A screen of Rubus idaeus berry fractions for bioactivity against Huntington’s disease identified salidroside, which was then produced by metabolically engineered microorganisms.
Abstract
Edible berries are considered to be among nature’s treasure chests as they contain a large number of (poly)phenols with potentially health-promoting properties. However, as berries contain complex (poly)phenol mixtures, it is challenging to associate any interesting pharmacological activity with a single compound. Thus, identification of pharmacologically interesting phenols requires systematic analyses of berry extracts. Here, raspberry (Rubus idaeus, var Prestige) extracts were systematically analyzed to identify bioactive compounds against pathological processes of neurodegenerative diseases. Berry extracts were tested on different Saccharomyces cerevisiae strains expressing disease proteins associated with Alzheimer’s, Parkinson’s, or Huntington’s disease, or amyotrophic lateral sclerosis. After identifying bioactivity against Huntington’s disease, the extract was fractionated and the obtained fractions were tested in the yeast model, which revealed that salidroside, a glycosylated phenol, displayed significant bioactivity. Subsequently, a metabolic route to salidroside was reconstructed in S. cerevisiae and Corynebacterium glutamicum. The best-performing S. cerevisiae strain was capable of producing 2.1 mm (640 mg L−1) salidroside from Glc in shake flasks, whereas an engineered C. glutamicum strain could efficiently convert the precursor tyrosol to salidroside, accumulating up to 32 mm (9,700 mg L−1) salidroside in bioreactor cultivations (yield: 0.81 mol mol−1). Targeted yeast assays verified that salidroside produced by both organisms has the same positive effects as salidroside of natural origin.
Berries are both an enjoyable and important part of a balanced diet providing energy, nutrients and dietary fiber, and contain a large number of compounds showing diverse health-promoting activities in humans (Seeram, 2012; Nile and Park, 2014). Edible berry species contain a diverse and concentrated portfolio of (poly)phenols such as anthocyanins, flavanols (epicatechin), and stilbenes (resveratrol), which are known to confer protection against oxidative stress and to have cytoprotective activities (Rodriguez-Mateos et al., 2014; Bensalem et al., 2015). In recent years, there is increasing evidence that natural phenols in berry fruits also show protective effects against metabolic disorders such as diabetes and obesity and against neurodegenerative diseases (ND; Yang and Kortesniemi, 2015; Tsuda, 2016; Rosado-Ramos et al., 2018). NDs comprise a heterogeneous group of debilitating disorders caused by the progressive loss of neuronal cells. The cell type affected by the neurodegenerative process determines the appearance of specific pathologies. Frequently, the loss of specific neuron populations is associated with the aggregation and/or formation of amyloid structures enriched with particular proteins. Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s diseases (HD), and amyotrophic lateral sclerosis (ALS), some of the major NDs, are characterized by the aggregation of Aβ-42, α-synuclein (α-Syn), huntingtin(HTT), and FUS (an RNA-binding protein), respectively, representing the hallmarks of these diseases (Mason and Giorgini, 2011; Lashuel et al., 2013; Masters et al., 2015; Zarei et al., 2015). Currently, there is no cure for NDs and therefore there is a strong interest in finding ways to treat these diseases. However, (poly)phenols have been shown to attenuate the deleterious effects of protein aggregation in NDs, rendering this group of natural products a promising source of compounds for ND treatment (Rosado-Ramos et al., 2018).
Typically, (poly)phenol concentrations in the naturally producing plant are quite low, which means that other strategies for a large-scale production have to be developed once a pharmacologically interesting compound has been identified. The microbial production of plant (poly)phenols has emerged as a true alternative to direct extraction from plants or (total) chemical synthesis (Milke et al., 2018). In particular, Saccharomyces cerevisiae, but also bacterial host systems such as Escherichia coli or Corynebacterium glutamicum, have been extensively engineered employing modern concepts of synthetic biology to produce plant (poly)phenols such as resveratrol, naringenin, quercetin, and calistephin in the last years (Lim et al., 2011; Koopman et al., 2012; Jones et al., 2017; Kallscheuer et al., 2017).
In this study, we followed a bioprospecting approach analyzing berry extracts from Rubus idaeus (var Prestige) as a representative species of Rubus genus to identify either novel compounds or known compounds with yet uncharacterized bioactivities in connection with NDs. This genus includes, e.g. raspberries and blackberries, which were placed at the top ranks of more than 1,000 polyphenol-rich foods consumed in the United States (Halvorsen et al., 2006). And we have been working with 15 species/varieties of this genus for its phytochemical composition and bioactivity (Dudnik et al., 2018). This multidisciplinary approach with experts from the fields of plant biology, cellular biology, microbiology, industrial biotechnology, and analytical chemistry covered the complete workflow from the preparation and fractionation of berry extracts, compound identification, and bioactivity testing to the reconstruction of metabolic pathways for identified compounds in microorganisms, production in bioreactors, and downstream processing.
RESULTS
Bioactivity Screening of R. idaeus (var Prestige) Extracts
Our study is based on the screening of R. idaeus (var Prestige) (poly)phenol-enriched extract for bioactivities against the cytotoxic effects of protein aggregation associated with major NDs, particularly AD, PD, HD, and ALS. Extracts were prepared as described in the “Material and Methods” section and tested for bioactivity using a yeast-based screening platform. The high degree of evolutionary conservation of fundamental biological processes among eukaryotes makes yeast a valuable model organism to identify lead molecules with health-promoting potential against NDs (Menezes et al., 2015; Oliveira et al., 2017). Our platform includes yeast strains heterologously expressing genes encoding Aβ-42, α-Syn, HTTpQ103 (HTT 17 N-terminal amino acids fused to 103 Gln residues), or FUS to assess bioactivity of R. idaeus (var Prestige) (poly)phenol-enriched extracts modulating pathological pathways associated with AD, PD, HD, and ALS, respectively (Outeiro and Lindquist, 2003; Willingham et al., 2003; Bharadwaj et al., 2010; Ju et al., 2011). The genes coding for the disease proteins are translationally fused to the green fluorescent protein (GFP) and the expression is under control of the Gal-inducible GAL1 promoter (Flick and Johnston, 1990).
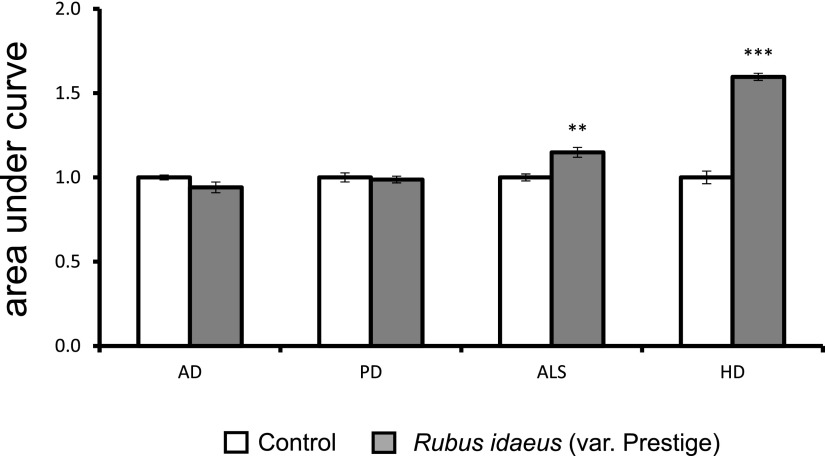
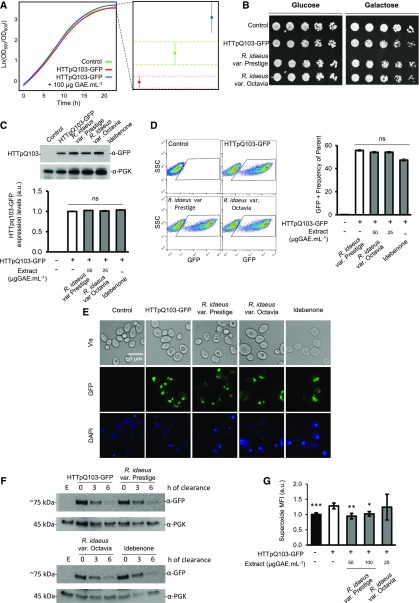
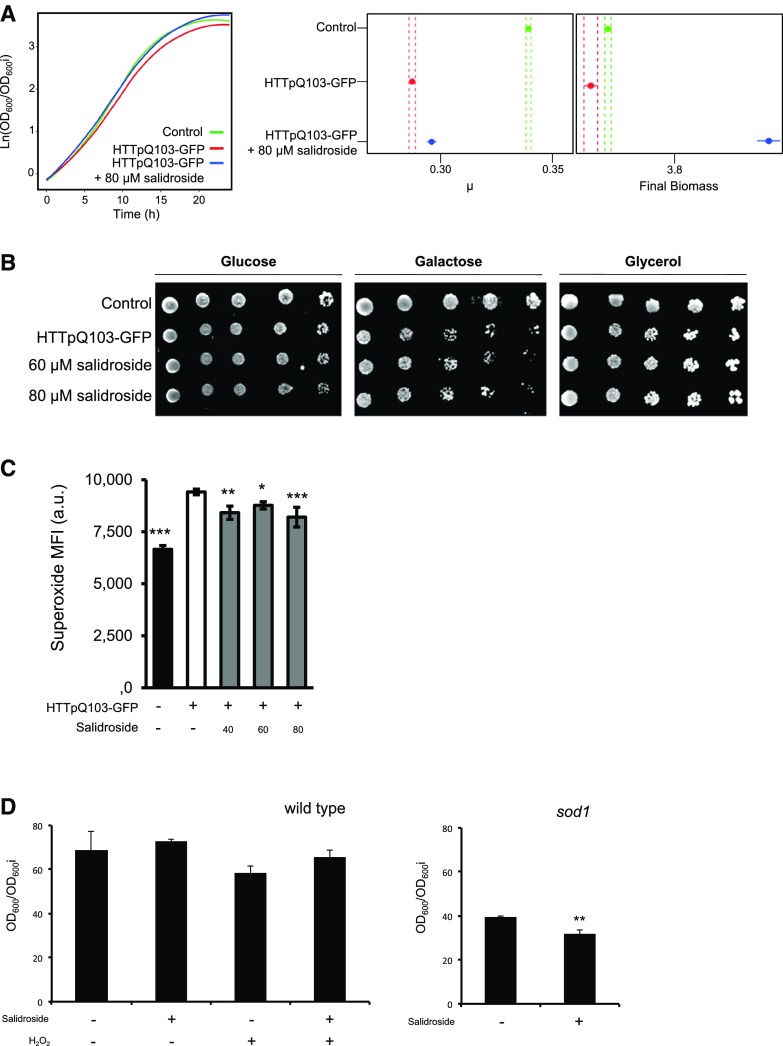
In the model systems tested, R. idaeus (var Prestige) extract was shown to be most potent in overcoming the deleterious effects of HTTpQ103 expression (Fig. 1). Therefore, the identified R. idaeus (var Prestige) bioactivity against HD was further characterized. Upon shifting to a Gal medium, growth of HTTpQ103-GFP-expressing cells was impaired in comparison to that of the control strain, as revealed by growth curve analysis and phenotypic growth assays (Fig. 2, A and B). The estimation of growth curve parameters using nonlinear parametric regressions (with 95% confidence intervals) indicated that expression of HTTpQ103-GFP decreases the final cell biomass, which was recovered by treatment with R. idaeus (var Prestige) (poly)phenol-enriched extract (Fig. 2A, right panel). Interestingly, R. idaeus (var Prestige)-mediated protection was efficient even when the extract was applied as a pretreatment before inducing HTTpQ103 expression with Gal (Fig. 2B). It is important to note that treatment of cells with a similar extract from R. idaeus (var Octavia) did not result in an observable cellular protection, indicating that a R. idaeus (var Prestige)-specific compound confers protection against HTTpQ103-GFP cytotoxicity (Fig. 2B, last spots in the right panel). The R. idaeus (var Octavia) extract was subsequently used as a negative control in further experiments. The investigation of R. idaeus (var Prestige) mode of action revealed that growth improvement mediated by the extract was not associated with any detectable difference in HTTpQ103 protein levels and subcellular distribution of the inclusions, as revealed by immunoblotting, flow cytometry, and fluorescence microscopy (Fig. 2, C–E). Idebenone, a synthetic analog of ubiquinone with a similar structure to (poly)phenols, known to reduce oxidative stress associated with neurodegenerative complications, was also unable to modulate these processes. We subsequently tested the ability of R. idaeus (var Prestige) phenolics to overcome HTTpQ103-GFP proteotoxicity by modulating its clearance. As shown in Figure 2F, HTTpQ103-GFP levels decrease at similar rates with or without extract incubation, indicating that this is not the modus operandi of R. idaeus (var Prestige) phenolics.
Figure 1.
R. idaeus (var Prestige) bioactivity against pathological processes of four major NDs.. Yeast models recapitulating Aβ-42, α-Syn, FUS, or HTTpQ103 proteotoxicity, respectively, were pregrown in SC raffinose medium and growth curves were obtained after 24 h incubation in SC Gal medium supplemented or not with R. idaeus (var Prestige) (poly)phenol-enriched extracts. The area under the curve calculated from the growth curve is shown. The values represent the mean ± se of at least three biological replicates. Statistical differences are denoted as **P < 0.01 and ***P < 0.001, relative to that in the control. AD, Alzheimer’s disease; ALS, amyotrophic lateral ; HD, Huntington´s disease; PD, Parkinson’s disease.
Figure 2.
R. idaeus (var Prestige) bioactivity against HD is specific for this species and associated with the reduction of superoxide radicals. S. cerevisiae W303-1A cells either expressing HTTpQ103-GFP or harboring the empty vector (control) were pregrown in SC raffinose medium. A, Growth curves of cells incubated in SC Gal medium for 24 h. The confidence interval for the final cell biomass is shown in the right panel. B, Representative panel of drop-serial dilutions assay, phenotypic growth assays on SC Glc and SC Gal media with and without R. idaeus (var Prestige) and R. idaeus (var Octavia) extract incubation. C and D, HTTpQ103-GFP gene expression after 18 h induction in SC Gal medium with and without R. idaeus (var Prestige) and R. idaeus (var Octavia) extract incubation was determined by: (C) immunoblotting and respective densitometry (below) with phosphoglycerate kinase as loading control and (D) flow cytometry, GFP fluorescence versus side scatter (left), corresponding GFP-positive frequency (right). Idebenone was used as control drug. E, HTTpQ103-GFP subcellular localization of the inclusions after 18 h induction in SC Gal medium was assessed by fluorescence microscopy with and without R. idaeus (var Prestige) and R. idaeus (var Octavia) extract incubation. Idebenone was used as control drug. F, Clearance of HTTpQ103-GFP evaluated by western blotting at the indicated time points with and without R. idaeus (var Prestige) and R. idaeus (var Octavia) extract incubation. Idebenone was used as control drug. Phosphoglycerate kinase was used for normalization. G, Superoxide median fluorescence intensity assessed by flow cytometry after 18-h induction in SC Gal medium with and without R. idaeus (var Prestige) and R. idaeus (var Octavia) extract incubation. For all graphs, data represent the mean ± se of at least three biological replicates. Statistical differences are denoted as *P < 0.05, **P < 0.01, and ***P < 0.001, relative to that in the HTTpQ103-GFP condition. PGK, Phosphoglycerate kinase; SSC, side scatter; ns, not statistically significant; Vis, visual spectrum; MFI, median fluorescence intensity; a.u., arbitrary units; GAE, gallic acid equivalent.
Oxidative stress is a hallmark shared by NDs, and HTT-induced oxidative damage has been linked to cell death (Goswami et al., 2006; Niedzielska et al., 2016; Van Raamsdonk et al., 2017). Our data show that HTTpQ103-GFP in yeast mimics redox imbalance observed in HD disease context (Browne et al., 1997, 1999), as indicated by the increase of superoxide anion production monitored by flow cytometry using the dihydroethidium (DHE) probe (Fig. 2G). R. idaeus (var Prestige), but not R. idaeus (var Octavia), reduced DHE signals to levels comparable to that of the control strain, indicating its potential to restore redox homeostasis impaired by expression of HTTpQ103-GFP.
Identification of Lead Compounds in Bioactive Fractions
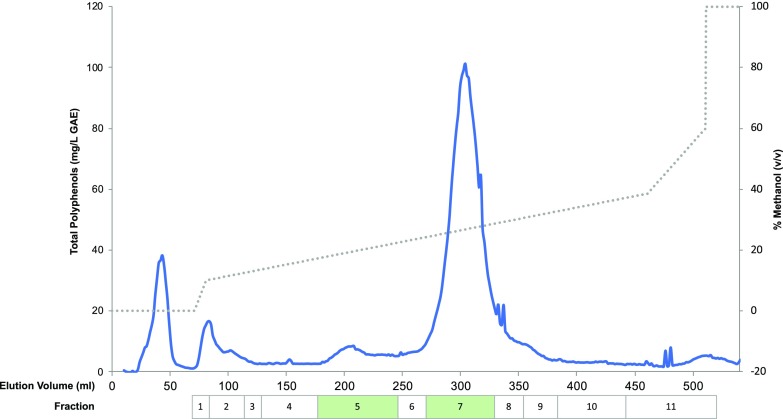
To identify the bioactive(s) compound(s) conferring protection to HD, the R. idaeus (var Prestige) extract was fractionated and 11 individual fractions (Fig. 3) were tested in the HD model as it has been done for the (poly)phenol-enriched extract. The growth assays revealed that fractions 5 and 7 still conferred protection to the HD model, suggesting that they contain the compound(s) conferring protection against HD. Alternatively, the bioactivity of the fractions may result from the synergistic effect of two or more compounds.
Figure 3.
Fractionation of the R. idaeus (var Prestige) extract. The chromatogram was obtained by quantification of total phenols (blue line, left y axis) for the fractions of R. idaeus (var Prestige) extracts. In total, 11 fractions were obtained by pooling the fractions comprising peaks of total phenols as indicated by the boxes. The boxes indicated in green correspond to fractions promoting the improvement of cellular growth in the yeast HD model as it was done for the (poly)phenol-enriched extract. GAE, gallic acid equivalent.
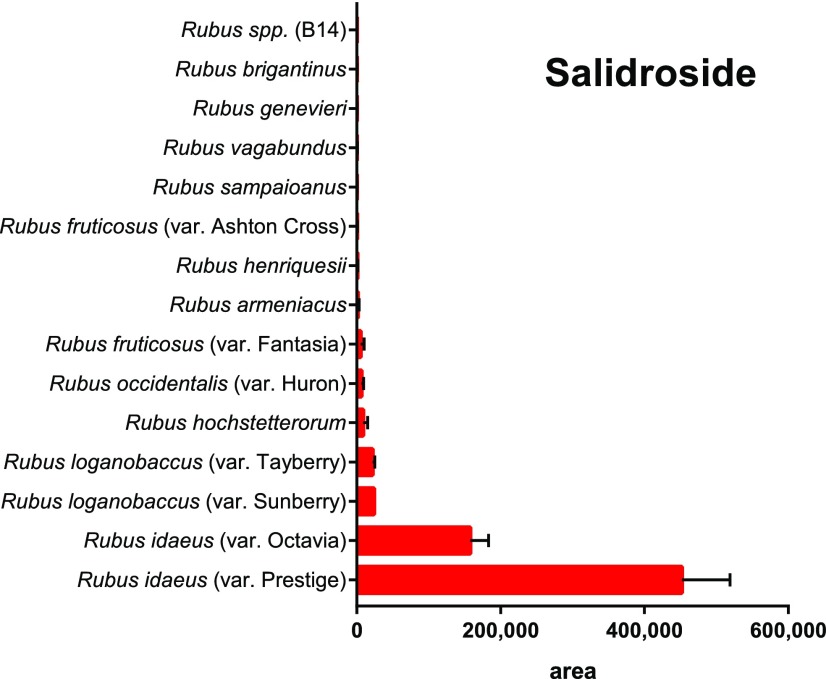
A total of 47 metabolites were found in fractions 5 (21 metabolites) and 7 (26 metabolites; Table 1) and these could be tentatively annotated by utilizing accurate mass, retention time, and MS/MS results acquired by liquid chromatography-mass spectrometry (LC-MS) in both electrospray ionization (ESI) positive and negative modes (Supplemental Table 1). To refine the number of lead molecules with potential protective effects on HD, the levels of compounds with tentative annotations in extracts of R. idaeus (var Prestige) were compared with that in 14 available Rubus species, which were tested in parallel, but that did not show any bioactivity toward HD (see Methods in Supplemental Material). The compounds with the highest relative abundance in R. idaeus (var Prestige) in comparison with that in other species or varieties were selected as tentative lead molecules (Supplemental Fig. S1). This reduced the number of lead molecules from 47 to five compounds that comprised glycosylated derivatives of hydroxybenzoates, phenylethanoids, or phenylpropanoids, namely a methoxybenzoyl hexoside, hydroxyphenylacetate glucoside, salidroside, and two benzoyl di-hexoside isomers (Supplemental Fig. S1). Whereas tentative annotations were provided based on the mass spectra only, definite annotation of salidroside (Supplmental Figs. S2 and S3) was confirmed against an analytical standard, which is only present in fraction 5. R. idaeus (var Prestige) contained the highest amount of salidroside compared to that in the other 14 analyzed Rubus species (Fig. 4). Interestingly, salidroside has been already suggested to confer a broad neuroprotective activity (Wang et al., 2015; Gao et al., 2016). Consequently, salidroside was selected for further experiments.
Table 1. Annotated compounds found in the HD bioactive fractions 5 and 7 of R. idaeus (var Prestige) in positive and negative modes using a Thermo Orbitrap XL LC-MS.
Additionally, the presence (+) or absence (−) of compounds in the fractions is noted.
| Retention Time (min) | m/z | Molecular Formula | Tentative Identification | Identity Level | Fractions | ||
|---|---|---|---|---|---|---|---|
| Positive Mode | Negative Mode | 5 | 7 | ||||
| 8.93 | — | 343.1029 [M-H]− | C15H20O9 | Methoxybenzoyl-hexoside | 3 | + | — |
| 9.17 | — | 299.0769 [M-H]− | C13H16O8 | Hydroxy-benzoyl-hexoside isomer A | 3 | + | — |
| 375.0928 | — | Unknown | 4 | + | — | ||
| 9.31 | 332.1354 [M+NH4]+ | 313.0923 [M-H]− | C14H18O8 | Hydroxyphenylacetate-hexoside | 3 | + | — |
| 359.0976 [M+FA-H]− | |||||||
| 10.10 | 205.0977 [M+H]+ | 203.0825 [M-H]− | C11H12N2O2 | Trp | 1 | + | — |
| 10.45 | — | 357.1186 [M+FA-H]− | C15H20O7 | Phenylpropanoyl-hexoside | 3 | + | — |
| 143.0350 | — | Unknown | 4 | + | — | ||
| 10.55 | — | 299.1132 [M-H]− | C14H18O8 | Salidroside | 1 | + | — |
| 345.1184 [M+FA-H]− | |||||||
| 10.72 | — | 341.0874 [M-H]− | C15H18O9 | Caffeoyl-glucoside | 2 | + | — |
| 11.03 | — | 427.1239 [M-H]− | C19H24O11 | Unknown | 4 | + | — |
| 11.08 | — | 299.0768 [M-H]− | C13H16O8 | Hydroxy-benzoyl-hexoside isomer B | 3 | + | — |
| 355.0666 [M-H]− | C15H16O10 | Coumaroyl-quinic acid | 2 | + | — | ||
| 11.41 | 464.1780 [M+NH4]+ | 445.1342 [M-H]− | C19H26O12 | Benzoyl-dihexoside isomer A | 3 | + | — |
| 469.1333 [M+Na]+ | |||||||
| 11.65 | — | 445.1346 [M-H]− | C19H26O12 | Benzoyl-dihexoside isomer B | 3 | + | — |
| 11.73 | 611.1619 M+ | 609.1446 [M+-2H]− | C27H31O16+ | Cyanidin 3-O-sophoroside | 1 | — | + |
| 12.00 | 757.2214 M+ | 755.2022 [M+-2H]− | C33H41O20+ | Cyanidin di-hexoside rhamnosyl | 2 | — | + |
| 12.19 | 449.1098 M+ | 447.0928 [M+-2H]− | C15H11O6+ | Cyanidin 3-O-glucoside | 1 | — | + |
| 12.37 | 595.1672 M+ | 593.1499 [M+-2H− | C27H31O15+ | Cyanidin 3-O-rutinoside | 1 | — | + |
| 12.40 | 629.174 [M+H]+ | 627.1552 [M-H]− | C15H12O7 | Taxifolin di-hexoside | 3 | — | + |
| 12.62 | — | 439.1815 | — | Unknown | 4 | — | + |
| 523.1812 | — | Unknown | 4 | — | + | ||
| 623.2181 | — | Unknown | 4 | — | + | ||
| 12.66 | 808.2535 [M+NH4]+ | 789.2075 [M-H]− | C33H42O22 | Unknown | 4 | — | + |
| 12.67 | 231.114 | — | — | Unknown | 4 | + | — |
| 302.1249 | — | — | Unknown | 4 | + | — | |
| — | 329.0873 | — | Unknown | 4 | + | — | |
| — | 577.1344 | — | Unknown | 4 | + | — | |
| — | 283.0821 | — | Unknown | 4 | + | — | |
| 12.72 | 467.1197 [M+H]+ | 465.1031 [M-H]− | C21H22O12 | Taxifolin-hexoside | 3 | — | + |
| 12.78 | 544.2408 [M+NH4]+ | — | C25H34O12 | Unknown | 4 | — | + |
| 12.99 | 317.1391 [M+H]+ | — | C18H20O5 | Unknown | 4 | — | + |
| 13.10 | — | 385.1135 [M-H]− | C17H22O10 | Sinapoyl-hexoside | 3 | — | + |
| — | 475.1451 [M-H]− | C20H28O13 | Hydroxyphenylacetate-dihexoside | 4 | — | + | |
| 13.15 | 434.2035 [M+NH4]+ | 461.1656 [M+FA-H]− | C19H28O10 | Benzyl alcohol-rutinoside | 3 | — | + |
| 13.22 | 464.1781 [M+NH4]+ | 445.1348 [M-H]− | C19H26O12 | Benzoyl di-hexoside isomer C | 3 | + | — |
| 491.1401 [M+FA-H]− | |||||||
| 267.067 | — | — | Unknown | 4 | + | — | |
| 13.40 | 291.0873 [M+H]+ | 289.0712 [M-H]− | C15H14O6 | (−)-epicatechin | 1 | — | + |
| 13.56 | — | 571.2025 [M-H]− | C26H36O14 | Unknown | 4 | — | + |
| 13.70 | — | 299.0770 [M-H]− | C13H16O8 | Unknown | 4 | — | + |
| 401.1444 | — | Unknown | 4 | — | + | ||
| 445.1347 [M-H]− | C19H26O12 | Benzoyl di-hexoside isomer D | 3 | — | + | ||
| 491.1400[M+FA-H]− | |||||||
| 14.11 | — | 783.0662 [M-2H]2− | C68H48O44 | Sanguiin H-10; | 2 | — | + |
| 1401.6058 [M-2H]2− | C123H80O78 | Lambertianin C | 2 | — | + | ||
| 14.16 | — | 371.0975 [M-H]− | C16H20O10 | Unknown | 4 | — | + |
| 14.40 | — | 934.0682 [M-2H]2− | C82H54O52 | Sanguiin H-6 | 2 | — | + |
| 14.80 | — | 455.1761 [M+FA-H]− | C17H30O11 | Unknown | 4 | — | + |
| 14.82 | — | 859.071 [M-2H]2− | C75H52O48 | Unknown Tannin | 3 | — | + |
Figure 4.
Salidroside areas detected during LC-MS/MS analysis in extracts of various Rubus species. Areas were obtained by integrating the selected ion chromatograms of m/z 345 at the retention time of 12.536 min of each extract analyzed in ESI negative mode. Data represent the mean ± se of at three technological replicates.
To confirm that salidroside indeed provides protection against HTTpQ103-GFP cytotoxicity, commercially available salidroside was also tested in the HD yeast model. Similar to the original R. idaeus (var Prestige) (poly)phenol-enriched extract, commercial salidroside improved growth of cells expressing HTTpQ103-GFP, particularly affecting the maximum growth rate of cells and final biomass of the cultures (Fig. 5A). The pretreatment with salidroside also protected cells from HTTpQ103-GFP cytotoxicity as revealed by the phenotypic growth assays (Fig. 5B). The data show that the mechanism by which the R. idaeus (var Prestige) (poly)phenol-enriched extract mediates cellular protection is associated with the removal of superoxide anions accumulated by the expression of HTTpQ103-GFP (Fig. 2G). In this scenario, we expected that mitochondrial functionality was affected (Indo et al., 2015) and wanted to evaluate if salidroside had the potential to improve cell growth again. Salidroside was therefore supplemented under nonfermentable growth conditions in the presence of glycerol as the sole carbon source, which requires full mitochondrial activity for cell viability. Under this growth conditions, salidroside pretreatment (80 µM) reversed the “sensitive” phenotype caused by HTTpQ103-GFP expression, indicating that it may prevent mitochondrial dysfunction. Remarkably, salidroside reduced superoxide anion production in all concentrations tested, which hints at a great potential for restoring redox homeostasis impaired by HTTpQ103-GFP (Fig. 5C). To test the specificity of salidroside bioactivity for HTTpQ103-GFP-induced cell damage, we evaluated the effect of salidroside on growth of control yeast cells (with no HTTpQ103-GFP expression) subjected to H2O2 as well on SOD1-defective mutants, which is characterized by defects in cellular antioxidant responses. SOD1 is the gene encoding for the yeast superoxide dismutase 1 (Rodrigues-Pousada et al., 2005), which is responsible for detoxifying superoxide yielding H2O2 and dioxygen. Salidroside had no effect on cells subjected to external oxidative stress or on conditions of intrinsic oxidative stress (Fig. 5D), underlining the assumed specificity toward HTTpQ103-GFP-induced cell damage.
Figure 5.
Salidroside is the bioactive compound of R. idaeus (var Prestige) against HD. S. cerevisiae W303-1A cells either expressing HTTpQ103-GFP or harboring the empty vector (control) were pregrown in SC raffinose medium. A, Growth curves of cells incubated in SC Gal medium for 24 h (left). The confidence interval for the maximum growth rate (μ) and final biomass is shown on the right. B, Cell growth assessed after 6 h induction in SC medium with Gal by phenotypic growth assays in SC medium with Glc, Gal, or glycerol. C, Superoxide production after 18 h induction in SC medium with Gal was determined by flow cytometry. D, Growth of the S. cerevisiae BY4741 wild type and sod1 mutant strains in the presence of H2O2 and/or salidroside in SC Glc medium for 24 h. Representative images are shown and the values in all graphs represent the mean ± se of at least three biological replicates. Statistical differences are denoted as *P < 0.05, **P < 0.01, and ***P < 0.001, relative to that in the HTTpQ103-GFP condition (C) or in the untreated condition (D). MFI, median fluorescence intensity; a.u., arbitrary units.
Salidroside was found in moderate levels (approximately 20 mg kg−1 fresh weight) in R. idaeus (var Prestige) berries collected in this study. No previous data were available regarding the effects of “environment” (E) and “genotype by environment” (G×E) on the abundance levels of this compound. Although there is potential to extract salidroside from red raspberries, it has to be ensured that salidroside levels are consistent across seasons and plant growth locations and that extraction methods are available to purify salidroside from a matrix rich in structurally closely related (poly)phenolics. When also considering seasonality of berry production, in particular the impact of climate change and seasonal weather extremes, other means for producing this compound need to be explored if these findings are to be translated to a commercial environment. In that regard, establishing a synthetic biosynthetic pathway toward salidroside in microorganisms and a process for its production appears to be a promising strategy for facile access to salidroside.
Pathway Reconstruction for Microbial Production of Salidroside in S. cerevisiae
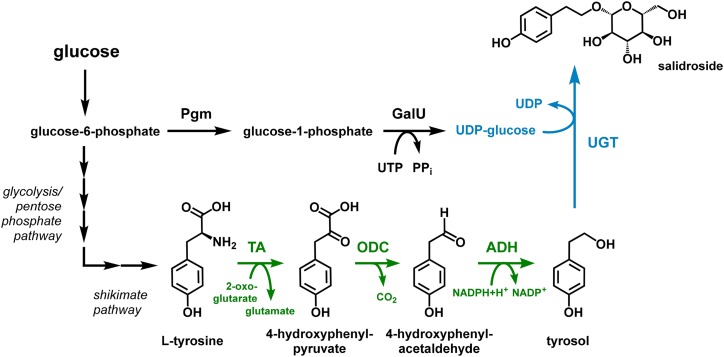
In plants of the Rhodiola genus, salidroside is synthesized from the aromatic amino acid l-Tyr in three enzymatic steps (Torrens-Spence et al., 2018). The involved glycosyltransferase, catalyzing the last step, uses uridine-diphosphate activated Glc (uridine diphosphate [UDP]-Glc) as donor for the sugar moiety. S. cerevisiae has an Ehrlich pathway as dedicated route for degrading aromatic amino acids, which is capable to catabolize l-Tyr directly to tyrosol, the aglycone of salidroside (Hazelwood et al., 2008; Fig. 6). This prompted us to choose this yeast for testing salidroside production by glycosylation of tyrosol obtained from the degradation of l-Tyr by using plant-derived UDP-Glc–dependent glycosyltransferases (UGT). In general, metabolic routes for biosynthesis and degradation of aromatic amino acids, including the Ehrlich pathway, are highly regulated in yeasts. Hence, as expected, a preliminary analysis of the basic S. cerevisiae S288C yeast strain BG (Eichenberger et al., 2017) showed only a very low accumulation of 0.02 mm (3 mg L−1) tyrosol in the total broth. To deregulate the biosynthesis of the precursor l-Tyr, we took advantage of feedback-resistant variants of l-Tyr-sensitive 3-deoxy-d-arabinoheptulosonate 7-P synthase (Aro4) and chorismate mutase (Aro7) of the aromatic amino acid-forming shikimate pathway (Luttik et al., 2008). The two respective genes ARO4K229L and ARO7T266I, encoding for these two enzymes, were integrated into the genome of the BG strain, replacing the ARO3 gene encoding a 3-deoxy-d-arabinoheptulosonate 7-P synthase isoenzyme feedback-regulated by l-Phe. This approach circumvents the natural feedback inhibition by l-Tyr, a strategy that was previously used in our labs to increase the carbon flow toward l-Tyr (Lehka et al., 2017). Relieving the feedback-inhibition resulted in a 10-fold increase in tyrosol concentration compared to that in the BG strain. To further improve the supply of tyrosol, we overexpressed two native genes, TYR1 encoding prephenate dehydrogenase, and ARO10 encoding a 2-oxo acid decarboxylase. These two genes were integrated into the yeast genome under the control of strong promoters, resulting in a strain, namely BG4, that produced more than 3.2 mm (442 mg L−1) of tyrosol in 72 h (Fig. 7). This strain was used for testing UGTs for salidroside production. For this purpose, two synthetic genes encoding the glycosyltransferases UGT72B14 and UGT73B6 from Rhodiola sachalinensis, which were previously applied to glycosylate tyrosol (Ma et al., 2007; Yu et al., 2011), were codon-optimized for expression in yeast and integrated into the strain setup. In addition, we performed a rapid in vitro screening of our in-house collection of purified UGTs for identifying variants with the ability to glycosylate tyrosol. A single UGT, OsUGT13 from Oryza sativa, showed a strong tyrosol glycosylation activity and the corresponding gene was therefore also synthesized as codon-optimized version for a heterologous gene expression in yeast. The three individual genes were cloned into yeast expression cassettes and introduced into the tyrosol producing strain BG4 by in vivo assembly of HRT plasmids as described earlier (Eichenberger et al., 2017). Surprisingly, application of the newly identified OsUGT13 resulted in much higher salidroside titers compared to those of the yeast variants expressing the two R. sachalinensis UGTs, as these two strains accumulated only very low amounts of salidroside (Fig. 7). In contrast, on a molar ratio, the yeast strain employing OsUGT13 converted more than two-thirds of the produced tyrosol to 2.1 mm (640 mg L−1) salidroside in 72 h. Notably, salidroside was the only product with no masses detected corresponding to possible side products such as the icariside D2 (tyrosol glycosylated at the phenolic hydroxyl group) or the double-glycosylated tyrosol derivative.
Figure 6.
Biosynthetic pathway exploited for salidroside production in S. cerevisiae and C. glutamicum. The direct salidroside precursor tyrosol can be obtained from the aromatic amino acid l-Tyr by an Ehrlich pathway (shown in green), which is naturally present in S. cerevisiae and which was functionally introduced into C. glutamicum by expression of the heterologous genes aro10 and yqhD encoding 2-oxo acid decarboxylase and alcohol dehydrogenase, respectively. The conversion of l-Tyr to 4-hydroxyphenylpyruvate was catalyzed by endogenous transaminases in both organisms. C. glutamicum was also engineered toward an increased supply of UDP-Glc required for the glycosylation of tyrosol catalyzed by UDP-Glc-dependent glycosyltransferases (shown in blue). To this end, genes coding for phosphoglucomutase and GalU were expressed for increasing the conversion of the glycolysis intermediate Glc-6-P to UDP-Glc. Pgm, phosphoglucomutase; TA, transaminase; ODC, 2-oxo acid decarboxylase; ADH, alcohol dehydrogenase.
Figure 7.
Salidroside production in engineered S. cerevisiae. S. cerevisiae BG4 strains expressing codon-optimized genes encoding the three indicated uridine-diphosphate–dependent glycosyltransferases were cultivated as described in the “Material and Methods” section. The final concentrations of the aromatic amino acids l-Ph and l-Tyr and of tyrosol and salidroside in the culture supernatant obtained after 72 h of cultivation are shown. Data represent the mean ± se of at three biological replicates.
Metabolic Engineering toward Salidroside Production in C. glutamicum
We decided to also establish the biosynthetic route to salidroside in C. glutamicum as this organism, already used for the million ton-scale production of the amino acids such as l-Glu and l-Lys, is characterized by a high tolerance toward toxic aromatic compounds (Kitade et al., 2018; Kogure and Inui, 2018). Not surprisingly, several C. glutamicum strains for the synthesis of aromatic plant natural products (Kallscheuer et al., 2016, 2017; Braga et al., 2018) and aromatic amino acids as their precursor molecules are available (Ikeda, 2006), rendering this prokaryotic platform organism a suitable alternative to yeast for salidroside production. Initially, the focus was put on the functional introduction of the glycosyltransferases UGT72B14 or UGT73B6 from R. sachalinensis for converting tyrosol to salidroside. As the metabolism of C. glutamicum is naturally incapable of providing tyrosol, salidroside synthesis was first tested by supplementing tyrosol. Unfortunately, no salidroside production could be observed in constructed strains expressing codon-optimized genes for the R. sachalinensis UGTs. Most likely, improper folding of the plant enzymes in C. glutamicum might abolish glycosyltransferase activity, which was also observed earlier during the expression of plant-derived genes (Kallscheuer et al., 2017). Previously, translational fusion of the protein of interest to the maltose-binding protein MalE from E. coli improved the solubility of the plant protein yielding functional enzymes in C. glutamicum. By following the same strategy, accumulation of low salidroside concentrations of up to 0.02 mm (6 mg L−1) from 20 mm supplemented tyrosol could be achieved with a C. glutamicum strain synthesizing the MalE-UGT73B6 fusion protein, whereas no salidroside formation could be detected in strain employing the MalE-UGT72B14 glycosyltransferase.
At this stage, it was speculated that low intracellular availability of the glycosyl-donor UDP-Glc probably limits tyrosol conversion by C. glutamicum, a problem encountered previously during disaccharide production with this microorganism (Padilla et al., 2004). Thus, the strain was additionally engineered toward increased UDP-Glc supply. UDP-Glc is obtained from Glc-1-P (G1P), an isomer of the glycolysis intermediate Glc-6-P (G6P) provided by phosphoglucomutase (Fig. 6). Subsequently, the uridine triphosphate (UTP):G1P uridylyltransferase (GalU) catalyzes the transfer of the uridine monophosphate (UMP) moiety from UTP to G1P yielding UDP-Glc (Weissborn et al., 1994). A C. glutamicum strain harboring MalE-UGT73B6 and additionally expressing the genes pgm and galU from E. coli accumulated 0.12 mm (37 mg L−1) salidroside from 20 mm tyrosol pointing toward a bottleneck at the stage of UDP-Glc supply. The overall yield of 0.01 mol salidroside per mol tyrosol was still very low, indicating that UGT73B6 might be not the best suited UGT for an application during salidroside production in C. glutamicum.
For an improved biotransformation of tyrosol, we then also tested the identified OsUGT13 from O. sativa, which allowed for the accumulation of the highest salidroside concentrations in cultivations of the engineered yeast strains. Functional introduction of the engineered MalE-OsUGT13 fusion protein into the C. glutamicum strain engineered for improved UDP-Glc supply allowed for a 60-fold increase in salidroside accumulation compared to that in the strain harboring MalE-UGT73B6. Under optimized expression conditions, 3.6 mm (1,100 g L−1) salidroside was produced from 20 mm tyrosol corresponding to a yield of 0.18 mol salidroside per mol tyrosol. The highest conversion yield of 0.74 mol salidroside per mol tyrosol with a final salidroside titer of 30 mm (8,900 g L−1) was observed with an initial tyrosol concentration of 40 mm (Supplemental Fig. S4). Tyrosol concentrations exceeding 40 mm had a negative effect on growth, which also negatively affected tyrosol conversion yields (Supplemental Fig. S4).
At this stage, salidroside production was still dependent on the supplementation of tyrosol as precursor molecule. For rendering the production economically more viable, we also tested production starting from l-Tyr. This compound is not only less expensive than tyrosol, but can also be naturally supplied by the host strain when using the mentioned strategies for overproduction of aromatic amino acids. For enabling salidroside production from l-Tyr, the Ehrlich pathway for supplying tyrosol was functionally integrated into C. glutamicum and combined with MalE-OsUGT13. This synthetic Ehrlich pathway is composed of the 2-oxo acid decarboxylase Aro10 from S. cerevisiae and the alcohol dehydrogenase YqhD from E. coli (Vuralhan et al., 2005; Jarboe, 2011; Fig. 6). For the initial transamination reaction, we relied on the native expression of the endogenous transaminases AroT and IlvE in C. glutamicum, which are both known to transaminate l-Tyr (Marienhagen et al., 2005). The heterologous pathway was found to be functional as C. glutamicum expressing aro10 and yqhD along with the gene encoding MalE-OsUGT13 were capable of producing 0.5 mm (150 mg L−1) salidroside when 20 mm l-Tyr was supplemented, whereas no salidroside was produced when the strain did not harbor aro10 and yqhD. Although a salidroside production strategy from l-Tyr was established, we decided to use the biotransformation strain with increased UDP-Glc supply for the up-scaling experiments to achieve product quantities sufficient for retesting salidroside in the yeast HD model.
Scale-up, Downstream Processing, and Retesting of Bioactivity
Both organisms, S. cerevisiae and C. glutamicum, could be successfully engineered toward producing salidroside. However, it remained to be demonstrated whether the microbially produced salidroside provokes the same beneficial effects in the HD model experiments as the commercially available salidroside analytical standard (purity > 98%). For that purpose, the microbial salidroside production was scaled up. In the case of C. glutamicum, biotransformations in two batch fermentations with initial concentrations of 40 g L−1 and 80 g L−1 Glc were performed, each in the presence of 40 mm tyrosol. The maximum biomass concentration of 24 g L−1 obtained with 80 g L−1 Glc was almost twice as high as the biomass concentration of 13 g L−1 obtained from 40 g L−1 Glc. The highest salidroside titer of 32 mm (9.7 g L−1) was achieved with 80 g L−1 of Glc, and under these conditions the salidroside yield was 0.81 mol salidroside per mol of tyrosol, which is 1.8-fold higher than the product yield of 0.43 obtained with 40 g L−1 of Glc. The best yield is only 10% higher than the yield of 0.74 obtained during shaking flask cultivations, indicating that at least for the biotransformation of tyrosol there is no large impact of the controlled cultivation conditions in the bioreactor. The cultivation of S. cerevisiae was scaled from the deep-well plate scale to the shaking flask scale for achieving a culture volume sufficient for salidroside purification.
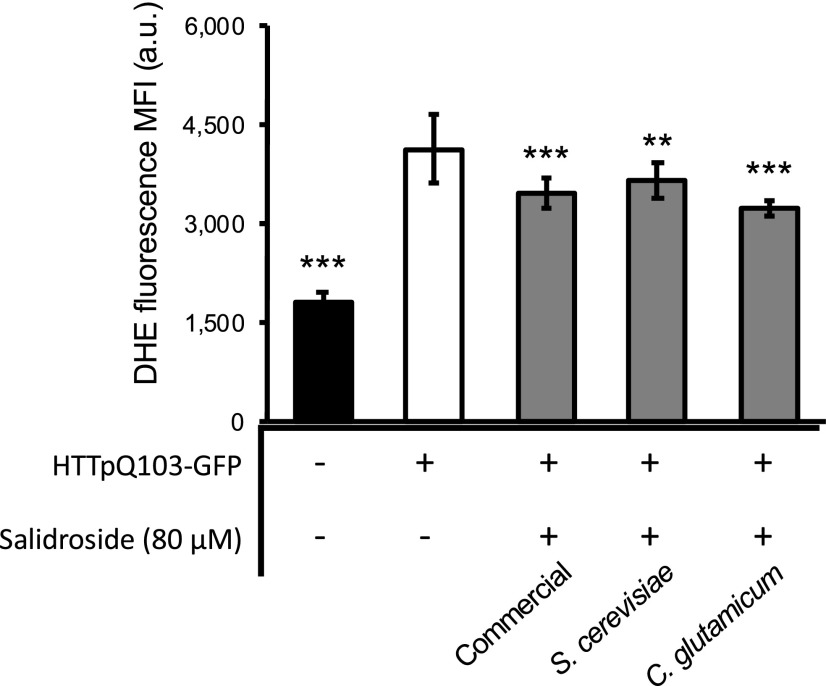
Salidroside was separately purified from the fermentation broth of S. cerevisiae and C. glutamicum (Supplemental Fig. S5). In direct comparison to the commercially available salidroside, the microbially produced and purified compound was tested in the HD yeast model to confirm that the microbially produced salidroside has the same beneficial bioactivity. With respect to the salidroside-mediated cell protection by reducing superoxide anions, the data indeed showed that salidroside produced with S. cerevisiae or C. glutamicum has very similar protective activities compared to that of the commercial, chemically synthesized compound (Fig. 8).
Figure 8.
Salidroside produced in S. cerevisiae and C. glutamicum mediated a similar protection as commercially available salidroside. W303-1A cells expressing the gene encoding HTTpQ103-GFP were pregrown in SC raffinose medium and superoxide production after 18 h induction in SC medium with Gal was determined by flow cytometry using a DHE probe. Data represent the mean ± se of at least three biological replicates. Statistical differences are denoted as **P < 0.01 and ***P < 0.001, relative to that in the HTTpQ103-GFP condition. a.u., arbitrary units. MFI, median fluorescence intensity; a.u., arbitrary units.
DISCUSSION
Plant natural products represent a rich source of bioactive compounds with numerous applications. This is reflected in the number of pharmaceuticals, nutraceuticals, and food additives brought to market, which are, to a large extent, natural plant products or derivatives thereof, or are mimics based on the structure (Marienhagen and Bott, 2013; Kallscheuer et al., 2019). Such compounds are also recognized as promising therapeutics for slowing down the course of NDs. Among these, HD is an inherited disorder resulting in the death of brain cells, which is associated with problems in coordination and mental abilities (Dayalu and Albin, 2015). HD is caused by alterations in the gene encoding HTT. This protein harbors a poly-Gln (poly-Q) sequence close to the N terminus, which consists of 10 to 35 Gln residues in the normal population but is expanded to 36 to 120 repeats in HD patients (MacDonald and The Huntington’s Disease Collaborative Research Group, 1993; DiFiglia et al., 1997). The severity of the disease directly increases with the increasing length of the poly-Q sequence, and pathological symptoms appear when the threshold of 37 Gln-encoding CAG codons is overcome. As a result, proteotoxic aggregates of poly-Q-expanded HTT, form in neuronal and glial cells. In our study, we showed that salidroside is an abundant bioactive compound in R. idaeus (var Prestige), whereas it is present in much lower amounts in 14 other Rubus species. Bioactivity of the R. idaeus (var Prestige) extract against HD could be traced back to salidroside as confirmed in the analogous bioactivity assays using salidroside, which was commercially available or produced in engineered S. cerevisiae and C. glutamicum strains.
Salidroside was also identified in the Chinese medicinal herb R. sachalinensis (Lu et al., 1980), which represents a major source of this compound (Xu et al., 1998; Wu et al., 2003; Zhou et al., 2007). It was shown to be a potent antioxidant on hydrogen-peroxide–induced apoptosis and beta-amyloid–induced oxidative stress in SH-SY5Y cells (Zhang et al., 2007, 2010). Moreover, salidroside shows neuroprotective effects against Glu excitotoxicity in a primary culture of rat hippocampal neurons and protects cells from hypoglycemia and serum limitation in the PC12 cell model (Yu et al., 2008). In Caenorhabditis elegans expressing poly-Q proteins, salidroside exerts its neuroprotective function against poly-Q-mediated toxicity via oxidative stress pathways (Xiao et al., 2014). However, the protective effect observed for C. elegans oxidative damage was general and not specific due to poly-Q-mediated toxicity. In this study, we could show that the protective action of salidroside is specific against HTTpQ103-GFP-induced cell damage and not generally against superoxide-driven damages.
The large potential of salidroside for pharmaceutical applications motivated us to design and introduce synthetic routes for its production into industrially relevant microorganisms. Yu and coworkers identified the genes coding for glycosyltransferases UGT72B14 and UGT73B6 and reported the ability of these enzymes to glycosylate tyrosol yielding salidroside (Yu et al., 2011). Interestingly, the natural pathway in R. sachalinensis supplying tyrosol remained unidentified for a long time. There were indications that tyrosol is produced from l-Tyr (Lan et al., 2013;, however, the complete metabolic route to tyrosol in Rhodiola sp. was elucidated only very recently (Torrens-Spence et al., 2018).
With enzymes from yeast and bacteria, a tyrosol biosynthetic pathway was reconstructed in E. coli, which was subsequently exploited for salidroside production by additional functional introduction of UGT73B6 into this strain (Bai et al., 2014). After 48 h, a product titer of 0.19 mm (57 mg L−1) salidroside could be achieved. In addition, 0.21 mm (63 mg L−1) of icariside D2, i.e. tyrosol glycosylated at the phenolic hydroxy group, was identified as a major side product. These results indicate a rather unspecific glycosylation of tyrosol by UGT73B6. With the glycosyltransferase OsUGT13 from rice we here identified an alternative UGT, which was more suitable for glycosylation of tyrosol both in S. cerevisiae and C. glutamicum. Recently, and after we had finished our screening of UGTs, another salidroside pathway was reconstituted in E. coli, using a combination of various plant genes (Chung et al., 2017). In this study, UGT85A1 from Arabidopsis (Arabidopsis thaliana), which showed essentially full conversion of tyrosol to salidroside, allowed for product titers of up to 0.97 mm (290 mg L−1) after 48 h of cultivation. Because Arabidopsis is not known to produce salidroside, these results demonstrate a relaxed specificity of some UGTs as it was also observed for the enzyme from O. sativa identified during screening of the UGT library. The same Arabidopsis enzyme (UGT85A1) was recently also functionally introduced into a tyrosol-accumulating S. cerevisiae strain, which was capable of producing 2.4 mm (732 mg L−1) salidroside during fed-batch fermentation (Jiang et al., 2018). The obtained salidroside titer is very similar to the product concentration of 2.1 mm (640 mg L−1) obtained in our study.
CONCLUSION
This study shows that an interdisciplinary team of experts from the fields of plant biology, industrial biotechnology, and analytical chemistry is able to develop a complete pipeline for discovery and microbial production of novel pharmacologically interesting compounds of plant origin within a very short time frame; here it took only 24 months from the identification of salidroside in R. idaeus (var Prestige) to lab-scale bioreactor production of this compound in engineered microorganisms. This approach may serve as a blueprint for future campaigns aiming at taking advantage of the chemical diversity of (plant) natural products.
Of course, salidroside, identified as a plant phenol with bioactivity against HD in this collaborative project, must be validated in cellular models with a higher degree of complexity and in preclinical animal models to really assess its pharmaceutical potential. Nevertheless, the microorganisms engineered in this study will ensure the availability of sufficient salidroside for this undertaking.
MATERIALS AND METHODS
Berry Sampling, Extraction Procedure, and Fractionation
Approximately 250 g of Rubus idaeus (var Prestige and Octavia) fresh ripe fruits were harvested manually in the field on June 21, 2014 in Invergowrie, Scotland, UK (56.46 latitude, −3.07 longitude). The samples were kept under cool conditions until transfer to −20°C storage. Approximately 150 g of the frozen fruits were weighed into a solvent-proof blender containing 450 mL of precooled 0.2% v/v formic acid in methanol solution. Samples were homogenized and subsequently filtered using Whatman filter paper no. 1 (GE Healthcare). A total of ten 1-mL aliquots of the filtrate were solvent-dried using a miVac duo centrifugal evaporator (Genevac) at room temperature for 4 h, followed by lyophilization, flushed with N2 and stored at −20°C until bioactivity assays. In parallel, six 50-mL aliquots of the remaining filtrate were stored at −80°C until fractionation.
The content of the six aliquots of the filtrate was evaporated using a centrifugal evaporator (Labconco), solubilized and combined. For the fractionations an ÄKTA Explorer preparative chromatography system (GE Healthcare) was used. For each fractionation (a total of eight replicates), 1 mg of gallic acid equivalent was injected into a 31-mL column containing a C18 silica resin (particle size 55–105 μm, pore size 125 Å) utilizing 0.1% formic acid v/v in H2O as mobile phase A and 100% methanol as mobile phase B. At a flow rate of 3 mL min−1 the following gradient was used: wash step 31 mL; 0%–10% solvent B in 10 mL; 10%–40% solvent B in 400 mL; 40%–60% solvent B in 50 mL; a regeneration step with 100% solvent B for 50 mL. Fractions exceeding 30 mL were split. The same fractions from different fractionation replicates were pooled and stored at −80°C. Total phenols of the fractions were determined as described below using the Folin-Ciocalteau method.
LC-MS Analysis of Fractionated Extracts
All obtained fractions were resuspended in 1 mL of 50:50 H2O: acetonitrile with 0.1% formic acid, subsequently diluted 5-fold with H2O and filtered using 0.45-µm filter vials (Thomson Instrument). Subsequently, samples were analyzed by LC-MS using an LC device composed of an Accela 600 quaternary pump and an Accela PDA detector coupled to a linear trap quadropole (LTQ) Orbitrap XL mass spectrometer (Thermo Fisher Scientific) with accurate mass capabilities, operated under the Xcalibur software package 2.0 as described previously in McDougall et al. (2014). Five microliters of the samples were injected into a Synergi Hydro RP-80Å 150 × 2 mm (Phenomenex) and autosampler and column temperatures were maintained at 4°C and 30°C, respectively. Samples were eluted at a flow rate of 0.3 mL min−1 using two mobile phases (A: 0.1% v/v formic acid in H2O; B: 0.1% formic acid in acetonitrile) with the following gradient: 0 min: 2% B; 2 min: 2% B; 5 min: 5% B; 25 min: 45% B; 26 min: 100% B; 29 min: 100% B; 30 min: 2% B; 35 min: 2% B. Mass detection was carried out in both positive and negative ESI modes. Data acquisition was performed in ESI full scan mode within the FT detector operating with a mass resolution of 30.000 (full width at half-maximum defined at m/z 400); the HESI probe temperature was set to 100°C, the capillary temperature at 275°C with sheath gas at 60 psi, and auxiliary gas at 30 psi. For the positive polarity, the source voltage was set at +4.5 kV, capillary voltage at 44 V, and tube lens voltage at 100 V. For negative polarity, the source voltage was set at −3.5 kV, capillary voltage at −44 V, and tube lens voltage at −100 V. In addition, the analysis was repeated in full-scan mode within the FT detector and followed by a data-dependent MS/MS of the three most intense ions within the LTQ ion trap detector using Helium as a collision gas, with a normalized collision energy of 45 mass isolation width of ± 1 m/z Activation Q of 0.25 and activation time of 30 ms, source voltage (set at 3.4 kV) in wide band activation mode. Full scan FT data are acquired in profile mode, whereas the DDA MS2 LTQ fragmentation spectra are acquired in centroid mode. A scan speed of 0.1 s and 0.4 s are applied in the LTQ and the Fourier transform mass spectrometer, respectively. The Automatic Gain Control was set to 1 × 105 and 5 × 105 for the LTQ and the Fourier transform mass spectrometer, respectively. Peaks present in the bioactive fractions were tentatively annotated by utilizing the accurate mass (<3 ppm), MS/MS fragmentation patterns, and reference to retention time for previously analyzed standards when available (Supplemental Table S1).
Total Quantification of Phenolic Compounds
The filtrates of Rubus extracts to be used in the cell assays were solvent-dried using a Speed-vac, resuspended in CH3COOH/H2O and subsequently subjected to solid phase extraction (Tavares et al., 2010). Total phenolic content of the samples were determined using the Folin-Ciocalteau method adapted to microplate readers (Tavares et al., 2010) with gallic acid as standard. Total phenolic content was expressed as µg GAE mL−1. The samples were aliquoted, freeze-dried, and stored at −20°C.
Yeast Plasmids, Strain, and Transformation for Bioactivity Screening
Plasmids and strains used in this study are listed in Supplemental Table S2. For construction of p426_GAL1pr-GFP-AB42, the fragment GFP-AB42 was removed from p416_GPD-GFP_AB42 using BamHI and SmaI and cloned into the p426_GAL1. For construction of p426_GAL1pr-FLAG-HTT103Q-GFP, the sequence GAL1pr-FLAG-HTTp103-GFP from p425GAL1_HTT103Q was amplified by PCR and cloned into the p426 vector using the In-Fusion Cloning Kit (TAKARA Clontech). W303-1A_FUS and W303-1A_T strains were obtained by transformation of W303-1A strain with plasmids pAG303_GAL1pr-FUS and pAG303_GAL1pr-ccdB, previously linearized with BstZ17I. Yeast transformation procedures were carried out using a lithium acetate method described in Gietz et al. (1995). Synthetic complete (SC) medium [0.67% (w/v) yeast nitrogen base without amino acids (YNB; Difco) and 0.79 g L−1 complete supplement mixture (CSM; QBiogene), containing 1% (w/v) raffinose, was used for growth of PD and ALS integrative models. Synthetic dropout CSM-URA medium [0.67% (w/v) YNB and 0.77 g L−1 single amino acid dropout CSM-URA (QBiogene) containing 1% (w/v) raffinose was used for growth of PD model. Synthetic dropout SC-LEU medium 0.6% (w/v) YNB and 0.54 g L−1 6-amino acid dropout CSM-ADE-HIS-LEU-LYS-TRP-URA (QBiogene) supplemented with standard concentrations of the required amino acids and containing 1% (w/v) raffinose was used for growth of the HD model. In all conditions, repression or induction of the expression of the disease-encoding gene was carried out in medium containing Glc (for repression) and Gal (for induction) at a final concentration of 2% (w/v). A preinoculum was prepared in raffinose medium and cultures were incubated overnight at 30°C under orbital shaking. Cells were diluted in fresh medium and cultures were incubated under the same conditions until the optical density at 600 nm (OD600) reached 0.5 ± 0.05 (log phase). Cell suspensions were further diluted according to the equation ODi × Vi = ODf/(2([t/gt]) × Vf, with ODi = initial optical density of the culture, Vi = initial volume of culture, ODf = final optical density of the culture, t = time (usually 16 h), gt = generation time of the strain, and Vf = final volume of culture to ensure synchronicity of the cells within the cultures. OD600 readings were performed in a 96-well microtiter plate using a Power Wave XS plate spectrophotometer (BioTek Instruments). For the phenotypic growth assays, yeast was grown as described to OD600 0.1 ± 0.01 and was inoculated to an OD600 0.2 ± 0.02 in medium supplemented or not with the indicated concentrations of Rubus extracts or the pure compounds. After 6 h, OD600 was adjusted to 0.05 ± 0.005, serial dilutions were performed with a ratio of 1:3, and 5 μL of each dilution was spotted onto solid medium containing Glc or Gal as the sole carbon source. Growth was recorded after 48 h incubation at 30°C. Images were acquired using Chemidoc XRS (Bio-Rad Laboratories) and Quantity-one software (Bio-Rad Laboratories). For the growth curves, yeast cultures were diluted to an OD600 of 0.12 ± 0.012 in fresh medium supplemented or not with the indicated concentrations of Rubus extracts, fractions, or the pure compounds in a 96-well microtiter plate. After 2 h of incubation at 30°C, cultures were further diluted to an OD600 of 0.03 ± 0.003 in medium containing Glc or Gal and supplemented or not with the indicated compounds. The cultures were then incubated at 30°C with shaking for 24 h and cellular growth was kinetically monitored hourly by measuring OD600. Data were modeled using nonlinear parametric regressions and growth parameters (final biomass, maximum growth rate, lag time, doubling time, and area under curve) as well as % of protection (area under curve relative to the control strain), all with 95% confidence intervals, and were estimated from the best fit model using RStudio (Version 0.99.902, GNU lesser general public license). Before performing the bioactivity assays, all extracts were tested for toxicity in the respective control strains. The higher, nontoxic concentration of each extract was then used for the bioactivity assays.
Flow Cytometry Analysis and Fluorescence Microscopy
Synchronized yeast cell cultures at OD600 of 0.2 ± 0.02 were exposed to the indicated concentrations of Rubus extracts or the pure compounds for 18 h in medium containing Glc or Gal. For analyzing superoxide production using a DHE probe, cells were incubated with 20 μg mL−1 of DHE for 30 min at 30°C protected from light. Flow cytometry analysis was performed using a CyFlow Cube 6 (Sysmex Partec), equipped with a blue solid state laser (488 nm), green fluorescence channel 530/30 nm, and orange red fluorescence channel 610/20 nm. Data analysis was performed using FlowJo software (Ver. 6.7, FlowJo). A minimum of 30,000 events was collected during each experiment. Results were expressed as median fluorescence intensity of DHE. In addition, yeast cells were visually inspected for the formation of intracellular inclusions of HTT by fluorescence microscopy using a DMRA2 fluorescence microscope (Leica) equipped with a CoolSNAP HQ CCD camera (1.3 MPx monochrome). Images were analyzed using the software ImageJ (University of WI-Madison).
Immunoblotting
Protein extraction from yeast cells by glass bead lysis and quantification of the total protein content of each sample was performed as described in Ausubel et al. (1992) and Gomes et al. (2006). Immunoblotting was carried out as described in Macedo et al. (2015). Antibodies against GFP (Antibodies Incorporated) and PGK (Life Technologies) were used.
Reverse Transcription Quantitative PCR
Reverse transcription quantitative PCR (RT-qPCR) analyses were performed according to the MIQE guidelines (Minimum Information for publication of Quantitative real-time PCR Experiments; Bustin et al., 2009). Total RNA was extracted using the E.N.Z.A. yeast RNA extraction Kit (OMEGA Bio-Tek). RNA (200–300 ng) was cleaned and reverse-transcribed with qScript cDNA superMix Kit (Quanta Biosciences). RT-qPCR was performed in a LightCycler 480 Instrument (Roche), using LightCycler 480 SYBR Green I Master (Roche) to evaluate expression of the HTTpolyQ103-GFP fusion gene. Relative standard curves were constructed for each gene, using triplicate serial dilutions of cDNA. The relative expression of these genes was calculated by the relative quantification method with efficiency correction, using LightCycler 480 (software version 1.5.0.39; Roche). ACT1 and PDA1 were used as reference genes. The results were expressed as fold change mRNA levels relative to that in the control (mRNA fold change) of at least three independent biological replicates.
Statistical Analysis
The results related to bioactivity (Figs. 1, 2, 5, and 8) reported in this study are the average of at least three independent biological replicates and are represented as the mean ± se. Differences among treatments were assessed by parametric Student’s t test or ANOVA with Tukey’s Honest Significant Difference multiple comparison test (α = 0.05) using SigmaStat 3.10 (Systat Software).
Salidroside Pathway Reconstruction in Microorganisms
Synthetic genes, based on the protein sequences of Rhodiola sachalinensis UGT72B14 (Acc. no. ACD87062) and UGT73B6 (Acc. no. AAS55083), as well as Oryza sativa OsUGT13 (XP_015622802), codon-optimized for expression in Saccharomyces cerevisiae or in Corynebacterium glutamicum ATCC13032, were synthesized by LifeTechnologies (GeneArt). All constructed strains and plasmids are listed in Supplemental Table S2. Oligonucleotides used for cloning are listed in Supplemental Table S3. In case of S. cerevisiae, genes were synthesized having a HindIII restriction site and an AAA Kozak sequence at the 5′ end and a SacII restriction site at the 3′ end. Genes were directly cloned into the yeast expression cassette of pEVE2176 (BC cassette with pGPD1-gene-tCYC1) for HRT plasmid assembly by in vivo homologous recombination as described in Eichenberger et al. (2018). For C. glutamicum, either synthetic genes (coding for the UGTs mentioned above and of Aro10 from S. cerevisiae and YqhD from Escherichia coli, all codon-optimized for expression in C. glutamicum) or genomic DNA of E. coli MG1655 served as template for gene amplification. For heterologous gene expression in C. glutamicum, provided synthetic genes were reamplified by PCR using primers containing unique restriction sites, which were subsequently used for cloning. E. coli DH5α or E. coli XL10 Gold cells (Agilent) were used for cloning of plasmids and were cultivated in LB medium (5 g L−1 yeast extract, 10 g L−1 tryptone, 5 g L−1 NaCl) with 100 mg L−1 ampicillin, 50 mg L−1 kanamycin or 100 mg L−1 spectinomycin. All constructed plasmids were finally verified by DNA sequencing at Eurofins MWG Operon.
Microbial Salidroside Production
The yeast strain BG4 used in this study is a derivative of the S288C strain NCYC 3608 (NCYC). This strain was optimized for the production of aromatic amino acid precursors in the shikimate pathway (Vanegas et al., 2018) and was additionally engineered toward an improved flow through the Ehrlich pathway (Mikkelsen et al., 2012). S. cerevisiae was grown in 96-well plates at 30°C, 5 cm shaking diameter, and 300 rpm for 72 h in SC medium containing 1.47 g L−1 Synthetic Complete (Kaiser) Drop Out: Leu, His, Ura (Formedium), 6.7 g L−1 Yeast Nitrogen Base Without Amino Acids, 20 g L−1 Glc, pH set to 5.8 with hydrochloric acid, and supplemented with 76 mg L−1 uracil for selection of the HRT plasmid. For extraction of relevant compounds, 150 μL culture broth was mixed with 150 μL acidified methanol (containing 1% hydrochloric acid), incubated for 10 min in a 96-well plate at 30°C, 5 cm shaking diameter, and 300 rpm and subsequently clarified by centrifugation at 4,000g for 5 min.
C. glutamicum was cultivated aerobically at 30°C in Brain Heart Infusion medium (for precultures) or in defined CGXII medium with Glc as sole carbon and energy source (Keilhauer et al., 1993) with kanamycin (25 mg L−1) and spectinomycin (100 mg L−1) when required for plasmid maintenance. For the production of salidroside the constructed strain C. glutamicum DelAro4 was used as a basis, as it is incapable of degrading aromatic compounds (Kallscheuer et al., 2016). Transformations were performed as described previously in Eggeling and Bott (2005). In the bioreactor cultivations, urea and MOPS were omitted because the pH is automatically controlled. Tyrosol was dissolved in DMSO. Heterologous gene expression was induced using 1 mm IPTG 2 h after inoculation. For cultivation experiments C. glutamicum was cultivated for 8–10 h in Brain Heart Infusion medium (first preculture) and was subsequently inoculated into 50 mL CGXII medium in 500-mL baffled Erlenmeyer flasks (second preculture) with an OD600 of 1 and cultivated overnight. The main cultures were inoculated to an OD600 of 1 in CGXII medium for production experiments. Bioreactor cultivations were performed in a 2-l DASGIP Parallel Bioreactor System (Eppendorf). The operating volume for fermentations was 1 L, the temperature set-point was maintained at 30°C, and the pH was automatically controlled at 7.0 by addition of 3 m NaOH or 3 m HCl. The dissolved oxygen was kept above 30% of saturation by using stirring-speed feedback-control ranging from 650 rpm until 1,500 rpm and a constant air-flow rate of 0.5 volume air per volume medium and min. Formation of foam was inhibited by addition of Antifoam 204 (Sigma-Aldrich). Biomass concentrations were determined by measuring the OD600. The obtained values were converted to biomass dry weight by using a previously prepared calibration curve.
Purification of Salidroside from Fermentation Broth
Salidroside was purified from the fermentation broths of S. cerevisiae and C. glutamicum cultivations. In the case of S. cerevisiae, the broth was first filtered through a 0.22-µm filter and then purified by reverse-phase chromatography using an ÄKTA Explorer (GE Healthcare). For this purpose, a 30-mL steel column, packed with preparative C18 resin (125 Å and 55–105 μm particle size; Waters) was used. Ethanol served as the eluting agent. Two mobile phases were applied (mobile phase A: water, mobile phase B: 40% v/v ethanol). During every run, a 2-mL sample was injected and the flow rate was kept constant at 3 mL min−1. In the first 40 min, 100% A was applied as washing step. Afterward, a gradient from 0% B to 100% B was applied for 60 min. Salidroside eluted between 69 and 78 min and a fraction of 15 mL (from 73 to 78 min) was collected in a 50-mL Falcon tube after each purification cycle. Afterward, the column was regenerated with 100% ethanol and equilibrated with water for the next step. The fermentation broth of C. glutamicum was centrifuged (for cell removal) followed by an ultrafiltration step (for removal of high Mr compounds) at 3,000g and for 10 min using a Centriprep YM-3 unit (Merck Millipore). Two mobile phases were used (mobile phase A: water, mobile phase B: 100% [v/v] ethanol). In every run, a 2-mL sample was injected and the flow rate was kept constant at 3 mL min−1. In the first 40 min, 100% A was applied as a washing step. Afterward, a gradient from 0% B to 100% B was applied for 150 min. Salidroside eluted between 69 and 80 min and a fraction of 9 mL was collected in a 15-mL Falcon tube after each chromatographic cycle. Afterward, the column was regenerated with 100% ethanol and equilibrated for the next step with water. After purification, the obtained Falcon tubes were dried by centrifugal vacuum concentration at 40°C in a RapidVap vacuum evaporation system with cold trap (Labconco) until complete drying.
HPLC Analysis for Quantification of Salidroside
Salidroside was quantified using a Shimadzu uHPLC system coupled to a SPD-M20A diode array detector. LC separation was carried out with a Kinetex 1.7u C18 100 Å pore size column (50 × 2.1 mm; Phenomenex) at 50°C. For elution, 0.1% (v/v) acetic acid (solvent A) and acetonitrile supplemented with 0.1% (v/v) acetic acid (solvent B) were applied as the mobile phases at a flow rate of 0.4 mL min−1. A gradient was used, where the amount of solvent B was increased stepwise: mins 0 to 6, 5%–30%; mins 6 to 7, 30%–50%; mins 7 to 8, 50 to 100%; and mins 8 to 8.5, 100% –5%. Area values were linear up to metabolite concentrations of at least 250 mg L−1.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers: KX262844.1 (RsUGT72B14, R. sachalinensis); AY547304.1 (RsUGT73B6. R. sachalinensis); NP_418458.1 (malE, E. coli); NP_415214.1 (pgm, E. coli); NP_415752.1 (galU, E. coli); NM_001180688.3 (aro10, S. cerevisiae), and NP_417484.1 (yqhD, E. coli).
Supplemental Data
The following supplementary materials are available.
Supplemental Figure S1. Compounds with highest relative areas detected in R. idaeus (var Prestige) compared with that in various Rubus species.
Supplemental Figure S2. Comparison among chromatographic, full MS results, and MS/MS results of salidroside analytical standard and the compound tentatively identified as salidroside from fraction 5a in ESI negative mode.
Supplemental Figure S3. Comparison among chromatographic, full MS results, and MS/MS results of salidroside analytical standard and the compound tentatively identified as salidroside from fraction 5a in ESI positive mode.
Supplemental Figure S4. Salidroside production from tyrosol with C. glutamicum.
Supplemental Figure S5. Chromatogram obtained during one chromatographic cycle for the purification of salidroside from the fermentation broth of S. cerevisiae or C. glutamicum.
Supplemental Table S1. Retention times and m/z values utilized for generating selected ion chromatograms for the semiquantification of the compounds reported in the bioactive fraction 5 and 7 of R. idaeus (var Prestige).
Supplemental Table S2. Strains and plasmids used in this study.
Supplemental Table S3. Oligonucleotides used for cloning and for RT-qPCR.
Supplemental Methods. Identification of a phenol against Huntington's disease in raspberries and its microbial production.
Acknowledgments
We express our gratitude to Dr. Rute Neves (Chr. Hansen A/S, Denmark), Prof. Dr. Jochen Förster and Dr. Alexey Dudnik (The Nova Nordisk Foundation Center for Biosustainability, Technical University of Denmark, Denmark), who coordinated the BacHBerry project. We also thank Prof. Dr. Ian Macreadie (Centre of Excellence for Alzheimer’s Disease Research & Care, School of Exercise, Biomedical & Health Sciences, Edith Cowan University, WA, Australia) for providing p416_GPD-GFP_AB42; Prof. Dr. Tiago Outeiro (University Medical Center Gottingen, Department of Neurodegeneration and Restorative Research, Germany) for providing W303-1A_Syn and W303-1A TU; Prof. Dr. Flaviano Giorgini (Department of Genetics and Genome Biology, University of Leicester, UK) for providing p425GAL1_HTT103Q; and Prof. Dr. Greg Petsko (Department of Biochemistry and Chemistry, Rosenstiel Basic Medical Sciences Research Center, Brandeis University, U.S.) for providing pYES_GAL1pr-FUS-GFP and pYES_CT.
Footnotes
This work was supported by the European Union Framework Program 7 “BacHBerry” (www.bachberry.eu), (Project No. FP7-613793). iNOVA4Health - UID/Multi/04462/2013, a program financially supported by Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência, through national funds and co-funded by FEDER under the PT2020 Partnership Agreement is acknowledged. This work was also supported by Fundação para a Ciência e Tecnologia (SFRH/BD/116597/2016 to R.R-R and IF/01097/2013 to C.N.d.S.). A.B., P.F., J.O., A.R.S., N.F., M.S., and I.R. thank the Portuguese Foundation for Science and Technology under the scope of the strategic funding of UID/BIO/04469 unit, COMPETE 2020 (POCI-01-0145-FEDER-006684) and BioTecNorte operation (NORTE-01-0145-FEDER-000004) funded by the European Regional Development Fund under the scope of Norte2020 - Programa Operacional Regional do Norte. M.B. and J.M. acknowledge the financial support of the BOOST fund PNP-EXPRESS kindly funded by the Bioeconomy Science Center (BioSC). The scientific activities of the BioSC were financially supported by the Ministry of Innovation, Science and Research of the German State of North Rhine-Westphalia within the framework of the NRW Strategieprojekt BioSC (No. 313/323-400-00213). J.M. would also like to thank the BMBF-funded project “BioLiSy” (Bioeconomic Lignan Synthesis, funding code 031A554) for financial support. D.M.S., A.F., and J.A.W. acknowledge additional part funding from the Rural & Environment Science & Analytical Services Division of the Scottish Government.
References
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman J, Smith JA, Struhl K (1992) Short protocols in molecular biology. John Wiley & Sons, New York [Google Scholar]
- Bai Y, Bi H, Zhuang Y, Liu C, Cai T, Liu X, Zhang X, Liu T, Ma Y (2014) Production of salidroside in metabolically engineered Escherichia coli. Sci Rep 4: 6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensalem J, Dal-Pan A, Gillard E, Calon F, Pallet V (2015) Protective effects of berry polyphenols against age-related cognitive impairment. Nutr Aging (Amst) 3: 89–106 [Google Scholar]
- Bharadwaj P, Martins R, Macreadie I (2010) Yeast as a model for studying Alzheimer’s disease. FEMS Yeast Res 10: 961–969 [DOI] [PubMed] [Google Scholar]
- Braga A, Oliveira J, Silva R, Ferreira P, Rocha I, Kallscheuer N, Marienhagen J, Faria N (2018) Impact of the cultivation strategy on resveratrol production from glucose in engineered Corynebacterium glutamicum. J Biotechnol 265: 70–75 [DOI] [PubMed] [Google Scholar]
- Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, Bird ED, Beal MF (1997) Oxidative damage and metabolic dysfunction in Huntington’s disease: Selective vulnerability of the basal ganglia. Ann Neurol 41: 646–653 [DOI] [PubMed] [Google Scholar]
- Browne SE, Ferrante RJ, Beal MF (1999) Oxidative stress in Huntington’s disease. Brain Pathol 9: 147–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, et al. (2009) The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55: 611–622 [DOI] [PubMed] [Google Scholar]
- Chung D, Kim SY, Ahn J-H (2017) Production of three phenylethanoids, tyrosol, hydroxytyrosol, and salidroside, using plant genes expressing in Escherichia coli. Sci Rep 7: 2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayalu P, Albin RL (2015) Huntington disease: Pathogenesis and treatment. Neurol Clin 33: 101–114 [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N (1997) Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science 277: 1990–1993 [DOI] [PubMed] [Google Scholar]
- Dudnik A, Almeida AF, Andrade R, Avila B, Bañados P, Barbay D, Bassard J-E, Benkoulouche M, Bott M, Braga A, Breitel D, Brennan R, et al. (2018) BacHBerry: BACterial Hosts for production of Bioactive phenolics from bERRY fruits. Phytochem Rev 17: 291–326 [Google Scholar]
- Eggeling L, Bott M, editors (2005) Handbook of Corynebacterium glutamicum. CRCPress, Taylor & Francis Group, Boca Raton, Florida
- Eichenberger M, Lehka BJ, Folly C, Fischer D, Martens S, Simón E, Naesby M (2017) Metabolic engineering of Saccharomyces cerevisiae for de novo production of dihydrochalcones with known antioxidant, antidiabetic, and sweet tasting properties. Metab Eng 39: 80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberger M, Hansson A, Fischer D, Dürr L, Naesby M (2018) De novo biosynthesis of anthocyanins in Saccharomyces cerevisiae. FEMS Yeast Res 18 [DOI] [PubMed] [Google Scholar]
- Flick JS, Johnston M (1990) Two systems of glucose repression of the GAL1 promoter in Saccharomyces cerevisiae. Mol Cell Biol 10: 4757–4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Zhou R, You X, Luo F, He H, Chang X, Zhu L, Ding X, Yan T (2016) Salidroside suppresses inflammation in a D-galactose-induced rat model of Alzheimer’s disease via SIRT1/NF-κB pathway. Metab Brain Dis 31: 771–778 [DOI] [PubMed] [Google Scholar]
- Gietz RD, Schiestl RH, Willems AR, Woods RA (1995) Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11: 355–360 [DOI] [PubMed] [Google Scholar]
- Gomes RA, Vicente Miranda H, Silva MS, Graça G, Coelho AV, Ferreira AE, Cordeiro C, Freire AP (2006) Yeast protein glycation in vivo by methylglyoxal. Molecular modification of glycolytic enzymes and heat shock proteins. FEBS J 273: 5273–5287 [DOI] [PubMed] [Google Scholar]
- Goswami A, Dikshit P, Mishra A, Mulherkar S, Nukina N, Jana NR (2006) Oxidative stress promotes mutant huntingtin aggregation and mutant huntingtin-dependent cell death by mimicking proteasomal malfunction. Biochem Biophys Res Commun 342: 184–190 [DOI] [PubMed] [Google Scholar]
- Halvorsen BL, Carlsen MH, Phillips KM, Bøhn SK, Holte K, Jacobs DR Jr., Blomhoff R (2006) Content of redox-active compounds (i.e. antioxidants) in foods consumed in the United States. Am J Clin Nutr 84: 95–135 [DOI] [PubMed] [Google Scholar]
- Hazelwood LA, Daran J-M, van Maris AJ, Pronk JT, Dickinson JR (2008) The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microbiol 74: 2259–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M. (2006) Towards bacterial strains overproducing L-tryptophan and other aromatics by metabolic engineering. Appl Microbiol Biotechnol 69: 615–626 [DOI] [PubMed] [Google Scholar]
- Indo HP, Yen H-C, Nakanishi I, Matsumoto K, Tamura M, Nagano Y, Matsui H, Gusev O, Cornette R, Okuda T, et al. (2015) A mitochondrial superoxide theory for oxidative stress diseases and aging. J Clin Biochem Nutr 56: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarboe LR. (2011) YqhD: A broad-substrate range aldehyde reductase with various applications in production of biorenewable fuels and chemicals. Appl Microbiol Biotechnol 89: 249–257 [DOI] [PubMed] [Google Scholar]
- Jiang J, Yin H, Wang S, Zhuang Y, Liu S, Liu T, Ma Y (2018) Metabolic engineering of Saccharomyces cerevisiae for high-level production of salidroside from glucose. J Agric Food Chem 66: 4431–4438 [DOI] [PubMed] [Google Scholar]
- Jones JA, Vernacchio VR, Collins SM, Shirke AN, Xiu Y, Englaender JA, Cress BF, McCutcheon CC, Linhardt RJ, Gross RA, et al. (2017) Complete biosynthesis of anthocyanins using E. coli polycultures. MBio 8: e00621–e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S, Tardiff DF, Han H, Divya K, Zhong Q, Maquat LE, Bosco DA, Hayward LJ, Brown RH Jr., Lindquist S, et al. (2011) A yeast model of FUS/TLS-dependent cytotoxicity. PLoS Biol 9: e1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallscheuer N, Vogt M, Stenzel A, Gätgens J, Bott M, Marienhagen J (2016) Construction of a Corynebacterium glutamicum platform strain for the production of stilbenes and (2S)-flavanones. Metab Eng 38: 47–55 [DOI] [PubMed] [Google Scholar]
- Kallscheuer N, Vogt M, Bott M, Marienhagen J (2017) Functional expression of plant-derived O-methyltransferase, flavanone 3-hydroxylase, and flavonol synthase in Corynebacterium glutamicum for production of pterostilbene, kaempferol, and quercetin. J Biotechnol 258: 190–196 [DOI] [PubMed] [Google Scholar]
- Kallscheuer N, Classen T, Drepper T, Marienhagen J (2019) Production of plant metabolites with applications in the food industry using engineered microorganisms. Curr Opin Biotechnol 56: 7–17 [DOI] [PubMed] [Google Scholar]
- Keilhauer C, Eggeling L, Sahm H (1993) Isoleucine synthesis in Corynebacterium glutamicum: Molecular analysis of the ilvB-ilvN-ilvC operon. J Bacteriol 175: 5595–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitade Y, Hashimoto R, Suda M, Hiraga K, Inui M (2018) Production of 4-hydroxybenzoic acid by an aerobic growth-arrested bioprocess using metabolically engineered Corynebacterium glutamicum. Appl Environ Microbiol 84: e02587–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure T, Inui M (2018) Recent advances in metabolic engineering of Corynebacterium glutamicum for bioproduction of value-added aromatic chemicals and natural products. Appl Microbiol Biotechnol 102: 8685–8705 [DOI] [PubMed] [Google Scholar]
- Koopman F, Beekwilder J, Crimi B, van Houwelingen A, Hall RD, Bosch D, van Maris AJ, Pronk JT, Daran J-M (2012) De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Microb Cell Fact 11: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan X, Chang K, Zeng L, Liu X, Qiu F, Zheng W, Quan H, Liao Z, Chen M, Huang W, et al. (2013) Engineering salidroside biosynthetic pathway in hairy root cultures of Rhodiola crenulata based on metabolic characterization of tyrosine decarboxylase. PLoS One 8: e75459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat Rev Neurosci 14: 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehka BJ, Eichenberger M, Bjørn-Yoshimoto WE, Vanegas KG, Buijs N, Jensen NB, Dyekjær JD, Jenssen H, Simon E, Naesby M (2017) Improving heterologous production of phenylpropanoids in Saccharomyces cerevisiae by tackling an unwanted side reaction of Tsc13, an endogenous double-bond reductase. FEMS Yeast Res 17: fox004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CG, Fowler ZL, Hueller T, Schaffer S, Koffas MA (2011) High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol 77: 3451–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Zheng J, Wang Z, Zhao L, Pei Y (1980) Study on the native Rhodiola sachalinensis: The extraction, separation and identification of salidroside. Chin Tradit Herbal Drugs 11: 147–148 [Google Scholar]
- Luttik MA, Vuralhan Z, Suir E, Braus GH, Pronk JT, Daran JM (2008) Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: Quantification of metabolic impact. Metab Eng 10: 141–153 [DOI] [PubMed] [Google Scholar]
- Ma L-Q, Liu B-Y, Gao D-Y, Pang X-B, Lü S-Y, Yu H-S, Wang H, Yan F, Li Z-Q, Li Y-F, et al. (2007) Molecular cloning and overexpression of a novel UDP-glucosyltransferase elevating salidroside levels in Rhodiola sachalinensis. Plant Cell Rep 26: 989–999 [DOI] [PubMed] [Google Scholar]
- MacDonald M; The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72: 971–983 [DOI] [PubMed] [Google Scholar]
- Macedo D, Tavares L, McDougall GJ, Vicente Miranda H, Stewart D, Ferreira RB, Tenreiro S, Outeiro TF, Santos CN (2015) (Poly)phenols protect from α-synuclein toxicity by reducing oxidative stress and promoting autophagy. Hum Mol Genet 24: 1717–1732 [DOI] [PubMed] [Google Scholar]
- Marienhagen J, Bott M (2013) Metabolic engineering of microorganisms for the synthesis of plant natural products. J Biotechnol 163: 166–178 [DOI] [PubMed] [Google Scholar]
- Marienhagen J, Kennerknecht N, Sahm H, Eggeling L (2005) Functional analysis of all aminotransferase proteins inferred from the genome sequence of Corynebacterium glutamicum. J Bacteriol 187: 7639–7646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Giorgini F (2011) Modeling Huntington disease in yeast: Perspectives and future directions. Prion 5: 269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Primers 1: 15056. [DOI] [PubMed] [Google Scholar]
- McDougall GJ, Conner S, Pereira-Caro G, Gonzalez-Barrio R, Brown EM, Verrall S, Stewart D, Moffet T, Ibars M, Lawther R, et al. (2014) Tracking (Poly)phenol components from raspberries in ileal fluid. J Agric Food Chem 62: 7631–7641 [DOI] [PubMed] [Google Scholar]
- Menezes R, Tenreiro S, Macedo D, Santos CN, Outeiro TF (2015) From the baker to the bedside: Yeast models of Parkinson’s disease. Microb Cell 2: 262–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen MD, Buron LD, Salomonsen B, Olsen CE, Hansen BG, Mortensen UH, Halkier BA (2012) Microbial production of indolylglucosinolate through engineering of a multi-gene pathway in a versatile yeast expression platform. Metab Eng 14: 104–111 [DOI] [PubMed] [Google Scholar]
- Milke L, Aschenbrenner J, Marienhagen J, Kallscheuer N (2018) Production of plant-derived polyphenols in microorganisms: Current state and perspectives. Appl Microbiol Biotechnol 102: 1575–1585 [DOI] [PubMed] [Google Scholar]
- Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M (2016) Oxidative stress in neurodegenerative diseases. Mol Neurobiol 53: 4094–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nile SH, Park SW (2014) Edible berries: Bioactive components and their effect on human health. Nutrition 30: 134–144 [DOI] [PubMed] [Google Scholar]
- Oliveira AV, Vilaça R, Santos CN, Costa V, Menezes R (2017) Exploring the power of yeast to model aging and age-related neurodegenerative disorders. Biogerontology 18: 3–34 [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S (2003) Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 302: 1772–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla L, Morbach S, Krämer R, Agosin E (2004) Impact of heterologous expression of Escherichia coli UDP-glucose pyrophosphorylase on trehalose and glycogen synthesis in Corynebacterium glutamicum. Appl Environ Microbiol 70: 3845–3854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Pousada C, Nevitt T, Menezes R (2005) The yeast stress response. Role of the Yap family of b-ZIP transcription factors. The PABMB Lecture delivered on 30 June 2004 at the 29th FEBS Congress in Warsaw. FEBS J 272: 2639–2647 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mateos A, Heiss C, Borges G, Crozier A (2014) Berry (poly)phenols and cardiovascular health. J Agric Food Chem 62: 3842–3851 [DOI] [PubMed] [Google Scholar]
- Rosado-Ramos R, Godinho-Pereira J, Figueira I, Jardim C, Garcia G, Menezes R (2018) Exploring the benefits of cellular models to uncover bioactive polyphenols for neurodegeneration. Curr Pharm Des 24: 2076–2106 [DOI] [PubMed] [Google Scholar]
- Seeram NP. (2012) Emerging research supporting the positive effects of berries on human health and disease prevention. J Agric Food Chem 60: 5685–5686 [DOI] [PubMed] [Google Scholar]
- Tavares L, Fortalezas S, Carrilho C, McDougall G, Stewart D, Ferreira R, Santos C (2010) Antioxidant and antiproliferative properties of strawberry tree tissues. J Berry Res 1: 3–12 [Google Scholar]
- Torrens-Spence MP, Pluskal T, Li F-S, Carballo V, Weng J-K (2018) Complete pathway elucidation and heterologous reconstitution of Rhodiola salidroside biosynthesis. Mol Plant 11: 205–217 [DOI] [PubMed] [Google Scholar]
- Tsuda T. (2016) Recent progress in anti-obesity and anti-diabetes effect of berries. Antioxidants 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas K, Larsen A, Eichenberger M, Fischer D, Mortensen U, Naesby M (2018) Indirect and direct routes to C-glycosylated flavones in Saccharomyces cerevisiae. Microb Cell Fact 17: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Vega IE, Brundin P (2017) Oxidative stress in neurodegenerative disease: Causation or association? Oncotarget 8: 10777–10778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuralhan Z, Luttik MA, Tai SL, Boer VM, Morais MA, Schipper D, Almering MJ, Kötter P, Dickinson JR, Daran J-M, et al. (2005) Physiological characterization of the ARO10-dependent, broad-substrate-specificity 2-oxo acid decarboxylase activity of Saccharomyces cerevisiae. Appl Environ Microbiol 71: 3276–3284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, He H, Chen L, Zhang W, Zhang X, Chen J (2015) Protective effects of salidroside in the MPTP/MPP+-induced model of Parkinson’s disease through ROS-NO-related mitochondrion pathway. Mol Neurobiol 51: 718–728 [DOI] [PubMed] [Google Scholar]
- Weissborn AC, Liu Q, Rumley MK, Kennedy EP (1994) UTP: Alpha-D-glucose-1-phosphate uridylyltransferase of Escherichia coli: Isolation and DNA sequence of the galU gene and purification of the enzyme. J Bacteriol 176: 2611–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham S, Outeiro TF, DeVit MJ, Lindquist SL, Muchowski PJ (2003) Yeast genes that enhance the toxicity of a mutant huntingtin fragment or α-synuclein. Science 302: 1769–1772 [DOI] [PubMed] [Google Scholar]
- Wu S, Zu Y, Wu M (2003) High yield production of salidroside in the suspension culture of Rhodiola sachalinensis. J Biotechnol 106: 33–43 [DOI] [PubMed] [Google Scholar]
- Xiao L, Li H, Zhang J, Yang F, Huang A, Deng J, Liang M, Ma F, Hu M, Huang Z (2014) Salidroside protects Caenorhabditis elegans neurons from polyglutamine-mediated toxicity by reducing oxidative stress. Molecules 19: 7757–7769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu C, Han A, Feng P, Su Z (1998) Strategies for the improvement of salidroside production in cell suspension cultures of Rhodiola sachalinensis. Plant Cell Rep 17: 288–293 [DOI] [PubMed] [Google Scholar]
- Yang B, Kortesniemi M (2015) Clinical evidence on potential health benefits of berries. Curr Opin Food Sci 2: 36–42 [Google Scholar]
- Yu H-S, Ma L-Q, Zhang J-X, Shi G-L, Hu Y-H, Wang Y-N (2011) Characterization of glycosyltransferases responsible for salidroside biosynthesis in Rhodiola sachalinensis. Phytochemistry 72: 862–870 [DOI] [PubMed] [Google Scholar]
- Yu S, Liu M, Gu X, Ding F (2008) Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol 28: 1067–1078 [DOI] [PubMed] [Google Scholar]
- Zarei S, Carr K, Reiley L, Diaz K, Guerra O, Altamirano PF, Pagani W, Lodin D, Orozco G, Chinea A (2015) A comprehensive review of amyotrophic lateral sclerosis. Surg Neurol Int 6: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z (2007) Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol 564: 18–25 [DOI] [PubMed] [Google Scholar]
- Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G, Wang Z (2010) Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int 57: 547–555 [DOI] [PubMed] [Google Scholar]
- Zhou X, Wu Y, Wang X, Liu B, Xu H (2007) Salidroside production by hairy roots of Rhodiola sachalinensis obtained after transformation with Agrobacterium rhizogenes. Biol Pharm Bull 30: 439–442 [DOI] [PubMed] [Google Scholar]