonary NETs Subgroups and Clinicopathologic Features.
| Clinicalopathologic Features | Carcinoid |
LCNEC |
SCLC |
All 3 Types |
Carcinoid vs. LCNEC |
LCNEC vs. SCLC |
Low to Intermediate vs. High Grade |
NSCLC vs. SCLC |
|---|---|---|---|---|---|---|---|---|
| (n = 23) | (n = 59) | (n = 35) | P value | P value | P value | P value | P value | |
| Age | ||||||||
| ≤55 years | 14 | 17 | 10 | .015 | .007 | .980 | .004 | .338 |
| >55 years | 9 | 42 | 25 | |||||
| Sex | ||||||||
| Male | 12 | 49 | 31 | .002 | .004 | .467 | .001 | .087 |
| Female | 11 | 10 | 4 | |||||
| Smoking status | ||||||||
| Never/unknown | 16 | 30 | 22 | .241 | .125 | .258 | .215 | .497 |
| Former/current | 7 | 29 | 13 | |||||
| Tumor site | ||||||||
| Left | 7 | 25 | 16 | .490 | .319 | .752 | .249 | .501 |
| Right | 16 | 34 | 19 | |||||
| Tumor size | ||||||||
| ≤3 cm | 16 | 31 | 18 | .319 | .161 | .917 | .131 | .557 |
| >3 cm | 7 | 28 | 17 | |||||
| Lymph node metastasis | ||||||||
| No | 17 | 37 | 18 | .219 | .337 | .283 | .174 | .142 |
| Yes | 6 | 22 | 17 | |||||
| TNM stage(8th) | ||||||||
| IA1-IB | 15 | 25 | 16 | .169 | .063 | .752 | .063 | .761 |
| IIA-IIIC | 8 | 34 | 19 |
Abbreviation: NETs: neuroendocrine tumors, LCNEC: large cell neuroendocrine carcinoma, SCLC: small cell lung carcinoma, NSCLC: non-small cell lung carcinoma; TNM: tumor-node-metastasis. Bold values indicate statistical significance (P < .05).
Then, we re-classified LCNEC and SCLC into the high-grade subgroup to compare them with the low- to intermediate-grade subgroup (carcinoid). The data showed that older age (mean age, 60.5 ± 9.1 vs. 48.1 ± 13.1 y, P = .004), male patients (85.1% vs. 52.2%, P = .001) and advanced stage (stage II and above: 54.3% vs. 34.8%, P = .063) were more common in the high-grade subgroup than those in the low- to intermediate-grade subgroup, which was consistent with the comparison between LCNEC and carcinoid. However, no significant difference was found in the comparison between LCNEC and SCLC. Furthermore, we combined carcinoid and LCNEC into NSCLC, and found that no significant difference existed in the comparison between NSCLC and SCLC.
LCNEC And SCLC Suffered Poorer Prognosis Than Carcinoid
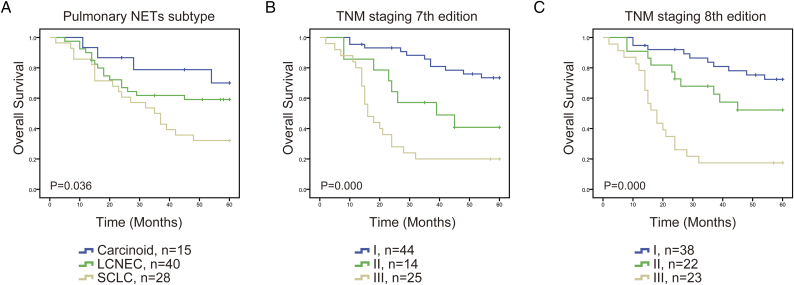
In survival analysis, we investigated the 5-year OS difference among carcinoid, LCNEC and SCLC. Up to 84 patients who received surgery before 2012, were enrolled in the analysis. The 5-year OS for the carcinoids, LCNECs, and SCLCs were 70.0%, 59.1%, and 32.1%, respectively (P = .036) (Figure 2A and Table 2). The data showed that the prognosis of LCNEC was poorer than that of carcinoid, but better than that of SCLC.
Figure 2.
The 5-year survival curves of the enrolled pulmonary NETs patients, obtained by Kaplan–Meier product limit estimator, were compared among different subtypes (A), TNM staging 7th edition (B) and TNM staging 8th edition (C).
Table 2.
The Analysis of Overall Survival Rate Between Pulmonary NETs Subgroups
| Tumor Type | No. | Median Survival |
5-year OS |
|---|---|---|---|
| (Months) | (%) | ||
| Carcinoid | 16 | NR | 70.0 |
| LCNEC | 40 | NR | 59.1 |
| SCLC | 28 | 35 | 32.1 |
Abbreviation: NETs: neuroendocrine tumors, LCNEC: large cell neuroendocrine carcinoma, SCLC: small cell lung carcinoma, NSCLC: non-small cell lung carcinoma; OS: overall survival, NR: not reached.
Currently, although there existed distinctive TNM stage for SCLC, no distinctive TNM stage for pulmonary NET has been released. For the reason that SCLC patients enrolled in this study were all in limited stage, we tried to apply 7th and 8th editions of the AJCC TNM stage for all these pulmonary NETs patients and to compare the prognostic value between these two editions. As shown in Figure 2, B and C, both 7th and 8th editions showed distinguished difference between carcinoids, LCNECs, and SCLCs (P = .000). To analyze the survival curve further, we figured out that in the 7th edition, the SCLC curve crossed the LCNEC curve during the first year after surgery, while in the 8th edition, no cross appeared among pulmonary NETs, which indicated that TNM 8th edition was superior in predicting the prognosis, compared with the TNM 7th edition in pulmonary NETs.
LCNEC Possessed Parallel Shifted Expression of Neuroendocrine-Related Markers With SCLC, Not Carcinoid
To explore the immunoprofile of neuroendocrine-related markers, we performed IHC to detect p53, CD117, SSR2A, SSR5 and PTEN expression in pulmonary NETs (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7). As shown in Table 3, p53 and CD117 expression was significantly higher in LCNECs than those in carcinoids (p53-positive: 67.8% vs. 34.8%, P = .006; CD117-positive: 39.0% vs. 0%, P = .000). Especially, all carcinoids showed negative CD117 expression. Conversely, both SSR2A/SSR5 expression scores and PTEN immunoreactivity levels were significantly lower in LCNECs than those in carcinoids (SSR2A-positive, 33.9% vs. 65.2%, P = .010; SSR5-positive, 6.8% vs. 30.4%, P = .005; PTEN-cytoplasm, 57.4% vs. 82.6%, P = .037).
Figure 3.
P53 expression. Negative: complete absent or ≤ 20% tumor cells expression. (A) 5% expression of tumor cells; (B) 10% expression of tumor cells.
Positive: >20% tumor cells expression. (C) 60% expression of tumor cells; (D) 100% expression of tumor cells
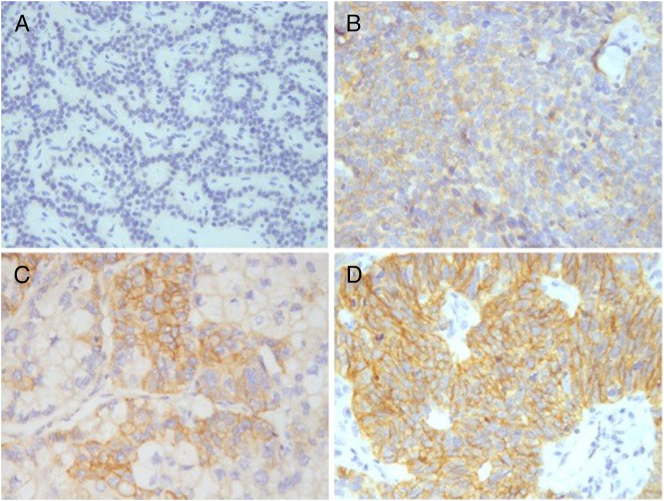
Figure 4.
CD117 expression. Negative: complete absent or cytoplasmic expression. (A) absent expression of tumor cells; (B) cytoplasmic expression of tumor cells.
Positive: ≥1% tumor cells membranes expression. (C) 40% expression of tumor cell membranes; (D) 100% expression of tumor cell membranes.
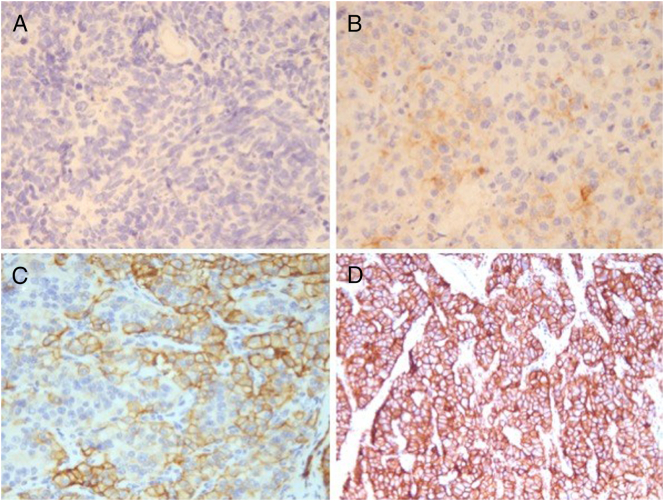
Figure 5.
SSR2A expression. Negative: scores 0 and 1. (A) score 0: absent expression of tumor cells; (B) score 1: cytoplasmic expression of tumor cells. Positive: scores 2 and 3. (C) score 2: ≤50% expression of tumor cell membranes; (D) score 3: >50% expression of tumor cell membranes.
Figure 6.
SSR5 expression. Negative: scores 0 and 1. (A) score 0: absent expression of tumor cells; (B) score 1: cytoplasmic expression of tumor cells.
Positive: scores 2 and 3. (C) score 2: ≤50% expression of tumor cell membranes; (D) score 3: >50% expression of tumor cell membranes.
Figure 7.
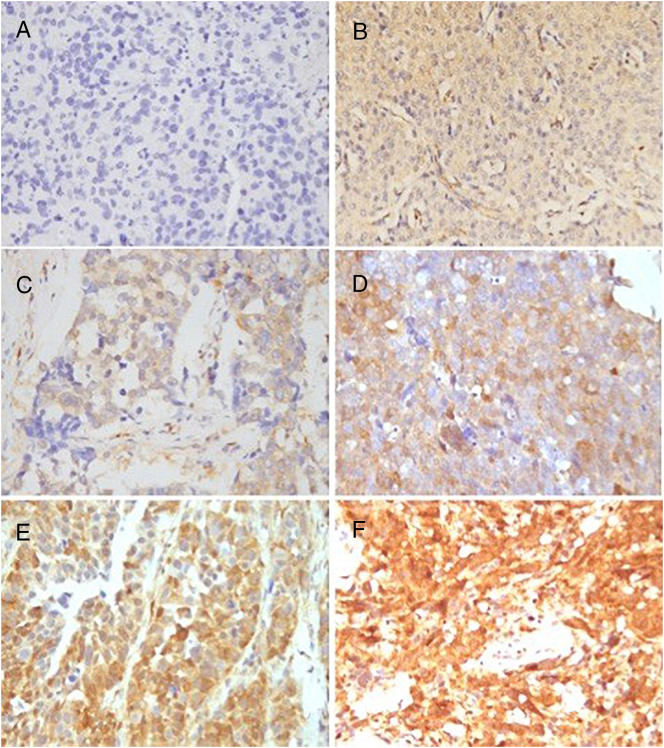
PTEN expression. Negative: score 0. (A) score 0: absent expression of tumor cells.
Low expression: scores 1–4. (B) score 1: the extent: 20%; the intensity: “+”; (C) score 2: the extent: 50%; the intensity: “+”; (D) score 4: the extent: 60%; the intensity: “++”
High expression: scores 6–9. (E) score 6: the extent:90%; the intensity: “++”;(F) score 9: the extent: 100%; the intensity: “+++”.
Table 3.
The Analysis of the Associations Between Pulmonary NETs Subgroups and Immunoprofile.
| NE-Related Markers | Carcinoid |
LCNEC |
SCLC |
All 3 Types |
Carcinoid vs. LCNEC |
LCNEC vs. SCLC |
Low to Intermediate vs. High Grade |
NSCLC vs. SCLC |
|---|---|---|---|---|---|---|---|---|
| (n = 23) | (n = 59) | (n = 35) | P value | P value | P value | P value | P value | |
| p53 | ||||||||
| Negative | 15 | 19 | 10 | .009 | .006 | .712 | .002 | .187 |
| Positive | 8 | 40 | 25 | |||||
| CD117 | ||||||||
| Negative | 23 | 36 | 18 | .000 | .000 | .363 | .000 | .032 |
| Positive | 0 | 23 | 17 | |||||
| SSR2A | ||||||||
| Negative | 8 | 39 | 24 | .017 | .010 | .806 | .005 | .254 |
| Positive | 15 | 20 | 11 | |||||
| SSR5 | ||||||||
| Negative | 16 | 55 | 35 | .000 | .005 | .115 | .000 | .023 |
| Positive | 7 | 4 | 0 | |||||
| PTEN | ||||||||
| Negative | 2 | 22 | 16 | .031 | .037 | .331 | .012 | .105 |
| Low expression | 2 | 3 | 0 | |||||
| High expression | 19 | 34 | 19 |
Abbreviation: NETs: neuroendocrine tumors, NE-related: neuroendocrine-related, LCNEC: large cell neuroendocrine carcinoma, SCLC: small cell lung carcinoma, NSCLC: non-small cell lung carcinoma. SSR2A: somatostatin receptor 2, SSR5: somatostatin receptor 5, PTEN: phosphatase and tensin homolog. Bold values indicate statistical significance (P < .05).
Then, in the high-grade subgroup, we found that p53 and CD117 expression was up-regulated, while SSR2A/SSR5 and PTEN expression were down-regulated significantly, compared with the low- to intermediate-grade subgroup (p53-positive, 69.1% vs. 34.8%, P = .002; CD117-positive, 42.6% vs. 0%, P = .000; SSR2A-positive, 33.0% vs. 65.2%, P = .005; SSR5-positive, 4.3% vs. 30.4%, P = .000; PTEN-cytoplasm, 56.4% vs. 82.6%, P = .012). In the comparison between NSCLC and SCLC, we found that only SSR5 expression was down-regulated, while CD117 expression was up-regulated significantly (SSR5-positive, 13.4% vs. 0%, P = .023; CD117-positive, 28.0% vs. 48.6%, P = .021), and the other markers expression showed no significant difference.
Discussion
The neuroendocrine carcinoma of the pulmonary has unique clinical and pathological feature. It is known to all that the high-grade pulmonary NETs (LCNEC and SCLC) suffered poorer prognosis than the low- to intermediate-grade NETs (TC and AC) [18]. The detailed molecular mechanisms of these biological and prognostic characteristics still remained unknown. In this study, we focused on clinical features, prognosis and immunoprofiles to analyze the difference between each subgroup and explored whether LCNEC shares some similarity with SCLC.
In the analysis of clinical features, we arrived at a conclusion that LCNEC patients, characteristic of old age, more males than females, and advanced stage, shared similarity with SCLC patients, rather than carcinoid patients. Moreover, when we re-classified patients into the low- to intermediate-grade subgroups and the high-grade subgroups, the same characteristics also could be revealed in the high-grade subgroups compared with the low- to intermediate-grade subgroups. However, when patients were re-classified into NSCLC subgroups and SCLC subgroups, these characteristics showed no significant statistical difference. Rekhtman reported that carcinoid occur in younger patients (mean age, 45–50 years), while the high-grade pulmonary NETs occur predominantly in older patients (mean age, 65 years). Furthermore, the high-grade pulmonary NETs patients are more frequent male [19]. Our results were consistent with the previous data. For the reason that the high-grade pulmonary NETs show high aggressive biological behavior [20], these patients might be prone to reaching advanced stage when being diagnosed. Therefore, the statistical analysis indicated that the clinical characteristics of LCNEC were much more similar to SCLC, than to carcinoid.
To analyze the prognostic difference between each subtype of pulmonary NETs, we applied Kaplan–Meier estimator and found that 5-year OS for the carcinoids, LCNECs, and SCLCs were 70.0%, 59.1%, and 32.1%, respectively (P = .036). As reported, carcinoid patients have the best prognosis (5-year survival ≥60%). LCNEC was considered a broad range of survival (15%–57%), in which the different treatment approaches and inclusion criteria might result [21]. The controversy in LCNEC survival also indicated the unknown of its exact biological features and targeted treatments. The most aggressive subtype of pulmonary NETs is SCLC, whose 5-year survival was almost 10–20% [22]. According to this study, the survival data was in accordance to the previous records. The fact that 5-year survival of LCNEC and SCLC was a little higher than the previous records might be ascribed to the limitation of the inclusion of patients who mostly received radical resection surgery in this study, which means these patients were almost at limited-stage. The patients in extensive-stage who lost operation opportunity might have suffered even poorer prognosis. The survival curve of carcinoid and SCLC in this study occupied the either end of the lifetime span and LCNEC was situated between these two subtypes. Although it is hard to define which end LCNEC was much closer to, the tendency would gradually be revealed with further exploration in-depth in future. Moreover, LCNEC patients were mostly advised to receive the chemotherapy regimen for NSCLC, which were different from the distinctive regimen for SCLC. Therefore, the post-operative chemotherapy was not taken into account in this study.
The p53 protein, as a tumor suppressor, plays a crucial role in the cell cycle regulation. Wild-type p53 inhibits tumor progression through the induction of cell cycle arrest and apoptosis [23], [24]. Brian and colleagues demonstrated that p53 was overexpressed in SCLC [6]. The similar results were also demonstrated in Şahan and colleagues' study in gastrointestinal and pancreatic neuroendocrine tumors [8]. Besides p53, the product of the c-kit gene, CD117 was frequently expressed in gastrointestinal stromal tumors [25]. In NETs, CD117 was also found up-regulated in the high-grade NETs, such as Merkel cell carcinoma [26], [27]. Araki and colleagues demonstrated that CD117 was overexpressed in 55% of the LCNEC and 46% of the SCLC tumor cells [28]. Pelosi and colleagues also analyzed CD117 expression difference among carcinoid, LCNEC and SCLC and found that CD117 was positive-membrane stained in 77% LCNECs, 67% SCLCs and 7% carcinoids, and positive cytoplasm-stained in 44% LCNEC, 70% SCLC 5% carcinoids [29]. These results indicated that CD117 was much more frequently over-expressed in the high-grade pulmonary NETs than the low- to intermediate-grade ones, which was in accordance with our data.
Somatostatin could inhibit several digestive fluid secretion and somatostatin receptors showed shifted expression in gastrointestinal NETs [30], [31]. The somatostatin receptors family includes SSR1 to SSR5, among which SSR2A and SSR5 were applied most frequently according to somatostatin analogue treatment [16], [32]. Tsuta and colleagues demonstrated that SSR1, 2A, 2B, 3 and 4 have a tendency toward decreased expression in well to poorly differentiated pulmonary NETs [33]. However, Kaemmerer and colleagues applied IHC and qRT-PCR in pulmonary NETs and revealed that SSR1 and 5 expression level gradually decreased toward the poorly differentiated tumor subgroups, while SSR2A was present almost the same among each subgroups [34]. Our data was consistent with Tsuta's results. The conflicts between our data and previous studies might result from different scoring standard, different antibodies and limited sample size. Further deeper investigation would be required to reveal the precise mechanism behind the data. Strikingly, our data showed that CD117 was entirely negative in pulmonary carcinoids and SSR5 was also totally negative in SCLC, which might be a good way to distinguish the types of pulmonary NETs. Definitely, the sample size needs to be enlarged to prove these results.
PTEN is another frequent mutated tumor suppressor, which serves as the regulator of the phosphoinositide 3-kinase (PI3K)/Akt/ mTOR and insulin signaling pathway [35]. Collaud and colleagues revealed that, compared with the carcinoids, the high-grade pulmonary NETs tumors presented a stronger loss of PTEN [11]. Wada and colleagues analyzed the PTEN expression in the NETs of multiple organs and found that PTEN immunoreactivity levels were significantly lower in the high-grade NETs subgroup, while p53 expression were significantly higher in the high-grade NETs subgroup, which was entirely consistent with our results in pulmonary NETs [10]. Our data also revealed that, with the elevation of differentiation grade, p53 expression level gradually increased, but PTEN expression level gradually decreased (Table 3). Therefore, considering the expressional difference of p53, CD117, SSR2A, SSR5 and PTEN in each pulmonary NETs subgroup, we speculated that LCNEC shared much more similarity with SCLC, than carcinoid, in immunoprofiles, which also indicated platinum, Imatinib or mTOR inhibitor treatment might be potential therapy for LCNEC.
To conclude, through the analysis of clinical features, overall survival, and immunoprofile between each type of pulmonary NETs, LCNECs shared more similarity with SCLC, rather than carcinoid, which might guide novel therapy for pulmonary NETs.
Acknowledgments
Acknowledgment
We were grateful to the support from the National Natural Science Foundation of China (81372313, 81401876 and 81872291).
Footnotes
Meeting Presentation: This content was presented at the IASLC 18th World Conference on Lung Cancer (WCLC 2017), Yokohama, Japan, October 15-18, 2017.
Conflicts of interest: All authors stated that no potential conflicts exist in this work.
Contributor Information
Yuan Ji, Email: ji.yuan@zs-hospital.sh.cn.
Di Ge, Email: gedi6902@hotmail.com.
References
- 1.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12:425–436. doi: 10.1016/j.jtho.2016.11.2222. [DOI] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 4.Kim KW, Kim HK, Kim J, Shim YM, Ahn MJ, Choi YL. Outcomes of curative-intent surgery and adjuvant treatment for pulmonary large cell neuroendocrine carcinoma. World J Surg. 2017;41:1820–1827. doi: 10.1007/s00268-017-3908-8. [DOI] [PubMed] [Google Scholar]
- 5.Derks JL, van Suylen RJ, Thunnissen E, den Bakker MA, Groen HJ, Smit EF, Damhuis RA, van den Broek EC, Speel EM, Dingemans AC. Chemotherapy for pulmonary large cell neuroendocrine carcinomas: does the regimen matter? Eur Respir J. 2017;49 doi: 10.1183/13993003.01838-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erler BS, Presby MM, Finch M, Hodges A, Horowitz K, Topilow AA, Matulewicz T. CD117, Ki-67, and p53 predict survival in neuroendocrine carcinomas, but not within the subgroup of small cell lung carcinoma. Tumour Biol. 2011;32:107–111. doi: 10.1007/s13277-010-0104-y. [DOI] [PubMed] [Google Scholar]
- 7.Thakur B, Ray P. p53 Loses grip on PIK3CA expression leading to enhanced cell survival during platinum resistance. Mol Oncol. 2016;10:1283–1295. doi: 10.1016/j.molonc.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Kimiloglu Sahan E, Erdogan N, Ulusoy I, Samet E, Akyildiz Igdem A, Gonullu D. P53, KI-67, CD117 expression in gastrointestinal and pancreatic neuroendocrine tumours and evaluation of their correlation with clinicopathological and prognostic parameters. Turk J Gastroenterol. 2015;26:104–111. doi: 10.5152/tjg.2015.1965. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Kuang Y, Qiu HB, Cao Z, Tu Y, Sheng Q, Eilers G, He Q, Li HL, Zhu M. Dual targeting of insulin receptor and KIT in imatinib-resistant gastrointestinal stromal tumors. Cancer Res. 2017;77:5107–5117. doi: 10.1158/0008-5472.CAN-17-0917. [DOI] [PubMed] [Google Scholar]
- 10.Wada H, Matsuda K, Akazawa Y, Yamaguchi Y, Miura S, Ueki N, Kinoshita A, Yoshiura K, Kondo H, Ito M. Expression of somatostatin receptor type 2A and PTEN in neuroendocrine neoplasms is associated with tumor grade but not with site of origin. Endocr Pathol. 2016;27:179–187. doi: 10.1007/s12022-016-9436-5. [DOI] [PubMed] [Google Scholar]
- 11.Collaud S, Tischler V, Atanassoff A, Wiedl T, Komminoth P, Oehlschlegel C, Weder W, Soltermann A. Lung neuroendocrine tumors: correlation of ubiquitinylation and sumoylation with nucleo-cytosolic partitioning of PTEN. BMC Cancer. 2015;15:74. doi: 10.1186/s12885-015-1084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151:193–203. doi: 10.1016/j.chest.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Gu J, Ding JY, Lu CL, Lin ZW, Chu YW, Zhao GY, Guo J, Ge D. Overexpression of CD88 predicts poor prognosis in non-small-cell lung cancer. Lung Cancer. 2013;81:259–265. doi: 10.1016/j.lungcan.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Volante M, Brizzi MP, Faggiano A, La Rosa S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C. Somatostatin receptor type 2A immunohistochemistry in neuroendocrine tumors: a proposal of scoring system correlated with somatostatin receptor scintigraphy. Mod Pathol. 2007;20:1172–1182. doi: 10.1038/modpathol.3800954. [DOI] [PubMed] [Google Scholar]
- 15.Han X, Ji Y, Zhao J, Xu X, Lou W. Expression of PTEN and mTOR in pancreatic neuroendocrine tumors. Tumour Biol. 2013;34:2871–2879. doi: 10.1007/s13277-013-0849-1. [DOI] [PubMed] [Google Scholar]
- 16.Konukiewitz B, Schlitter AM, Jesinghaus M, Pfister D, Steiger K, Segler A, Agaimy A, Sipos B, Zamboni G, Weichert W. Somatostatin receptor expression related to TP53 and RB1 alterations in pancreatic and extrapancreatic neuroendocrine neoplasms with a Ki67-index above 20. Mod Pathol. 2017;30:587–598. doi: 10.1038/modpathol.2016.217. [DOI] [PubMed] [Google Scholar]
- 17.Zavalhia LS, Romitti M, Netto GC, dos Santos GT, Meurer RT, Hilbig A, Michalowski MB, Barbosa Coutinho LM, de Castro Ribeiro M. Evaluation of the expression of C-kit (CD117) in ependymomas and oligodendrogliomas. Dis Markers. 2012;33:61–68. doi: 10.3233/DMA-2012-0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grand B, Cazes A, Mordant P, Foucault C, Dujon A, Guillevin EF, Barthes Fle P, Riquet M. High grade neuroendocrine lung tumors: pathological characteristics, surgical management and prognostic implications. Lung Cancer. 2013;81:404–409. doi: 10.1016/j.lungcan.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628–1638. doi: 10.5858/2009-0583-RAR.1. [DOI] [PubMed] [Google Scholar]
- 20.Fisseler-Eckhoff A, Demes M. Neuroendocrine tumors of the lung. Cancer. 2012;4:777–798. doi: 10.3390/cancers4030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis WD. Advances in neuroendocrine lung tumors. Ann Oncol. 2010;21(Suppl 7):vii65–vii71. doi: 10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]
- 22.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paik KH, Park YH, Ryoo BY, Yang SH, Lee JC, Kim CH, Ki SS, Kim JM, Park MJ, Ahn HJ. Prognostic value of immunohistochemical staining of p53, bcl-2, and Ki-67 in small cell lung cancer. J Korean Med Sci. 2006;21:35–39. doi: 10.3346/jkms.2006.21.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38(Suppl. 5):S39–S51. doi: 10.1016/s0959-8049(02)80602-5. [DOI] [PubMed] [Google Scholar]
- 26.Su LD, Fullen DR, Lowe L, Uherova P, Schnitzer B, Valdez R. CD117 (KIT receptor) expression in Merkel cell carcinoma. Am J Dermatopathol. 2002;24:289–293. doi: 10.1097/00000372-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Feinmesser M, Halpern M, Kaganovsky E, Brenner B, Fenig E, Hodak E, Sulkes J, Okon E. c-kit expression in primary and metastatic merkel cell carcinoma. Am J Dermatopathol. 2004;26:458–462. doi: 10.1097/00000372-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Araki K, Ishii G, Yokose T, Nagai K, Funai K, Kodama K, Nishiwaki Y, Ochiai A. Frequent overexpression of the c-kit protein in large cell neuroendocrine carcinoma of the lung. Lung Cancer. 2003;40:173–180. doi: 10.1016/s0169-5002(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 29.Pelosi G, Masullo M, Leon ME, Veronesi G, Spaggiari L, Pasini F, Sonzogni A, Iannucci A, Bresaola E, Viale G. CD117 immunoreactivity in high-grade neuroendocrine tumors of the lung: a comparative study of 39 large-cell neuroendocrine carcinomas and 27 surgically resected small-cell carcinomas. Virchows Arch. 2004;445:449–455. doi: 10.1007/s00428-004-1106-1. [DOI] [PubMed] [Google Scholar]
- 30.Mehta S, de Reuver PR, Gill P, Andrici J, D'Urso L, Mittal A, Pavlakis N, Clarke S, Samra JS, Gill AJ. Somatostatin receptor SSTR-2a expression is a stronger predictor for survival than Ki-67 in pancreatic neuroendocrine tumors. Medicine. 2015;94 doi: 10.1097/MD.0000000000001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaemmerer D, Trager T, Hoffmeister M, Sipos B, Hommann M, Sanger J, Schulz S, Lupp A. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget. 2015;6:27566–27579. doi: 10.18632/oncotarget.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerasimou G, Moralidis E, Gotzamani-Psarrakou A. Somatostatin receptor imaging with (111)In-pentetreotide in gastro-intestinal tract and lung neuroendocrine tumors-Impact on targeted treatment. Hell J Nucl Med. 2010;13:158–162. [PubMed] [Google Scholar]
- 33.Tsuta K, Wistuba II, Moran CA. Differential expression of somatostatin receptors 1-5 in neuroendocrine carcinoma of the lung. Pathol Res Pract. 2012;208:470–474. doi: 10.1016/j.prp.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Kaemmerer D, Specht E, Sanger J, Wirtz RM, Sayeg M, Schulz S, Lupp A. Somatostatin receptors in bronchopulmonary neuroendocrine neoplasms: new diagnostic, prognostic, and therapeutic markers. J Clin Endocrinol Metab. 2015;100:831–840. doi: 10.1210/jc.2014-2699. [DOI] [PubMed] [Google Scholar]
- 35.Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A, delle Fave G. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28:245–255. doi: 10.1200/JCO.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]